Abstract
Background:
Prevalence of metabolic syndrome (MetS) in HIV-infected patients is very limited in the Ghanaian setting and may vary across the globe by the different study populations and criteria used.
Aim:
We investigated the prevalence of MetS among HIV-infected patients receiving highly active antiretroviral therapy (HAART) at the St. Dominic Hospital, Akwatia, Ghana.
Patients and Methods:
This cross-sectional study recruited 433 HIV-infected patients (294 on HAART and 139 HAART-naïve) from the period of February 2013 to December 2013. Information on the demographic, clinical, anthropometric characteristics were obtained and lipid profile for each patient was assessed. MetS was assessed based on the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III), World Health Organization (WHO) and International Diabetes Federation (IDF) criteria.
Results:
The prevalence of MetS was 24.5% according to WHO criteria, 48.3% by NCEP-ATP III criteria, and 42.3% by IDF criteria. In general, participants on HAART were significantly associated with higher prevalence of MetS compared to those without HAART (P < 0.05) irrespective of the criteria used. Prevalence of clustering components of MetS was significantly higher among those on HAART when risk scores of 2 and above were used compared with those not on HAART (P < 0.05).
Conclusion:
HAART recipient developed MetS as indicated by dyslipidemia, high blood pressure, and abnormal body fat. It is incumbent on health giver to incorporate MetS assessment as a part of treatment and management plan in patients receiving HAART.
Keywords: Highly active antiretroviral therapy, HIV patient, metabolic syndrome, prevalence
INTRODUCTION
The introduction and widespread use of highly active antiretroviral therapy (HAART) in the mid 1990's has led to a dramatic decline in immunodeficiency-related events including causes of death in HIV-infected individuals.1,2,3 Long-term toxicity has been recognized with a variety of metabolic abnormalities including dyslipidemia, insulin resistance, and changes in body fat being frequently associated with protease inhibitor-based therapy.4 Metabolic syndrome (MetS) is an aggregation of central obesity and metabolic abnormalities that confers an increased risk of cardiovascular disease and type 2 diabetes.5,6
From previous studies, the prevalence of MetS among HIV-infected patients ranges from 17.0% to 45.4%.7,8,9 The varying rate of prevalence has been associated with difference in criteria used and sampled population.9
HAART-naïve patients have been shown to exhibit dyslipidemia, with increased levels of triglycerides (TG) and decreased total cholesterol and high-density lipoprotein (HDL) cholesterol.10 When on HAART, a characteristic dyslipidemic pattern with a further increase in TG, total cholesterol, and low-density lipoprotein cholesterol has been described.8 MetS encompasses disturbances in glucose, insulin, and lipid metabolism associated with abdominal obesity.11 There is a growing concern that metabolic complications associated with HIV-infection and HAART use may lead to increased risk for cardiovascular events; hence, the emergence of CVD as a cause of morbidity and death in the HIV-infected population.12,13
Currently, there are limited studies published in the literature investigating the association between HAART use and MetS in sub-Saharan Africa. With the scaling up of HAART use in Ghana, such adverse effects need to be investigated to formulate policy guidelines for people giving care to HIV-infected patients. The present study aims to assess the prevalence of MetS in HIV-infected patients on HAART and HAART-naïve patients at the St. Dominic Hospital, Akwatia in Ghana.
PATIENTS AND METHODS
This cross-sectional study was conducted among HIV-infected patients seeking HIV care at the HIV clinic of the St. Dominic Hospital, Akwatia, Ghana from February 2013 to December 2013.
Four hundred and thirty three HIV-infected subjects aged 18 years or above were recruited for the study. They consisted of 294 subjects who have been on a combination of HAART (3-lamivudine [3TC]/nevirapine [NVP]/tenofovir [TDF], 3TC/efavirenz [EFV]/TDF, 3TC/TDF/lopinavir, zidovudine [AZT]/3TC/EFV, and AZT/3TC/NVP) for at least 3 months and 139 who were HAART-naïve. All participants completed a written informed consent form and a pretested questionnaire. The content of the questionnaire included information on their sociodemographics, duration of infection, current HIV drug regimen, duration of drug regimen, and smoking status. Ethical approval for the study was granted by the Committee on Human Research, Publications and Ethics (CHRPE), Kwame Nkrumah University of Science and Technology (KNUST).
All HIV-infected subjects who were 18 years or above who consented to be part of the study were recruited. Subjects with documented medical history of comorbidities such as diabetes, tuberculosis, and hypertension were excluded from the study. Pregnant women were also excluded from the study.
Five milliliters (5 mL) of blood samples was be drawn into vacutainer® after an overnight fast. The serum was then be separated immediately, and the serum was used to assay for total cholesterol, HDL-cholesterol, and TG using the Mindray BC200 Chemistry auto-analyzer.
Blood pressure (BP) was measured using an automated BP monitor (Omron HEM711DLX, UK) after patients were seated for 10 min. The weight of the subjects was measured in the upright position to the nearest 0.5 kg using a weight measuring scale (Seca, Hamburg, Deutschland, Germany). Height was measured without shoes to the nearest 0.1 m using a well-calibrated wall-mounted rule. Body mass index (BMI) was calculated based on weight in kilograms divided by the square of the height in meters (kg/m2). Waist circumference (WC) was measured to the nearest 0.1 cm horizontally at the narrowest point between the lower end of the rib cage and iliac crest. Hip circumference was measured to the nearest 0.1 cm at the greatest horizontal circumference below the iliac crest at the level of greater trochanter (the widest portion on the buttocks). Waist and hip circumference were measured with an inelastic tape measure. Waist to hip ratio (WHR) was calculated from the waist and hip circumference.
BMI were classified based on the World Health Organization (WHO) definition for adults as underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), and obese (>30 kg/m2) (WHO 2000).14 Male subjects with a WC of <94, 94–101.9, and ≥102 cm were classified as normal weight, overweight, and obese, respectively, while female subjects were classified in the same obesity categories on the basis of WC < 80, 80–87.9, and ≥88 cm. Male subjects with WHR <0.90, 0.90–0.99, and ≥1.0 were classified as normal weight, overweight, or obese, respectively, while female subjects were classified in the same categories on the basis of WHR of <0.80, 0.80–0.84, and ≥0.85 (WHO, 2000; Croft et al., 1995).14
National Cholesterol Education Program (NCEP), Adult Treatment Panel III (ATP III) criteria:
According to the NCEP-ATP III guidelines (2005). MetS was defined as having three or more of the following criteria: (i) Waist measurement ≥80 cm for women and ≥90 cm for men; (ii) TG level of 1.69 mmol/L; (iii) HDL-C of 1.29 mmol/L for women and 1.03 mmol/L for men HDL-C; (iv) elevated BP (systolic ≥130 mmHg or diastolic ≥85 mm Hg using the average of two seated measurements); and (v) fasting glucose = 5.56 mmol/L.
Statistical analysis
Data were entered into Microsoft Excel 2010 and cleaned for double entries. Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad Software, San Diego California, USA, www.graphpad.com). Categorical variables are presented in frequency (proportion) and test of association between proportions was done using Chi-square test. Continuous variables were tested using Student's t-test expressed as a mean ± standard deviation for all levels of comparison, a P < 0.05 was considered as statistically significant.
Ethical consideration
Ethical approval was sought for and granted by the CHRPE, KNUST. Verbal informed consent was obtained from all subjects.
RESULTS
Sociodemographic characteristics of study participants stratified by HAART status are as shown in Table 1. Out of the total of 433 study subjects, 179 (41.3%) were males with 254 (58.7%) being females. The majority (71.4%) of the subjects were married with 4.4% being widowed. A high number 202 (46.7%) of the patients had between 1 and 5 years duration of infection. Out of this, 61.2% were on HAART compared 15.8% HAART-naïve (P < 0.0001). The common combination of drugs used by patients was 3TC/EFV/TDF (33.3%), AZT/3TC/EFV (29.6%), and AZT/3TC/NVP (20.6%). Most (98.3%) of the study patients had never smoked cigarettes before while 1.2% and 0.5% were current and past smokers, respectively [Table 1].
Table 1.
Sociodemographic characteristics of study population stratified by highly active antiretroviral therapy status
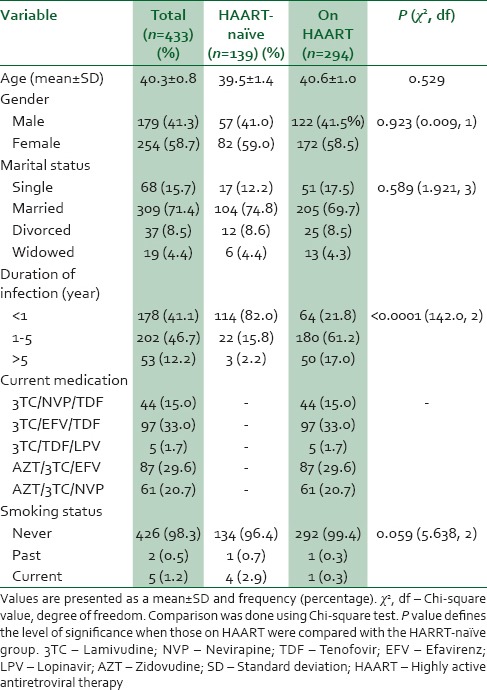
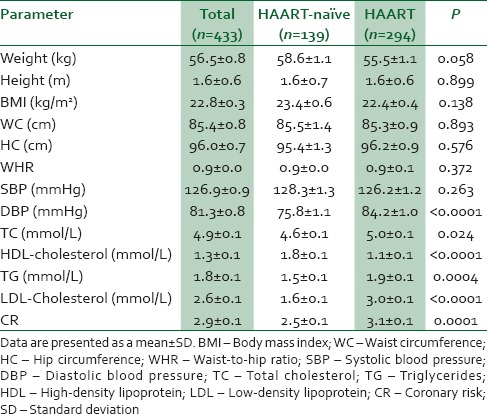
Table 2 shows anthropometric and biochemical measurement of study participants stratified by treatment status. There was no statistically significant different between anthropometric measurements of patients on HAART and their HAART-naïve counterpart (P > 0.05). The mean diastolic BP as estimated for patients on HAART was significantly higher compared to HAART-naïve (P < 0.0001). Dyslipidemia was significantly associated with the patient on HAART compared to HAART-naïve (P < 0.05). Patient on HAART had an increased coronary risk compared to their HAART-naïve counterparts (P = 0.0001).
Table 2.
Anthropometric and biochemical characteristics of the study population stratified by highly active antiretroviral therapy status
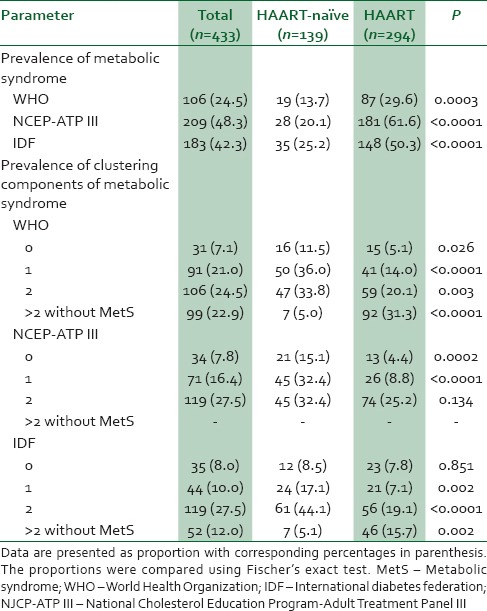
The prevalence of MetS among the study population was 24.5%, 48.3%, and 42.3% for WHO, NCEP-ATP III, and IDF criteria, respectively. Irrespective of the classification criterion the study observed a significantly higher prevalence of MetS among patients on HAART compared to their HAART-naïve counterparts. The prevalence of clustering components of MetS was also significantly higher in the HAART study participants when risk scores of 2 or above were compared with those not on HAART (P < 0.05) [Table 3].
Table 3.
Prevalence of metabolic syndrome and metabolic risk scores among the population stratified by treatment profile
DISCUSSION
MetS involves a cluster of risk factors leading to cardiovascular diseases and other health-related morbidities.15 Though the introduction of HAART has proved an indubitable success, the prevalence of insulin resistance, BP, fat redistribution, and dyslipidemia has markedly increased after its global scaling up.16 We investigated the prevalence of MetS among HIV-infected patients receiving HAART at the St. Dominic Hospital, Akwatia, Ghana.
Accumulative evidence indicates that MetS could be associated with different HAART use.17 However, reports are inconsistent with some published research studies.18 Despite these disparities, this study found a high prevalence of MetS among patients on HAART compared to their HAART-naïve counterparts. The finding of this study is consistent with findings by Wand et al.17
The strength of this study is the comparative use of the three different criteria to assess the prevalence of MetS. The trend of the prevalence of MetS was significantly higher using NCEP-ATP III criteria (48.3%) followed by IDF (42.3%) and then WHO (24.5%) criteria [Table 3]. Prevalence per the NCEP-ATP III criteria observed in this study is extremely higher compared to 17% reported by Jericó et al.,7 18%,19 and 45.4%.20 Similar disparity was observed with several other published.18,21
Again some studies also reported a prevalence of 14%19 and 11.4%22 per the IDF criteria which is far lower compared to our prevalence (42.3%). A current work done among Cameroonian patients observed a prevalence of 32.8% according to IDF and 30.2% according to NCEP-ATP III.23 Although there is a scarcity of data on the use of WHO criteria in assessing MetS in HIV patients, this study found a prevalence rate to be 24.5%. The possible explanation for the varying prevalence rate of MetS in this study and previously published work may be explained by the different criteria, duration of exposure on HAART, and the different sampled population size used.9
The use of combination of drugs such as 3TC/EFV/TDF and AZT/3TC/EFV and AZT/3TC/NVP by HIV patients in this study buttress the explanation made by Fiala et al.24 that HAART such as zidovudine, efavirenz, and indinavir induce toxicity through induction of cardiomyocyte and endothelial cell apoptosis leading to endothelial dysfunction and vascular damage and hence MetS.24 Some observed that HAART may increase resting energy expenditure, fat oxidation, and food intake in patients with HAART-associated lipodystrophy.25
The major components of MetS using NCEP-ATP III are known to include three or more of factors such as abdominal obesity, hypertriglyceridemia, low HDL cholesterol, high BP, and high fasting glucose. Coincidently, the most significant component of MetS among patients on HAART was high BP, hypertriglyceridemia, hypercholesterolemia, and low HDL cholesterol which in no doubt confirmed that high prevalence observed with NCEP-ATP III criteria. This pattern was largely similar to that demonstrated in other studies of HIV-infected patients.7,26,27
Increase cardiovascular risk among patients on HAART as assessed by Framingham equation buttress our findings that HIV-infected subjects on HAART may be associated with increased prevalence of MetS. Our study demonstrated that MetS could range from 24.5% to 48.3%. Compared with other studies that observed a pool of prevalence range from 17% to 45.5%7,8,9 our study reported the transient significant difference.
CONCLUSION
The results of this study indicate that the prevalence rate of MetS is high among HIV patients on HAART. HAART patients are not secured from the emerging epidemic of MetS as demonstrated by their hypertension, hypertriglyceridemia, and low HDL-cholesterol in the present study. The high prevalence of Mets along with additional data from other studies supports the notion that HAART may be an independent predisposing factor of MetS in HIV-infected patients. Not only will clinicians need the outcome of the study to guide the choice of drugs used for treating HIV patients but also the need for HIV patients to adhere to dietary adjustment and modification to help reduce future risk of obesity, which is a component of MetS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Krentz HB, Kliewer G, Gill MJ. Changing mortality rates and causes of death for HIV-infected individuals living in Southern Alberta, Canada from 1984 to 2003. HIV Med. 2005;6:99–106. doi: 10.1111/j.1468-1293.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 2.Pacheco AG, Tuboi SH, May SB, Moreira LF, Ramadas L, Nunes EP, et al. Temporal changes in causes of death among HIV-infected patients in the HAART era in Rio de Janeiro, Brazil. J Acquir Immune Defic Syndr. 2009;51:624–30. doi: 10.1097/QAI.0b013e3181a4ecf5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grinsztejn B, Luz PM, Pacheco AG, Santos DV, Velasque L, Moreira RI, et al. Changing mortality profile among HIV-infected patients in Rio de Janeiro, Brazil: Shifting from AIDS to non-AIDS related conditions in the HAART era. PLoS One. 2013;8:e59768. doi: 10.1371/journal.pone.0059768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–8. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan S, Schouten JT, Atkinson B, Brown T, Wohl D, McComsey GA, et al. Metabolic syndrome before and after initiation of antiretroviral therapy in treatment-naive HIV-infected individuals. J Acquir Immune Defic Syndr. 2012;61:381–9. doi: 10.1097/QAI.0b013e3182690e3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract 2014. 2014:943162. doi: 10.1155/2014/943162. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Jericó C, Knobel H, Montero M, Ordoñez-Llanos J, Guelar A, Gimeno JL, et al. Metabolic syndrome among HIV-infected patients: Prevalence, characteristics, and related factors. Diabetes Care. 2005;28:132–7. doi: 10.2337/diacare.28.1.132. [DOI] [PubMed] [Google Scholar]
- 8.Hansen BR, Petersen J, Haugaard SB, Madsbad S, Obel N, Suzuki Y, et al. The prevalence of metabolic syndrome in Danish patients with HIV infection: The effect of antiretroviral therapy. HIV Med. 2009;10:378–87. doi: 10.1111/j.1468-1293.2009.00697.x. [DOI] [PubMed] [Google Scholar]
- 9.Worm SW, Friis-Møller N, Bruyand M, D’Arminio Monforte A, Rickenbach M, Reiss P, et al. High prevalence of the metabolic syndrome in HIV-infected patients: Impact of different definitions of the metabolic syndrome. AIDS. 2010;24:427–35. doi: 10.1097/QAD.0b013e328334344e. [DOI] [PubMed] [Google Scholar]
- 10.Souza SJ, Luzia LA, Santos SS, Rondó PH. Lipid profile of HIV-infected patients in relation to antiretroviral therapy: A review. Rev Assoc Med Bras. 2013;59:186–98. doi: 10.1016/j.ramb.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, et al. American Heart Association. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 12.Biron A, Bobin-Dubigeon C, Volteau C, Piroth L, Perré P, Leport C, et al. Metabolic syndrome in French HIV-infected patients: Prevalence and predictive factors after 3 years of antiretroviral therapy. AIDS Res Hum Retroviruses. 2012;28:1672–8. doi: 10.1089/AID.2012.0048. [DOI] [PubMed] [Google Scholar]
- 13.Feleke Y, Fekade D, Mezegebu Y. Prevalence of highly active antiretroviral therapy associated metabolic abnormalities and lipodystrophy in HIV infected patients. Ethiop Med J. 2012;50:221–30. [PubMed] [Google Scholar]
- 14.World Health Organization: Obesity: Preventing and managing the global epidemic: Report of a WHO consultation. (1-253).World Health Org Tech Rep Ser. 2000;894:I–xii. [PubMed] [Google Scholar]
- 15.Alberti KGMM, Zimmet P, Shaw J. Metabolic syndrome - A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabetic Medicine. 2006;23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 16.Barbaro G. Metabolic and cardiovascular complications of highly active antiretroviral therapy for HIV infection. Curr HIV Res. 2006;4:79–85. doi: 10.2174/157016206775197664. [DOI] [PubMed] [Google Scholar]
- 17.Wand H, Calmy A, Carey DL, Samaras K, Carr A, Law MG, et al. Metabolic syndrome, cardiovascular disease and type 2 diabetes mellitus after initiation of antiretroviral therapy in HIV infection. AIDS. 2007;21:2445–53. doi: 10.1097/QAD.0b013e3282efad32. [DOI] [PubMed] [Google Scholar]
- 18.Mondy K, Overton ET, Grubb J, Tong S, Seyfried W, Powderly W, et al. Metabolic syndrome in HIV-infected patients from an urban, midwestern US outpatient population. Clin Infect Dis. 2007;44:726–34. doi: 10.1086/511679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samaras K, Wand H, Law M, Emery S, Cooper D, Carr A. Prevalence of metabolic syndrome in HIV-infected patients receiving highly active antiretroviral therapy using International Diabetes Foundation and Adult Treatment Panel III criteria: Associations with insulin resistance, disturbed body fat compartmentalization, elevated C-reactive protein, and [corrected] hypoadiponectinemia. Diabetes Care. 2007;30:113–9. doi: 10.2337/dc06-1075. [DOI] [PubMed] [Google Scholar]
- 20.Gazzaruso C, Sacchi P, Garzaniti A, Fratino P, Bruno R, Filice G. Prevalence of metabolic syndrome among HIV patients. Diabetes Care. 2002;25:1253–4. doi: 10.2337/diacare.25.7.1253. [DOI] [PubMed] [Google Scholar]
- 21.Estrada V, Martínez-Larrad MT, González-Sánchez JL, de Villar NG, Zabena C, Fernández C, et al. Lipodystrophy and metabolic syndrome in HIV-infected patients treated with antiretroviral therapy. Metabolism. 2006;55:940–5. doi: 10.1016/j.metabol.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 22.Bernal E, Masiá M, Padilla S, Martín-Hidalgo A, Gutiérrez F. Prevalence and characteristics of metabolic syndrome among HIV-infected patients from a Mediterranean cohort. Med Clin (Barc) 2007;128:172–5. doi: 10.1157/13098391. [DOI] [PubMed] [Google Scholar]
- 23.Dimodi H, Etame L, Nguimkeng B, Mbappe F, Ndoe N, Tchinda J, et al. Prevalence of metabolic syndrome in hiv-infected cameroonian patients. World Journal of AIDS. 2014;4:85–92. [doi: 10.4236/wja.2014.41011] [Google Scholar]
- 24.Fiala M, Murphy T, MacDougall J, Yang W, Luque A, Iruela-Arispe L, et al. HAART drugs induce mitochondrial damage and intercellular gaps and gp120 causes apoptosis. Cardiovasc Toxicol. 2004;4:327–37. doi: 10.1385/ct:4:4:327. [DOI] [PubMed] [Google Scholar]
- 25.Sutinen J, Yki-Järvinen H. Increased resting energy expenditure, fat oxidation, and food intake in patients with highly active antiretroviral therapy-associated lipodystrophy. Am J Physiol Endocrinol Metab. 2007;292:E687–92. doi: 10.1152/ajpendo.00219.2006. [DOI] [PubMed] [Google Scholar]
- 26.Bonfanti P, Ricci E, de Socio G, Zeme D, Carradori S, Penco G, et al. Metabolic syndrome: A real threat for HIV-positive patients?: Results from the SIMONE study. J Acquir Immune Defic Syndr. 2006;42:128–31. doi: 10.1097/01.qai.0000219775.20174.2d. [DOI] [PubMed] [Google Scholar]
- 27.Jacobson DL, Tang AM, Spiegelman D, Thomas AM, Skinner S, Gorbach SL, et al. Incidence of metabolic syndrome in a cohort of HIV-infected adults and prevalence relative to the US population (National Health and Nutrition Examination Survey) J Acquir Immune Defic Syndr. 2006;43:458–66. doi: 10.1097/01.qai.0000243093.34652.41. [DOI] [PubMed] [Google Scholar]


