Abstract
Contemporary endodontics has seen an unprecedented advance in technology and materials. This article aimed to review some of the challenges and advances in the following sections: (1) endodontic imaging, (2) root canal preparation, (3) root canal disinfection, (4) root canal filling, and (4) regenerative endodontic procedures (REPs). Jointly, these advances are aimed at improving the state of the art and science of root canal treatment.
Keywords: Canal preparation, disinfection, endodontic imaging, filling, regeneration
INTRODUCTION
The past couple of decades have witnessed one of the most rapid and extensive technological evolutions in dentistry. This period has presented some remarkable developments of endodontic technologies. The current article is aimed to concisely review some of these advances pertinent to endodontic imaging, root canal preparation, root canal disinfection, root filling, and regenerative endodontic procedures (REPs).
ENDODONTIC IMAGING
Analog and digital imaging modalities are available for use in diagnostic endodontic imaging. The National Council for Radiation Protection (NCRP) report #145 recommends the use of the fastest speed sensor with rectangular collimation to conform to the as low as reasonably achievable (ALARA) principle while capturing analog images. Analog imaging presents several disadvantages: Need for repeat exposure in suboptimal image capture situations, inability to enhance images interactively, wet processing issues, and difficulty in acquiring/transmitting images electronically, all of which have resulted in the adoption of digital technology. Digital capture systems include electronic sensors [digital radiography (DR)] such as a charge-coupled device (CCD) or a complementary metal oxide semiconductor (CMOS) while indirect systems use photostimulable phosphor (PSP) plates. This is known as computed radiography (CR). The advantages of digital imaging include significant dose reduction, relatively faster image acquisition, ability to enhance images, elimination of wet processing, easier transmission, and archival of images.
Currently hardwired and wireless sensors are available for use. DR offers the highest spatial and contrast resolution but the latitude is limited. CR offers wider latitude. Both have been shown to be as good as intraoral film or better for endodontic diagnoses. Most have active areas that are slightly smaller than film but more than sufficient for endodontic purposes. Advantages of CMOS over CCD include a lower manufacturing cost, need for a lesser amount of electrical energy for functioning, and comparable spatial/contrast resolution for diagnostic purposes. Presently available wireless sensors, including the newly introduced CMOS-APS sensors (Wifi Schick Elite sensor, Sirona Dental Inc., Long Island City, NY, USA) are less bulky and transmit signals via a thinner wire that enables wireless transmission and lasts for about 100 exposures.
PSP plates are wireless and activated by adding an impurity to the phosphor, thus rendering it sensitive to incident radiation. Charges are generated and stored in the form of a latent image following exposure. Exposing to white light would erase the plates and allow reusability. PSP plates require almost the same amount of exposure as an F-speed film with rectangular collimation. Plates are significantly less expensive than CCD or CMOS sensors. However, in endodontics instantaneous chairside acquisition of images with relatively high contrast and spatial resolution is required, which is best served by CMOS sensors.[1] Since multiple images are frequently needed, use of a sensor that requires the least amount of radiation to produce a high quality image is optimal in endodontics.
The processing of film carries several new recommendations as well. Transitioning to DR will help clinicians get into compliance fairly easily. For periapical and bitewing radiography, rectangular collimation should be used whenever possible because a round field beam used with a rectangular image receptor produces unnecessary radiation exposure to the patient. The human visual system is seriously limited in terms of the shades of gray it can view at any one point in time. Therefore, adjusting the display properties of the image optimizes visualization of the signal of interest. Images must be saved in a universal Digital Imaging and Communication in Medicine (DICOM) format for best fidelity and ease of transmission between imaging systems of different vendors. The compression scheme used does not result in loss of image data. Most newer systems permit a 16-bit depth (216 shades of gray) capture for an image. The file size is large and therefore, stored in a compressed format without the loss of diagnostic information (8-bit data).
The advent of cone beam computed tomography (CBCT) has resulted in widespread adoption of this technology for three-dimensional image capture/processing. Computed tomography greatly enhances diagnostic yield in certain situations where two-dimensional conventional radiographic studies have limitations. However, care should be exercised not to prescribe CBCTs for all endodontic procedures due to the fact that the radiation dose to the patient is significantly higher than those from conventional studies.[2] Apart from this, the presence of artifacts, noise, and lower spatial resolution as compared to conventional radiographs preclude the generation of useful images in several clinical scenarios. It is imperative that recommendations from the updated position paper on the use of CBCT in endodontics be carefully followed to keep doses to the minimum while maximizing diagnostic information from such cases. Several CBCT units are available with varying fields of view (FOV). Shortest scan times should be used with the smallest field of view and the smallest available voxel size without compromising on the signal to noise ratio but without a massive reduction in radiation as this would seriously degrade the signal quality. Voxel sizes range 76-500 microns but endodontic applications require voxel sizes of less than 200 microns for optimal spatial resolution. Those that employ smaller fields of view have smaller voxel dimensions. The existing literature supports the use of CBCT in clinical endodontics for selected diagnostic tasks, on a case-by-case basis, following a thorough clinical evaluation.
Few clinical studies have validated the use of CBCT in endodontics with the help of ground truth. Most are in vitro/ex vivo studies, the results of which cannot be extrapolated to the clinical scenario. Care must be exercised in the use of CBCT in pediatric patients, in view of the fact that the American Academy of Oral and Maxillofacial Radiology (AAOMR) and American Association of Endodontists (AAE) support the Image Gently Campaign led by the Alliance for Radiation Safety in Pediatric Imaging to help minimize the radiation dose to children. Some of the potential applications of CBCT include diagnoses related to the following: Initial diagnosis where nonspecific signs and symptoms exist, dental anomalies and developmental disturbances, presence of anatomic variations, calcified canals, broken instruments, vertical root fractures, failure of prior treatment, nonsurgical and surgical retreatments, select cases of trauma, resorption (external and internal), and implant placement.[3,4]
ROOT CANAL PREPARATION
All treatment steps in endodontics need to be assessed under the premise of antimicrobial effectiveness[5] and canal preparation is no exception. Root canal preparation serves to remove intracanal tissue (in vital cases) and necrotic material including microbial biofilms (in necrotic cases). In addition, an adequately shaped canal accepts irrigation solutions as well as interappointment medication and is ultimately filled optimally.
Engine-driven instrumentation continues to be used more frequently by endodontists compared to hand instruments.[6] At this point, several trends are observed in the marketplace:
Application of more flexible alloys, which not only promises better canal negotiation but also extend the fatigue life.
Practice of reciprocation motion and potentially reduction of the number of instruments used per patient.
Introduction of instruments that are designed to instrument a larger area of the canal wall and decrease the need for coronal flaring.
The following section will explore these trends and summarize expectations for the future. Table 1 provides an overview of current nickel-titanium rotary instruments.
Table 1.
Nickel-titanium root canal preparation instruments introduced after 2010
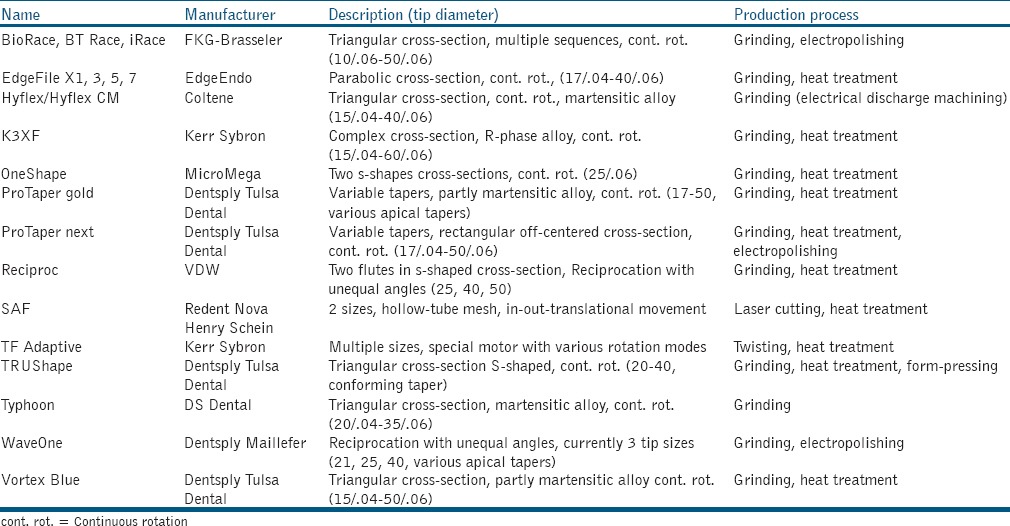
Due to its specific metallurgical properties, nickel-titanium (NiTi) alloy can be manufactured so that it is, for example, at body temperature, predominantly either in austenitic or martensitic crystal configuration.[7] These two crystal configurations have distinctly different properties, with austenite being less flexible but allowing up to 7% recoverable elastic deformation range.[8] Conversely, martensite can be dead-soft and very flexible but only allows about 2% linear strain before nonrecoverable plastic deformation occurs.[9] With these differences in flexibility, distinct differences in fatigue resistance are observed: martensitic files have significantly extended lifespans.[9] Some martensitic instruments are designed to have deformations removed during sterilization cycles while reprocessing; however, regularly certain residual deformation still remains.[10] The development of specific heat treatment and production methods continues, which includes the fine tuning of the crystal conversion temperature so that instruments may for instance be very flexible when on the shelf while assuming a different shape and behaving more rigidly when placed in a root canal.
Currently, most practitioners use electric motors to power rotary instruments. These motors are also undergoing development. The ability to set a torque limit is common to most electric motors but many models currently allow reciprocating action. While this is not entirely new,[11] several NiTi instruments have been developed entirely for reciprocation motion with unequal angles of rotation. Reciprocation movement has been shown to be efficient and safe[12] In particular, fatigue lifespan of a file is extended with reciprocation design.[13] Irrigation efficiency in infected root canal systems may be facilitated by instrumentation via mechanical force[14] and perhaps a scraping action of instruments along the canal walls.[15] Toward this several techniques were initiated in the last few years, beginning with the so-called self-adjusting file.[16]
Clinical observations suggest that not only healing of apical periodontitis but also extended mechanical function of teeth is an important endodontic outcome.[17,18] Since it is well-understood that the major factor that increases fracture susceptibility in endodontically treated teeth is the removal of bulk dentin during access[19,20] and canal preparation,[21,22] strategies are being developed that retain more dentin,[23] specifically in the coronal root third[24,25] during shaping. One strategy to achieve this goal is to limit coronal flaring and perhaps the so-called maximum fluted diameter (MFD). A more radical change would occur if a completely noninstrumental technique without the use of any canal preparation was to be adopted. Such a technique was experimented years ago[26] requiring an airtight connection to the access cavity; currently, a noninstrumental canal disinfection system, based on ultrasonic activation, is being researched in vitro.[27]
In summary, most current instruments perform well when used judiciously; apical canal transportation, a measure of shaping quality, is typically under 150 μm.[28] Flexibility and resistance to fatigue of the instruments are increasing. Current and future developments of instruments and strategies are aimed to provide antibiofilm effects and remove less radicular dentin structure. As shaping alone is not sufficient to reduce microbial loads, adequate irrigation strategies will continue to complement canal preparation.[29]
ROOT CANAL DISINFECTION
Complexities of the root canal systems, in addition to the structure and composition of the dentin, are key challenges for effective disinfection in endodontics. Topical antimicrobial such as sodium hypochlorite is commonly used in root canal treatment to combat microbial biofilms. The inability of antimicrobials to eliminate biofilm bacteria in the anatomical complexities and uninstrumented portions of the root canal would compromise their efficacy in root canal treatment. Irrigation dynamics deals with how irrigants flow, penetrate, and exchange within the root canal space and the forces produced by them. Unfortunately, the widely used syringe-based irrigation displayed a passive flow of irrigant at the apical region, 1-3 mm beyond the exit of the needle. The syringe-based method also failed to generate optimum levels of shear stresses on the canal wall, which is significant for disinfecting root canal biofilms. Thus, steps taken to improve the delivery of irrigant (irrigation dynamics) within the root canal system are crucial to achieve the maximum efficacy out of the antimicrobials. The current advances in endodontic disinfection are aimed toward:
Improving the fluid dynamics during root canal irrigation — improving bubble dynamics and activating intensified cavitational bubbles are some of the mechanisms by which fluid dynamics can be improved.
Developing newer antimicrobials, which demonstrate potent antibiofilm effect over sodium hypochlorite.[30]
Antibacterial nanoparticles
Nanoparticles (NPs) are microscopic particles with one or more dimensions in the range of 1-100 nm. It is established that NPs have properties that are very unique from their bulk counterparts. Antibacterial NP has been found to have a broad spectrum of antimicrobial activity and far lower propensity to induce microbial resistance. The electrostatic interaction between positively charged NPs and negatively charged bacterial cells, and the accumulation of large number of NPs on the bacterial cell membrane have been associated with the leading to the loss of membrane permeability and rapid loss of membrane function.[30] The ability of some of the tested NPs to rapidly eliminate biofilm bacteria needs further improvement. However, when sealers are loaded with NP, they displayed a superior ability to diffuse the antibacterial component deep into the dentin.[31] Studies have stressed their role as an intracanal medicament than an irrigant, and to improve the antimicrobial effectiveness of root canal sealers.[32] Currently, functionalized NPs are being developed to eliminate bacteria more specifically without damaging the host cells (targeted antibacterial efficacy) and to repair previously infected dentin matrix.[33,34]
Antimicrobial photodynamic therapy
Antimicrobial photodynamic therapy (APDT) is a two-step procedure that involves the application of a photosensitizer (PS) (step-1), followed by light illumination (step 2) of the sensitized tissue, which would generate a toxic photochemistry on the target cell, leading to microbial killing. Currently, APDT is considered not an alternative but a possible supplement to the existing protocols for root canal disinfection.[30] In an approach to adapt and improve the antimicrobial efficacy of APDT in endodontics, recent research has developed novel formulations of photosensitizers that displayed effective penetration into dentinal tubules, anatomical complexities, and antibiofilm properties. Well-designed clinical studies are currently warranted to examine the prospects for APDT in root canal disinfection.[35,36]
Photon-induced photoacoustic streaming
Photon-induced photoacoustic streaming (PIPS) is based on the direct shock wave generated by a erbium:YAG (Er:YAG) laser (Fidelis AT; Fotona, Ljubljana, Slovenia) in a liquid irrigant. The laser system is equipped with a fiberoptic delivery tip and subablative parameters to produce the desired effect. When activated in a limited volume of fluid, the high absorption of Er:YAG wavelength combined with the high peak power derived from the short pulse duration resulted in an enhanced bubble dynamics, which improved the irrigant flow dynamics within the root canal. The current literature presents conflicting findings on this technology.[37,38,39] This demands further evaluation and modifications of this technology to optimize therapeutic efficacy through the root canal system.[40]
Gentlewave irrigation
Gentlewave (GW) (Sonendo, Laguna Hills, CA, USA) has been developed and tested for root canal irrigation. It delivers sodium hypochlorite into the root canal under pressure through a specialized handpiece, which is activated by a broad spectrum of acoustic waves. At the same time, suction removes the outflowing fluid through the handpiece. A silicon ring surrounding the extremity of the handpiece creates a tight seal with the artificially created flat tooth surface. This establishes a vented and closed-loop fluid flow within the root canal.[27] This system is expected to enhance irrigation dynamics in minimally enlarged root canals. Studies are currently being performed to assess the ability of the GW system to disinfect the root canal biofilms.[37]
In summary, many advanced antimicrobial strategies are being tested and developed to enhance antibiofilm efficacy within the root canal system. These techniques are focused toward potent antibiofilm methods and optimized irrigant delivery systems to achieve essential goals in root canal treatment. Further clinical research is required in this area.
ADVANCES IN ROOT FILLING
To complete a root canal treatment in a mature tooth, the root canal system is filled with synthetic materials. A predictable alternative to this technique is currently elusive. It may be possible in the future to attract pulp-like tissue into the cleaned and shaped root canals.[41] However, the translation of tissue engineering concepts to everyday clinics has not yet been made and thus, the current focus to improve the conventional approach to fill the root canals will still remain.
Primarily, the root-filling is expected to provide a hermetic seal against microorganisms, be tissue-friendly, easy to apply, monitor, and retrieve in case of treatment failure. These requirements are not always met by current filling materials.[42] The core of the problem with current materials is that a so-called hermetic seal is not easy to achieve.
Why is a hermetic seal difficult to achieve in root canals? Any material that is used to fill the small anatomical intricacies of a tooth needs to be applied in a plastic state. Later, this material should be dimensionally stable. With the synthetic materials used in dentistry, this means that some form of physical or chemical reaction takes place between the application of a filling material and its final state. In the context of filling root canals, we encounter the problem that the configuration factor (C-factor), which is the ratio between bonded to nonbonded area of a filling material, is extremely high in the root canals.[43,44] This means that the volume shrinkage is more detrimental to the cohesiveness of the tooth and filling in this environment than in the crown of the tooth. When a material alters its state, it usually changes its dimensions. For root-filling materials in particular, dimensional changes should be kept minimal. While most current sealers, especially those based on an epoxy resin or silicone, are dimensionally stable[45] the core material is not. In current root-filling techniques, gutta-percha (essentially polyisoprene with a high zinc oxide filler content) is used as a core material. It is heated to become plastic, and applied in conjunction with a sealer. However, it was well-recognized that thermoplasticized gutta-percha shrinks upon cooling.[46]
Some newer concepts: Two related concepts have evolved over the recent years that might improve and simplify root-filling procedures. The first approach is to use a calcium silicate cement-based sealer.[47,48] These sealers are initially flowable and express bioactive properties, i.e., they promote Ca/P precipitation in a wet environment. As the root canal system is inherently wet,[49] the use of bioactive filling materials is logical.[48] The interface that forms between sealer and root canal wall is calcium phosphate and thus, mimics nature. Since calcium-silicate cements set hard, a core material is still necessary, which remains to be gutta-percha. Consequently, root-fillings with calcium-silicate cements still have two interfaces:
Between the sealer and the canal wall, and
Between the sealer and the gutta-percha.[50]
Hence, calcium silicate sealers per se do not conclusively solve the root-filling conundrum.
In a recent approach, bioactive particles were embedded in the matrix (polycaprolactone) of the core filling material.[51] This matrix material was thermoplasticized and used as the sole material to fill root canals, thus reducing the interface between filling and tooth to one, with rather promising results in vitro. However, polycaprolactone appears not to be an ideal material for root-fillings, as it is biodegradable.[52] A later approach used nanometric bioactive glass particles of the 45S5 type embedded in the gutta-percha matrix.[53] In contrast to conventional gutta-percha, this material showed immediate sealing properties when applied in heated form. A premarket radiopaque material was later introduced and tested for its self-adhesiveness to the root dentin[54] and tissue compatibility.[55] The initial in vitro results were promising, yet the material has to be scrutinized clinically before final recommendation.
Complex application schemes[56] and uncontrolled/extended thermal shrinkage[57,58] are some of the challenges in current root-filling. Newer nanomaterial-based approaches are showing promise for the future.
REGENERATIVE ENDODONTICS
The treatment of immature teeth with pulpal necrosis represents a major clinical challenge. The untoward advent of pulpal necrosis arrests the developmental process in immature teeth.[59] The challenges related to treating these cases far exceed the technical challenges of debriding and obturating a large root canal space with thin dentinal walls, and lacking an apical constriction. These teeth have been traditionally treated with apexification procedures. Although these procedures are quite successful in arresting the infectious process and resolving apical periodontitis, they fail to promote continued root development and normal physiological pulpal responses. Thus, immature teeth remain with thin fragile dentinal walls becoming susceptible to fractures and lower survival. Further, implants are contraindicated in patients undergoing cranioskeletal development. Thus, there has been an unmet need to provide adequate treatment to patients with immature teeth with pulpal necrosis.
The field of regenerative endodontics emerged in early 2000s with the publication of two remarkable case reports.[60,61] Since then, there have been more than 200 published cases demonstrating that these procedures allow unprecedented results.[59,62,63] These include:
Resolution of apical periodontitis and signs and symptoms of pulpal inflammation;
Radiographic evidence of continued root development and apical narrowing; and
Restoration of vitality responses.
Importantly, these published cases demonstrate that REPs address the unmet need of promoting normal physiological development and responses in immature teeth diagnosed with pulpal necrosis.
In most REPs, clinicians rely on creating bleeding from the apical region that passively fills the canal space and forms a blood clot. However, it was not until 2011 that a clinical study demonstrated that the influx of apical blood into disinfected root canals allowed a significant transfer of stem cells into the root canal system. This was a very important pivoting moment in this young field of regenerative endodontics since it established that these procedures were in fact, stem cell-based procedures. The realization that stem cells were present in root canals during these procedures propelled researchers to investigate the effect of various steps usually employed on these procedures on stem cell fate.
The balance between adequate disinfection and stem cell survival, proliferation, and differentiation represents an important first barrier to be overcome. The resolution of infection and the disease process remains the primary goal of any endodontic procedure. However, it has become obvious that the philosophy of disinfecting the root canal at all costs typically advocated in traditional root canal therapy had to be modified to a “biocompatible disinfection” strategy. For example, sodium hypochlorite remains the most used disinfectant in endodontics.[64] However, its use at full concentration of 6% denatures crucial growth factors in the dentin[65] and results in residual detrimental effects greatly affecting stem cell survival and differentiation potential.[66,67,68] These effects can be largely avoided with the use of the concentration of 1.5% followed by 17% EDTA.[68,69] Another example is the long-lasting detrimental effects of using high concentrations of antibiotic pastes (approximately 1 g/mL) as intracanal medicament on stem cells. At this concentration, triple antibiotic paste (minocycline, metronidazole, and ciprofloxacin) have long-lasting effects on stem cell survival through both direct and indirect mechanisms.[70,71] This undesirable carryover effect can be greatly avoided by the use calcium hydroxide as intracanal medicament[70,71] or the use of these pastes in lower concentrations (<1 mg/mL) while maintaining their desirable antibacterial effect.[72,73] Therefore, there has been significant advancement in understanding how to adapt currently used disinfection protocols to the reality of stem cell-based therapies.
Apart from biocompatible disinfection, many other frontiers in regenerative endodontic research are being currently investigated. These involves tissue engineering strategies that include the evaluation of suitable scaffolds, growth factors, and harvested stem cells to be used in pulpal regeneration.[74] Importantly, many of the advances from translational research have been transferred to clinical practice such as the use of platelet-rich plasma,[75,76] platelet fibrin,[77] and a gelatin hydrogel[78] as scaffolds in patients. In addition, a groundbreaking clinical trial is currently in process in Japan. This trial involves harvesting stem cells from a donor site followed by ex vivo expansion, sorting, and autotransplantation into a recipient tooth to promote the regeneration of the once lost functional pulp-dentin complex.[79] These elegant studies highlight the current status and sophistication of REPs.
In summary, significant advances in regenerative endodontics allow better understanding of a multitude of factors that govern stem cell-mediated regeneration and repair of the damaged pulp-dentin complex. Translational research is proving to be crucial in making these procedures more predictable while pushing the boundaries of future procedures that are likely to involve the direct clinical manipulation of scaffolds, growth factors, and stem cells.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Nair MK, Nair UP. Digital and advanced imaging in endodontics: A review. J Endod. 2007;33:1–6. doi: 10.1016/j.joen.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 2.ADA Council on Scientific Affairs. An update on radiographic practices: Information and recommendations. ADA Council on Scientific Affairs. J Am Dent Assoc. 2001;132:234–8. doi: 10.14219/jada.archive.2001.0161. [DOI] [PubMed] [Google Scholar]
- 3.American Association of Endodontists; American Academy of Oral and Maxillofacial Radiolog. Use of cone-beam computed tomography in endodontics Joint Position Statement of the American Association of Endodontists and the American Academy of Oral and Maxillofacial Radiology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:234–7. doi: 10.1016/j.tripleo.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Tyndall DA, Price JB, Tetradis S, Ganz SD, Hildebolt C, Scarfe WC. American Academy of Oral and Maxillofacial Radiology. Position statement of the American Academy of Oral and Maxillofacial Radiology on selection criteria for the use of radiology in dental implantology with emphasis on cone beam computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:817–26. doi: 10.1016/j.oooo.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Ørstavik D, Pitt Ford T. Essentials in Endodontology. 2nd ed. Oxford: Blackwell Munksgaard; 2008. pp. 1–488. [Google Scholar]
- 6.Bird DC, Chambers D, Peters OA. Usage parameters of nickel-titanium rotary instruments: A survey of endodontists in the United States. J Endod. 2009;35:1193–7. doi: 10.1016/j.joen.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 7.Thompson SA. An overview of nickel-titanium alloys used in dentistry. Int Endod J. 2000;33:297–310. doi: 10.1046/j.1365-2591.2000.00339.x. [DOI] [PubMed] [Google Scholar]
- 8.Otsuka K, Ren X. Physical metallurgy of Ti-Ni-based shape memory alloys. Progr Mat Sci. 2005;50:511–678. [Google Scholar]
- 9.Shen Y, Zhou HM, Zheng YF, Peng B, Haapasalo M. Current challenges and concepts of the thermomechanical treatment of nickel-titanium instruments. J Endod. 2013;39:163–72. doi: 10.1016/j.joen.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Peters OA, Gluskin AK, Weiss RA, Han JT. An in vitro assessment of the physical properties of novel Hyflex nickel-titanium rotary instruments. Int Endod J. 2012;45:1027–34. doi: 10.1111/j.1365-2591.2012.02067.x. [DOI] [PubMed] [Google Scholar]
- 11.Hülsmann M, Peters OA, Dummer PM. Mechanical preparation of root canals: Shapinhg goals, techniques and means. Endod Topics. 2005;10:30–76. [Google Scholar]
- 12.Yared G. Canal preparation using only one Ni-Ti rotary instrument: Preliminary observations. Int Endod J. 2008;41:339–44. doi: 10.1111/j.1365-2591.2007.01351.x. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Higueras JJ, Arias A, de la Macorra JC. Cyclic fatigue resistance of K3, K3XF, and twisted file nickel-titanium files under continuous rotation or reciprocating motion. J Endod. 2013;39:1585–8. doi: 10.1016/j.joen.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Koch J, Borg J, Mattson A, Olsen K, Bahcall J. An in vitro comparative study of intracanal fluid motion and wall shear stress induced by ultrasonic and polymer rotary finishing files in a simulated root canal model. ISRN Dent 2012. 2012:764041. doi: 10.5402/2012/764041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters OA, Schönenberger K, Laib A. Effects of four Ni-Ti preparation techniques on root canal geometry assessed by micro computed tomography. Int Endod J. 2001;34:221–30. doi: 10.1046/j.1365-2591.2001.00373.x. [DOI] [PubMed] [Google Scholar]
- 16.Metzger Z, Teperovich E, Zary R, Cohen R, Hof R. The self-adjusting file (SAF). Part 1: Respecting the root canal anatomy--a new concept of endodontic files and its implementation. J Endod. 2010;36:679–90. doi: 10.1016/j.joen.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 17.Ng YL, Mann V, Gulabivala K. A prospective study of the factors affecting outcomes of nonsurgical root canal treatment: Part 1: Periapical health. Int Endod J. 2011;44:583–609. doi: 10.1111/j.1365-2591.2011.01872.x. [DOI] [PubMed] [Google Scholar]
- 18.Ng YL, Mann V, Gulabivala K. A prospective study of the factors affecting outcomes of non-surgical root canal treatment: Part 2: Tooth survival. Int Endod J. 2011;44:610–25. doi: 10.1111/j.1365-2591.2011.01873.x. [DOI] [PubMed] [Google Scholar]
- 19.Reeh ES, Messer HH, Douglas WH. Reduction in tooth stiffness as a result of endodontic and restorative procedures. J Endod. 1989;15:512–6. doi: 10.1016/S0099-2399(89)80191-8. [DOI] [PubMed] [Google Scholar]
- 20.Krishan R, Paqué F, Ossareh A, Kishen A, Dao T, Friedman S. Impacts of conservative endodontic cavity on root canal instrumentation efficacy and resistance to fracture assessed in incisors, premolars, and molars. J Endod. 2014;40:1160–6. doi: 10.1016/j.joen.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Lang H, Korkmaz Y, Schneider K, Raab WH. Impact of endodontic treatments on the rigidity of the root. J Dent Res. 2006;85:364–8. doi: 10.1177/154405910608500416. [DOI] [PubMed] [Google Scholar]
- 22.Zelic K, Vukicevic A, Jovicic G, Aleksandrovic S, Filipovic N, Djuric M. Mechanical weakening of devitalized teeth: Three-dimensional finite element analysis and prediction of tooth fracture. Int Endod J. 2015;48:850–63. doi: 10.1111/iej.12381. [DOI] [PubMed] [Google Scholar]
- 23.Gluskin AH, Peters CI, Peters OA. Minimally invasive endodontics: Challenging prevailing paradigms. Br Dent J. 2014;216:347–53. doi: 10.1038/sj.bdj.2014.201. [DOI] [PubMed] [Google Scholar]
- 24.Degerness RA, Bowles WR. Dimension, anatomy and morphology of the mesiobuccal root canal system in maxillary molars. J Endod. 2010;36:985–9. doi: 10.1016/j.joen.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Garala M, Kuttler S, Hardigan P, Steiner-Carmi R, Dorn S. A comparison of the minimum canal wall thickness remaining following preparation using two nickel-titanium rotary systems. Int Endod J. 2003;36:636–42. doi: 10.1046/j.1365-2591.2003.00704.x. [DOI] [PubMed] [Google Scholar]
- 26.Lussi A, Nussbächer U, Grosrey J. A novel noninstrumented technique for cleansing the root canal system. J Endod. 1993;19:549–53. doi: 10.1016/S0099-2399(06)81284-7. [DOI] [PubMed] [Google Scholar]
- 27.Haapasalo M, Wang Z, Shen Y, Curtis A, Patel P, Khakpour M. Tissue dissolution by a novel multisonic ultracleaning system and sodium hypochlorite. J Endod. 2014;40:1178–81. doi: 10.1016/j.joen.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 28.Peters OA, Paque F. Current developments in rotary root canal instrument technology and clinical use: A review. Quintessence Int. 2010;41:479–88. [PubMed] [Google Scholar]
- 29.Peters OA, Paque F. Shaping the root canal system to promote disinfection. In: Cohenca N, editor. Disinfection of root canal systems. 1st ed. Ames IA, Oxford UK: Wiley Blackwell; 2014. pp. 91–108. [Google Scholar]
- 30.Kishen A. Advanced therapeutic options for endodontic biofilms. Endod Topics. 2010;22:99–123. [Google Scholar]
- 31.Kishen A, Shi Z, Shrestha A, Neoh KG. An investigation on the antibacterial and antibiofilm efficacy of cationic nanoparticulates for root canal disinfection. J Endod. 2008;34:1515–20. doi: 10.1016/j.joen.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 32.Wu D, Fan W, Kishen A, Gutmann JL, Fan B. Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. J Endod. 2014;40:285–90. doi: 10.1016/j.joen.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 33.Shrestha A, Kishen A. Antibiofilm efficacy of photosensitizer-functionalized bioactive nanoparticles on multispecies biofilm. J Endod. 2014;40:1604–10. doi: 10.1016/j.joen.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Shrestha A, Hamblin MR, Kishen A. Photoactivated rose bengal functionalized chitosan nanoparticles produce antibacterial/biofilm activity and stabilize dentin-collagen. Nanomedicine. 2014;10:491–501. doi: 10.1016/j.nano.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.George S, Kishen A. Photophysical, photochemical, and photobiological characterization of methylene blue formulations for light-activated root canal disinfection. J Biomed Opt. 2007;12:034029. doi: 10.1117/1.2745982. [DOI] [PubMed] [Google Scholar]
- 36.George S, Kishen A. Augmenting the antibiofilm efficacy of advanced noninvasive light activated disinfection with emulsified oxidizer and oxygen carrier. J Endod. 2008;34:1119–23. doi: 10.1016/j.joen.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 37.Peters OA, Bardsley S, Fong J, Pandher G, Divito E. Disinfection of root canals with photon-initiated photoacoustic streaming. J Endod. 2011;37:1008–12. doi: 10.1016/j.joen.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Pedullà E, Genovese C, Campagna E, Tempera G, Rapisarda E. Decontamination efficacy of photon-initiated photoacoustic streaming (PIPS) of irrigants using low-energy laser settings: An ex vivo study. Int Endod J. 2012;45:865–70. doi: 10.1111/j.1365-2591.2012.02044.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhu X, Yin X, Chang JW, Wang Y, Cheung GS, Zhang C. Comparison of the antibacterial effect and smear layer removal using photon-initiated photoacoustic streaming aided irrigation versus a conventional irrigation in single-rooted canals: An in vitro study. Photomed Laser Surg. 2013;31:371–7. doi: 10.1089/pho.2013.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.George R, Chan K, Walsh LJ. Laser-induced agitation and cavitation from proprietary honeycomb tips for endodontic applications. Lasers Med Sci. 2015;30:1203–8. doi: 10.1007/s10103-014-1539-y. [DOI] [PubMed] [Google Scholar]
- 41.Mao JJ, Kim SG, Zhou J, Ye L, Cho S, Suzuki T, et al. Regenerative endodontics: Barriers and strategies for clinical translation. Dent Clin North Am. 2012;56:639–49. doi: 10.1016/j.cden.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gatewood RS. Endodontic materials. Dent Clin North Am. 2007;51:695–712, vii. doi: 10.1016/j.cden.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Bouillaguet S, Troesch S, Wataha JC, Krejci I, Meyer JM, Pashley DH. Microtensile bond strength between adhesive cements and root canal dentin. Dent Mater. 2003;19:199–205. doi: 10.1016/s0109-5641(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 44.Tay FR, Loushine RJ, Lambrechts P, Weller RN, Pashley DH. Geometric factors affecting dentin bonding in root canals: A theoretical modeling approach. J Endod. 2005;31:584–9. doi: 10.1097/01.don.0000168891.23486.de. [DOI] [PubMed] [Google Scholar]
- 45.Ørstavik D, Nordahl I, Tibballs JE. Dimensional change following setting of root canal sealer materials. Dent Mater. 2001;17:512–9. doi: 10.1016/s0109-5641(01)00011-2. [DOI] [PubMed] [Google Scholar]
- 46.Schilder H, Goodman A, Aldrich W. The thermomechanical properties of gutta-percha. Part V. Volume changes in bulk gutta-percha as a function of temperature and its relationship to molecular phase transformation. Oral Surg Oral Med Oral Pathol. 1985;59:285–96. doi: 10.1016/0030-4220(85)90169-0. [DOI] [PubMed] [Google Scholar]
- 47.Huffman BP, Mai S, Pinna L, Weller RN, Primus CM, Gutmann JL, et al. Dislocation resistance of ProRoot Endo Sealer, a calcium silicate-based root canal sealer, from radicular dentine. Int Endod J. 2009;42:34–46. doi: 10.1111/j.1365-2591.2008.01490.x. [DOI] [PubMed] [Google Scholar]
- 48.Prati C, Gandolfi MG. Calcium silicate bioactive cements: Biological perspectives and clinical applications. Dent Mater. 2015;31:351–70. doi: 10.1016/j.dental.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Papa J, Cain C, Messer HH. Moisture content of vital vs endodontically treated teeth. Endod Dent Traumatol. 1994;10:91–3. doi: 10.1111/j.1600-9657.1994.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 50.Tay FR, Pashley DH. Monoblocks in root canals - A hypothetical or a tangible goal. J Endod. 2007;33:391–8. doi: 10.1016/j.joen.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alani A, Knowles JC, Chrzanowski W, Ng YL, Gulabivala K. Ion release characteristics, precipitate formation and sealing ability of a phosphate glass-polycaprolactone-based composite for use as a root canal obturation material. Dent Mater. 2009;25:400–10. doi: 10.1016/j.dental.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 52.Tay FR, Pashley DH, Yiu CK, Yau JY, Yiu-fai M, Loushine RJ, et al. Susceptibility of a polycaprolactone-based root canal filling material to degradation. II. Gravimetric evaluation of enzymatic hydrolysis. J Endod. 2005;31:737–41. doi: 10.1097/01.don.0000155225.40794.79. [DOI] [PubMed] [Google Scholar]
- 53.Mohn D, Bruhin C, Luechinger NA, Stark WJ, Imfeld T, Zehnder M. Composites made of flame-sprayed bioactive glass 45S5 and polymers: Bioactivity and immediate sealing properties. Int Endod J. 2010;43:1037–46. doi: 10.1111/j.1365-2591.2010.01772.x. [DOI] [PubMed] [Google Scholar]
- 54.Marending M, Bubenhofer SB, Sener B, De-Deus G. Primary assessment of a self-adhesive gutta-percha material. Int Endod J. 2013;46:317–22. doi: 10.1111/j.1365-2591.2012.02117.x. [DOI] [PubMed] [Google Scholar]
- 55.Belladonna FG, Calasans-Maia MD, Novellino Alves AT, de Brito Resende RF, Souza EM, Silva EJ, et al. Biocompatibility of a self-adhesive gutta-percha-based material in subcutaneous tissue of mice. J Endod. 2014;40:1869–73. doi: 10.1016/j.joen.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 56.Schilder H. Filling root canals in three dimensions. Dent Clin North Am. 1967:723–44. [PubMed] [Google Scholar]
- 57.Lottanti S, Tauböck TT, Zehnder M. Shrinkage of backfill gutta-percha upon cooling. J Endod. 2014;40:721–4. doi: 10.1016/j.joen.2013.09.043. [DOI] [PubMed] [Google Scholar]
- 58.Lee CQ, Chang Y, Cobb CM, Robinson S, Hellmuth EM. Dimensional stability of thermosensitive gutta-percha. J Endod. 1997;23:579–82. doi: 10.1016/S0099-2399(06)81126-X. [DOI] [PubMed] [Google Scholar]
- 59.Diogenes A, Henry MA, Teixeira FB, Hargreaves KM. An update on clinical regenerative endodontics. Endod Topics. 2013;28:2–23. [Google Scholar]
- 60.Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: New treatment protocol? J Endod. 2004;30:196–200. doi: 10.1097/00004770-200404000-00003. [DOI] [PubMed] [Google Scholar]
- 61.Iwaya SI, Ikawa M, Kubota M. Revascularization of an immature permanent tooth with apical periodontitis and sinus tract. Dent Traumatol. 2001;17:185–7. doi: 10.1034/j.1600-9657.2001.017004185.x. [DOI] [PubMed] [Google Scholar]
- 62.Kontakiotis EG, Filippatos CG, Tzanetakis GN, Agrafioti A. Regenerative endodontic therapy: A data analysis of clinical protocols. J Endod. 2015;41:146–54. doi: 10.1016/j.joen.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 63.Hargreaves KM, Diogenes A, Teixeira FB. Treatment options: Biological basis of regenerative endodontic procedures. Pediatr Dent. 2013;35:129–40. [PubMed] [Google Scholar]
- 64.Harrison JW, Hand RE. The effect of dilution and organic matter on the anti-bacterial property of 5.25% sodium hypochlorite. J Endod. 1981;7:128–32. doi: 10.1016/S0099-2399(81)80127-6. [DOI] [PubMed] [Google Scholar]
- 65.Zhao S, Sloan AJ, Murray PE, Lumley PJ, Smith AJ. Ultrastructural localisation of TGF-beta exposure in dentine by chemical treatment. Histochem J. 2000;32:489–94. doi: 10.1023/a:1004100518245. [DOI] [PubMed] [Google Scholar]
- 66.Trevino EG, Patwardhan AN, Henry MA, Perry G, Dybdal-Hargreaves N, Hargreaves KM, et al. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J Endod. 2011;37:1109–15. doi: 10.1016/j.joen.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 67.Casagrande L, Demarco FF, Zhang Z, Araujo FB, Shi S, Nör JE. Dentin-derived BMP-2 and odontoblast differentiation. J Dent Res. 2010;89:603–8. doi: 10.1177/0022034510364487. [DOI] [PubMed] [Google Scholar]
- 68.Galler KM, D’Souza RN, Federlin M, Cavender AC, Hartgerink JD, Hecker S, et al. Dentin conditioning codetermines cell fate in regenerative endodontics. J Endod. 2011;37:1536–41. doi: 10.1016/j.joen.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 69.Martin DE, De Almeida JF, Henry MA, Khaing ZZ, Schmidt CE, Teixeira FB, et al. Concentration-dependent effect of sodium hypochlorite on stem cells of apical papilla survival and differentiation. J Endod. 2014;40:51–5. doi: 10.1016/j.joen.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 70.Ruparel NB, Teixeira FB, Ferraz CC, Diogenes A. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J Endod. 2012;38:1372–5. doi: 10.1016/j.joen.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 71.Althumairy RI, Teixeira FB, Diogenes A. Effect of dentin conditioning with intracanal medicaments on survival of stem cells of apical papilla. J Endod. 2014;40:521–5. doi: 10.1016/j.joen.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 72.Sabrah AH, Yassen GH, Liu WC, Goebel WS, Gregory RL, Platt JA. The effect of diluted triple and double antibiotic pastes on dental pulp stem cells and established Enterococcus faecalis biofilm. Clin Oral Investig. 2015;19:2059–66. doi: 10.1007/s00784-015-1423-6. [DOI] [PubMed] [Google Scholar]
- 73.Sabrah AH, Yassen GH, Gregory RL. Effectiveness of antibiotic medicaments against biofilm formation of enterococcus faecalis and porphyromonas gingivalis. J Endod. 2013;39:1385–9. doi: 10.1016/j.joen.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 74.Albuquerque MT, Valera MC, Nakashima M, Nör JE, Bottino MC. Tissue-engineering-based strategies for regenerative endodontics. J Dent Res. 2014;93:1222–31. doi: 10.1177/0022034514549809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sachdeva GS, Sachdeva LT, Goel M, Bala S. Regenerative endodontic treatment of an immature tooth with a necrotic pulp and apical periodontitis using platelet-rich plasma (PRP) and mineral trioxide aggregate (MTA): A case report. Int Endod J. 2015;48:902–10. doi: 10.1111/iej.12407. [DOI] [PubMed] [Google Scholar]
- 76.Torabinejad M, Turman M. Revitalization of tooth with necrotic pulp and open apex by using platelet-rich plasma: A case report. J Endod. 2011;37:265–8. doi: 10.1016/j.joen.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 77.Shivashankar VY, Johns DA, Vidyanath S, Kumar MR. Platelet rich fibrin in the revitalization of tooth with necrotic pulp and open apex. J Conserv Dent. 2012;15:395–8. doi: 10.4103/0972-0707.101926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagy MM, Tawfik HE, Hashem AA, Abu-Seida AM. Regenerative potential of immature permanent teeth with necrotic pulps after different regenerative protocols. J Endod. 2014;40:192–8. doi: 10.1016/j.joen.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 79.Nakashima M, Iohara K. Mobilized dental pulp stem cells for pulp regeneration: Initiation of clinical trial. J Endod. 2014;40(Suppl):S26–32. doi: 10.1016/j.joen.2014.01.020. [DOI] [PubMed] [Google Scholar]


