Abstract
When challenged with a variety of inflammatory threats, multiple systems across the body undergo physiological responses to promote defense and survival. The constellation of fever, anorexia, and fatigue is known as the acute illness response, and represents an adaptive behavioral and physiological reaction to stimuli such as infection. On the other end of the spectrum, cachexia is a deadly and clinically challenging syndrome involving anorexia, fatigue, and muscle wasting. Both of these processes are governed by inflammatory mediators including cytokines, chemokines, and immune cells. Though the effects of cachexia can be partially explained by direct effects of disease processes on wasting tissues, a growing body of evidence shows the central nervous system (CNS) also plays an essential mechanistic role in cachexia. In the context of inflammatory stress, the hypothalamus integrates signals from peripheral systems, which it translates into neuroendocrine perturbations, altered neuronal signaling, and global metabolic derangements. Therefore, we will discuss how hypothalamic inflammation is an essential driver of both the acute illness response and cachexia, and why this organ is uniquely equipped to generate and maintain chronic inflammation. First, we will focus on the role of the hypothalamus in acute responses to dietary and infectious stimuli. Next, we will discuss the role of cytokines in driving homeostatic disequilibrium, resulting in muscle wasting, anorexia, and weight loss. Finally, we will address mechanisms and mediators of chronic hypothalamic inflammation, including endothelial cells, chemokines, and peripheral leukocytes.
Keywords: Hypothalamus, Acute illness response, Cachexia, Neuroinflammation
1. Introduction
Sickness behaviors and their associated metabolic responses are among the most ubiquitous and readily identifiable aspects of acute and chronic illness. In the context of acute threats, the onset of fever, anorexia, lethargy, and catabolism of lean body tissues all evolved as important defenses to promote survival. However, with chronic and ongoing inflammation, these same defenses prove to be a double-edged sword, leading to neurodegeneration, psychiatric conditions, and cachexia. Cachexia is an important predictor of morbidity and mortality in a diverse array of conditions ranging from infectious disease to cancer. Though a large body of work has focused on the direct interaction of cytokines and inflammatory mediators with muscle tissue in the development of cachexia, these direct effects do not account for all aspects of body mass alterations in acute and chronic illness. An increasing body of work suggests the central nervous system (CNS) is a key mechanistic force in the pathogenesis of cachexia by sensing inflammation, integrating information from peripheral organ systems, and evoking downstream changes in body mass and metabolism.
Decades of investigation provide ample evidence that the CNS functions as both a receiver and amplifier of peripheral inflammatory insults, thereby orchestrating a behavioral and metabolic program that leads to wasting and debilitation if prolonged. As a demonstration of neuronal signaling leading to amplified response to inflammation, intracerebroventricular (ICV) injection of proinflammatory cytokines potently induces anorexia, lethargy, and catabolism at doses far below the threshold for response with peripheral injection [1–4]. Collectively, existing data support a model wherein peripheral inflammatory insults are amplified and modified within the mediobasal hypothalamus (MBH), creating a paracrine inflammatory milieu that in turn initiates and sustains alterations in the activity of neuronal populations that regulate appetite and metabolism. The attenuated blood brain barrier (BBB) and dynamic regulation of vascular access found in this region is undoubtedly one of the reasons this brain area is highly sensitive to a number of metabolic and inflammatory signaling molecules [5]. This review will focus on recent insights in how stimuli of hypothalamic inflammation, including dietary, infectious, and neoplastic sources, are sensed in the CNS and translated peripherally into illness responses and cachexia.
1.1. Central nervous system control of body mass and energy homeostasis
Within the MBH, there are several neuronal populations in various nuclei responsible for regulation of appetite, body mass, and energy homeostasis. These populations include anorexigenic and orexigenic neuronal subsets, which decrease and increase appetite and food intake respectively. Key anorexigenic populations include pro-opiomelanocortin (POMC) and cocaine and amphetamine regulated transcript (CART) expressing neurons [6]. POMC is a precursor polypeptide that can be differentially processed for production of αMSH, ACTH, and opioid peptides βendorphin and [met]enkephalin [7]. POMC neurons release the anorexic neurotransmitter αMSH, which binds to type 4 melanocortin receptors (MC4R) in a number of downstream sites in the brain to decrease appetite and energy storage. CART is a neuropeptide first discovered as a transcriptional target of cocaine and amphetamine exposure, but was subsequently found to act as an endogenous psychostimulant that increases locomotor activity and decreases food intake [8]. Conversely, two key orexigenic populations include neurons expressing agouti-related peptide (AgRP) and neuropeptide Y (NPY). AgRP was first characterized because of a curious connection between pigmentation and metabolic phenotypes, both of which act through melanocortin receptors: mice carrying the dominant Agouti allele known as lethal yellow not only demonstrate a yellow pigmentation, but are profoundly obese [9]. Further characterization determined that the counter-regulatory neuropeptide AgRP functions as an antagonist of the MCR4 receptor and AgRP neurons directly inhibit POMC neuronal activity, producing increased appetite and energy storage [10,11]. NPY was first identified as a neuropeptide structurally and biologically similar to the intestinal peptide YY (PYY), and is a potent orexigen in the brain and autonomic nervous system [12,13]. Both anorexigenic and orexigenic neuronal circuitry are affected by physiologic cues and by pathophysiologic signals such as cytokines and pathogen-associated molecular patterns (PAMPs) [14]. Therefore, the MBH is a key central target of cytokine signaling and provides an important primary neuronal substrate linking inflammation to muscle catabolism, anorexia, and other sickness responses [15–19].
2. The brain-muscle axis: a model for hypothalamic mechanisms of illness responses and cachexia
In states of illness, the body initiates several processes to mobilize energy stores and provide anabolic building blocks for the acute phase response. While these processes are integral to normal immune physiology, they can result in pathologic homeostatic disequilibrium, where the body assumes a state of energy wasting. As a result, tissue breakdown occurs in organs important to activities of daily living. Muscle is an abundant source of amino acids, and often the main target of pathologic tissue breakdown in disease. Selective catabolism of skeletal muscle is one of the hallmarks of cachexia. It is well known that prolonged systemic inflammation causes skeletal muscle breakdown [20–22]. While much of the literature has focused on catabolic pathways within muscle itself, a growing body of evidence indicates the CNS is a key mediator in this process [23–25]. In particular, the hypothalamic-pituitary (HPA) axis promotes catabolism of carbohydrates, lipids, and proteins in peripheral tissue in response to cytokines that enter the CNS. As a result, adipose tissue undergoes lipolysis, and proteolysis occurs in skeletal muscle [25]. However, skeletal muscle proteolysis occurs selectively over lipolysis in states of neuroinflammation [26].
2.1. Skeletal muscle catabolism is mediated by hypothalamic cytokines
A series of studies demonstrate that through the actions of the prototypical inflammatory cytokine IL-1β, a cascade of signaling is initiated in the hypothalamus, resulting in both local and systemic changes in gene expression, protein synthesis, and neuroendocrine signaling [23,24,26,27]. In a rodent model of cancer cachexia, hypothalamic expression of IL-1β mRNA was increased. When IL-1β was administered ICV, animals experienced profound muscle wasting. Importantly, this effect did not occur when IL-1β was administered IP [26], supporting the hypothesis that central, rather than peripheral inflammation is the main instigator of muscle catabolism in this model of cachexia.
While hypothalamic IL-1β alone can induce muscle atrophy [26], other pro-inflammatory cytokines are implicated in this process. However, there is conflicting evidence regarding the significance of these cytokines, and additional research is needed to fully elucidate their mechanisms [27]. For example, IL-6 is well established as an important mediator in muscle metabolism [28,29] but its role within the hypothalamus is not well known. Although systemic inflammation increases levels of IL-6 in the hypothalamus in concordance with altered energy homeostasis [30], the effect of ICV IL-6 on skeletal muscle has not been examined. Furthermore, TNFα can directly induce skeletal muscle catabolism [31–33] and produces anorexia when administered centrally [34,35], yet ICV administration of TNFα in rodents does not induce skeletal muscle thermogenesis [34]. However, follow-up studies are lacking, and thus the roles of hypothalamic TNFα, IL-6, or other proinflammatory cytokines in skeletal muscle catabolism remain largely unknown. As such, this remains an important area of research in the pathophysiology of cachexia.
2.2. Role of neuroendocrine modulation of muscle mass
Following the onset of hypothalamic inflammation, a neuroendocrine signaling cascade is initiated, including marked activation of the HPA axis [5]. These neuroendocrine mediators can act directly on skeletal muscle, or act on other organs to amplify the inflammatory signal. It is also likely that CNS mediated activation of the sympathetic nervous system contributes to the disordered muscle metabolism in cachexia, including inhibition of protein synthesis as well as proteolysis within skeletal muscle and myofibril breakdown [26]. Endogenous glucocorticoids such as cortisol (or corticosterone in rodents) are important in their ability to modify metabolism of fats, carbohydrates, and proteins, as well as exert control over immune response in times of stress. The chief regulation of glucocorticoid release in humans is at the level of the hypothalamus and pituitary, which produce corticotrophin releasing hormone (CRH) and adrenocorticotrophic hormone (ACTH) respectively. CRH, manufactured in the paraventricular nucleus (PVN) of the hypothalamus, provides the initiating signal of the HPA axis by promoting release of ACTH from corticotroph cells of the anterior pituitary. Direct stimulation by ACTH causes production and release of cortisol from the zona fasiculata of the adrenal cortex [36]. Broadly speaking, stress is the key activator of the HPA axis. This system is widely evolutionarily conserved as a method of mounting a defense against stressors including infection and starvation, largely by shifting targets of anabolism and catabolism. While the immune system must undergo tremendous anabolism during stress, with increased production of granulocytes and acute phase proteins, other systems must undergo catabolism in order to provide a supply of biomolecules and energy, particularly in the face of stress-induced anorexia. Skeletal muscle is a major site of protein storage, and serves as a primary target of glucocorticoids. Thus, upon HPA axis activation, skeletal muscle undergoes catabolism to increase plasma levels of free amino acids [26]. These amino acids can be used either for production of proteins such as acute phase reactants, or can enter as Krebs cycle intermediates and serve as gluconeogenic substrates in the liver [37]. This process is mediated by proteosomal degradation of muscle protein, with E3 ubiquitin ligases, Muscle Atrophy F-box (MAFBx), and Muscle Specific Ring Finger Protein 1 (MuRF1) playing key roles [38,39].
In the early phases of acute illness, the catabolism of skeletal muscle provides an important energy substrate upon which other defenses can be built. However, when inflammation persists in the context of subacute and chronic disease, this mobilization of protein from skeletal muscle leads to substantial atrophy and functional impairment. The clinical archetype of “matchstick limbs” seen in Cushing’s disease, a syndrome resulting from ACTH-secreting pituitary adenomas, serves as a classic illustration of skeletal muscle derangements evoked by the HPA axis [40]. However, Cushing’s disease is only one of many conditions known to cause neuroendocrine muscle wasting. Compelling evidence demonstrates that the CNS invokes peripheral muscle wasting via the HPA axis in both cancer and diabetes, with hypothesized roles in numerous other chronic illnesses [27,41]. As such, the CNS not only is tied to the global metabolic dysregulation and behavioral aspects of the illness response, but it is also able to employ neuroendocrine signaling to invoke an indirect pathway of disease-mediated skeletal muscle atrophy.
3. Mechanisms of hypothalamic inflammation in metabolic derangements: insights from high fat diet and obesity
Consistent with a wide variety of other pathologies, hypothalamic inflammation occurs in both acute and chronic stages. In both instances, the hypothalamus acts in a feed-forward loop to propagate inflammatory responses in the periphery, including changes in behavior and metabolism. To understand how signals arising from the periphery can induce disordered systemic metabolism via a hypothalamic relay, it is illustrative to examine obesity as a systemic inflammatory disorder. In particular, exposure to high fat diet (HFD) is widely studied as a cause of hypothalamic inflammation that in turn leads to significant alterations in body mass regulation and energy homeostasis. Specifically, HFD exposure causes acute inflammation and gliosis in the MBH, which alters metabolic signaling in this part of the brain [42]. HFD exposure therefore represents one of several acute inflammatory insults capable of inducing global metabolic derangements via hypothalamic signaling.
Multiple forms of dietary stressors have been investigated for their ability to induce hypothalamic inflammation and downstream alterations in metabolism, including high sucrose diet, high polyunsaturated fatty acid diet, and high saturated fat diet [43,44]. Though all of these diets are capable of producing obesity in experimental models, hypothalamic inflammation and gliosis only consistently ensues in the context of high saturated fat diets. These CNS manifestations substantially precede the onset of overt changes in weight and body mass, occurring within 1–4 days of the onset of a high saturated fat diet [42,45]. A key site of inflammation is the arcuate nucleus, where HFD produces reactive gliosis, increased expression of inflammatory genes IL-1β, IL-6, and TNFα, astrocyte injury, and eventually POMC neuronal injury [42,46]. Further studies indicate that, similarly to peripheral macrophages, microglia of the MBH exhibit an M1-dominant inflammatory response in the presence of saturated fatty acids [46].
To date, studies investigating the mechanisms of HFD-induced hypothalamic inflammatory changes have identified two main pathways: NF-κB signaling and endoplasmic reticulum stress. The hypothalamus demonstrates a different pattern of NF-κB activity compared to peripheral systems, including higher expression of both IKKβ and NF-κB inhibitory protein IKBα, with an overall suppression of NF-κB activity [47]. However, with HFD exposure, this dynamic is altered to significantly increase NF-κB activity in the MBH. Forced suppression of NF-κB signaling via MBH-targeted IKKβ knockout results in decreased dietary intake, while MBH-targeted constitutive NF-κB activation results in central insulin and leptin resistance. This same study identified hypothalamic endoplasmic reticulum stress as both an upstream inducer and downstream event of NF-κB signaling [47]. Further studies confirm the importance of NF-κB to hypothalamic energy homeostasis. In both leptin deficient and diet-induced obese (DIO) mice, pharmacologic and genetic inhibition of IKKβ/NF-κB signaling in the arcuate nucleus results in improvements in glucose tolerance and hypothalamic insulin signaling, as well as increasing energy expenditure [48].
The importance of hypothalamic inflammation rests primarily with the fact that the hypothalamus is a central regulator of whole-body metabolism, which produces substantial downstream consequences. Central administration of the saturated fat palmitic acid not only induces a program of hypothalamic inflammation, including increased local expression of cytokines IL-6, IL-1β, and TNFα, but it also leads to decreases in leptin-induced mRNA expression related to gluconeogenesis, glucose transport, and lipogenesis in the liver [49]. These peripheral pathological outcomes are abrogated by reduction of hypothalamic inflammation. For example, hypothalamus-specific inhibition of Toll-like receptor 4 (TLR4) and TNFα both result in improved insulin sensitivity in the liver, resulting in decreases in hepatic steatosis and gluconeogenesis [50]. However, TLR4-mediated and TNFα-mediated hypothalamic signaling lead to divergent downstream consequences, with only TLR4 signaling inhibition leading to loss of body mass.
Overall, these findings demonstrate that hypothalamic inflammation results as a consequence of high exposure to saturated fat, and through inflammatory changes and recruitment of glial cell populations is able to alter both peripheral metabolism and behavior. As such, HFD models provide a compelling example of how peripherally derived inflammatory stimuli can induce systemically significant pathophysiological changes in the hypothalamus. In contrast to infectious and neoplastic sources of inflammation, however, HFD eventually produces changes which inhibit anorexigenic POMC and CART neurons, while increasing expression of orexigenic NPY and AgRP. Therefore, hypothalamic inflammation is a potent inducer of peripheral pathophysiological states, the manifestations of which vary substantially with the type and duration of inflammatory stimulus. Through what is in many ways a similar mechanism, other inflammatory insults of the hypothalamus provoke the familiar anorexia, fever, and weight loss known collectively as the acute illness response.
4. Hypothalamic inflammation and the acute illness response
The most commonly experienced cause of the acute illness response is logically that which the system directly evolved to combat: the immediate threat of infection. The quintessential traits of acute inflammation and sickness were described as early as Roman antiquity; however, many molecular mechanisms linking inflammation to sickness behavior remain incompletely understood. Even though it is clear that acute inflammation resulting from infection can be deadly, as in sepsis, it is also increasingly clear that acute phase responses are essential to survival. Thus, a significant and growing body of work has focused on understanding the peripheral and central mediators of the response to acute infection, and determining whether each individual step of the process is beneficial or deleterious. The hypothalamus is a vital component of the system responsible for sensing and responding to infectious stimuli, serving as an upstream effector of fever, mobilization of energy stores, and initiation of sickness-associated behaviors. As such, research has focused on two arms of this system: first, how the hypothalamus responds to infectious stimuli on a molecular and signaling level, and second, how hypothalamic sensing of threats leads to downstream manifestations of sickness.
In its role as a sensor of acute infectious stimuli, the hypothalamus employs a diverse array of danger and pathogen associated molecular pattern (DAMP and PAMP) receptors, as well as being robustly responsive to cytokines and chemokines. Peripheral or central injection of viral or bacterial PAMPs or pro-inflammatory cytokines produces neuronal activation in several brain regions, particularly in nuclei that make up the MBH and its associated vascular structures, collectively known as the median eminence [51–53]. Within this region, two key appetite regulating populations of centrally projecting neurons alter their roles as effectors during inflammatory responses: the anorexigenic POMC and CART, and the orexigenic AgRP and NPY.
Much of the current understanding of acute hypothalamic inflammation derives from experiments using lipopolysaccharide (LPS), a PAMP isolated from the outer membrane of gram-negative bacteria. LPS binds to TLR4 to induce canonical NF-κB signaling, which alters gene transcription to produce a myriad of cytokines, chemokines, and stress response proteins. Both ICV and IP LPS produce acute sickness responses, suggesting it can act through direct interactions with the hypothalamus as well as indirect pathways from the periphery (although with far greater potency after direct CNS administration). Upon exposure to LPS, animals develop sickness-associated anorexia, and orexigenic signaling via NPY decreases at the transcriptional level [54,55]. Similarly, AgRP secretion is decreased following LPS exposure, even though its mRNA levels are increased [17]. Conversely, the appetite inhibiting pathways are activated following LPS exposure. With acute LPS stimulation, POMC neurons are activated, and MC4R and POMC mRNA levels increase [56,57]. Accordingly, both pharmacologic inhibition of MC4R signaling with AgRP, and repression of POMC neuron activation both abrogate the anorexia response following LPS exposure [57–59].
Similar to HFD exposure, the molecular mechanism of LPS-induced hypothalamic inflammation involves NF-κB signaling via TLR4 and endoplasmic reticulum stress [60]. Specifically, TLR4, MyD88, and CD14 are critical to the initiation of sickness behaviors: mice with genetic deletion of any of these proteins demonstrate reduced anorexia in response to IP LPS compared to wild type [54,61]. Fever, anorexia, and hypothalamic inflammation responses to LPS exposure involve the signaling intermediate atypical protein kinase C, whereas hypoactivity and weight loss seem to be mediated by separate pathways [62]. Additionally, inducible nitric oxide synthase (iNOS) is induced by LPS exposure in the MBH, where nitric oxide inhibits orexigenic neurons via a STAT dependent mechanism independent of prostaglandin synthesis [63,64]. Obviously, these are important but not exclusive molecular signaling pathways whereby PAMP exposure is translated into behavior, nor is the MBH the only brain region involved. Indeed, it is clear that the brainstem has both redundant and exclusive roles relative to the MBH (for example), but discussion of the entirety of the CNS response to inflammation is beyond the scope of this review.
Though a large body of work has focused exclusively on LPS as an instigator of hypothalamic inflammation of infectious etiologies, viral proteins and nucleic acids are less explored, yet potent inducers of central inflammation. In a study by Jang et al., viral and bacterial components, Tat and LPS respectively, were compared in their effects on hypothalamic inflammation and resultant sickness behavior. Following IP exposure to Tat or LPS, NF-κB was acutely activated in the MBH, which induced hypothalamic production of the pro-inflammatory cytokines IL-1β, IL-6, and TNFα. In corticotroph AtT20 cells, NF-κB served as a transcriptional regulator of increased POMC expression following exposure to LPS, Tat, and pro-inflammatory cytokines IL-1β and TNFα. Hypothalamic injection of LPS or Tat both caused a significant reduction in food intake and body weight. However, these effects were prevented by blockade of NF-κB signaling via IKK inhibitory peptide, as well as by blockade of melanocortin signaling via administration of AgRP. Furthermore, specific IKKβ knockout in POMC neurons attenuated anorexia following LPS and Tat exposure. Hypothalamic NF-κB was also activated by high doses of ICV leptin and serves as a transcriptional regulator of leptin-stimulated POMC expression, suggesting NF-κB is common signaling pathway for all three stimuli [57]. In addition to viral proteins, the viral double stranded RNA mimetic poly I:C causes fever, malaise, anorexia, and hypoactivity [65–67]. While the fever induced by poly I:C depends partially on IL-1β and IFNα, the mechanism of viral induction of sickness behavior is not yet fully understood – however, this PAMP clearly activates inflammatory signaling and HPA axis activation at the level of the hypothalamus [65,68,69].
Importantly, the hypothalamus regulates exposure to inflammatory stimuli differently from peripheral sites of surveillance. While LPS priming decreases the magnitude of cytokine release in the hypothalamus in subsequent LPS exposures, it has the opposite effect in the spleen, where increased levels of IL-1β and IL-1R are induced by repeated exposures [70]. Furthermore, IP LPS exposure time-dependently increases STAT3 phosphorylation in both the hypothalamus and liver, but the hypothalamus demonstrates a relatively more acute and transient signaling response compared to the liver [71]. Most intriguing of all is the recent discovery that microglia have a distinct ontogeny from the peripheral immune system, which may explain why it differs in its responses to inflammatory events [72]. Despite the long-held assumption that microglia originate in bone marrow, akin to other monocytes, fate mapping demonstrates that microglia are seeded from the embryonic yolk sac and remain as a self-sustaining population throughout life. While other tissue macrophages arise from this same embryonic event, the key difference is that only the peripheral tissues continue to recruit from hematopoietic stem cells of the bone marrow [73]. Microglia may recruit from the peripheral monocyte pool during times of profound pathology, such as major insults to the BBB, but they remain an isolated and selfrenewing population in most physiological states. This discovery is important, as the distinct lineage of microglia suggests they are likely to have distinct molecular signaling pathways to detect and propagate inflammation. These combined data suggest acute hypothalamic inflammation is a process robustly induced by a variety of stimuli, proceeds through distinct signaling modalities compared to peripheral tissues, and requires a combination of signal initiation and signal propagation events. A variety of signaling mediators and anatomical considerations make hypothalamic inflammation a particularly unique and a targetable niche of acute sickness responses and cachexia alike.
4.1. The role of cytokines in acute hypothalamic inflammation and cachexia
While cytokines are key signaling mediators in normal immune responses, high levels are damaging and can lead to sustained inflammation with many detrimental effects. In cancer, higher levels of cytokines correlate with poorer outcomes [74]. Furthermore, elevated circulating levels of TNFα and IL-6 in cardiac cachexia are the strongest predictor for pathological weight loss [75,76]. While cytokines have significant effector functions in various organs including muscle [77], it is their action in the CNS, specifically the hypothalamus, that is the primary driver of the behavioral features of cachexia [78]. In spite of the presence of the mostly impermeable BBB, there are several ways cytokines can enter the brain from the circulation. First, since circumventricular organs lack a BBB, most cytokines can enter the hypothalamus by volume diffusion [79]. In addition, some cytokines, including IL-6 [80], TNFα [81], and IL-1β [82], can cross through various transporter systems. Lastly, circumventricular organs and the choroid plexus contain macrophage-like cells that express TLRs, allowing cytokines to exert their functions without entering the brain parenchyma [83].
Dozens of different cytokines have been implicated in cachexia. However, in the hypothalamus, the most robust data supports roles for TNFα and IL-1β in cachexia and sickness behavior [84,85].
4.1.1. TNFα
Although TNFα has direct effects on various target tissues [84,86,87], its most potent actions in regard to cachexia occur in the hypothalamus. Within the hypothalamus, the actions of TNFα alone lead to anorexia, thermogenesis, and increased respiratory rate [34,88,89]. Exposure of ventromedial hypothalamic slice cultures to TNFα causes decreased firing rate of neurons in that nucleus [90]. In rodents, ICV injection of TNFα leads to decreased food intake and increased respiratory quotient [89]. These symptoms correlate with increased expression of mediators of the JAK/STAT signaling pathway within the hypothalamus, indicating this signaling mechanism is an important mediator in sickness behavior. Furthermore, ICV injection of TNFα leads to reduced food intake and weight loss. In addition to the JAK/STAT signaling pathway, it also was determined that TNFα signaling within the hypothalamus leads to increased thermogenesis in brown adipose tissue via βadrenergic signaling [34]. Further studies are necessary to determine the specificity of different TNFα signaling pathways within the hypothalamus in cachexia.
4.1.2. IL-1β
IL-1β is established as one of the primary molecules in neuroimmune signaling. While IL-1β can act on numerous different types of cultured cells found within the brain, functional IL-1 receptors are primarily localized to endothelial cells and certain neuronal populations, including those within the ARC [91]. It is well documented that systemic inflammation increases IL-1β activity in the hypothalamus [26,92,93]. IP injection of LPS induced increased IL-1β expression in endothelial cells of the PVN [92]. Furthermore, IL-1β is an important cytokine in the hypothalamic relay leading to skeletal muscle catabolism [26]. In rodents, chronic ICV administration of IL-1β induces skeletal muscle wasting [26]. In the same study, development of carcinoma-induced cachexia was associated with increased hypothalamic IL-1β expression. In addition, knockout of endothelial IL-1 receptor attenuated expression of IL-1β, TNF-α, and IL-6 mRNA in the brain in a mouse model of chronic stress [94].
5. From acute to chronic: how hypothalamic inflammation contributes to transition from sickness to cachexia
5.1. Pathological transition from acute to chronic inflammation
Although IL-1β and TNFα potently induce acute anorexia, animals rapidly desensitize to continuous ICV administration [95–98]. This tachyphylaxis indicates these cytokines are not sufficient to produce sustained catabolism, suggesting other mediators are necessary to maintain and amplify the inflammatory signal. While the mechanism of chronic hypothalamic inflammation in cachexia is not fully understood, several candidates have been identified. The cytokine leukemia inhibitory factor (LIF) is expressed in the ARC, and can induce anorexia in inflammatory disease [2,99,100]. In contrast to IL-1β and TNFα, chronic administration of LIF does not induce tachyphylaxis [101–103]. However, the neuroinflammatory actions of LIF appear to overlap with IL-1β synergistically, suggesting that it may be a secondary amplifier of inflammatory response rather than the sole mediator of chronic hypothalamic inflammation [104].
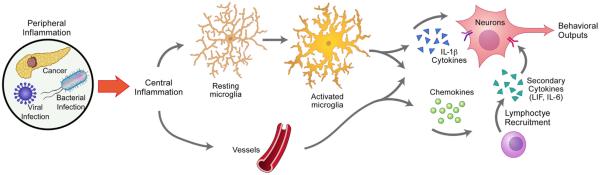
While most studies focus on the altered signaling of neurons within the hypothalamus in producing sickness response, an emerging body of evidence suggests non-neuronal cells play an important role in the transition from acute to chronic inflammation in the CNS in cachexia. In particular, activation of hypothalamic microglia and endothelium, followed by secondary recruitment of peripheral leukocytes, may lead to the observable changes in neuronal activity (Fig. 1). Specifically, cytokines initiate a cascade involving endothelial activation, followed by increased expression of cell adhesion molecules, secretion of chemokines, and recruitment of peripheral leukocytes. While the role of these mediators in the hypothalamus is not well known, they represent an important area for future research in the field of cachexia.
Fig. 1.
Initiation, amplification, and perpetuation of hypothalamic inflammation. Repeated CNS exposure to primary inflammatory cytokines results in tachyphylaxis; therefore, additional pathophysiological steps must be involved to maintain chronic inflammation in cachexia. Upon entering the CNS, cytokines bind to PAMP receptors and activate numerous types of cells, including glia and hypothalamic vascular endothelial cells. Microglia are recruited and activated by PAMPs, which results in increased release of cytokines and chemokines. Endothelial cell activation in vasculature results in secretion of IL-1β and additional cytokines, chemokine secretion, and expression of cell adhesion molecules. Chemokines and adhesion molecules in turn recruit leukocytes from the periphery. These cells secrete secondary cytokines, such as IL-6 and LIF. These pathways ultimately converge on neurons to elicit neuroendocrine, metabolic, and behavioral changes.
5.2. A reactive endothelium and leukocyte recruitment: new frontiers in neuroinflammatory mechanisms of cachexia
When crossing from the periphery to the CNS, cytokines and other inflammatory molecules first encounter endothelial cells, which express high levels of cytokine receptors. This interaction leads to secretion of additional cytokines, expression of selectins, production of chemokines, and peripheral lymphocyte recruitment. While the role of these mediators has not been studied extensively in the hypothalamus specifically, there is evidence to suggest they play a key role in the sequelae of cachexia (discussed further below). Further studies are necessary to determine possible variations in these immune response proteins within the hypothalamus. Furthermore, these mediators have not yet been targeted in the context of cachexia, presenting an unexplored opportunity for therapeutic intervention.
5.2.1. An activated endothelium
Upon exposure to inflammatory products, vascular endothelial cells enhance their primary role in the circulatory system by activating pathways designed to combat pathogens. Endothelial cells express TLRs that serve as activation switches in response to a variety of DAMPs and PAMPs, leading to several phenotypic changes. Upon activation, inter-epithelial junctions are down-regulated, allowing for immune cells to access tissue and fight pathogens. Furthermore, endothelial cells can produce cytokines and molecules such as metalloproteinases that are directly toxic to pathogens, as well as adhesion molecules to promote leukocyte migration and extravasation. In the CNS, neuroinflammation activates endothelial cells and other cells associated with the BBB. If the neuroinflammation persists, this can cause BBB breakdown, which is associated with several pathologies such as Alzheimer’s disease [105], Parkinson’s disease [106], vascular dementia [107], stroke [108], and multiple sclerosis [109].
5.2.1.1. Cytokines and the hypothalamic endothelium
All of the major cytokines involved in cachexia play a role in activating endothelial cells. It is well known that increased expression of cytokines selectively occurs within endothelial cells of circumventricular organs of the hypothalamus during systemic inflammation [83,92,93]. For example, injection of LPS leads to increased expression of IL-1β in endothelial cells of the hypothalamus [93]. This induces the production of numerous immune response elements, including cytokines, adhesion molecules, matrix metalloproteinases, and coagulation factors [110]. In addition, IL-1β activates cyclooxygenase in cerebral endothelium, leading to production of the pyrogenic arachidonic acid metabolite prostaglandin E2 [111]. Furthermore, upon exposure to LPS, IL-1β is a key mediator to microglial production of the vasodilator and neuromodulator nitric oxide, via increased biosynthesis of iNOS [112].
Additional cytokines play important roles in endothelial activation as well. In cerebral microvascular endothelial cells, TNFα exposure induces Rho activation and myosin light chain phosphorylation, leading to a gradual increase in permeability and loss of endothelial junctions [113]. IL-6 is produced locally by brain endothelium upon LPS injection [114] and can be further induced by IL-1β [115]. Lastly, although it does not cross the BBB [116], TGF-β accumulates in cerebral endothelium [117] and is reported to increase BBB permeability during inflammation [118].
While endothelial activation in response to cytokines is not exclusive to the hypothalamus, the circumventricular structures – including the MBH – make this organ uniquely equipped to sense, amplify, and respond to inflammatory molecules. First, the MBH is a highly vascularized structure lacking a BBB, containing mainly fenestrated capillaries, which provides circulating materials direct access to the parenchyma. Second, due to its adjacency to the third ventricle, the MBH is in direct contact with the CSF, furthering its exposure to circulating solutes within the CNS. Third, within the ependymal lining of the third ventricle are specialized cells called tanycytes. These cells are found exclusively within the ventricular lining of the hypothalamus, and extend processes deep into the parenchyma [119]. Although relatively little is known about tanycytes, evidence suggests they are heavily involved in energy homeostasis and hypothalamic neuroendocrine signaling [120]. However, the role of these cells in cachexia has not been investigated. Lastly, as described previously, the hypothalamus is the feeding center of the CNS, and many of these areas, including the PVN and ARC, express receptors for these cytokines [91,121–123].
Extensive vasculature and ample access to cytokine exposure make the hypothalamus an ideal location for signal amplification via endothelial signaling. Furthermore, in response to cytokines, cell adhesion molecules are upregulated and chemokines are secreted, subsequently leading to peripheral immune cell inflltration. All of these factors make the hypothalamus prone to perpetuation of inflammation, which is critical for development of cachexia.
5.2.1.2. Cell adhesion molecules
Endothelial adhesion molecules function to tether circulating leukocytes to vascular endothelium, as well as facilitate rolling and migration into tissue. There are several different adhesion molecules expressed on leukocytes and endothelial cells, which fall into two broad categories: selectins, which are expressed on endothelial cells (with the exception of L-selectin) and integrins, which are expressed on leukocytes [124].
Cellular adhesion molecules have very low levels of basal expression and are only upregulated in inflammatory conditions [125]. While expression of these molecules is necessary for an appropriate immune response, they can be markers of pathologic inflammation. Increased expression of adhesion molecules is reported in numerous systemic inflammatory diseases, such as atherosclerosis [126], heart failure [127] inflammatory bowel disease [128], allergy [129], renal disease [130], COPD [131], and cancer [132]. While the role of adhesion molecules in local immune responses has been studied extensively in nearly every condition that causes cachexia, their role in the pathophysiology of sickness behavior and cachexia itself is less well known. In patients with cancer cachexia, P-selectin polymorphisms are predictive of increased muscle wasting [133]. In an accompanying rodent model of cachexia, the P-selectin gene was a top early-induced gene [133]. Furthermore, a follow-up study found P-selectin polymorphisms were associated with increased risk of developing cachexia in pancreatic cancer patients [134]. These studies assessed the role of P-selectin in skeletal muscle, rather than the CNS. No studies have investigated the expression and function of P-selectin or other adhesion molecules within the hypothalamus in cachexia. However, these adhesion molecules are highly upregulated in other portions of the brain during acute and chronic inflammation [135–137]. Furthermore, in rodent models of liver inflammation, cellular adhesion molecules within the CNS are implicated as important mediators of sickness behavior [136–138]. Liver disease is often associated with high plasma concentrations cytokines and endotoxins, which results in increased expression of cellular adhesion molecules, chemokines, and subsequent leukocyte recruitment. Kerfoot et al. reported that in mice with cholestatic inflammatory liver disease, there were increased levels of endothelial adhesion molecule VCAM-1. This correlated with increased levels of monocytes within brain parenchyma. Furthermore, blockade of leukocyte trafficking molecules α-4 integrin and P-selectin abolished this effect [136]. In a follow-up study administration of the same blocking antip combination resulted in a decrease in sickness behavior, quantified by social interaction, in mice with inflammatory liver disease [137].
These results, along with substantial evidence indicating adhesion molecules play a prominent role in other neuroinflammatory diseases such as multiple sclerosis [139], Alzheimer’s disease [140], and stroke [141], make it reasonable to suspect these molecules are important in the pathophysiology of cachexia. Furthermore, viral models of cachexia suggest leukocyte recruitment into the CNS, which is mediated by chemokines and adhesion molecules, also has an important role in sickness behavior [142,143] (see Section 5.2.3).
5.2.2. Chemokines
Chemokines are small proteins that attract and activate immune cells. They are involved in virtually all pathologies with an inflammatory component. While their role in the immune response is well known, it was only recently discovered that they are prominent mediators of CNS response to stress. In the CNS, chemokine receptors are upregulated on astrocytes [144], microglia [145], and neurons [146] during neuroinflammation. In states of stress, hypothalamic chemokines play an important role in the pathophysiology of cachexia [147]. ICV administration of numerous chemokines into the rat brain, including IL-8/CXCL8, IP-10/CXCL10, CCL2 and RANTES/CCL5, decrease short-term food intake [148]. During states of stress, these chemokines are expressed mainly in circumventricular organs [147]. Furthermore, CXCL8 expression in the PVN is implicated in signaling of stress hormones, such as ACTH and CRH [149].
Various chemokines cause a leukocyte response in the CNS via migration from the periphery into the brain parenchyma. While the role of these molecules in the hypothalamus in cachexia is not yet known, previous studies show they are important in endothelial activation and maintenance of neuroinflammation. For example, Wu et al. showed that genetic deletion of CCR2 in mice prevented endothelial activation and leukocyte recruitment into the CNS after ICV LPS injection [150]. Similarly, when CCR2 is knocked out of microglia, monocyte inflltration into the CNS is decreased during systemic inflammation. This subsequently results in decreased sickness behavior, at least as measured by diminished social interaction [137].
Furthermore, CXCL10 is associated with T-cell responses, and regulates the migration of T-cells into the brain parenchyma in response to various neuroinflammatory states [142,151]. It is induced by IFN-Y [143] and in the CNS is expressed almost exclusively in astrocytes [143,152]. While the role of CXCL10 in the hypothalamus has not been studied, mice infected IP with the parasite Trypanosoma brucei showed increased CXCL10 expression in the hypothalamus [153]. Furthermore, mice with global CXCL10 deletion show decreased mortality in lymphocytic choriomeningitis virus (LCMV) infection, a viral model of cachexia (see Section 5.2.3) [142,143]. However, these knockout studies did not assess hypothalamic inflltration or sickness behavior. Future studies should investigate whether CCR2 or other chemokine knockouts can attenuate additional inflammation or alleviate cachexia symptoms.
High levels of chemokines within the brain, along with increased expression of adhesion molecules on endothelial cells suggest a mechanism of recruiting peripheral cells into the CNS during neuroinflammation and a potential role for these cells in cachexia. While the role of peripheral leukocytes has not been studied in most forms of cachexia, for over 20 years immunologists have been studying a murine model of viral CNS infection that induces profound wasting. This wasting is mediated almost entirely by inflltration of peripheral lymphocytes into the CNS. As described in the following section, this model presents a powerful means for investigating the role of leukocytes in viral cachexia, and a framework for studying these cells in other causes of sickness behavior.
5.2.3. Leukocyte recruitment
While leukocyte trafficking into the CNS is evident in several neurological diseases that result in cachexia, their role in the pathophysiology of hypothalamus mediated sickness behavior is unknown. However, studies of LCMV infection demonstrate that peripheral leukocytes enter the CNS and play an important role in maintaining cachexia [154–156]. T-cells release a number of cytokines capable of producing an illness response when injected centrally, including IL-6 and LIF [157,158]. In animals inoculated ICV with LCMV, anorexia and lethargy are maintained by MHC II restricted CD4+ cells [154]. Mice treated with anti-CD8 antibodies or HLA Class I knockout then infected with LCMV experienced non-lethal chronic wasting, losing approximately 25% body weight over the course of 32 days before recovering [154].
While the LCMV model is used extensively from the immunology standpoint to study persistent infections, it has rarely been used to study cachexia [156,159]. Kampershroer and Quinn infected CD8+ T-cell deficient mice with LCMV via ICV inoculation and found that wasting was dependent on IFN-γ and IL-1, with IL-6 contributing to symptoms [159]. However, in contrast to previous cachexia literature, wasting was not dependent on TNFα. In a recent study, Stamm et al. injected WT mice with LCMV virus IV at different doses and sought to identify dose effects and further characterize mediators of wasting. The authors found a positive correlation between weight loss and viral dose, but were unable to attenuate symptoms with IFN-γ or IL-1 blockade [156]. Nevertheless, these studies show the LCMV model is a potent inducer of cachexia and these intriguing results suggest it can be a means of making new discoveries in the mechanisms and treatment of cachexia.
Future studies are needed to determine whether lymphocyte recruitment in LCMV models is a byproduct of elevated numbers of lymphocytes recruited systemically to fight infection, or a mediator of hypothalamic inflammation and an integral part of the mechanism of cachexia. In addition to LCMV, other mouse models of sickness behavior demonstrate that systemic inflammation induces leukocyte recruitment in the CNS. In models of inflammatory liver disease, monocytes were found in various regions of the brain and produced TNFα, amplifying the neuroinflammatory response [136,137]. Blocking entry of these molecules to the CNS attenuated sickness behavior. In contrast to LCMV studies, the leukocyte population entering the CNS was almost exclusively monocytes. Unfortunately, the hypothalamus was not assessed as a potential location for leukocyte recruitment. Future studies are needed to investigate whether monocytes play a role in the hypothalamus in inflammatory liver disease.
Lastly, in a rodent model of chronic stress, monocytes were reported to inflltrate the CNS, including the PVN [94,160]. Future studies will be necessary to assess whether leukocyte recruitment is specific to the hypothalamus and determine the characteristics and effector functions of these cells.
Recent studies demonstrate that peripheral leukocytes enter the brain in cachexia and play a role in the neuroinflammatory response that causes sickness behavior. In addition to the studies mentioned above, studies in cancer immunology have shown that leukocytes play an important role in modulating inflammatory immune response in the brain [161]. However, the specificity and characteristics of these cells have yet to be fully elucidated. Further studies are needed to determine if these cells enter the hypothalamus, and the role of their effector functions.
6. Concluding remarks
Whether in the context of acute or chronic inflammatory insults, a growing body of evidence implicates the hypothalamus as a driving force in the pathophysiology of the acute illness response and cachexia. The hypothalamus orchestrates orexigenic and anorexigenic drives in both physiological and pathophysiological states. A variety of dietary stressors, infectious stimuli, and chronic illnesses are able to alter the behavior of these cell populations. Anatomical considerations such as the attenuated BBB of the median eminence allow direct sensing of DAMPs and PAMPS, as well as indirect sensing of threats via peripheral immune responses giving rise to cytokines. Hypothalamic exposure to any of numerous inflammatory stimuli triggers an acute illness response, caused by IL-1β and TNF-α, leading to fever, lethargy, anorexia, and weight loss. These molecules act acutely by binding to receptors on circumventricular neuronal populations, such as POMC and AgRP, triggering a feed-forward loop involving skeletal muscle catabolism and lipolysis. While cytokines are important mediators of acute illness responses, their effects are rapidly attenuated and undergo tachyphylaxis over time, implicating additional mediators in chronic hypothalamic inflammation. The key paracrine and autocrine signaling pathways local to the hypothalamic neurons and glia remain yet to be fully elucidated, but the recent discovery of the separate ontogeny of microglia from peripheral immune cells provides an exciting opportunity for understanding how inflammation differs in the CNS. Furthermore, cytokines can also act on endothelial cells within the rich capillary supply of the hypothalamus, leading to further production of cytokines, increased expression of cell adhesion molecules such as selectins and integrins, and secretion of chemokines. Subsequently, peripheral leukocytes are recruited into the CNS, where they secrete cytokines and amplify neuroinflammation. While LCMV and chronic liver inflammation rodent models provide insight on the role of peripheral leukocytes in cachexia, no studies have addressed their function within the hypothalamus. Further studies and characterization are needed to determine whether these cells enter the MBH or adjacent structures in states of inflammation. Even though many unknowns persist, one thing remains clear: the hypothalamus is crucial in both the acute sickness response and cachexia, and an improved understanding of its role in these processes could prove essential in uncovering the elusive therapeutic solution for these conditions.
References
- 1.Bodnar RJ, Pasternak GW, Mann PE, Paul D, Warren R, Donner DB. Mediation of anorexia by human recombinant tumor necrosis factor through a peripheral action in the rat. Cancer Res. 1989;49:6280–4. [PubMed] [Google Scholar]
- 2.Grossberg AJ, Scarlett JM, Zhu X, Bowe DD, Batra AK, Braun TP, et al. Arcuate nucleus proopiomelanocortin neurons mediate the acute anorectic actions of leukemia inhibitory factor via gp130. Endocrinology. 2010;151:606–16. doi: 10.1210/en.2009-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrence CB, Rothwell NJ. Anorexic but not pyrogenic actions of interleukin-1 are modulated by central melanocortin-3/4 receptors in the rat. J. Neuroendocrinol. 2001;13:490–5. doi: 10.1046/j.1365-2826.2001.00660.x. [DOI] [PubMed] [Google Scholar]
- 4.Sonti G, Ilyin SE, Plata-Salaman CR. Anorexia induced by cytokine interactions at pathophysiological concentrations. Am. J. Physiol. 1996;270:R1394–402. doi: 10.1152/ajpregu.1996.270.6.R1394. [DOI] [PubMed] [Google Scholar]
- 5.Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int. J. Obes. Relat. Metab. Disord. 2001;25(Suppl. 5):S63–7. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- 6.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and Normal weight. Nature. 2006;443:289–95. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 7.Millington GW. The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutr. Metab. (Lond.) 2007;4:18. doi: 10.1186/1743-7075-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy KG. Dissecting the role of cocaine- and amphetamine-regulated transcript (CART) in the control of appetite. Brief Funct. Genomic Proteomic. 2005;4:95–111. doi: 10.1093/bfgp/4.2.95. [DOI] [PubMed] [Google Scholar]
- 9.Ollmann MM, Lamoreux ML, Wilson BD, Barsh GS. Interaction of Agouti protein with the melanocortin 1 receptor in vitro and in vivo. Genes Dev. 1998;12:316–30. doi: 10.1101/gad.12.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD. Integration of NPY AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron. 1999;24:155–63. doi: 10.1016/s0896-6273(00)80829-6. [DOI] [PubMed] [Google Scholar]
- 11.Pritchard LE, Armstrong D, Davies N, Oliver RL, Schmitz CA, Brennand JC, et al. Agouti-related protein (83-132) is a competitive antagonist at the human melanocortin-4 receptor: no evidence for differential interactions with pro-opiomelanocortin-derived ligands. J. Endocrinol. 2004;180:183–91. doi: 10.1677/joe.0.1800183. [DOI] [PubMed] [Google Scholar]
- 12.Tatemoto K, Carlquist M, Mutt V. Neuropeptide Y—a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–60. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- 13.Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, et al. Neuropeptide Y. acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat. Med. 2007;13:803–11. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- 14.McCusker RH, Kelley KW. Immune-neural connections: how the immune system’s response to infectious agents influences behavior. J. Exp. Biol. 2013;216:84–98. doi: 10.1242/jeb.073411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marks DL, Ling N, Cone RD. Role of the central melanocortin system in cachexia. Cancer Res. 2001;61:1432–8. [PubMed] [Google Scholar]
- 16.Scarlett JM, Jobst EE, Enriori PJ, Bowe DD, Batra AK, Grant WF, et al. Regulation of central melanocortin signaling by interleukin-1 beta. Endocrinology. 2007;148:4217–25. doi: 10.1210/en.2007-0017. [DOI] [PubMed] [Google Scholar]
- 17.Scarlett JM, Zhu X, Enriori PJ, Bowe DD, Batra AK, Levasseur PR, et al. Regulation of agouti-related protein messenger ribonucleic acid transcription and peptide secretion by acute and chronic inflammation. Endocrinology. 2008;149:4837–45. doi: 10.1210/en.2007-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wisse BE, Frayo RS, Schwartz MW, Cummings DE. Reversal of cancer anorexia by blockade of central melanocortin receptors in rats. Endocrinology. 2001;142:3292–301. doi: 10.1210/endo.142.8.8324. [DOI] [PubMed] [Google Scholar]
- 19.Wisse BE, Ogimoto K, Tang J, Harris MK, Jr, Raines EW, Schwartz MW. Evidence that lipopolysaccharide-induced anorexia depends upon central, rather than peripheral, inflammatory signals. Endocrinology. 2007;148:5230–7. doi: 10.1210/en.2007-0394. [DOI] [PubMed] [Google Scholar]
- 20.Mitch WE, Goldberg AL. Mechanisms of muscle wasting—the role of the ubiquitin–proteasome pathway. N. Engl. J. Med. 1996;335:1897–905. doi: 10.1056/NEJM199612193352507. [DOI] [PubMed] [Google Scholar]
- 21.Reid MB, Li YP. Cytokines and oxidative signalling in skeletal muscle. Acta Physiol. Scand. 2001;171:225–32. doi: 10.1046/j.1365-201x.2001.00824.x. [DOI] [PubMed] [Google Scholar]
- 22.Frost RA, Lang CH. Skeletal muscle cytokines: regulation by pathogen-associated molecules and catabolic hormones. Curr. Opin. Clin. Nutr. Metab. Care. 2005;8:255–63. doi: 10.1097/01.mco.0000165003.16578.2d. [DOI] [PubMed] [Google Scholar]
- 23.Braun TP, Marks DL. The regulation of muscle mass by endogenous glucocorticoids. Front. Physiol. 2015;6:12. doi: 10.3389/fphys.2015.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braun TP, Szumowski M, Levasseur PR, Grossberg AJ, Zhu X, Agarwal A, et al. Muscle atrophy in response to cytotoxic chemotherapy is dependent on intact glucocorticoid signaling in skeletal muscle. PLoS One. 2014;9:e106489. doi: 10.1371/journal.pone.0106489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johns N, Stephens NA, Fearon KC. Muscle wasting in cancer. Int. J. Biochem. Cell Biol. 2013;45:2215–29. doi: 10.1016/j.biocel.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 26.Braun TP, Zhu X, Szumowski M, Scott GD, Grossberg AJ, Levasseur PR, et al. Central nervous system inflammation induces muscle atrophy via activation of the hypothalamic-pituitary-adrenal axis. J. Exp. Med. 2011;208:2449–63. doi: 10.1084/jem.20111020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun TP, Grossberg AJ, Krasnow SM, Levasseur PR, Szumowski M, Zhu XX, et al. Cancer- and endotoxin-induced cachexia require intact glucocorticoid signaling in skeletal muscle. FASEB J. 2013;27:3572–82. doi: 10.1096/fj.13-230375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J. 2002;16:1335–47. doi: 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- 29.Munoz-Canoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? FEBS J. 2013;280:4131–48. doi: 10.1111/febs.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senaris RM, Trujillo ML, Navia B, Comes G, Ferrer B, Giralt M, et al. Interleukin-6 regulates the expression of hypothalamic neuropeptides involved in Normal weight in a gender-dependent way. J. Neuroendocrinol. 2011;23:675–86. doi: 10.1111/j.1365-2826.2011.02158.x. [DOI] [PubMed] [Google Scholar]
- 31.Li Y-P, Reid MB. NF-κB mediates the protein loss induced by TNF-α: in differentiated skeletal muscle myotubes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279(4):1165–70. doi: 10.1152/ajpregu.2000.279.4.R1165. [DOI] [PubMed] [Google Scholar]
- 32.Glass DJ. Signaling pathways perturbing muscle mass. Curr. Opin. Clin. Nutr.Metab. Care. 2010;13:225–9. doi: 10.1097/mco.0b013e32833862df. [DOI] [PubMed] [Google Scholar]
- 33.Dalla Libera L, Sabbadini R, Renken C, Ravara B, Sandri M, Betto R, et al. Apoptosis in the skeletal muscle of rats with heart failure is associated with increased serum levels of TNF-α: and sphingosine. J. Mol. Cell. Cardiol. 2001;33:1871–8. doi: 10.1006/jmcc.2001.1453. [DOI] [PubMed] [Google Scholar]
- 34.Arruda AP, Milanski M, Romanatto T, Solon C, Coope A, Alberici LC, et al. Hypothalamic actions of tumor necrosis factor alpha provide the thermogenic core for the wastage syndrome in cachexia. Endocrinology. 2010;151:683–94. doi: 10.1210/en.2009-0865. [DOI] [PubMed] [Google Scholar]
- 35.Ramos EJ, Suzuki S, Marks D, Inui A, Asakawa A, Meguid MM. Cancer anorexia-cachexia syndrome: cytokines and neuropeptides. Curr. Opin. Clin. Nutr. Metab. Care. 2004;7:427–34. doi: 10.1097/01.mco.0000134363.53782.cb. [DOI] [PubMed] [Google Scholar]
- 36.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002;53:865–71. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 37.Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- 38.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–8. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu N, Yoshikawa N, Ito N, Maruyama T, Suzuki Y, Takeda S, et al. Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab. 2011;13:170–82. doi: 10.1016/j.cmet.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Pleasure DE, Walsh GO, Engel WK. Atrophy of skeletal muscle in patients with Cushing’s syndrome. Arch. Neurol. 1970;22:118–25. doi: 10.1001/archneur.1970.00480200024002. [DOI] [PubMed] [Google Scholar]
- 41.Hu Z, Wang H, Lee IH, Du J, Mitch WE. Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice. J. Clin. Invest. 2009;119:3059–69. doi: 10.1172/JCI38770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 2012;122:153–62. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuente-Martin E, Garcia-Caceres C, Diaz F, Argente-Arizon P, Granado M, Barrios V, et al. Hypothalamic inflammation without astrogliosis in response to high sucrose intake is modulated by neonatal nutrition in male rats. Endocrinology. 2013;154:2318–30. doi: 10.1210/en.2012-2196. [DOI] [PubMed] [Google Scholar]
- 44.Pimentel GD, Lira FS, Rosa JC, Oller do Nascimento CM, Oyama LM, Harumi Watanabe RL, et al. High-fat fish oil diet prevents hypothalamic inflammatory profile in rats. ISRN Inflamm. 2013;2013:419823. doi: 10.1155/2013/419823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guyenet SJ, Nguyen HT, Hwang BH, Schwartz MW, Baskin DG, Thaler JP. High-fat diet feeding causes rapid, non-apoptotic cleavage of caspase-3 in astrocytes. Brain Res. 2013;1512:97–105. doi: 10.1016/j.brainres.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014;9:2124–38. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benzler J, Ganjam GK, Pretz D, Oelkrug R, Koch CE, Legler K, et al. Central inhibition of IKKbeta/NF-kappaB signaling attenuates high-fat diet-induced obesity and glucose intolerance. Diabetes. 2015;64:2015–27. doi: 10.2337/db14-0093. [DOI] [PubMed] [Google Scholar]
- 49.Cheng L, Yu Y, Szabo A, Wu Y, Wang H, Camer D, et al. Palmitic acid induces central leptin resistance and impairs hepatic glucose and lipid metabolism in male mice. J. Nutr. Biochem. 2015;26:541–8. doi: 10.1016/j.jnutbio.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 50.Milanski M, Arruda AP, Coope A, Ignacio-Souza LM, Nunez CE, Roman EA, et al. Inhibition of hypothalamic inflammation reverses diet-induced insulin resistance in the liver. Diabetes. 2012;61:1455–62. doi: 10.2337/db11-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elmquist JK, Scammell TE, Jacobsen CD, Saper CB. Distribution of Fos-like immunoreactivity in the rat brain following intravenous lipopolysaccharide administration. J. Comp. Neurol. 1996;371:85–103. doi: 10.1002/(SICI)1096-9861(19960715)371:1<85::AID-CNE5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 52.Matsuwaki T, Eskilsson A, Kugelberg U, Jonsson JI, Blomqvist A. Interleukin-1beta induced activation of the hypothalamus-pituitary-adrenal axis is dependent on interleukin-1 receptors on non-hematopoietic cells. Brain Behav. Immun. 2014;40:166–73. doi: 10.1016/j.bbi.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 53.Tolchard S, Hare AS, Nutt DJ, Clarke G. TNF alpha mimics the endocrine but not the thermoregulatory responses of bacterial lipopolysaccharide (LPS): correlation with FOS-expression in the brain. Neuropharmacology. 1996;35:243–8. doi: 10.1016/0028-3908(96)00002-0. [DOI] [PubMed] [Google Scholar]
- 54.von Meyenburg C, Hrupka BH, Arsenijevic D, Schwartz GJ, Landmann R, Langhans W. Role for CD14 TLR2, and TLR4 in bacterial product-induced anorexia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287:R298–305. doi: 10.1152/ajpregu.00659.2003. [DOI] [PubMed] [Google Scholar]
- 55.Sergeyev V, Broberger C, Hokfelt T. Effect of LPS administration on the expression of POMC, NPY, galanin, CART and MCH mRNAs in the rat hypothalamus. Brain Res. Mol. Brain Res. 2001;90:93–100. doi: 10.1016/s0169-328x(01)00088-2. [DOI] [PubMed] [Google Scholar]
- 56.Borges BC, Rorato R, Avraham Y, Castro M, Vorobiav L, et al. Leptin resistance and desensitization of hypophagia during prolonged inflammatory challenge. Am. J. Physiol. Endocrinol. Metab. 2011;300:E858–69. doi: 10.1152/ajpendo.00558.2010. [DOI] [PubMed] [Google Scholar]
- 57.Jang PG, Namkoong C, Kang GM, Hur MW, Kim SW, Kim GH, et al. NF-kappaB activation in hypothalamic pro-opiomelanocortin neurons is essential in illness- and leptin-induced anorexia. J. Biol. Chem. 2010;285:9706–15. doi: 10.1074/jbc.M109.070706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sartin JL, Marks DL, McMahon CD, Daniel JA, Levasseur P, Wagner CG, et al. Central role of the melanocortin-4 receptors in appetite regulation after endotoxin. J. Anim. Sci. 2008;86:2557–67. doi: 10.2527/jas.2008-0916. [DOI] [PubMed] [Google Scholar]
- 59.Huang QH, Hruby VJ, Tatro JB. Role of central melanocortins in endotoxin-induced anorexia. Am. J. Physiol. 1999;276:R864–71. doi: 10.1152/ajpregu.1999.276.3.R864. [DOI] [PubMed] [Google Scholar]
- 60.Denis RG, Arruda AP, Romanatto T, Milanski M, Coope A, Solon C, et al. TNF-alpha transiently induces endoplasmic reticulum stress and an incomplete unfolded protein response in the hypothalamus. Neuroscience. 2010;170:1035–44. doi: 10.1016/j.neuroscience.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 61.Ogimoto K, Wisse BE. MyD88 is a key mediator of anorexia, but not weight loss, induced by lipopolysaccharide and interleukin-1 beta. Endocrinology. 2006;147:4445–53. doi: 10.1210/en.2006-0465. [DOI] [PubMed] [Google Scholar]
- 62.Thaler JP, Choi SJ, Sajan MP, Ogimoto K, Nguyen HT, Matsen M, et al. Atypical protein kinase C activity in the hypothalamus is required for lipopolysaccharide-mediated sickness responses. Endocrinology. 2009;150:5362–72. doi: 10.1210/en.2009-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riediger T, Cordani C, Potes CS, Lutz TA. Involvement of nitric oxide in lipopolysaccharide induced anorexia. Pharmacol. Biochem. Behav. 2010;97:112–20. doi: 10.1016/j.pbb.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 64.Borner T, Pinkernell S, Lutz TA, Riediger T. Lipopolysaccharide inhibits ghrelin-excited neurons of the arcuate nucleus and reduces food intake via central nitric oxide signaling. Brain Behav. Immun. 2012;26:867–79. doi: 10.1016/j.bbi.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 65.Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287(4):759–66. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- 66.Cunningham C, Campion S, Teeling J, Felton L, Perry VH. The sickness behaviour and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded RNA (poly I:C) Brain Behav. Immun. 2007;21:490–502. doi: 10.1016/j.bbi.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 67.Traynor TR, Majde JA, Bohnet SG, Krueger JM. Sleep and Normal temperature responses in an acute viral infection model are altered in interferon type I receptor-deficient mice. Brain Behav. Immun. 2006;20:290–9. doi: 10.1016/j.bbi.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 68.Murray C, Griffin EW, O’Loughlin E, Lyons A, Sherwin E, Ahmed S, et al. Interdependent and independent roles of type I interferons and IL-6 in innate immune, neuroinflammatory and sickness behaviour responses to systemic poly I:C. Brain Behav. Immun. 2015;48:274–86. doi: 10.1016/j.bbi.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Milton NG, Self CH, Hillhouse EW. Effects of pyrogenic immunomodulators on the release of corticotrophin-releasing factor-41 and prostaglandin E2 from the intact rat hypothalamus in vitro. Br. J. Pharmacol. 1993;109:88–93. doi: 10.1111/j.1476-5381.1993.tb13535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.del Rey A, Randolf A, Wildmann J, Besedovsky HO, Jessop DS. Re-exposure to endotoxin induces differential cytokine gene expression in the rat hypothalamus and spleen. Brain Behav. Immun. 2009;23:776–83. doi: 10.1016/j.bbi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamawaki Y, Kimura H, Hosoi T, Ozawa K. MyD88 plays a key role in LPS-induced Stat3 activation in the hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R403–10. doi: 10.1152/ajpregu.00395.2009. [DOI] [PubMed] [Google Scholar]
- 72.Ginhoux F, Lim S, Hoeffel G, Low D, Huber T. Origin and differentiation of microglia. Front. Cell Neurosci. 2013;7:45. doi: 10.3389/fncel.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seelaender M, Batista M, Jr, Lira F, Silverio R, Rossi-Fanelli F. Inflammation in cancer cachexia: to resolve or not to resolve (is that the question?) Clin. Nutr. 2012;31:562–6. doi: 10.1016/j.clnu.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 75.Anker SD, Ponikowski PP, Clark AL, Leyva F, Rauchhaus M, Kemp M, et al. Cytokines and neurohormones relating to Normal composition alterations in the wasting syndrome of chronic heart failure. Eur. Heart J. 1999;20:683–93. doi: 10.1053/euhj.1998.1446. [DOI] [PubMed] [Google Scholar]
- 76.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N. Engl. J. Med. 1990;323:236–41. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 77.Argiles JM, Busquets S, Stemmler B, Lopez-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat. Rev. Cancer. 2014;14:754–62. doi: 10.1038/nrc3829. [DOI] [PubMed] [Google Scholar]
- 78.Grossberg AJ, Scarlett JM, Marks DL. Hypothalamic mechanisms in cachexia. Physiol. Behav. 2010;100:478–89. doi: 10.1016/j.physbeh.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vitkovic L, Konsman JP, Bockaert J, Dantzer R, Homburger V, Jacque C. Cytokine signals propagate through the brain. Mol. Psychiatry. 2000;5:604–15. doi: 10.1038/sj.mp.4000813. [DOI] [PubMed] [Google Scholar]
- 80.Banks WA, Kastin AJ, Gutierrez EG. Penetration of interleukin-6 across the murine blood–brain barrier. Neurosci. Lett. 1994;179:53–6. doi: 10.1016/0304-3940(94)90933-4. [DOI] [PubMed] [Google Scholar]
- 81.Gutierrez EG, Banks WA, Kastin AJ. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J. Neuroimmunol. 1993;47:169–76. doi: 10.1016/0165-5728(93)90027-v. [DOI] [PubMed] [Google Scholar]
- 82.Banks WA, Ortiz L, Plotkin SR, Kastin AJ. Human interleukin (IL) 1 alpha, murine IL-1 alpha and murine IL-1 beta are transported from blood to brain in the mouse by a shared saturable mechanism. J. Pharmacol. Exp. Ther. 1991;259:988–96. [PubMed] [Google Scholar]
- 83.Quan N, Whiteside M, Herkenham M. Time course and localization patterns of interleukin-1beta messenger RNA expression in brain and pituitary after peripheral administration of lipopolysaccharide. Neuroscience. 1998;83:281–93. doi: 10.1016/s0306-4522(97)00350-3. [DOI] [PubMed] [Google Scholar]
- 84.Dantzer R, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duan K, Yu W, Lin Z, Tan S, Bai X, Gao T, et al. Endotoxemia-induced muscle wasting is associated with the change of hypothalamic neuropeptides in rats. Neuropeptides. 2014;48:379–86. doi: 10.1016/j.npep.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 86.Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett. 2008;582:117–31. doi: 10.1016/j.febslet.2007.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am. J. Physiol. Cell Physiol. 2004;287:C834–43. doi: 10.1152/ajpcell.00579.2003. [DOI] [PubMed] [Google Scholar]
- 88.Bernardini R, Kamilaris TC, Calogero AE, Johnson EO, Gomez MT, Gold PW, et al. Interactions between tumor necrosis factor-alpha, hypothalamic corticotropin-releasing hormone, and adrenocorticotropin secretion in the rat. Endocrinology. 1990;126:2876–81. doi: 10.1210/endo-126-6-2876. [DOI] [PubMed] [Google Scholar]
- 89.Romanatto T, Cesquini M, Amaral ME, Roman EA, Moraes JC, Torsoni MA, et al. TNF-alpha acts in the hypothalamus inhibiting food intake and increasing the respiratory quotient—effects on leptin and insulin signaling pathways. Peptides. 2007;28:1050–8. doi: 10.1016/j.peptides.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 90.Katafuchi T, Motomura K, Baba S, Ota K, Hori T. Differential effects of tumor necrosis factor-alpha and -beta on rat ventromedial hypothalamic neurons in vitro. Am. J. Physiol. 1997;272:R1966–71. doi: 10.1152/ajpregu.1997.272.6.R1966. [DOI] [PubMed] [Google Scholar]
- 91.Ericsson A, Liu C, Hart RP, Sawchenko PE. Type 1 interleukin-1 receptor in the rat brain: distribution, regulation, and relationship to sites of IL-1-induced cellular activation. J. Comp. Neurol. 1995;361:681–98. doi: 10.1002/cne.903610410. [DOI] [PubMed] [Google Scholar]
- 92.Ching S, Zhang H, Belevych N, He L, Lai W, Pu XA, et al. Endothelial-specific knockdown of interleukin-1 (IL-1) type 1 receptor differentially alters CNS responses to IL-1 depending on its route of administration. J. Neurosci. 2007;27:10476–86. doi: 10.1523/JNEUROSCI.3357-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Quan N, He L, Lai W. Endothelial activation is an intermediate step for peripheral lipopolysaccharide induced activation of paraventricular nucleus. Brain Res. Bull. 2003;59:447–52. doi: 10.1016/s0361-9230(02)00951-6. [DOI] [PubMed] [Google Scholar]
- 94.Wohleb ES, Patterson JM, Sharma V, Quan N, Godbout JP, Sheridan JF. Knockdown of interleukin-1 receptor type-1 on endothelial cells attenuated stress-induced neuroinflammation and prevented anxiety-like behavior. J. Neurosci. 2014;34:2583–91. doi: 10.1523/JNEUROSCI.3723-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hill AG, Jacobson L, Gonzalez J, Rounds J, Majzoub JA, Wilmore DW. Chronic central nervous system exposure to interleukin-1 beta causes catabolism in the rat. Am. J. Physiol. 1996;271:R1142–8. doi: 10.1152/ajpregu.1996.271.5.R1142. [DOI] [PubMed] [Google Scholar]
- 96.Schafers M, Lee DH, Brors D, Yaksh TL, Sorkin LS. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J. Neurosci. 2003;23:3028–38. doi: 10.1523/JNEUROSCI.23-07-03028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Riemsdijk IC, Baan CC, Loonen EH, Knoop CJ, Navarro Betonico G, Niesters HG, et al. T cells activate the tumor necrosis factor-alpha system during hemodialysis, resulting in tachyphylaxis. Kidney Int. 2001;59:883–92. doi: 10.1046/j.1523-1755.2001.059003883.x. [DOI] [PubMed] [Google Scholar]
- 98.Takahashi N, Brouckaert P, Fiers W. Induction of tolerance allows separation of lethal and antitumor activities of tumor necrosis factor in mice. Cancer Res. 1991;51:2366–72. [PubMed] [Google Scholar]
- 99.Carlson CD, Bai Y, Jonakait GM, Hart RP. Interleukin-1 beta increases leukemia inhibitory factor mRNA levels through transient stimulation of transcription rate. Glia. 1996;18:141–51. doi: 10.1002/(SICI)1098-1136(199610)18:2<141::AID-GLIA6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 100.Gayle D, Ilyin SE, Flynn MC, Plata-Salaman CR. Lipopolysaccharide (LPS)- and muramyl dipeptide (MDP)-induced anorexia during refeeding following acute fasting: characterization of brain cytokine and neuropeptide systems mRNAs. Brain Res. 1998;795:77–86. doi: 10.1016/s0006-8993(98)00280-7. [DOI] [PubMed] [Google Scholar]
- 101.Beretta E, Dhillon H, Kalra PS, Kalra SP, Central LIF. gene therapy suppresses food intake, Normal weight, serum leptin and insulin for extended periods. Peptides. 2002;23:975–84. doi: 10.1016/s0196-9781(02)00021-9. [DOI] [PubMed] [Google Scholar]
- 102.Metcalf D, Gearing DP. Fatal syndrome in mice engrafted with cells producing high levels of the leukemia inhibitory factor. Proc. Natl. Acad. Sci. U. S. A. 1989;86:5948–52. doi: 10.1073/pnas.86.15.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mori M, Yamaguchi K, Honda S, Nagasaki K, Ueda M, Abe O, et al. Cancer cachexia syndrome developed in nude mice bearing melanoma cells producing leukemia-inhibitory factor. Cancer Res. 1991;51:6656–9. [PubMed] [Google Scholar]
- 104.Nicola NA, Babon JJ. Leukemia inhibitory factor (LIF) Cytokine Growth Factor Rev. 2015 doi: 10.1016/j.cytogfr.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kalaria RN. The blood-brain barrier and cerebral microcirculation in Alzheimer disease. Cerebrovasc. Brain Metab. Rev. 1992;4:226–60. [PubMed] [Google Scholar]
- 106.Whitton PS. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br. J. Pharmacol. 2007;150:963–76. doi: 10.1038/sj.bjp.0707167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Popescu BO, Toescu EC, Popescu LM, Bajenaru O, Muresanu DF, Schultzberg M, et al. Blood-brain barrier alterations in ageing and dementia. J. Neurol. Sci. 2009;283:99–106. doi: 10.1016/j.jns.2009.02.321. [DOI] [PubMed] [Google Scholar]
- 108.Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34:806–12. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- 109.Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult. Scler. 2003;9:540–9. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- 110.Puhlmann M, Weinreich DM, Farma JM, Carroll NM, Turner EM, Alexander HR., Jr Interleukin-1beta induced vascular permeability is dependent on induction of endothelial tissue factor (TF) activity. J. Transl. Med. 2005;3:37. doi: 10.1186/1479-5876-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, et al. Interleukin-1[beta]-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–5. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 112.Kim Y-J, Hwang S-Y, Oh E-S, Oh S, Han I-O. IL-1β an immediate early protein secreted by activated microglia, induces iNOS/NO in C6 astrocytoma cells through p38 MAPK and NF-κ B pathways. J. Neurosci. Res. 2006;84:1037–46. doi: 10.1002/jnr.21011. [DOI] [PubMed] [Google Scholar]
- 113.Peng J, He F, Zhang C, Deng X, Yin F. Protein kinase C-o: signals P115RhoGEF phosphorylation and RhoA activation in TNF-α:-induced mouse brain microvascular endothelial cell barrier dysfunction. J. Neuroinflamm. 2011;8:28–30. doi: 10.1186/1742-2094-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fabry Z, Fitzsimmons KM, Herlein JA, Moninger TO, Dobbs MB, Hart MN. Production of the cytokines interleukin 1 and 6 by murine brain microvessel endothelium and smooth muscle pericytes. J. Neuroimmunol. 1993;47:23–34. doi: 10.1016/0165-5728(93)90281-3. [DOI] [PubMed] [Google Scholar]
- 115.Katerina D-Z, Alexander E. The Blood-Brain Barrier in Health and Disease. Vol. 1. CRC Press; 2015. Inflammatory mediators and the blood-brain barrier; pp. 239–88. [Google Scholar]
- 116.Kastin AJ, Akerstrom V, Pan W. Circulating TGF-beta1 does not cross the intact blood-brain barrier. J. Mol. Neurosci. 2003;21:43–8. doi: 10.1385/JMN:21:1:43. [DOI] [PubMed] [Google Scholar]
- 117.Pan W, Vallance K, Kastin AJ. TGFalpha and the blood-brain barrier: accumulation in cerebral vasculature. Exp. Neurol. 1999;160:454–9. doi: 10.1006/exnr.1999.7215. [DOI] [PubMed] [Google Scholar]
- 118.Ronaldson PT, Demarco KM, Sanchez-Covarrubias L, Solinsky CM, Davis TP. Transforming growth factor-beta signaling alters substrate permeability and tight junction protein expression at the blood-brain barrier during inflammatory pain. J. Cereb. Blood Flow Metab. 2009;29:1084–98. doi: 10.1038/jcbfm.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rodriguez EM, Blazquez JL, Guerra M. The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: the former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides. 2010;31:757–76. doi: 10.1016/j.peptides.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 120.Langlet F. Tanycytes: a gateway to the metabolic hypothalamus. J. Neuroendocrinol. 2014;26:753–60. doi: 10.1111/jne.12191. [DOI] [PubMed] [Google Scholar]
- 121.Nadeau S, Rivest S. Effects of circulating tumor necrosis factor on the neuronal activity and expression of the genes encoding the tumor necrosis factor receptors (p55 and p75) in the rat brain: a view from the blood-brain barrier. Neuroscience. 1999;93:1449–64. doi: 10.1016/s0306-4522(99)00225-0. [DOI] [PubMed] [Google Scholar]
- 122.Utsuyama M, Hirokawa K. Differential expression of various cytokine receptors in the brain after stimulation with LPS in young and old mice. Exp. Gerontol. 2002;37:411–20. doi: 10.1016/s0531-5565(01)00208-x. [DOI] [PubMed] [Google Scholar]
- 123.Yamakuni H, Minami M, Satoh M. Localization of mRNA for leukemia inhibitory factor receptor in the adult rat brain. J. Neuroimmunol. 1996;70:45–53. doi: 10.1016/s0165-5728(96)00097-5. [DOI] [PubMed] [Google Scholar]
- 124.Rossi B, Angiari S, Zenaro E, Budui SL, Constantin G. Vascular inflammation in central nervous system diseases: adhesion receptors controlling leukocyte-endothelial interactions. J. Leukoc. Biol. 2011;89:539–56. doi: 10.1189/jlb.0710432. [DOI] [PubMed] [Google Scholar]
- 125.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- 126.Davies MJ, Gordon JL, Gearing AJ, Pigott R, Woolf N, Katz D, et al. The expression of the adhesion molecules ICAM-1, VCAM-1 PECAM, and E-selectin in human atherosclerosis. J. Pathol. 1993;171:223–9. doi: 10.1002/path.1711710311. [DOI] [PubMed] [Google Scholar]
- 127.Chung I, Choudhury A, Patel J, Lip GY. Soluble, platelet-bound, and total P-selectin as indices of platelet activation in congestive heart failure. Ann. Med. 2009;41:45–51. doi: 10.1080/07853890802227089. [DOI] [PubMed] [Google Scholar]
- 128.Arijs I, De Hertogh G, Machiels K, Van Steen K, Lemaire K, Schraenen A, et al. Mucosal gene expression of cell adhesion molecules, chemokines, and chemokine receptors in patients with inflammatory bowel disease before and after Infliximab treatment. Am. J. Gastroenterol. 2011;106:748–61. doi: 10.1038/ajg.2011.27. [DOI] [PubMed] [Google Scholar]
- 129.Amin K. The role of mast cells in allergic inflammation. Respir. Med. 2012;106:9–14. doi: 10.1016/j.rmed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 130.Rabelink TJ, de Boer HC, van Zonneveld AJ. Endothelial activation and circulating markers of endothelial activation in kidney disease. Nat. Rev. Nephrol. 2010;6:404–14. doi: 10.1038/nrneph.2010.65. [DOI] [PubMed] [Google Scholar]
- 131.Romano SJ. Selectin antagonists: therapeutic potential in asthma and COPD. Treat. Respir. Med. 2005;4:85–94. doi: 10.2165/00151829-200504020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Mol. Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tan BH, Fladvad T, Braun TP, Vigano A, Strasser F, Deans DA, et al. P-selectin genotype is associated with the development of cancer cachexia. EMBO Mol. Med. 2012;4:462–71. doi: 10.1002/emmm.201200231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Avan A, Avan A, Le Large TY, Mambrini A, Funel N, Maftouh M, et al. AKT1 and SELP polymorphisms predict the risk of developing cachexia in pancreatic cancer patients. PLoS One. 2014;9:e108057. doi: 10.1371/journal.pone.0108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Love S, Barber R. Expression of P-selectin and intercellular adhesion molecule-1 in human brain after focal infarction or cardiac arrest. Neuropathol. Appl. Neurobiol. 2001;27:465–73. doi: 10.1046/j.1365-2990.2001.00356.x. [DOI] [PubMed] [Google Scholar]
- 136.Kerfoot SM, Nguyen H, Ajuebor MN, Kubes P, Le T, et al. TNF-alpha-secreting monocytes are recruited into the brain of cholestatic mice. Hepatology. 2006;43:154–62. doi: 10.1002/hep.21003. [DOI] [PubMed] [Google Scholar]
- 137.Le T, Swain MG. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J. Neurosci. 2009;29:2089–102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Swain MG. Liver-brain interactions in inflammatory liver diseases: implications for fatigue and mood disorders. Brain Behav. Immun. 2014;35:9–20. doi: 10.1016/j.bbi.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 139.Cayrol R, Wosik K, Berard JL, Dodelet-Devillers A, Ifergan I, Kebir H, et al. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat. Immunol. 2008;9:137–45. doi: 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
- 140.Liu G, Jiang Y, Wang P, Feng R, Jiang N, Chen X, et al. Cell adhesion molecules contribute to Alzheimer’s disease: multiple pathway analyses of two genome-wide association studies. J. Neurochem. 2012;120:190–8. doi: 10.1111/j.1471-4159.2011.07547.x. [DOI] [PubMed] [Google Scholar]
- 141.Yilmaz G, Granger DN. Cell adhesion molecules and ischemic stroke. Neurol. Res. 2008;30:783–93. doi: 10.1179/174313208X341085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Christensen JE, de Lemos C, Moos T, Christensen JP, Thomsen AR. CXCL10 is the key ligand for CXCR3 on CD8+ effector T cells involved in immune surveillance of the lymphocytic choriomeningitis virus-infected central nervous system. J. Immunol. 2006;176:4235–43. doi: 10.4049/jimmunol.176.7.4235. [DOI] [PubMed] [Google Scholar]
- 143.Christensen JE, Simonsen S, Fenger C, Sorensen MR, Moos T, Christensen JP, et al. Fulminant lymphocytic choriomeningitis virus-induced inflammation of the CNS involves a cytokine-chemokine-cytokine-chemokine cascade. J. Immunol. 2009;182:1079–87. doi: 10.4049/jimmunol.182.2.1079. [DOI] [PubMed] [Google Scholar]
- 144.Ransohoff RM, Hamilton TA, Tani M, Stoler MH, Shick HE, Major JA, et al. Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J. 1993;7:592–600. doi: 10.1096/fasebj.7.6.8472896. [DOI] [PubMed] [Google Scholar]
- 145.Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 146.Banisadr G, Rostene W, Kitabgi P, Parsadaniantz SM. Chemokines and brain functions. Curr. Drug Targets Inflamm. Allergy. 2005;4:387–99. doi: 10.2174/1568010054022097. [DOI] [PubMed] [Google Scholar]
- 147.Rostene W, Guyon A, Kular L, Godefroy D, Barbieri F, Bajetto A, et al. Chemokines and chemokine receptors: new actors in neuroendocrine regulations. Front. Neuroendocrinol. 2011;32:10–24. doi: 10.1016/j.yfrne.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 148.Plata-Salaman CR, Borkoski JP. Chemokines/intercrines and central regulation of feeding. Am. J. Physiol. 1994;266:R1711–5. doi: 10.1152/ajpregu.1994.266.5.R1711. [DOI] [PubMed] [Google Scholar]
- 149.Licinio J, Wong ML, Gold PW. Neutrophil-activating peptide-1/interleukin-8 mRNA is localized in rat hypothalamus and hippocampus. Neuroreport. 1992;3:753–6. doi: 10.1097/00001756-199209000-00008. [DOI] [PubMed] [Google Scholar]
- 150.Wu F, Zhao Y, Jiao T, Shi D, Zhu X, Zhang M, et al. CXCR2 is essential for cerebral endothelial activation and leukocyte recruitment during neuroinflammation. J. Neuroinflamm. 2015;12:98. doi: 10.1186/s12974-015-0316-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Klein RS, Lin E, Zhang B, Luster AD, Tollett J, Samuel MA, et al. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J. Virol. 2005;79:11457–66. doi: 10.1128/JVI.79.17.11457-11466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mills Ko E, Ma JH, Guo F, Miers L, Lee E, Bannerman P, et al. Deletion of astroglial CXCL10 delays clinical onset but does not affect progressive axon loss in a murine autoimmune multiple sclerosis model. J. Neuroinflamm. 2014;11:105. doi: 10.1186/1742-2094-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Amin DN, Rottenberg ME, Thomsen AR, Mumba D, Fenger C, Kristensson K, et al. Expression and role of CXCL10 during the encephalitic stage of experimental and clinical African trypanosomiasis. J. Infect. Dis. 2009;200:1556–65. doi: 10.1086/644597. [DOI] [PubMed] [Google Scholar]
- 154.Doherty PC, Hou S, Southern PJ. Lymphocytic choriomeningitis virus induces a chronic wasting disease in mice lacking class I major histocompatibility complex glycoproteins. J. Neuroimmunol. 1993;46:11–7. doi: 10.1016/0165-5728(93)90228-q. [DOI] [PubMed] [Google Scholar]
- 155.Lin AA, Tripathi PK, Sholl A, Jordan MB, Hildeman DA. Gamma interferon signaling in macrophage lineage cells regulates central nervous system inflammation and chemokine production. J. Virol. 2009;83:8604–15. doi: 10.1128/JVI.02477-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stamm A, Valentine L, Potts R, Premenko-Lanier M. An intermediate dose of LCMV clone 13 causes prolonged morbidity that is maintained by CD4+ T cells. Virology. 2012;425:122–32. doi: 10.1016/j.virol.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mahic M, Kalland ME, Aandahl EM, Torgersen KM, Tasken K. Human naturally occurring and adaptive regulatory T cells secrete high levels of leukaemia inhibitory factor upon activation. Scand. J. Immunol. 2008;68:391–6. doi: 10.1111/j.1365-3083.2008.02148.x. [DOI] [PubMed] [Google Scholar]
- 158.Vanderlocht J, Hendriks JJ, Venken K, Stinissen P, Hellings N. Effects of IFN-beta, leptin and simvastatin on LIF secretion by T lymphocytes of MS patients and healthy controls. J. Neuroimmunol. 2006;177:189–200. doi: 10.1016/j.jneuroim.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 159.Kamperschroer C, Quinn DG. The role of proinflammatory cytokines in wasting disease during lymphocytic choriomeningitis virus infection. J. Immunol. 2002;169:340–9. doi: 10.4049/jimmunol.169.1.340. [DOI] [PubMed] [Google Scholar]
- 160.Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, et al. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J. Neurosci. 2011;31:6277–88. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sugihara AQ, Rolle CE, Lesniak MS. Regulatory T cells actively Inflltrate metastatic brain tumors. Int. J. Oncol. 2009;34:1533–40. doi: 10.3892/ijo_00000282. [DOI] [PubMed] [Google Scholar]