Abstract
Objectives
This study evaluates the variation in practice patterns associated with contrast-induced acute kidney injury (CI-AKI) and identifies clinical practices that have been associated with a reduction in CI-AKI.
Background
Contrast-induced acute kidney injury (AKI) is a recognized as a complication of invasive cardiovascular procedures and is associated with cardiovascular events, prolonged hospitalization, end-stage renal disease, and all-cause mortality. Reducing the risk of CI-AKI is a patient safety objective set by the National Quality Forum.
Methods
We prospectively collected quantitative and qualitative data from 10 centers, which participate in the Northern New England Cardiovascular Disease Study Group PCI Registry. Quantitative data were collected from the PCI registry. Qualitative data were obtained through clinical team meetings to map care processes related to CI-AKI and focus groups to understand attitudes towards CI-AKI prophylaxis. Fixed and random effects modeling were conducted to test the differences across centers.
Results
Significant variation in rates of CI-AKI were found across ten medical centers. Both fixed effects and mixed effects logistic regression demonstrated significant variabiltiy across centers even after adjustment for baseline co-variates (p<0.001 for both modeling approaches). We found patterns in reported processes and clinical leadership that were attributable to centers with lower rates of CI-AKI. These included: reducing NPO time to 4 hours prior to case, and standardizing volume administration protocols in combination with administering 3–4 high doses of N-acetylcysteine (1200 mg) for each patient.
Conclusions
These data suggest that clinical leadership and institution-focused efforts to standardize preventive practices can help reduce the incidence of CI-AKI.
Keywords: Contrast Media, acute kidney injury, patient safety, quality improvement
INTRODUCTION
Contrast-induced acute kidney injury (CI-AKI) results from exposure to radio-contrast agents given during invasive radiographic procedures. Radio-contrast has been hypothesized to cause AKI through direct toxicity and via hemodynamic changes.[1, 2] CI-AKI occurs in 3–14% of patients and is associated with an increased in-hospital and long-term mortality.[3, 4] CI-AKI is the third most common cause of AKI in hospitalized patients.[5] On average, patients developing CI-AKI stay 3.5 more days in the hospital costing patients and payers over $7,500 in additional hospital costs.[6]
Reducing the prevalence of CI-AKI is a patient safety objective set forth by the National Quality Forum.[7] However, since 2006, preventive measures to reduce CI-AKI have been applied inconsistently.[8] Reports of systematic efforts to apply this evidence in care settings are lacking in the literature. Specifically, the implementation of multi-disciplinary quality improvement care teams to adopt effective evidence-based guidelines to reduce CI-AKI has been underused as a method to address this problem. Weisbord and colleagues found in the Veterans Administration system that 16% of high-risk patients, that were eligible to receive prophylatic fluids, failed to receive intravenous (IV) fluid expansion. When IV fluids were employed, their dose and timing of administration varied significantly by treating specialty and procedure. Furthermore, contra-indicated agents including NSAIDs and COX-2 inhibitors, were prescribed in 8% of patients. These data suggested there is a considerable opportunity to improve the clinical effectiveness of CI-AKI prevention measures.[9]
We hypothesized variation in PCI practice patterns and presence of institutional protocols explains the variability in CI-AKI outcomes in northern New England. To accomplish this aim, we conducted baseline focus groups of multidisciplinary care teams at ten medical centers performing percutaneous coronary interventions (PCI) in Vermont, New Hampshire, and Maine. We hypothesized that patient safety might be improved by reducing CI-AKI through modifiable practice patterns and prophylactic strategies.
METHODS
Study setting
Baseline procedural data was collected prospectively on 7,287 consecutive PCI patients between July 1, 2008 and June 30, 2009 at ten medical centers in northern New England. The cases are recorded in the PCI registry maintained by the Northern New England Cardiovascular Disease Study Group (NNECDSG), a voluntary regional consortium of clinicians, hospital administrators, and health care research personnel who seek to improve continually the quality, safety, effectiveness, and cost of medical interventions in cardiovascular disease [10, 11]. The NNECDSG has Institutional Review Board approval for data collection and analysis at all participating centers. Additional Institutional Review Board approval was obtained for the multi-disciplinary focus groups at all participating centers.
Determination of CI-AKI Incidence
All data collected in the NNECDSG PCI Registry are collected prospectively using the same definitions for all variables across centers. The last pre-PCI serum creatinine and highest post-PCI serum creatinine between the PCI procedure and discharge were used to determine the incidence of CI-AKI across medical centers. In an effort to move toward standardization of definitions, we used the Acute Kidney Injury Network definition for CI-AKI: ≥0.3 (mg/dL) or ≥50% increase in serum creatinine from baseline within 48 hours.[12] Multivariable logistic regression was used to calculate adjusted rates of CI-AKI, adjusting for: age, gender, priority of procedure (non-urgent, urgent, emergent), congestive heart failure, diabetes, use of pre-PCI intra-aortic balloon pump, and baseline estimated glomerular filtration rate based on National Kidney Foundation definitions using the Modification of Diet in Renal Disease (MDRD) equation (mL/min/1.73 m2): 186 x (serum creatinine mg/dL)−1.154 x (age)−0.203 x (0.742 for women) x (0.180 for African American).[13–15] Multilevel mixed-effects logistic regression (both fixed and random effects modeling) was conducted to test the differences across centers adjusting for the same covariates listed above.
Process Mapping of CI-AKI Prophylaxis
Baseline multidisciplinary clinical team meetings were conducted at all medical centers. Each center organized their multidisciplinary team to include interventional cardiologists, cardiac catheterization lab managers and technicians, nursing representation from the ICU and/or holding areas, cardiology administration, and nephrology. Participants provided written informed consent to participate in the focus group according to each institution’s IRB protocol. Each multi-disciplinary team was asked to explain the process of care for an in-patient undergoing PCI (already on the hospital service) from the time PCI was recommended through one week after discharge. A research coordinator facilitated the meetings and the teams were then asked about any differences that typically happen for a same-day admission patient. Detailed information about key aspects of the center’s process on CI-AKI prophylaxis was collected. The teams were asked to describe their attitudes towards CI-AKI and prevention of CI-AKI. Teams were blinded to their rank-ordered adjusted rates of CI-AKI to prevent bias in reporting.
Each meeting was videotaped by a videographer and moderated by an experienced facilitator. Videotaped interviews were standardized and conducted in the same process for all centers. Ascertainment of center protocols or standing order sets relevant to CI-AKI prophylaxis was collected (if available). Table 2 reports “Variable” when standard order sets or protocols was non-existent or order sets had a “write-in” order for volume administration, N-acetylcysteine, or sodium bicarbonate (i.e., the use of preventive therapies was at the discretion of the provider and not a team-wide mandate). Field notes were recorded. A third party transcribed each tape void of identifiable information. Videotapes were destroyed after transcription. The transcription was provided to the research team without names or identifiable information on the participants.
Table 2.
Process and procedural practices for each site by rate of acute kidney injury
| Center | A | B | C | D | E | F | G | H | I | J |
|---|---|---|---|---|---|---|---|---|---|---|
| CI-AKI | 1.9 | 3.6 | 4.0 | 4.6 | 5.5 | 7.0 | 7.1 | 8.8 | 9.8 | 10.1 |
| Pre-PCI | ||||||||||
| NPO | Mandated 4hrs | Optional 2hrs | Midnight | Midnight | Midnight | Midnight | Midnight | Midnight | Midnight | Midnight |
| IV NaCl | Yes | Mandated | Optional | Optional | Optional | Optional | Optional | Yes | Optional | Optional |
| IV NaHCO3 | Mandated | No | Optional | Optional | No | Optional | Optional | Optional | No | Optional |
| N-AC | 1200mg | 1200mg | 600mg | 1200mg | 600mg | 600mg | 1200mg | 600mg | 1200mg | 1200mg |
| Peri-PCI | ||||||||||
| Contrast agent | Iopamidol | Iopamidol | Iohexol Iodixanol |
Iodixanol | Iodixanol | Iohexol Iodixanol |
Iodixanol | Ioversol | Iopromide Ioxaglate Iodixanol |
Iopamidol Iodixanol |
| Osmolality | Low | Low | Low, Iso | Iso | Iso | Low, Iso | Iso | Low | Low, Iso | Low, Iso |
| Post-PCI | ||||||||||
| IV NaCl | No | Mandated | Optional | Optional | Optional | Optional | Optional | Yes | Optional | Optional |
| IV NaHCO3 | Mandated | No | Optional | Optional | No | Optional | Optional | Optional | No | Optional |
CI-AKI = adjusted rates of contrast-induced acute kidney injury; NPO = pre-anesthesia orders (eating and drinking); IV = intravenous; NaCl: 0.9% normal saline; PCI = percutaneous coronary intervention; NaHCO3 = sodium bicarbonate (1000mL D5W mixed with 150 mEq NaHCO3) given at 3mL/Kg/hr for 1 hour pre-PCI and 1mL/Kg/hr for 6 hours post-PCI; Iopamidol = Isovue; Iohexol = Omnipaque; Iodixanol = Visipaque; Ioxaglate = Hexabrix; Mandated = a standardized and mandated institutional or cardiology practice protocol were the practice was routinely implemented; Yes = use of this practice as an optional selection for care, not mandated; No = this option was not available or used at the center.
Qualitative analysis
Qualitative analysis was performed using the grounded theory approach.[16] In this approach, theory is created from the data and categories are not specified a priori. We used open coding to develop initial themes, followed by axial and selective coding to develop matrixes across medical centers. We defined aggressive CI-AKI prophylaxis as indicated by the presence of clinical leaders who devised a CI-AKI prophylactic protocol, implemented such protocol at their institution and had a heightened awareness of the need for CI-AKI prevention through volume expansion or sparing use of contrast.
RESULTS
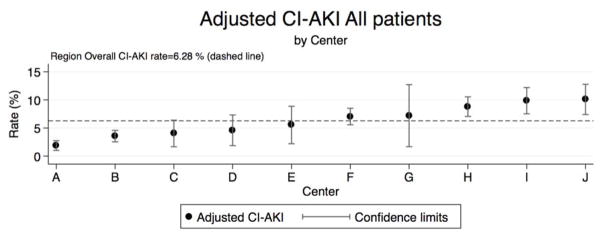
Adjusted rates of CI-AKI were measured across 7,286 consecutive patients undergoing PCI at ten medical centers and varied from 1.9% to 10.1% percent representing a fivefold difference in the incidence of CI-AKI (Figure 1). Adjusted rates of CI-AKI are reported for patients undergoing PCI by center; patients with a history of dialysis prior to PCI were excluded from the adjusted rates of CI-AKI. The regional average rate of CI-AKI was 6.28%. Baseline patient and disease characteristics are reported in Table 1 rank-ordered by the adjusted rate of CI-AKI, denoted as A-J. Both fixed effects and mixed effects logistic regression demonstrated significant variabiltiy across centers even after adjustment for baseline co-variates (p<0.001 for both modeling approaches). We report the adjusted rates of CI-AKI stratified by eGFR≥60 and <60 (mL/min/m2) in the online appendix.
Figure 1. Adjusted rates of CI-AKI by rank and center.
Adjusted rates of CI-AKI by center are rank ordered with 95% confidence intervals. The regional rate of CI-AKI was 6.28% (red dashed line).
Table 1.
Patient and Disease Characteristics Across Centers
| Center | A | B | C | D | E | F | G | H | I | J |
|---|---|---|---|---|---|---|---|---|---|---|
| CI-AKI | 1.9 | 3.6 | 4.0 | 4.6 | 5.5 | 7.0 | 7.1 | 8.8* | 9.8 | 10.1 |
| CIN (≥25% or ≥0.5 mg/dL ΔSCr) | 4.0 | 5.1 | 7.1 | 6.9 | 8.1 | 10.4 | 7.5 | 10.8 | 15.7 | 12.2 |
| Age (mean±sd) | 62±11 | 63±12 | 60±12 | 61± 13 | 62±11 | 65±12 | 65±11 | 64±12 | 62±12 | 65±13 |
| Female (%) | 32. 5 | 30.4 | 26.8 | 28.5 | 27.5 | 29.1 | 36.1 | 27.9 | 27.6 | 29.0 |
| BMI (%) | 31±7 | 30±6 | 30±6 | 32±7 | 31±7 | 30±6 | 29±6 | 30±6 | 30±6 | 30±6 |
| Diabetes (%) | 26.3 | 31.0 | 25.9 | 35.8 | 23.8 | 29.7 | 20.9 | 29.0 | 24.8 | 34.6 |
| Hypertension (%) | 64.2 | 67.9 | 85.2 | 78.2 | 61.2 | 70.2 | 72.1 | 69.8 | 70.8 | 81.7 |
| Hypercholesterolemia (%) | 69.3 | 89.2 | 79.8 | 79.5 | 67.8 | 71.3 | 81.4 | 76.0 | 78.3 | 82.4 |
| COPD (%) | 8.1 | 13.0 | 22.9 | 13.9 | 5.5 | 11.6 | 8.1 | 10.2 | 18.0 | 15.7 |
| PVD (%) | 8.2 | 25.0 | 16.0 | 13.9 | 8.8 | 15.5 | 24.4 | 7.0 | 14.3 | 22.8 |
| Angina at rest (%) | 17.4 | 53.4 | 43.8 | 80.7 | 77.8 | 22.6 | 51.4 | 87.6 | 52.9 | 78.8 |
| Smoker | 20.2 | 31.4 | 35.5 | 29.8 | 29.7 | 18.1 | 26.7 | 20.8 | 28.8 | 24.7 |
| Prior PCI (%) | 29.6 | 34.7 | 30.1 | 43.1 | 23.8 | 31.3 | 33.7 | 29.7 | 32.9 | 43.7 |
| Prior CABG (%) | 15.5 | 18.8 | 10.2 | 19.2 | 10.3 | 15.5 | 16.3 | 15.6 | 11.5 | 20.4 |
| ST-elevation myocardial infarction | 37.4 | 61.9 | 67.2 | 34.1 | 65.3 | 70.3 | 51.6 | 71.8 | 63.6 | 51.1 |
| Out-patient PCI | 5.4 | 0.3 | 0.3 | 0.7 | 0.7 | 0.4 | 1.2 | 0.2 | 1.0 | 1.7 |
| Pre-procedure IABP (%) | 0.0 | 0.7 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 0.1 | 0.0 | 0.7 |
| No. of treated vessels (mean±SD) | 1.1±0. 3 | 1.2±0.4 | 1.1±0.4 | 1.2±0.4 | 1.1±0.3 | 1.2±0.4 | 1.2±0.5 | 1.2±0.4 | 1.1±0.3 | 1.2±0.4 |
| No. of stents used (mean±SD) | 1.4±1. 1 | 1.5±1.1 | 1.1±0.6 | 1.7±1.1 | 1.1±0.6 | 1.7±1.3 | 1.6±0.8 | 1.4±0.9 | 1.2±0.7 | 1.5±0.9 |
| Success of procedure | 96.5 | 91.9 | 94.3 | 98.0 | 96.0 | 91.0 | 96.5 | 93.2 | 93.3 | 93.8 |
| Completeness of revascularization | 86.6 | 95.7 | 79.4 | 96.6 | 71.6 | No data | 80.2 | 83.9 | 95.6 | 95.0 |
| eGFR<60 (mL/min/m2) (%) | 12.4 | 19.9 | 14.8 | 27.2 | 15.6 | 22.9 | 15.3 | 17.5 | 20.5 | 28.6 |
| Fixed effects multivariable logistic | 1.00 (ref) | 2.13 (1.14–3.98) | 2.40 (1.01–5.69) | 3.46 (1.43–8.35) | 3.53 (1.56–7.97) | 3.54 (1.95–6.40) | 4.01 (1.47–10.95) | 5.97 (3.33–10.70) | 6.76 (3.71–12.32) | 6.28 (3.36–11.75) |
CI-AKI = adjusted rates of contrast-induced acute kidney injury defined using the AKIN criteria as 0.3 mg/dL or ≥50% increase in serum creatinine; CIN: adjusted rates of contrast-induced nephropathy defined as ≥25% or ≥0.5 mg/dL increase in serum creatinine; BMI: body mass index; COPD: chronic obstructive pulmonary disease; PVD: peripheral vascular disease; PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft surgery; IABP: intra-aortic balloon pump; eGFR: estimated glomerular filtration rate.
Based on 50% capture of post-PCI serum creatinine.
Common processes of care for patients were identified from focus groups of providers at each medical center. These processes were used to develop a matrix of procedural practice hypothesized to be associated with CI-AKI (Table 2). The rates of CI-AKI are reported based on all patients undergoing PCI at the center without pre-PCI dialysis, the process-level data in Table 2 were developed in response to patients eligible for volume expansion or CI-AKI prophylaxis (serum creatinine ≥1.5 mg/dL or eGFR<60 mL/min/m2). The matrix was rank-ordered according to the adjusted CI-AKI incidence across centers. Table 3 represents a second matrix on performing checks in the process of care.
Table 3.
Additional processes for each site by rate of acute kidney injury
| Center | A | B | C | D | E | F | G | H | I | J |
|---|---|---|---|---|---|---|---|---|---|---|
| CI-AKI | 1.9 | 3.6 | 4.0 | 4.6 | 5.5 | 7.0 | 7.1 | 8.8 | 9.8 | 10.1 |
| Pre-PCI | ||||||||||
| Check Pre-PCI Labs | + | + | + | + | + | + | + | + | + | + |
| Stop Metformin | + | + | + | + | + | + | + | + | + | + |
| Stop Diuretics | + | + | + | + | + | + | + | + | + | + |
| Peri-PCI | ||||||||||
| Cath-lab Time-out | + | + | + | + | + | + | + | + | + | + |
| Check Fluids Given | − | − | − | − | − | − | − | − | − | + |
| MACD Calculated | − | + | + | + | + | + | + | − | + | + |
| MACD Used | − | − | − | + | − | + | + | − | + | + |
| Contrast Sparing Device | − | + | + | + | + | − | + | − | − | + |
| LV Gram Avoided | + | − | − | |||||||
| Staging | − | + | + | + | + | + | + | + | + | + |
| Post-PCI | ||||||||||
| Check Post-PCI Labs Next AM | + | + | + | + | + | + | + | − | + | + |
| Post-discharge follow-up | − | − | − | − | − | − | − | − | − | − |
| Clinical Champion* | + | + | − | − | − | − | − | − | − | − |
CI-AKI = adjusted rates of contrast-induced acute kidney injury; PCI = percutaneous coronary intervention; MACD = maximum allowable contrast dose (5mL contrast x body weight, kg/serum creatinine, mg/dL);
Defined by the presence of clinical leadership championing action towards implementing CI-AKI protocols and order sets. Staging = stopping a case to complete at a later date to prevent renal injury due reaching or exceeding the MACD; Cells with a + = Yes; − = No; empty = not reported.
The practice with the lowest adjusted rate of CI-AKI prevention in our region was identified as Center A. Center A incorporated a standardized protocol for CI-AKI prevention in 2005. The protocol included by-passing the midnight NPO orders to incorporate NPO orders limited to 4-hours prior to the procedure. All patients with chronic kidney disease (eGFR<60 mL/min/m2) were started on an intravenous (IV) sodium bicarbonate protocol at 3mL/kg/hr for one hour prior to the procedure. In addition, IV normal saline was given to patients until the sodium bicarbonate protocol was started approximately 1 hour prior to the procedure. If a case was delayed, the sodium bicarbonate rate was reduced to 1mL/kg/hr until the case was performed. IV sodium bicarbonate was continued after PCI at a rate of 1mL/kg/hr for 6 hours. Patients were also given 1200mg of N-acetylcysteine orally two times before the case and two times after. Iopamidol contrast was used on all procedures.
The practice with the second lowest adjusted rate of CI-AKI was Center B. Similar to Center A, Center B had an established volume administration protocol, used 1200mg N-acetylcysteine twice before PCI and twice after PCI, and all cases were conducted with Iopamidol. Center B also had an option to by-pass the NPO orders after midnight and changing the NPO status to only 2-hours pre PCI. However, Center B differed from Center A in that they used only IV normal saline for volume expansion before and after PCI.
Standardizing practice reduces variation. The main contributor to success from our two centers with the lowest rates of CI-AKI was an evidence-based standardized CI-AKI prophylaxis protocol. Center B called this their:
“Power Protocol.”
Center A sought to
“Keep it simple so everyone knows what to do: hydrate patients and alkalinize the kidneys.”
Centers A and B were the only centers to rank significantly below the regional average; all other centers ranged between 4% and 10.1% of the post-procedure CI-AKI rate. All centers routinely had patients NPO after midnight, with the exception of Center F, which occasionally had patients NPO after 6AM on the day of the procedure. All centers had variable volume administration practice, most using physician written orders or electronic written orders without a standardized protocol. Most other centers used a lower dose N-acetylcysteine at 600mg (as opposed to the 1200mg) and sometimes this was ordered only three times (once pre and twice post) as opposed to four times (two pre and two post). There was also variability in use of contrast agent; the two centers with the lowest CI-AKI rates used the same contrast agent on all patients (regardless of high or low risk of CI-AKI), while other centers would use a low-osmolar contrast agent for low-risk patients and iso-osmolar agent for high-risk patients. The findings suggest that low adjusted rates of CI-AKI may be obtained with clinical leadership and aggressive prophylaxis through volume expansion.
Key patterns from centers with the lowest incidence of CI-AKI were identified as potential targets for CI-AKI prevention at other centers (Table 4). Four practice patterns were identified: 1) changing NPO liquid orders from midnight to 2–4 hours prior to case; 2) standardization of volume administration protocols; 3) use of high dose N-acetylcysteine (1200 mg); and 4) Iopamidol low-osmolar contrast agent.
Table 4.
Change Strategies For Evaluation and Further Consideration
| NPO with clear fluids allowed up to 2–4 hours prior to PCI |
| N-acetylcysteine 1200mg PO q12° x 4, first dose 6pm night before PCI: 1200mg dose @ 6PM, 6AM, 6PM, 6AM |
| Standardization of volume administration prophylaxis |
| IV NS @ 1.5mL/kg/hr at 10pm night before PCI, continue until IV NaHCO3 protocol begins |
| IV NaHCO3 @ 3mL/kg/hr for 1 hour pre-PCI (reduce to 1mL/kg/hr if PCI is delayed) |
| IV NaHCO3 @ 1mL/kg/hr for 6 hours post-PCI |
| Recommendation: Post-discharge follow-up at 3–5 days to determine CI-AKI, if persistent weekly labs until resolved |
| Iopamidol low-osmolar contrast agent |
CI-AKI = contrast-induced acute kidney injury; PCI = percutaneous coronary intervention; IV=intravenous; NS = normal saline; NaHCO3 = sodium bicarbonate (1000mL D5W mixed with 150 mEq NaHCO3).
Clinical leadership was a key driver in Centers A and B. The above tools of CI-AKI prophylaxis may be markers for the level of aggressiveness in attitudes towards CI-AKI prophylaxis and proactive clinical leadership. One observation from the focus groups was the level of aggressiveness and attitudes towards implementing and using of these prophylactic tools and proactive leadership (championing) by clinical faculty that drove institutional changes resulting in aggressive CI-AKI prophylaxis protocols. Both Centers A and B demonstrated aggressive attitudes towards CI-AKI prophylaxis during the baseline focus groups; these aggressive attitudes were also captured through use of standard order sets to drive the use of aggressive volume administration (Centers A and B), use of N-acetylcysteine (centers A and B), and sodium bicarbonate (Center A). Both centers had interventional cardiologists who proposed changes to their standard order sets and implemented those changes. Center A called this protocol the “CI-AKI Prophylaxis”, Center B called it their “Power Protocol”. While these tools may be useful in preventing CI-AKI, we must also look at the aggressive prophylactic culture of the practicing operators to obtain an understanding of true CI-AKI prophylaxis. It may require both quality improvement tools and active leadership with attention to detail, volume administration status of the patient prior to - and after - the intervention, and sparing use of contrast to improve the rates of CI-AKI.
We identified collective quality improvement efforts that may explain the collection of processes and protocols aimed at CI-AKI prevention. Through systematic focus groups, we ascertained each center’s culture, processes and protocols for CI-AKI prevention. The major changes that emerged were the following: reducing NPO after midnight orders to 2–4 hours prior to PCI, volume expansion protocols, use of N-acetylcysteine, contrast agent, and other procedural factors such as the limited use of an LV gram for patients with eGFR<60. We considered these trends in practice and clinical leadership to exist within each center collectively. This collective approach may suggest that any of these prophylactic efforts may not be individually driving the prevention of CI-AKI. The role of the clinical champion as described above was one element that emerged from these focus groups and was attributed to the top performing centers. It is believed that the emergence of this trend in clinical leadership may well encompass a collective group of culture, process, and attitudes that were collective approaches towards the prevention of CI-AKI and deserves further investigation.
DISCUSSION
In our investigation of ten medical centers performing routine PCI, we found 5-fold variability in adjusted rates of CI-AKI. By means of facilitating focus groups and collecting qualitative data, we were able to identify key attributes of centers with the lowest rates of CI-AKI in our region. The practice identified in Center A was achieved from establishing a simple CI-AKI prophylaxis protocol including NPO orders for only 4 hours, Iopamidol contrast, a standardized sodium bicarbonate protocol (3mL/kg/hr for 1 hour pre- and 1mL/kg/hr for 6 hours post-PCI) and use of 1200mg N-acetylcysteine two times pre- and post-PCI.[17]
Volume administration
The discussion over volume administration has been an on-going debate in the literature, ranging from a volume administration protocol with 0.45% normal saline for 12 hours before and after angiography[18] to a sodium bicarbonate protocol with isotonic (154 mEq/L) infusion of sodium bicarbonate before and after Iopamidol administration (370 mg iodine/mL). The largest randomized trial by Maioli is consistent with our qualitative findings, where the two centers with the lowest rates of CI-AKI both used standardized protocols for CI-AKI prevention, but one used sodium bicarbonate and the other used normal saline – both with successful results.[19] Alkalinization of the urine with sodium bicarbonate has been shown to reduce labile iron dependent free oxygen radicals to a larger degree than saline alone.[17] Both protocols have demonstrated reductions in CI-AKI in multiple trials and subsequent meta-analyses. Most recently, a meta-analysis by Kunadian et al., demonstrated the superiority of sodium bicarbonate over normal saline with a 67% risk reduction for CI-AKI, yet no difference in other clinical endpoints.[20] In our qualitative analysis the practice with the lowest rates of CI-AKI used a protocol for sodium bicarbonate, yet the second lowest incidence of CI-AKI had success using a normal saline protocol. While other centers used the sodium bicarbonate protocol from time to time, there was a lack of standardization of this approach for all patients. Current evidence suggests that volume administration should remain a central focus for preventing CI-AKI.
N-acetylcysteine
The most supportive evidence for high dose N-acetylcysteine was published by Marenzi et al. and demonstrated high dose N-acetylcysteine at 1200mg significantly reduced CI-AKI (3%) compared to low dose (600mg, 8%) or placebo (33%) after primary angioplasty.[21] Trivedi et al., (2009) used a meta-analysis to compare randomized trials of high dose N-acetylcysteine (a daily dose greater than 1200mg or a single dose within 4 hours of contrast exposure greater than 600mg). Results showed high dose N-acetylcysteine significantly reduced the occurrence of CI-AKI by 54% when compared to low dose N-acetylcysteine (HR: 0.46; 95%CI: 0.33–0.63).[22] However, the largest trial to date, the ACT trial with 2,308 patients, recently reported no effect of N-acetylcysteine over placebo (RR: 1.00; 95%CI: 0.81–1.25) suggesting this agent may have no efficacy in preventing CI-AKI.[23] It should be noted that our reported research was conducted prior to the ACT trial public release and represented practice patterns from the current best evidence. Although N-acetylcysteine has been postulated to cause an artificial decline in serum creatinine,[24, 25] recent evidence showed that in the absence of contrast, N-acetylcysteine had no effect on serum creatinine or cystatin C.[26]
Combination therapy
Prevention of CI-AKI has been investigated through the combination of interventions. Recently, Brown and colleagues reported the results of a meta-analysis evaluating randomized trials using a combination of volume administration and N-acetylcysteine. They demonstrated the combination of sodium bicarbonate and N-acetylcysteine provided additional prevention of CI-AKI over normal saline with or without N-acetylcysteine.[27] This finding from clinical trials was supported by the experience of Center A, whereby a simple, yet concrete protocol of combination prophylaxis of IV sodium bicarbonate and high dose N-acetylcysteine (1200 mg) resulted in the lowest rate of CI-AKI in northern New England. However, this should be balanced with the recent release of the ACT trial[23] and may suggest the more aggressive approach to CI-AKI prophylaxis was driven by clinical leadership and center culture.
Contrary to some of our a priori hypotheses, there were CI-AKI techniques that did not prevail in the best-practice approach. Surprisingly, automated contrast devices were not a key attribute of reducing CI-AKI despite initial evidence demonstrating significantly less use of contrast and a 31.1% risk reduction for CI-AKI over hand-held devices.[28] Nor was the systematic use of the maximum allowable contrast dose (MACD) a key factor for CI-AKI prevention.[29, 30]
Limitations
Careful readers will note that serum creatinine may have a delayed latency prior to reaching its peak. In fact, Maioli reported that when CI-AKI is assessed up to 5-days post PCI, 40% of the patients had not reached AKI by 48 hours.[19] Therefore, we may be underestimating the incidence of AKI in this study. The definition of AKI by 48 hours is, however, consistent with real world practice. Second, We have chosen to use the Acute Kidney Injury Network definition of CI-AKI.[12] The diagnosis of CI-AKI using this definition has undergone substantial evaluation and the field of AKI is moving towards standardizing the definition of AKI in all clinical contexts using the ≥0.3 (mg/dL) or ≥50% increase in serum creatinine. Some experts believe that ≥25% increase in serum creatinine is too low of an elevation in the acute setting and ≥0.5 (mg/dL) misses patients with AKI. We have included the 25%, 0.5 (mg/dL) and 25% or 0.5 (mg/dL) definitions in Table 1 for comparison to previously reported randomized controlled trials and the Contrast-Induce Nephropathy Consensus[31] and re-evaluated the inference from the results. The result does not change with respect to the centers with the best rates of CI-AKI; changes include Center “D” to swap with “C” and Center “G” to move into the “E” position Upon re-evaluation of these two changes, the inference from the results remains the same with added support on the use of high-dose N-acetylcysteine at 1200mg. Third, we recognize the importance of contrast volume, however it is not currently reported in our registry or other national registries at this time. The NNECDSG and other registries such as the NCDR are working towards including contrast volume in future data collection forms. However, we can assume contrast exposure based on the number of vessels intervened upon and the number of stents deployed. Fourth, we used a quantitative approach to summarize center characteristics and to develop risk-adjusted rates of CI-AKI. We used qualitative methods to develop matrices to characterize the variation in the underlying practice. The qualitative data from Table 2 and 3 represent data collected from focus groups and therefore is captured as center-level data. This limits the ability to conduct sophisticated regression analyses to ascertain cause and effect. While this is a limitation, there are strengths associated with having the qualitative data to ascertain process-level characteristics that may be the underlying reason for variation in adjusted rates of CI-AKI. Through the use of these methods, we suggest several strategies that may account for lower rates of CI-AKI. Further investigation with patient-level data is needed to confirm the hypotheses generated from the qualitative findings. Centers reported their practice patterns, but evidence was not collected prospectively to determine whether these practices were in fact conducted on the floor or in the catheterization laboratory. However, this self-reporting provides a snapshot that allows for a useful comparison across practices, the goal of this study. In addition, these initial findings can serve as the hypotheses for future more detailed investigation of the processes of care for patients undergoing PCI.
Conceptual framework for prevention of CI-AKI in Northern New England
The NNE has chosen to evaluate the relationship of high-intensity quality improvement efforts on patient safety and CI-AKI. This report summarizes the initial steps in understanding the variation in adjusted CI-AKI rates and practice patterns in the region. We now plan to start a high-intensity quality improvement intervention based on the NNE and Vermont Oxford frameworks.[32–35] High-intensity quality improvement interventions will be focused at each center and will include training in continuous quality improvement methods to engage clinical practice and evaluation of that practice through 1) evidence based medicine, 2) collaborative learning, 3) developing a habit for systems thinking, and 4) developing a habit for change, and 5) robust data systems to assess and manage process changes.
CONCLUSIONS
In conclusion, we have identified best practices associated with a reduction in CI-AKI. Future research should aim to validate these findings and determine if the reduction in CK-AKI translates into clinically meaningful outcomes.
Supplementary Material
Figure 2a: Adjusted rates of CI-AKI by rank and center for patients with eGFR≥60 (mL/min/m2). Adjusted rates of CI-AKI by center are rank ordered with 95% confidence intervals based on the CI-AKI rank for all patients. The regional rate of CI-AKI among patients with eGFR≥60 was 4.43% (red dashed line). This figure shows that the top two center rankings on CI-AKI and CI-AKI for patients with eGFR≥60 did not change; centers E, F, and H dropped slightly in their ranking.
Figure 2b: Adjusted rates of CI-AKI by rank and center for patients with eGFR<60 (mL/min/m2). Adjusted rates of CI-AKI by center are rank ordered with 95% confidence intervals based on the CI-AKI rank for all patients. The regional rate of CI-AKI among patients with eGFR≥60 was 12.30% (red dashed line). This figure shows that the top ranking center remained the lowest ranking center; however center B and H increased in their rank with respect to the other centers, while centers C, E, G, I, and J dropped in their ranking position.
Acknowledgments
SOURCES OF FUNDING
This project was supported by grant number K01 HS018443 (Dr. Brown) from the Agency for Healthcare Research and Quality and K24 DK078204 (Dr. Sarnak) from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality or the National Institute of Health.
References
- 1.Bui KL, Horner JD, Herts BR, Einstein DM. Intravenous iodinated contrast agents: risks and problematic situations. Cleveland Clinic journal of medicine. 2007;74:361–4. 7. doi: 10.3949/ccjm.74.5.361. [DOI] [PubMed] [Google Scholar]
- 2.Tumlin J, Stacul F, Adam A, Becker CR, Davidson C, Lameire N, et al. Pathophysiology of contrast-induced nephropathy. The American journal of cardiology. 2006;98:14K–20K. doi: 10.1016/j.amjcard.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 3.McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368–75. doi: 10.1016/s0002-9343(97)00150-2. [DOI] [PubMed] [Google Scholar]
- 4.Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–64. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 5.Finn WF. The clinical and renal consequences of contrast-induced nephropathy. Nephrol Dial Transplant. 2006;21:i2–10. doi: 10.1093/ndt/gfl213. [DOI] [PubMed] [Google Scholar]
- 6.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–70. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 7.National Quality Forum. Safe practices for better healthcare 2006 Update : a consensus report. Washington, DC: National Quality Forum; 2007. [Google Scholar]
- 8.Elicker BM, Cypel YS, Weinreb JC. IV contrast administration for CT: a survey of practices for the screening and prevention of contrast nephropathy. Ajr. 2006;186:1651–8. doi: 10.2214/AJR.05.0407. [DOI] [PubMed] [Google Scholar]
- 9.Weisbord SD, Bruns FJ, Saul MI, Palevsky PM. Provider use of preventive strategies for radiocontrast nephropathy in high-risk patients. Nephron Clin Pract. 2004;96:c56–62. doi: 10.1159/000076400. [DOI] [PubMed] [Google Scholar]
- 10.Malenka DJ, O’Connor GT. A regional collaborative effort for CQI in cardiovascular disease. Northern New England Cardiovascular Study Group. The Joint Commission journal on quality improvement. 1995;21:627–33. doi: 10.1016/s1070-3241(16)30191-2. [DOI] [PubMed] [Google Scholar]
- 11.O’Connor GT, Malenka DJ, Quinton H, Robb JF, Kellett MA, Jr, Shubrooks S, et al. Multivariate prediction of in-hospital mortality after percutaneous coronary interventions in 1994–1996. Northern New England Cardiovascular Disease Study Group. Journal of the American College of Cardiology. 1999;34:681–91. doi: 10.1016/s0735-1097(99)00267-3. [DOI] [PubMed] [Google Scholar]
- 12.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical care (London, England) 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anonymous. Executive summary-K/DOQI clinical practice guidelines. Am J Kidney Dis. 2002;39:S17–31. [Google Scholar]
- 14.Best PJ, Reddan DN, Berger PB, Szczech LA, McCullough PA, Califf RM. Cardiovascular disease and chronic kidney disease: insights and an update. American heart journal. 2004;148:230–42. doi: 10.1016/j.ahj.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA. 1996;275:1489–94. [PubMed] [Google Scholar]
- 16.Corbin JM, Strauss AL. Basics of qualitative research : techniques and procedures for developing grounded theory. 3. Los Angeles, Calif: Sage Publications, Inc; 2008. [Google Scholar]
- 17.Merten GJ, Burgess WP, Gray LV, Holleman JH, Roush TS, Kowalchuk GJ, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. JAMA. 2004;291:2328–34. doi: 10.1001/jama.291.19.2328. [DOI] [PubMed] [Google Scholar]
- 18.Solomon R, Werner C, Mann D, D’Elia J, Silva P. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. The New England journal of medicine. 1994;331:1416–20. doi: 10.1056/NEJM199411243312104. [DOI] [PubMed] [Google Scholar]
- 19.Maioli M, Toso A, Leoncini M, Gallopin M, Tedeschi D, Micheletti C, et al. Sodium bicarbonate versus saline for the prevention of contrast-induced nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Journal of the American College of Cardiology. 2008;52:599–604. doi: 10.1016/j.jacc.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 20.Kunadian V, Zaman A, Spyridopoulos I, Qiu W. Sodium bicarbonate for the prevention of contrast induced nephropathy: A meta-analysis of published clinical trials. Eur J Radiol. 2010 doi: 10.1016/j.ejrad.2009.12.015. In Press. [DOI] [PubMed] [Google Scholar]
- 21.Marenzi G, Assanelli E, Marana I, Lauri G, Campodonico J, Grazi M, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. The New England journal of medicine. 2006;354:2773–82. doi: 10.1056/NEJMoa054209. [DOI] [PubMed] [Google Scholar]
- 22.Trivedi H, Daram S, Szabo A, Bartorelli AL, Marenzi G. High-dose N-acetylcysteine for the prevention of contrast-induced nephropathy. Am J Med. 2009;122:874, e9–15. doi: 10.1016/j.amjmed.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 23.Acetylcystein for the Prevention of Contrast-Induced nephropaThy (ACT) Trial: a Pragmatic Multicenter Randomized Trial to Evaluate the Efficacy of Acetylcysteine for the Prevention of Renal Outcomes in Patients Undergoing Coronary and Vascular Angiography. Circulation. 2010;122:2219. [Google Scholar]
- 24.Duong MH, MacKenzie TA, Malenka DJ. N-acetylcysteine prophylaxis significantly reduces the risk of radiocontrast-induced nephropathy: comprehensive meta-analysis. Catheter Cardiovasc Interv. 2005;64:471–9. doi: 10.1002/ccd.20342. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann U, Fischereder M, Kruger B, Drobnik W, Kramer BK. The value of N-acetylcysteine in the prevention of radiocontrast agent-induced nephropathy seems questionable. J Am Soc Nephrol. 2004;15:407–10. doi: 10.1097/01.asn.0000106780.14856.55. [DOI] [PubMed] [Google Scholar]
- 26.Rehman T, Fought J, Solomon R. N-Acetylcysteine Effect on Serum Creatinine and Cystatin C Levels in CKD Patients. Clin J Am Soc Nephrol. 2008;3:1610–4. doi: 10.2215/CJN.01560408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown JR, Block CA, Malenka DJ, O’Connor GT, Schoolwerth AC, Thompson CA. Sodium bicarbonate plus N-acetylcysteine prophylaxis: a meta-analysis. JACC Cardiovasc Interv. 2009;2:1116–24. doi: 10.1016/j.jcin.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Call J, Sacrinty M, Applegate R, Little W, Santos R, Baki T, et al. Automated contrast injection in contemporary practice during cardiac catheterization and PCI: effects on contrast-induced nephropathy. The Journal of invasive cardiology. 2006;18:469–74. [PubMed] [Google Scholar]
- 29.Brown JR, Robb JF, Malenka DJ. Does “Safe” Dosing of Iodinated Contrast Prevent Contrast-Induced Acute Kidney Injury. Circulation. 2009;120:S435. doi: 10.1161/CIRCINTERVENTIONS.109.910638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marenzi G, Assanelli E, Campodonico J, Lauri G, Marana I, De Metrio M, et al. Contrast volume during primary percutaneous coronary intervention and subsequent contrast-induced nephropathy and mortality. Annals of internal medicine. 2009;150:170–7. doi: 10.7326/0003-4819-150-3-200902030-00006. [DOI] [PubMed] [Google Scholar]
- 31.McCullough PA, Adam A, Becker CR, Davidson C, Lameire N, Stacul F, et al. Epidemiology and prognostic implications of contrast-induced nephropathy. The American journal of cardiology. 2006;98:5K–13K. doi: 10.1016/j.amjcard.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Horbar JD. The Vermont-Oxford Neonatal Network: integrating research and clinical practice to improve the quality of medical care. Seminars in perinatology. 1995;19:124–31. doi: 10.1016/s0146-0005(05)80032-1. [DOI] [PubMed] [Google Scholar]
- 33.Likosky DS, Nugent WC, Ross CS. Improving outcomes of cardiac surgery through cooperative efforts: the northern new England experience. Seminars in cardiothoracic and vascular anesthesia. 2005;9:119–21. doi: 10.1177/108925320500900203. [DOI] [PubMed] [Google Scholar]
- 34.Nugent WC. Building and supporting sustainable improvement in cardiac surgery: the Northern New England experience. Seminars in cardiothoracic and vascular anesthesia. 2005;9:115–8. doi: 10.1177/108925320500900202. [DOI] [PubMed] [Google Scholar]
- 35.Kasper JF, Plume SK, O’Connor GT. A methodology for QI in the coronary artery bypass grafting procedure involving comparative process analysis. Qrb. 1992;18:129–33. doi: 10.1016/s0097-5990(16)30519-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 2a: Adjusted rates of CI-AKI by rank and center for patients with eGFR≥60 (mL/min/m2). Adjusted rates of CI-AKI by center are rank ordered with 95% confidence intervals based on the CI-AKI rank for all patients. The regional rate of CI-AKI among patients with eGFR≥60 was 4.43% (red dashed line). This figure shows that the top two center rankings on CI-AKI and CI-AKI for patients with eGFR≥60 did not change; centers E, F, and H dropped slightly in their ranking.
Figure 2b: Adjusted rates of CI-AKI by rank and center for patients with eGFR<60 (mL/min/m2). Adjusted rates of CI-AKI by center are rank ordered with 95% confidence intervals based on the CI-AKI rank for all patients. The regional rate of CI-AKI among patients with eGFR≥60 was 12.30% (red dashed line). This figure shows that the top ranking center remained the lowest ranking center; however center B and H increased in their rank with respect to the other centers, while centers C, E, G, I, and J dropped in their ranking position.