Abstract
Background:
One of the most common sexual-transmitted infections among women is human papillomavirus (HPV) infection which is associated with genital cancers. Different studies in Iran reported various prevalences, and combining their results could be important for health policy makers. This study aims to determine the total prevalence of HPV infection as well as its related genotypes, particularly HPV16 and HPV18 among Iranian healthy women.
Methods:
Searching the Scientific Information Database, Iranmedex, Magiran, Irandoc, PubMed, Google Scholar, Scopus, and ScienceDirect databanks using relevant keywords and excluding duplicates and irrelevant evidence followed by applying exclusion criteria and quality assessment, eligible articles were selected. Standard error of the prevalence was calculated based on binomial distribution. Random effects model was used because of the high heterogeneity among the results.
Results:
Of 14 studies entered into the systematic review, 24 pieces of evidence reported the HPV prevalence among 7655 healthy and noncancerous women in different Provinces of Iran. Total prevalence of HPV, 9.4% (95% confidence interval [CI]: 6.8–12.02); HPV16, 2.03% (95% CI: 1.3–2.8); HPV18, 1.7% (95% CI: 0.9–2.5); and other genotypes of HPV, 5.3% (95% CI: 3.6–6.9) were estimated.
Conclusions:
Our meta-analysis showed that the total prevalence of HPV and its high-risk genotypes (16 and 18) among healthy noncancerous Iranian women was very high.
Keywords: Cervix, genotype, human papillomavirus, Iran, polymerase chain reaction, prevalence
INTRODUCTION
One of the most common sexually transmitted infections worldwide with a high pathogenicity occurs by human papillomavirus (HPV)[1,2,3,4] which is considered as the main risk factor for cervical cancer.[4] Although most HPV infections are self-limited, some other nononcogenic types (especially 6 and 11) develop genital warts. Moreover, persistent infections (HPV oncogenic types) can lead to cervical, vaginal, penile, anal, oropharyngeal and probably skin and lung cancers.[5] HPV infection can develop as latent, subclinical, and clinical forms.[2] It also has a high tendency to squamous epithelial cells with clinical manifestations differ from benign hyperplasic proliferative epithelial lesions (wart, swelling, or dermal masses) to invasive cancers.[6] It takes 10 years from occurring HPV infection to developing malignancy.[7] HPV prevalence rate decreases with age, but relative contribution of persistent infections increases over time.[8] Therefore, there is a high burden of morbidity and mortality in the developing countries mainly due to the lack of organized screening programs.[9]
Out of 100 HPV genotypes, forty genotypes are genital types[10] dividing into two types: Low risk and high risk.[2] Among these genotypes, 13 (HPV16, HPV18, HPV31, HPV33, HPV35, HPV39, HPV45, HPV51, HPV52, HPV56, HPV58, HPV59, and HPV66) are carcinogenic (high-risk types) while other types are low risk or with unknown risk,[8] and HPV16 and HPV18 are two common types.[11] HPV16, HPV18, and HPV16/18 are identifiable in 50–60%, 10–20%,[8] and 70% of cervical tumor specimens, respectively.[3,7,8,12,13]
About 10–20% of women without cervical dysfunction are infected with HPV. The corresponding figures for sub-Saharan Africa, Eastern Europe, and Latin America are 24%, 21%, and 16%, respectively.[11] HPV infection is more common in developing countries (42.4%) than among developed countries (22.6%).[14] Approximately 233.9 million women in reproductive age worldwide, 32.03 million Asian women, and 3.71 million Iranian women are HPV infected.[1] According to the WHO reports, out of four women, three women develop HPV infection at least once in their lifetime. Therefore, HPV is a common infection can affect each woman and is not related to any specific populations.[10]
Comprehensive review of national and international databases identifies different evidence regarding HPV infection among Iranian women indicating various prevalence estimates. It is necessary to combine these results using statistical methods. This study aims to estimate the total prevalence of HPV infection and its most risky genotypes among Iranian women using meta-analysis.
METHODS
The current study is a systematic review and meta-analysis regarding estimation of HPV infection prevalence and its hazardous genotypes among Iranian women conducted by the review of literature.
Search strategy
To find published electronic studies until February 20, 2015, national (Scientific Information Database, Iranmedex, Magiran, and Irandoc) and international (PubMed, Google Scholar, Scopus, and ScienceDirect) databases were reviewed. The following keywords and their Farsi equivalents were applied during searching processes:
“Prevalence,” “seroprevalence,” “frequency,” “seroepidemiology,” “human papillomavirus,” “HPV,” “pap smear,” “cervical cancer,” “PCR,” “polymerase chain reaction,” “HPV16,” “HPV18,”“high-risk genotypes,” “Iran.”
Our search was performed between February 21 and 29, 2015. To increase the search sensitivity, we investigated the lists of references belonged to the reviewed studies. One of our research team members randomly evaluated this search and reported no omitting of any relevant study. We also interviewed some experts and research centers in the study field to find probable unpublished studies.
Study selection
Full text or abstract of all articles, documents, and reports identified during our systematic search was extracted. We first exclude all duplicates. Then, irrelevant papers were removed after reviewing titles, abstracts, and full texts. To prevent reprint bias, investigating the results with regard to identification of any repeated study was taken into consideration.
Quality assessment
After selection of papers based on the titles and contents, their quality assessment was carried out using special checklists[15] derived from STROBE checklist,[16] containing 12 questions regarding different aspects of methodology such as appropriate sample size and sampling strategy, study population, data collection methods and tools, variable definition and methods of deal with samples, statistical tests, study objectives, and illustration and reporting of the results according to the study aims. One score was assigned to each question and studies achieved at least eight scores[15] were eligible to enter into the final meta-analysis.
Inclusion criteria
All Persian and English studies passed the evaluation processes and obtained the required quality scores and also estimated the HPV infection prevalence as well as its high-risk genotypes among healthy and noncancerous Iranian women based on cervical samples were selected.
Exclusion criteria
Studies did not report the prevalence of HPV infection, its high risk genotypes or sample size, those carried out among men, cancerous women, abnormal pap smears or saliva samples, duplicates or repeated results, studies reported in congresses or meetings without full-text presentation, case–control or clinical trials whose results did not reliably estimate the prevalence rate, and finally, articles without minimum quality assessment scores were excluded from the systematic review and meta-analysis.
Data extraction
Study title, first author name, study date, sample size and sampling design, total prevalence of HPV and its genotypes, place of study, language of publication, and mean age of studied women were extracted from each study and entered into Excel Spreadsheet.
Meta-analysis
All data analysis was performed by STATA SE Version 11 software (Stata Corporation, College Station, TX, USA). Standard error of the prevalence was calculated according to the binomial distribution equation. Cochrane test and I2 index were used to detect the degree of heterogeneity between the results of different studies. If Q statistics showed statistical significance (P < 0.1) or when I2 index was near to one, we considered that the heterogeneity between the results is considerable so that pooling the results by random effects model would be required. Accordingly, prevalence of HPV infection as well as its genotypes was estimated using random effects model. In addition, using Bayesian analysis, we adjusted all point estimates to control the random variations. Moreover, we conducted sensitivity analysis to determine studies with the most influence on the heterogeneity. At least 20% change in the I2 index following the exclusion of each study indicated a significant influence of that study results in the heterogeneity. We also illustrated point and pooled estimates as well as their 95% confidence intervals (CIs) using forest plots. In these graphs, box size referred to study weight and crossed line represented 95% CI.
RESULTS
Searching national and international databases, 4737 primary studies were identified. Of them, 286 articles were duplicated; 4065 irrelevant articles were excluded after advanced search; 256 papers were omitted following title and abstract review; 107 irrelevant studies were removed after full-text review, and finally, 11 articles did not satisfy the inclusion/exclusion criteria or did not achieve enough quality scores. During the review of references, two eligible studies were added to the systematic review process. Finally, 14 papers were entered into the meta-analysis [Figure 1 and Table 1].
Figure 1.
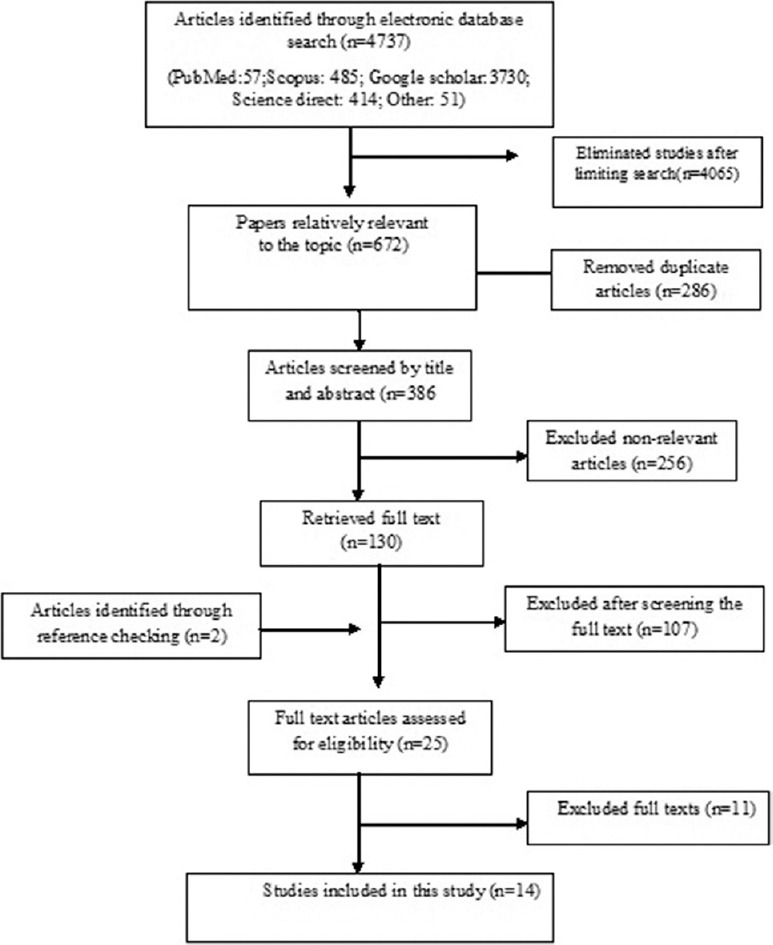
Literature search and review flowchart for selection of primary studies
Table 1.
Baseline characteristics of included primary studies in meta-analysis of prevalence of human papillomavirus among Iranian healthy women
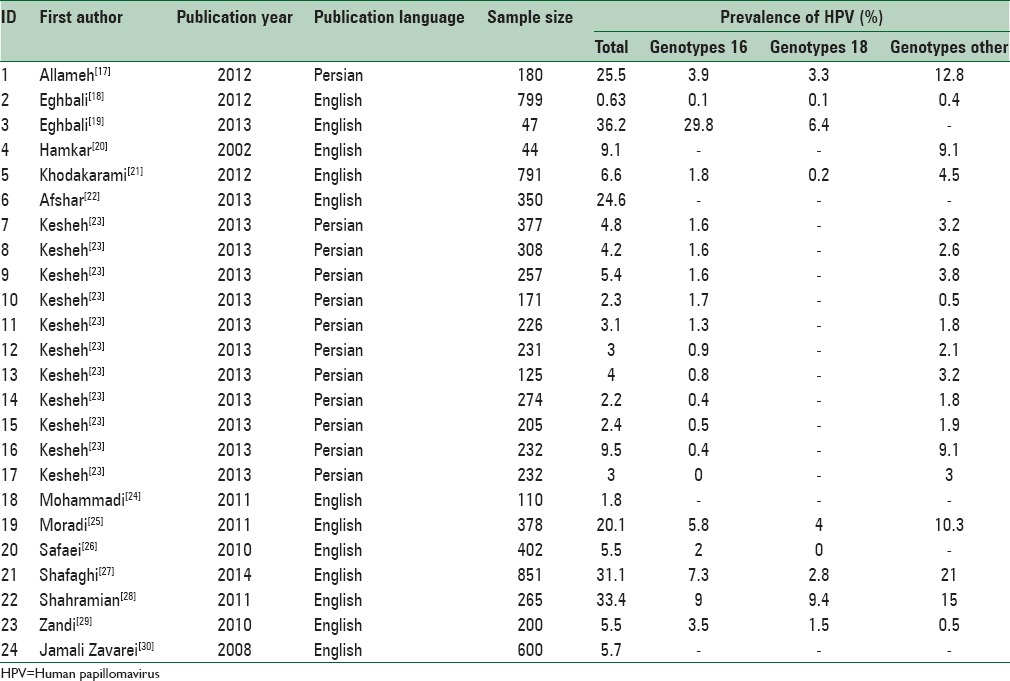
Of 14 finally selected studies, 24 pieces of evidence were reported with regard to HPV infection prevalence among healthy (noncancerous) women's pap smear specimens from different Provinces in Iran (Mobinikeshe in 2013 in a Persian-written study reported HPV infection rate among women in 11 Provinces in Iran). These studies have been published from 2002 to 2014, 12 of which written in English. Type of the study, sampling method, age groups, and mean age of the participants were reported in three, six, seven, and four papers, respectively. Sample size had not been reported in one study and revealed after contact with corresponding author. All of the above studies used polymerase chain reaction as diagnostic method.
Total prevalences of HPV infection among women were differed from 0.63% in Eghbali study carried out in 2012 among 799 women to 36.2% in Eghbali study carried out among 47 women in 2013. Having adjusted by Bayesian analysis, the corresponding figures were 0.6 (Eghbali study) to 29.8% (Yousefzadeh study). Moreover, twenty studies reported the prevalence of HPV16 genotype among healthy pap smear specimens varied from zero in the study conducted by Mobinikeshe in 2013 among 232 samples to 29.8% in Eghbali study. Bayesian analysis limited these results to 0.1% (Eghbali) and 5.9% (Yousefzadeh). Prevalence of HPV-18 genotype was estimated in nine pieces of evidence from zero in the study carried out by Safaei in 2010 among 402 participants to 9.4% reported by Shahramian in 2011 among 265 women. These estimates changed after Bayesian analysis from 0.1% in Eghbali study to 3.4% in Shahramian study.
Among the entered studies, 19 pieces of evidence reported various HPV genotypes other than 16 and 18. Because of these high variations, reporting results in accordance with these genotypes was worthless. Prevalences of such genotypes varied between 0.4% in Eghbali study conducted among 799 women in 2012 and 21% in study carried out by Yousefzadeh among 851 participants in 2014. Bayesian analysis is adjusted these results as of 0.4% (Eghbali) and 18.8% (Yousefzadeh).
Due to the heterogeneity observed among the primary studies, we applied sensitivity analysis to identify studies mostly influenced the degree of heterogeneity. The study conducted by Eghbali among 799 women with HPV prevalence of 0.63 mostly affected the heterogeneity. Having excluded this study, the heterogeneity index changed from 97% to 96.3%. Continuing the sensitivity analysis showed that changes in the I2 index were inconsiderable. Therefore, meta-analysis was conducted using all studies entered into the final process.
As illustrated in Figures 2–5 and Table 2, prevalences of HPV infection and 16, 18, and other genotypes among Iranian healthy women using random effects model were estimated as of 9.4%, 2.03%, 1.7%, and 5.3%, respectively.
Figure 2.
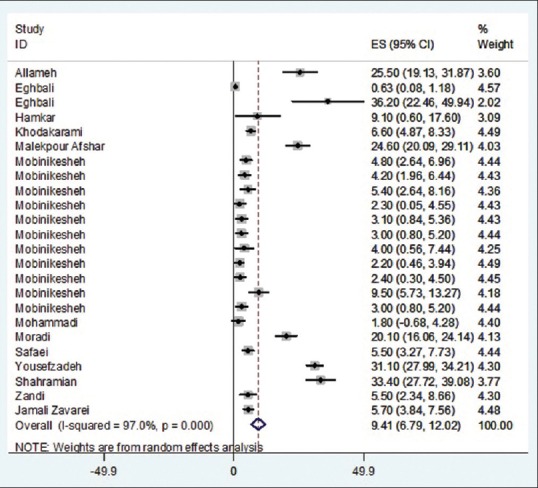
Prevalence of human papillomavirus overall in Iranian healthy women in per primary study and pooled estimate
Figure 5.
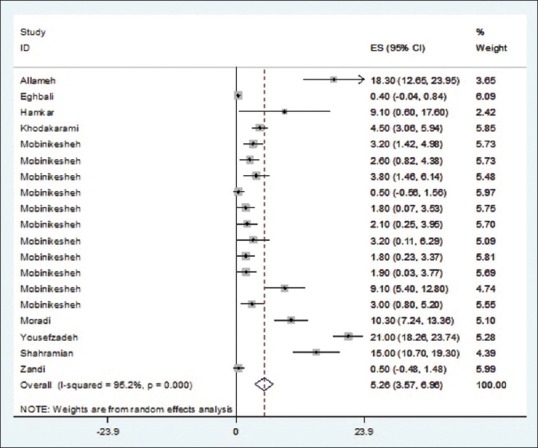
Prevalence of human papillomavirus other genotype in Iranian healthy women in per primary study and pooled estimate
Table 2.
Pooled estimated of human papillomavirus prevalence and its high-risk genotypes in Iranian healthy women, according to the result of meta-analysis
Figure 3.
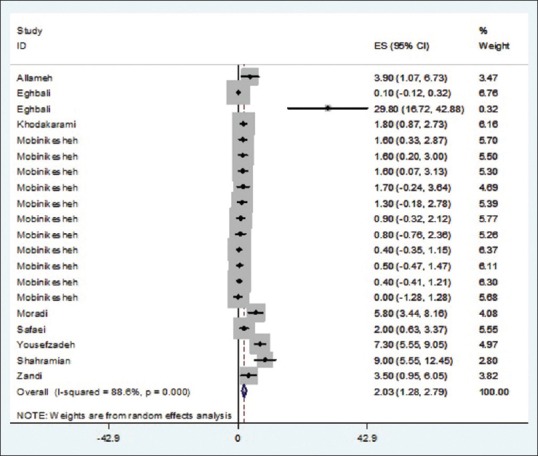
Prevalence of human papillomavirus genotype 16 in Iranian healthy women in per primary study and pooled estimate
Figure 4.
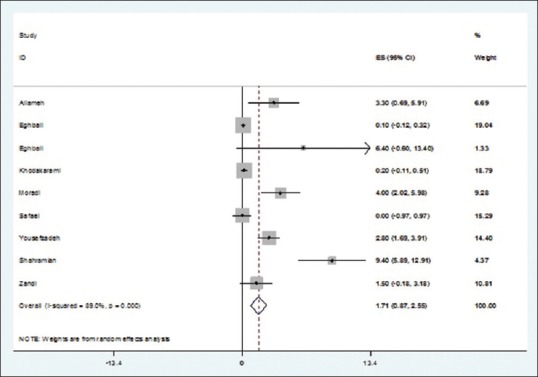
Prevalence of human papillomavirus genotype 18 in Iranian healthy women in per primary study and pooled estimate
DISCUSSION
In this systematic review and meta-analysis, we estimated the total prevalence of HPV as well as its high-risk genotypes (HPV16 and HPV18) among Iranian healthy women as of 9.4%, 2.03%, and 1.7%, respectively.
In a study conducted in Romania among 1000 women aged between 17.3 and 57 years referred to Brasov Gynecology Hospital, prevalences of HPV infection with 35 different HPV genotypes were reported. HPV16 was the most common type (26.01%). Total prevalence of infection was significantly lower among women under 25. Moreover, HPV16 and HPV18 genotypes were more common among women with cervical abnormalities. Prevalences of high- and low-risk genotypes among these abnormal women were 48.2% and 3%, respectively.[31]
In a Turkish study carried out among 2161 normal and abnormal cervical cytological specimens, HPV infection prevalence was estimated as 12.6% most of whom carried HPV6 (5%), HPV18 (2.9%), and HPV16 (2.4%) genotypes.[32] Total prevalence of HPV among 725 women in Benin, Western Africa, was reported as 33.2%. Moreover, high-risk genotypes consisted about 88% of the infections, i.e. frequencies of HPV16 and HPV18 were 17.6% and 14.7%, respectively. The odds ratio of abnormal cytology among HPV-positive women was 2.9 times greater than that of HPV-negative women.[33] Among 60,775 18–79-year-old Korean women, prevalence of HPV infection was reported as 34.2%. Among HPV positives, 87.7% had only one type of infection while 12.3% had different types of HPV infection. The most common genotypes were HPV16 (26%), HPV52 (25.5%), HPV58 (12.3%), HPV18 (7.1%), and HPV56 (4.9%).[34]
In a study conducted in Portugal, total HPV infection prevalence was reported as 19.4%; most of them were 18–24-year-old women. About 16.5% of normal cytological specimens were infected. Approximately 76.5% of women were infected with HPV infection particularly with HPV16 (3.8%). Moreover, a statistically significant relationship (P < 0.001) was observed between high-risk HPV infection and abnormal cytology.[35] Prevalence of HPV infection among 899 Pakistani married women aged between 15 and 59 years was reported as 2.8%. Prevalence of HPV16 among women with or without abnormal cytology was 0.5% and 9.1%, respectively. In addition, number of partners, age difference between couples, other characteristics of husbands such as extra-marriage contacts, and their regular absents from the home were related with HPV infection prevalence.[36] In another study in Spanish among 2362 women, 34 different genotypes were identified. The most common genotypes were HPV16 (19.18%), HPV53 (11.26%), HPV58 (7.66%), and HPV18 (4.02%). HPV16 and HPV18 were detected among 24.3% and 5.1% of the high-risk infections, respectively. Both HPV16 and HPV18 were responsible for 30% of the high-risk infections in Spanish clinical centers.[37]
In a study carried out in China with a sample size of 4987, total prevalence of HPV and prevalence of high-risk HPV genotype were 13.3% and 10.2%, respectively. HPV52 (3.1%), HPV16 (2.5%), HPV58 (2.1%), HPV68 (1%), and HPV81 (0.9%) were the most common genotypes.[38] Moreover, in a study in Thailand, 29 different types of HPV were observed among 1662 women with average age of 43.4 years. Total prevalence of HPV and its prevalence among general population was 8.7% and 7.8%, respectively. Among HPV-positive samples, 44.8% were high-risk types. HPV infection was more common among 20–39-year-old women and then gradually decreased.[39] Roteli-Martins et al. in 2011 reported the total HPV infection among 3204 healthy 15–25-year-old women in Brazil, Canada, and the USA as of 26.6%. The prevalence of oncogenic HPV was 21.7% (25% in Brazil, 16.5% in Canada, and 19.1% in the USA). The most common types were HPV16 (5.2%), HPV51 (3.3%), HPV52 (3.3%), HPV31 (2.9%), HPV66 (2.3%), and HPV39 (2%). HPV infection was strongly associated with sexual behavior.[40]
According to the results of the current study, prevalence of HPV infection among healthy and noncancerous Iranian women was considerably lower than that reported among Roman, Brazilian, Canadian, American, Spanish, African, Portuguese, and Korean women. It was also slightly lower than the prevalences reported in Turkey and China. HPV infection rate in Iran was also higher than that of reported in Pakistan and Thailand. These differences might be due to different mean age of the participants, sampling selection methods, and variation in the prevalence of sexual behaviors and number of sexual partners. It should be noted that low age during marriage, first sexual contact in lower ages (<15), multiple sexual partners, and tobacco smoking were mostly related to HPV infection.[14] In addition, factors such as characteristics of the sexual partners, concomitance of sexual contact, and alcohol and drug abuse were the main risk factors of HPV infection.[3] In a meta-analysis, two-third of women with cervical cancer had HPV16 (51%) or HPV18 (16.2%) genotypes.[41]
In another meta-analysis, 16.88% (95% CI: 15.53%–18.31%) of patients with bladder cancer were infected with HPV. In addition, HPV infection increased the risk of bladder cancer more than 2.8 folds.[42]
HPV infection is a risk factor for invasive cervical cancer, and HPV16 and HPV18 genotypes are considered as carcinogenic factors for cervical cancer. Moreover, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 66 genotypes were also reported as high-risk genotypes of HPV infection. Among all HPV genotypes, 16 and 18 genotypes are the most common types of HPV associated with cervical cancer.[23,43]
The heterogeneity observed between the results of the primary studies is one of the limitations of this systematic review and meta-analysis; therefore, we had to apply the random effects model to overcome this problem. Another limitation of the current study is that the most of the primary studies did not report factors associated with HPV infection. Even mean age of the participants did not report in many studies; therefore, we could not investigate the HPV infection risk factors during this meta-analysis. Further studies detecting the prevalence of all genotypes are suggested to be better applied for this reason.
Our meta-analysis presented evidence regarding HPV infection and its high-risk genotypes (16 and 18) among healthy Iranian women for policymakers. It should be noted that cervical cancer preventing programs have been planned according to control of HPV infection.
CONCLUSIONS
This meta-analysis showed that the prevalence of HPV and its high-risk genotypes among healthy Iranian women are considerable. Although it is less common in compare with the most other countries, the main part of cervical, breast, and bladder cancers among women can be attributed to this virus.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Jahdi F, Khademi K, Merghati Khoei E, Haghani H, Yazdanpanahi Z. Evaluation of the individual and medical factors associated with genital human papillomavirus in Iranian women. Scimetr. 2014;2:e17495. [Google Scholar]
- 2.Juckett G, Hartman-Adams H. Human papillomavirus: Clinical manifestations and prevention. Am Fam Physician. 2010;82:1209–13. [PubMed] [Google Scholar]
- 3.Javanbakht M, Gorbach PM, Amani B, Walker S, Cranston RD, Datta SD, et al. Concurrency, sex partner risk, and high-risk human papillomavirus infection among African American, Asian, and Hispanic women. Sex Transm Dis. 2010;37:68–74. doi: 10.1097/OLQ.0b013e3181bcd3e7. [DOI] [PubMed] [Google Scholar]
- 4.Farzaneh F, Shirvani HE, Barouti E, Salehpour S, Khodakarami N, Alizadeh K. Knowledge and attitude of women regarding the human papillomavirus (HPV) infection, its relationship to cervical cancer and prevention methods. Med J Malaysia. 2011;66:468–73. [PubMed] [Google Scholar]
- 5.Reiter PL, Pendergraft WF, 3rd, Brewer NT. Meta-analysis of human papillomavirus infection concordance. Cancer Epidemiol Biomarkers Prev. 2010;19:2916–31. doi: 10.1158/1055-9965.EPI-10-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shukla S, Bharti AC, Mahata S, Hussain S, Kumar R, Hedau S, et al. Infection of human papillomaviruses in cancers of different human organ sites. Indian J Med Res. 2009;130:222–33. [PubMed] [Google Scholar]
- 7.Azam F, Shams-ul-Islam M. Prevention of human papillomavirus infection with vaccines. J Pak Med Assoc. 2010;60:676–81. [PubMed] [Google Scholar]
- 8.Iftner T, Eberle S, Iftner A, Holz B, Banik N, Quint W, et al. Prevalence of low-risk and high-risk types of human papillomavirus and other risk factors for HPV infection in Germany within different age groups in women up to 30 years of age: An epidemiological observational study. J Med Virol. 2010;82:1928–39. doi: 10.1002/jmv.21910. [DOI] [PubMed] [Google Scholar]
- 9.Garland SM, Smith JS. Human papillomavirus vaccines: Current status and future prospects. Drugs. 2010;70:1079–98. doi: 10.2165/10898580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Kawana K, Yasugi T, Taketani Y. Human papillomavirus vaccines: Current issues & future. Indian J Med Res. 2009;130:341–7. [PubMed] [Google Scholar]
- 11.Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 12.Shikary T, Bernstein DI, Jin Y, Zimet GD, Rosenthal SL, Kahn JA. Epidemiology and risk factors for human papillomavirus infection in a diverse sample of low-income young women. J Clin Virol. 2009;46:107–11. doi: 10.1016/j.jcv.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishna SK, Jethwa AS. Human Papillomavirus infections in adults and children. Am J Epidemiol Infect Dis. 2013;1:11–9. [Google Scholar]
- 14.Vinodhini K, Shanmughapriya S, Das BC, Natarajaseenivasan K. Prevalence and risk factors of HPV infection among women from various provinces of the world. Arch Gynecol Obstet. 2012;285:771–7. doi: 10.1007/s00404-011-2155-8. [DOI] [PubMed] [Google Scholar]
- 15.Moosazadeh M, Nekoei-Moghadam M, Emrani Z, Amiresmaili M. Prevalence of unwanted pregnancy in Iran: A systematic review and meta-analysis. Int J Health Plann Manage. 2014;29:e277–90. doi: 10.1002/hpm.2184. [DOI] [PubMed] [Google Scholar]
- 16.Elm EV, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke P, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Prev Med. 2007;45:247–51. doi: 10.1016/j.ypmed.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Allameh T, Moghim S, Farahbod F. Reviewing the prevalence of human papillomavirus (HPV) in married women aged 18-60 years with normal Pap smear referring to gynecology clinics in hospitals affiliated to Isfahan University of Medical Sciences, Iran. J Isfahan Med Sch. 2012;29:2048–5. [Google Scholar]
- 18.Eghbali SS, Amirinejad R, Obeidi N, Mosadeghzadeh S, Vahdat K, Azizi F, et al. Oncogenic human papillomavirus genital infection in Southern Iranian women: Population-based study versus clinic-based data. Virol J. 2012;9:194. doi: 10.1186/1743-422X-9-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eghbali M, Sadeghi F, Mirinargesi M. Frequency of human papillomavirus among pregnant women by PCR technique. Int J Mol Clin Microbiol. 2013;2:285–8. [Google Scholar]
- 20.Hamkar R, Mokhtari Azad T, Mohammadi M, Seyedirashti S, Severini A, Nategh R. Prevalence of human papillomavirus in Mazandaran province, Islamic Republic of Iran. East Mediterr Health J. 2002;8:805–11. [PubMed] [Google Scholar]
- 21.Khodakarami N, Clifford GM, Yavari P, Farzaneh F, Salehpour S, Broutet N, et al. Human papillomavirus infection in women with and without cervical cancer in Tehran, Iran. Int J Cancer. 2012;131:E156–61. doi: 10.1002/ijc.26488. [DOI] [PubMed] [Google Scholar]
- 22.Afshar RM, Mollaie HR, Fazlalipour M, Arabzadeh SA. Prevalence and type distribution of human papillomavirus infection using the INNo-Lipa assay, Kerman, Southeast Iran. Asian Pac J Cancer Prev. 2013;14:5287–91. doi: 10.7314/apjcp.2013.14.9.5287. [DOI] [PubMed] [Google Scholar]
- 23.Kesheh MM, Kaffashi A, Bagheri G, Karami MS, Mohammadi M, Naji A. Identification of human papillomavirus type 16 among Thinprep samples from 11 provinces of Iran. Iran J Obstet Gynecol Infertil. 2013;16:22–8. [Google Scholar]
- 24.Mohammadi A, Daneshbod K, Ghaffari N, Masoudian M, Rezvaninia S, Mohsenpour Z, et al. Evaluation of human papillomavirus infection among women using Pap smear and PCR in Shiraz, Iran. J Life Sci. 2011;5:784–8. [Google Scholar]
- 25.Moradi A, Bakhshandeh Nosrat S, Besharat S. Molecular epidemiology of high-risk types of human papillomaviruses (16, 18) in Pap-smear, the North East of Iran. Iran J Cancer Prev. 2011;4:135–40. [PMC free article] [PubMed] [Google Scholar]
- 26.Safaei A, Khanlari M, Momtahen M, Monabati A, Robati M, Amooei S, et al. Prevalence of high-risk human papillomavirus types 16 and 18 in healthy women with cytologically negative Pap smear in Iran. Indian J Pathol Microbiol. 2010;53:681–5. doi: 10.4103/0377-4929.72030. [DOI] [PubMed] [Google Scholar]
- 27.Shafaghi B, Jarollahi A, Yousefzadeh B, Ameri A, Moghadam S, Mostafavi M. Human papillomavirus prevalence and types among Iranian women attending regular gynecological visits. Rep Radiother Oncol. 2013;1:73–9. [Google Scholar]
- 28.Shahramian I, Heidari Z, Mahmoudzadeh-Sagheb H, Moradi A, Forghani F. Prevalence of HPV infection and high risk HPV genotypes (16, 18), among monogamous and polygamous women, in Zabol, Iran. Iran J Public Health. 2011;40:113–21. [PMC free article] [PubMed] [Google Scholar]
- 29.Zandi K, Eghbali SS, Hamkar R, Ahmadi S, Ramedani E, Deilami I, et al. Prevalence of various human papillomavirus (HPV) genotypes among women who subjected to routine Pap smear test in Bushehr city (South West of Iran) 2008-2009. Virol J. 2010;7:65. doi: 10.1186/1743-422X-7-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jamali Zavarei M, Hamkar R, Dana VG, Delforoosh M, Shojamoradi MH, Gilani MM. Prevalence of HPV infection and its association with cytological abnormalities of Pap smears in Tehran. Iran J Public Health. 2008;37:101–6. [Google Scholar]
- 31.Moga MA, Irimie M, Oanta A, Pascu A, Burtea V. Type-specific prevalence of human papillomavirus by cervical cytology among women in Brasov, Romania. Asian Pac J Cancer Prev. 2014;15:6887–92. doi: 10.7314/apjcp.2014.15.16.6887. [DOI] [PubMed] [Google Scholar]
- 32.Abike F, Bingöl B, Yılmaz A, Temizkan O, Tapısız ÖL, Dunder Ý. HPV infection and HPV subtypes in normal and abnormal cervical cytology in Turkish women. J Virol Microbiol. 2013:1–7. Article ID 640873. [Google Scholar]
- 33.Piras F, Piga M, De Montis A, Zannou AR, Minerba L, Perra MT, et al. Prevalence of human papillomavirus infection in women in Benin, West Africa. Virol J. 2011;8:514. doi: 10.1186/1743-422X-8-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee EH, Um TH, Chi HS, Hong YJ, Cha YJ. Prevalence and distribution of human papillomavirus infection in Korean women as determined by restriction fragment mass polymorphism assay. J Korean Med Sci. 2012;27:1091–7. doi: 10.3346/jkms.2012.27.9.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pista A, de Oliveira CF, Cunha MJ, Paixao MT, Real O. CLEOPATRE Portugal Study Group Prevalence of human papillomavirus infection in women in Portugal: The CLEOPATRE Portugal study. Int J Gynecol Cancer. 2011;21:1150–8. doi: 10.1097/IGC.0b013e31821dd3b2. [DOI] [PubMed] [Google Scholar]
- 36.Raza SA, Franceschi S, Pallardy S, Malik FR, Avan BI, Zafar A, et al. Human papillomavirus infection in women with and without cervical cancer in Karachi, Pakistan. Br J Cancer. 2010;102:1657–60. doi: 10.1038/sj.bjc.6605664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomez-Roman JJ, Echevarria C, Salas S, González-Morán MA, Perez-Mies B, García-Higuera I, et al. A type-specific study of human papillomavirus prevalence in cervicovaginal samples in three different Spanish regions. APMIS. 2009;117:22–7. doi: 10.1111/j.1600-0463.2008.00009.x. [DOI] [PubMed] [Google Scholar]
- 38.Ye J, Cheng X, Chen X, Ye F, Lü W, Xie X. Prevalence and risk profile of cervical human papillomavirus infection in Zhejiang province, Southeast China: A population-based study. Virol J. 2010;7:66. doi: 10.1186/1743-422X-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chansaenroj J, Lurchachaiwong W, Termrungruanglert W, Tresukosol D, Niruthisard S, Trivijitsilp P, et al. Prevalence and genotypes of human papillomavirus among Thai women. Asian Pac J Cancer Prev. 2010;11:117–22. [PubMed] [Google Scholar]
- 40.Roteli-Martins CM, de Carvalho NS, Naud P, Teixeira J, Borba P, Derchain S, et al. Prevalence of human papillomavirus infection and associated risk factors in young women in Brazil, Canada, and the United States: A multicenter cross-sectional study. Int J Gynecol Pathol. 2011;30:173–84. doi: 10.1097/PGP.0b013e3181f38dfe. [DOI] [PubMed] [Google Scholar]
- 41.Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: A meta-analysis. Br J Cancer. 2003;88:63–73. doi: 10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li N, Yang L, Zhang Y, Zhao P, Zheng T, Dai M. Human papillomavirus infection and bladder cancer risk: A meta-analysis. J Infect Dis. 2011;204:217–23. doi: 10.1093/infdis/jir248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serrano B, Alemany L, Tous S, Bruni L, Clifford GM, Weiss T, et al. Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infect Agent Cancer. 2012;7:38. doi: 10.1186/1750-9378-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]


