Abstract
Aims:
To compare the surface microhardness, setting time, and elemental and topographic changes of mineral trioxide aggregate (MTA) and calcium-enriched mixture (CEM) in contact with acidic, neutral, and alkaline solutions.
Subjects and Methods:
For evaluating 24-h and 28-day surface microhardness using Vickers test and initial setting time using Gillmore apparatus, glass molds were filled manually or ultrasonically, either with CEM or MTA and randomly immersed in solutions with acidic, neutral, and alkaline pH (5.4, 7.4, and 9.4, respectively). Topographic changes of the samples as well as energy dispersive X-ray spectra were examined using the scanning electron microscopy.
Statistical Analysis Used:
Data were analyzed using the Kruskal-Wallis, Mann-Whitney, Wilcoxon, one- and two-way ANOVA, Tukey's post hoc, and t-tests.
Results:
After 28 days, there was an increase in the microhardness for all samples (without statistical significance [P > 0.05]), except for the samples of CEM in acidic environment (P > 0.05). The setting time of MTA samples was statistically higher than CEM samples (P ≤ 0.001). The setting time of both biomaterials was significantly higher in acidic pH than other groups (P ≤ 0.005). Surface topography and elemental constituents of biomaterials were altered in different solutions.
Conclusion:
The surface microhardness, setting time, and elemental and topographic properties of MTA and CEM were affected by different solutions. CEM exhibited quicker setting time than MTA; however, acidic solution negatively influenced both of them.
Keywords: Biomaterials, calcium-enriched mixture, endodontics, hardness, mineral trioxide aggregate, pH, ultrasonics
INTRODUCTION
Mineral trioxide aggregate (MTA) is an endodontic repair biomaterial. Despite the advantages such as biocompatibility, sealability, and hard-tissue forming capacity,[1] some drawbacks are mentioned, including poor handling, long setting time,[2] and high price. The physical/chemical properties of MTA are affected in different pH levels.[3,4]
Calcium-enriched mixture (CEM) has been developed with similar clinical indications as MTA but with different compositions.[5] CEM is capable of forming hydroxyapatite in aqueous environments.[6] In comparison with MTA, CEM offers the advantages of favorable handling, more clinician-friendly setting time, and reasonable price.[5]
Physical characteristics of hydrophilic biocements can be affected by several factors such as powder/liquid ratio, method of mixing, pressure used for condensation, the type of storage media, the pH level of the environment, the time lapse between mixing and evaluation, thickness of the material, humidity, and temperature.[1] Some studies showed increased compressive strength and surface microhardness of ultrasonically compacted MTA samples compared to hand-mixed samples,[7,8] while others found poorer physical properties due to incorporation of more air into MTA.[9,10]
Instead, hydroxyapatite precipitation on the surface of MTA is responsible for its physical/chemical reactions to the surrounding environment, in addition to its biologic properties.[11,12] Hydroxyapatite crystals can be best created in contact with physiologic solutions (i.e., simulated body fluid [SBF]/phosphate-buffered solution) by the reaction between calcium ions from MTA and phosphorus from the solutions.[13]
Variations in the pH of the host tissues would adversely affect the properties of MTA; Lee et al. showed reduction in the hydration byproducts of MTA in acidic pH.[14] In addition, a scanning electron microscopy (SEM) indicated more porosity and unhydrated structure at a pH level of 10.4.[15] Furthermore, reduction in push out bond strength, sealability, and hardness has been reported at acidic or highly alkaline pH levels.[4,16,17,18] Most of the studies in this regard created the acidic/alkaline environments by utilizing buffered butyric acid or potassium hydroxide with the desired pH levels, respectively; not considering the effect of interstitial fluid as the buffering agent.
To date, few studies have been conducted regarding the effect of different pH values on the physical properties of ProRoot MTA and CEM. The purpose of this investigation was to compare the physical characteristics of these two endodontic biomaterials including setting time, surface microhardness, topographic and elemental level changes in contact with acidic, neutral, and alkaline solutions, using hand and ultrasonic condensation techniques, during different periods. The null hypothesis was that buffering acidic/alkaline solutions with SBF have an impact on the physical properties of the MTA and CEM cements.
SUBJECTS AND METHODS
Sample preparation
White ProRoot MTA (Dentsply, Tulsa, OK, USA) and CEM cement (BioniqueDent, Tehran, Iran) were mixed with water at a ratio of 3:1 by weight and were packed into cylindrical Plexi glass molds. Half of the CEM sample was manually packed (n = 36) using a hand condenser (Hu-Friedy, Chicago, IL, USA) and the other half was condensed using an ultrasonic tip (n = 36) in a Suprasson P5 Booster unit (Satelec, ActeÓn Group, France) held lightly against the same hand condenser at each increment for 10 s. All the MTA samples were manually packed (n = 36) with the similarly sized hand condenser. All the fillings were flushed with the surface of the blocks.
Environmental preparation
To assure of obtaining exact pH values by buffering an acid and base with SBF, a pilot study was carried out. Then, the specimens were immersed in one of these solutions in separate glass vessels:
Neutral pH: 1-deionized water, 2-SBF; pH = 7.4.
Acidic pH: Butyric acid buffered by SBF; pH = 5.4.
Alkaline pH: Ca(OH)2 buffered by SBF; pH = 9.4.
Then, the vessels were transported to an incubator with 37°C temperature and 95% humidity.
Surface microhardness
Glass molds (5 mm × 3 mm) were packed with cements (n = 72 for CEM cement, half manually and the other half ultrasonically condensed, and n = 36 for MTA, all manually condensed). The molds were immersed in the above-mentioned solutions. After 24 h and 28 days, the samples were slightly polished with sand papers with varying grit sizes (800-, 1500-, and 3000-grit) and gently cleaned and dried by air spray. The Vicker's test was performed for each sample using microhardness tester (HVS-1000, Taiwan) after fuchsine dye staining for obtaining more readable indentations. For each surface, a full load of 50 g for 10 s was applied 3 times with a square-based pyramid-shaped diamond indenter at three distant points. Resulting indentation was measured with a microscope (×40 magnification), and the average of the Vicker's hardness values was calculated for each specimen.
The data were analyzed; Shapiro-Wilk and Levene's tests were used for evaluating normal distribution and equality of error variances, respectively, and the Kruskal-Wallis, Mann-Whitney, and Wilcoxon tests were used to detect any significant difference among the groups. The level of significance was set at 0.05.
Scanning electron microscopy-energy dispersive X-ray analysis
The chemical composition and ultrastructure of the precipitate samples were studied using the SEM-energy dispersive X-ray (EDAX) after thin conductive coating of samples with gold. The chemical composition analysis was carried out at 15 kV and 100 μA of probe current. One sample from each cement, each of different periods and different solution was taken to be evaluated under SEM (Tescan, Czech Republic).
Setting time
Evaluation of setting time was based on ISO6876:2002 criteria. The prepared materials were packed into 36 resin molds (24 for CEM, half manually and the other half ultrasonically condensed, and 12 for MTA, all manually condensed). For both cements, four groups each containing three specimens were prepared; pH of the each group was provided from sponges soaked in acidic, neutral (both SBF and deionized water), and alkaline solutions in close contact with the materials’ surface. To record the initial setting time, a Gillmore apparatus (Taksaz-ideh, Tehran, Iran) was used with 100 g load on a flat-end indenter with a diameter of 2 mm, starting 30 min after initiation of mixing, with 5 min intervals until the indenter needle failed to make any indentation. One- and Two-way ANOVA, Tukey's SD, and t-test were used for statistical analysis at a significance level of 0.05. The dependent variable (setting time) was normally distributed as assessed by Shapiro-Wilk and Leven's tests.
RESULTS
Surface microhardness
Evaluation of hardness values of each separate group showed a significant difference among solutions. [CEM/ultrasonically packed/24 h (P = 0.024); CEM/manually packed/24 h (P = 0.001); CEM/ultrasonically packed/28 days (P = 0.001); CEM/manually packed/28 days (P = 0.001); and MTA/24 h (P = 0.001); MTA/28 days (P = 0.06)]. However, there was no significant difference among solutions while comparing the two materials at the same time interval (P > 0.05).
After 28 days, there was an increase in the mean values of microhardness for all samples in all environments (P > 0.05), except for manually/ultrasonically packed samples of CEM in acidic environment (P = 0.1).
Analyzing packing techniques, ultrasonication had the most impact on the hardness of CEM samples during the first 24 h (P = 0.016).
Comparing both manually packed cements at each evaluated time intervals, CEM samples showed the highest mean values of microhardness in alkaline solution (P = 0.2); however, MTA samples exhibited the highest values of hardness at deionized water (P = 0.1).
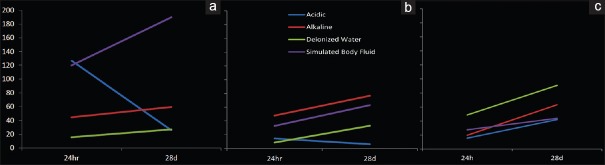
Among all the samples, the lowest and highest mean values of microhardness were related to 28-day manually packed samples of CEM cement, immersed in acidic solution (6.22 ± 1.99) and 28-day ultrasonically packed CEM samples, immersed in SBF (189.75 ± 56.72), respectively [Figure 1].
Figure 1.
Surface microhardness of calcium-enriched mixture and mineral trioxide aggregate samples; (a) ultrasonically packed calcium-enriched mixture, (b) manually packed calcium-enriched mixture, and (c) mineral trioxide aggregate
Scanning electron microscopy-energy dispersive X-ray analysis
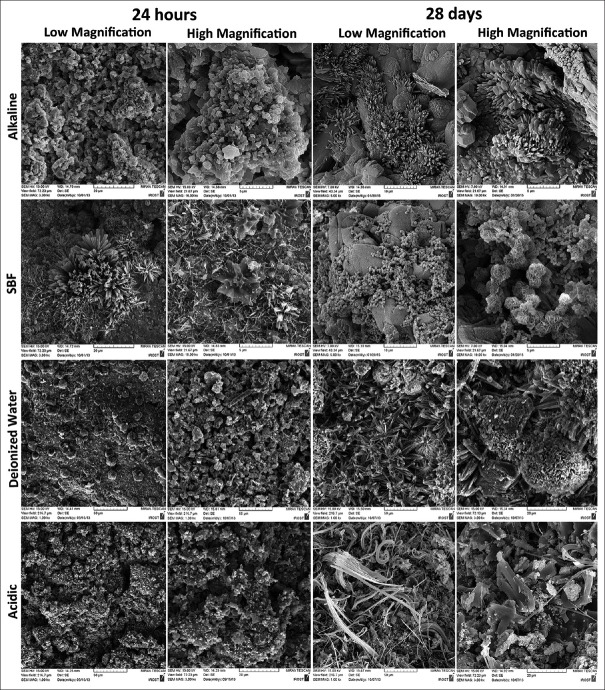
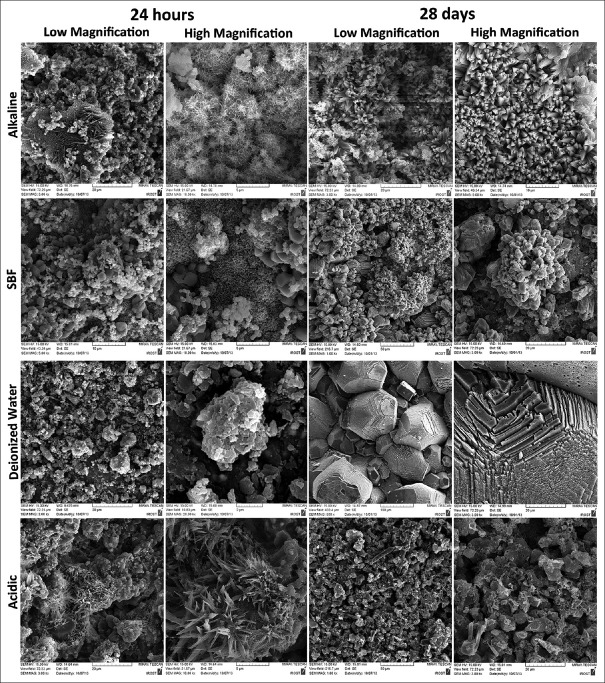
Scanning electron micrographs of CEM and MTA are shown in Figures 2 and 3; EDAX spectra of CEM cement and MTA are shown in Figures 4 and 5, respectively.
Figure 2.
Scanning electron micrographs of calcium-enriched mixture cement, hydrated after 24 h and 28 days, in alkaline, simulated body fluid, deionized water, and acidic solutions
Figure 3.
Scanning electron micrographs of mineral trioxide aggregate cement, hydrated after 24 h and 28 days in alkaline, simulated body fluid, deionized water, and acidic solutions
Figure 4.
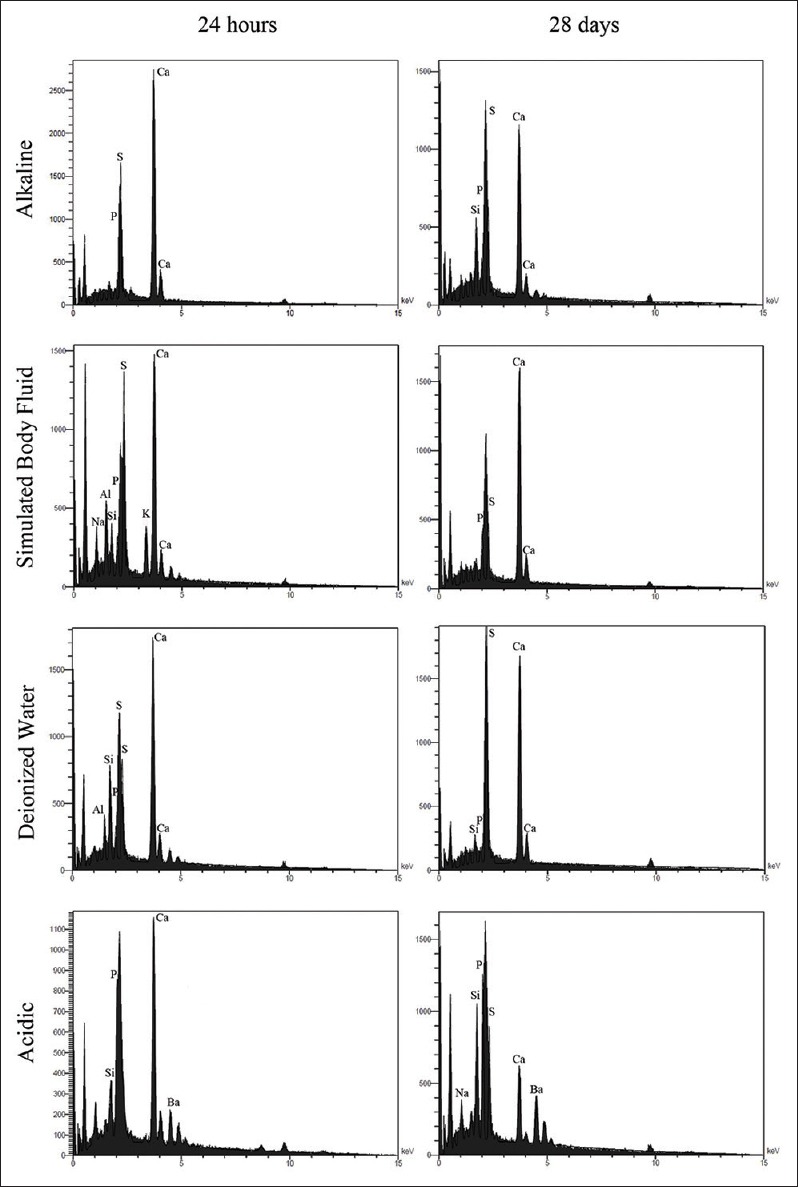
Energy dispersive X-ray spectra of calcium-enriched mixture cement hydrated after 24 h and 28 days in alkaline, simulated body fluid, deionized water, and acidic solutions
Figure 5.
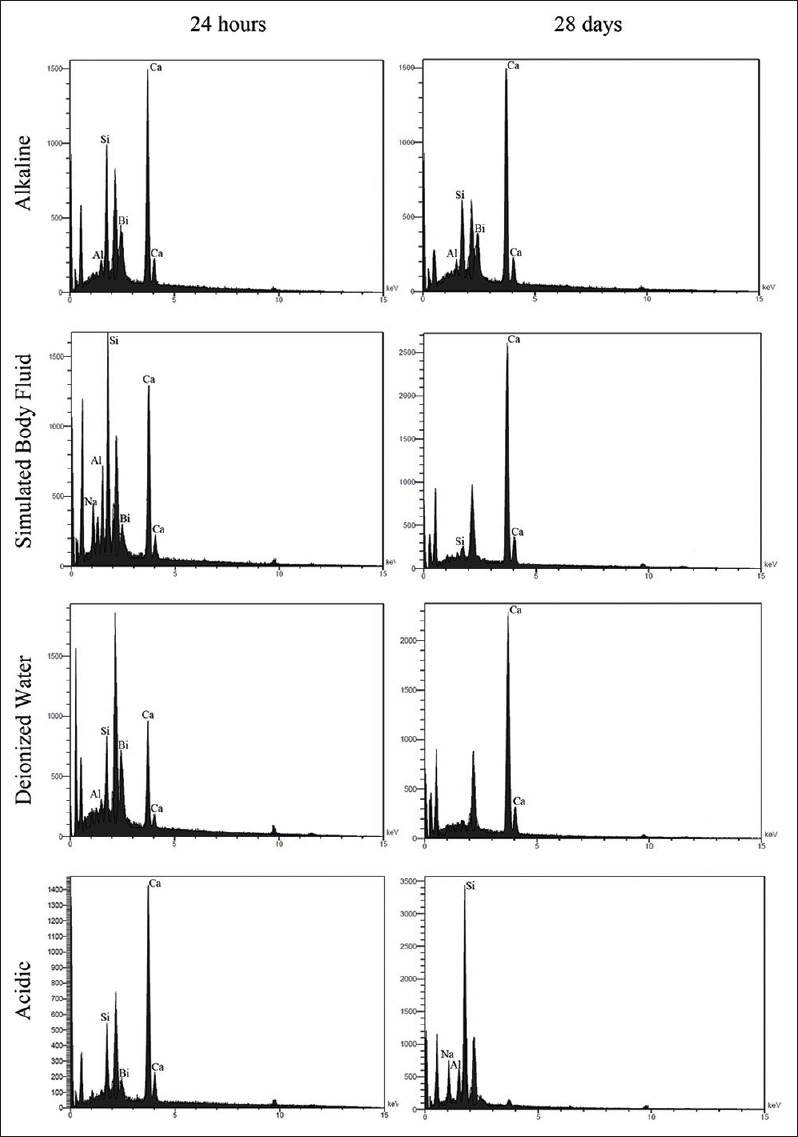
Energy dispersive X-ray spectra of mineral trioxide aggregate cement hydrated after 24 h and 28 days in alkaline, simulated body fluid, deionized water, and acidic solutions
Calcium-enriched mixture cement, alkaline solution
In samples kept in alkaline solution for 24 h, the crystals are spherical and polygonal, gathered together in some places. After 28 days, clusters of polygonal flower-shaped crystals, being dispersed among other polygonal and cubic-shaped ones along with concentric prismatic precipitates are seen in a relatively homogeneous surface. It seems that with passage of time, the particles acquire a more distinct shape.
Calcium-enriched mixture cement, simulated body fluid
Samples immersed in SBF for 24 h exhibited scattered acicular precipitates that are thicker and more concentric in some places accompanying smaller amounts of spherical-, polygonal-, and cauliflower-shaped crystals. At day 28, numerous tiny spherical crystals with few numbers of bigger-layered polygonal precipitates among them could be detected. In higher magnification, it seems that some globular precipitates are gatherings of thinner sheet-like crystals wrapped together to form a flower.
Calcium-enriched mixture cement, deionized water
Twenty-four-hour samples showed large stratified cubic crystals accompanied by few small spherical particles. Twenty-eight-day samples revealed prismatic acicular crystals interlocked with each other at a constant angle and direction, besides globular clusters of polygonal crystals in the shape of a solid flower, and some stratified angular particles were detected.
Calcium-enriched mixture cement, acidic solution
Twenty-four-hour samples showed small spherical crystals dispersed throughout the surface, while after 28 days, the particles grew more in length and were tortuous. Moreover, larger polygonal crystals with rounded angles could be detected [Figure 2]. EDAX analysis showed that in acidic pH, a great reduction in Ca and increase in P, Si, S, and Na levels took place after 28 days.
Mineral trioxide aggregate, alkaline solution
Samples of 24 h showed many globular clusters of acicular crystals that together created a view of a coral. Some spherical crystals were scattered throughout the surface. After 28 days, these particles seemed to grow in size regularly, and created concentric prismatic crystals in the shape of cauliflowers, indicating ettringite because of the presence of Ca, Al, and Si.
Mineral trioxide aggregate, simulated body fluid
In 24-h SBF specimen, tiny spherical and much larger polygonal crystals with more concentration at some places could be seen. After 28 days, these particles gathered and regulated to exhibit small and large stratified precipitates and concentric polygonal crystals in the shape of a flower.
Mineral trioxide aggregate, deionized water
After 24 h, bundles of small and big polygonal crystals were detected. Twenty-eight-day samples revealed very large stratified polygonal- and cubic-shaped crystals, mainly composed of O, C, and Ca, characteristic of calcium hydroxide precipitates.
Mineral trioxide aggregate, acidic solution
Samples of 24 h indicated some collections of small and big crystals with rounded edges. Besides, few acicular crystals as the shape of a coral could be seen sporadically; while 28-day specimen was characterized by few thick needle-shaped crystals scattered among polygonal ones [Figure 3]. EDAX analysis showed that in acidic pH, a great reduction in Ca and increase in Si, Al, Na, O, and S occurred after 28 days.
Setting time
The mean values of initial setting time of MTA samples were statistically higher than CEM samples (P = 0.001). The mean values of initial setting time of both cements were significantly higher in acidic pH compared to other three groups (deionized water, P = 0.024; SBF, P = 0.004; alkaline, P = 0.001) [Figure 6].
Figure 6.
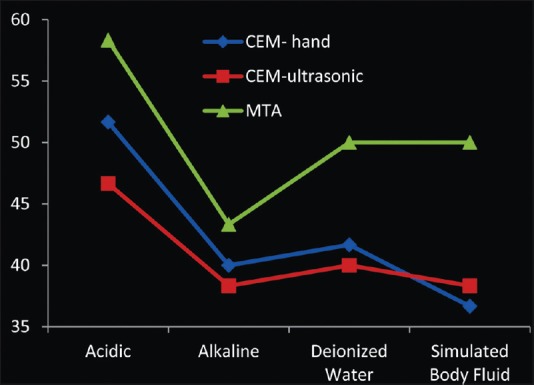
Setting time of mineral trioxide aggregate and calcium-enriched mixture cements
DISCUSSION
This investigation was among the rare ones comprehensively evaluating the impact of all the four environments, namely acidic, alkaline, deionized water, and SBF on the physical characteristics of MTA and CEM cement, using hand/ultrasonic condensation techniques, during different periods. The null hypothesis of this study was confirmed in the sense that surface microhardness, setting time, and elemental and topographic properties of MTA and CEM were influenced by solutions different in pH over time. Microhardness values for all samples in all environments increased, although manually/ultrasonically packed samples of CEM in acidic environment were exception. The initial setting time of MTA was statistically higher than CEM. Moreover, the initial setting time of both cements was significantly higher in acidic pH compared to other groups.
As physical behavior of biomaterials could be a result of changes in the chemical aspect of the material, EDAX was conducted in this study to associate the findings related to physical aspects. However, a drawback to this method would be introducing the formed crystals as separate elements rather than the exact compound structures. Microanalysis revealed that Ca/S/Si/P were the major elements of CEM, with phosphorus being the main compartment, whereas the amount of latter element is very low in MTA,[5] which is consistent with EDAX results of the present study. Another difference is the existence of Bi in MTA, contrary to CEM.
Immersion of the cements in different environmental fluids results in chemically/morphologically different precipitates in the surface and variation in nucleation rates.[12,19] In case of infection or calcium hydroxide therapy, the pH of the solution surrounding the MTA/CEM can be changed.[17,20] To best mimic the clinical conditions in the present study, in one hand, all the samples were totally immersed in the solutions in contrast to the previous studies using moistened gauzes; on the other hand, the acidic/alkaline solutions were buffered with the SBF. Moreover, we used calcium hydroxide for better stimulating the alkaline medium present in the real situations of multiple-session treated teeth.
Although the micrographs of SEM analysis are obtained from the topography of the surface of the material, they could be an index of the hydration process taking place at deeper layers. In contrast to the previous studies, spindle-shaped crystals, indicating ettringite, were observed in MTA samples exposed to acidic pH.[3] This can be attributed to the more clinical similarity of the solution we used in this study that was butyric acid buffered with SBF. A noteworthy difference between MTA and CEM exposed to deionized water was the presence of crystals other than cubic-shaped ones (calcium hydroxide) in CEM specimen, indicating hydroxyapatite.[19] This finding is due to the existence of endogenous phosphorous apart from the exogenous element being provided from the surrounding solutions which resulted in better hydration of the material and obtaining more surface hardness values in comparison with MTA, and with the passage of time. We detected more hydroxyapatite and globular crystals on the surface of the CEM treated in alkaline and SBF solutions; which can be indicative of more similarity of surface composition of CEM to the surrounding dentin.[6]
Better hydration could greatly enhance the microhardness of the cement and its setting process. There are two universal tests for measuring the surface hardness of the materials, including Knoop and Vickers tests, with the latter being the more popular one for biomaterials.[21] In the present study, a rise in microhardness values of CEM/MTA samples was seen after 28 days, except for acidic group in CEM. It can be justified that the strength of the material bulk increases with the passage of time due to the balance between expansive cracking and self-healing, which takes place inside the material. Curing in water elicits early formation of ettringite, prismatic acicular precipitates, which act as interlocking particles in the mass of the cement and subsequently enhances the strength.[22,23] During the hydration process, calcified byproducts dissolve gradually. Ettringite dissolution occurs in a faster rate at lower pH.[24] EDAX results of both CEM/MTA samples hydrated at acidic environment showed great reduction in Ca and an increase in the level of Si with the passage of time. With taking all these into consideration, it can be assumed that acidic environment can be detrimental to the hardness of tricalcium silicate cements, which is in accordance with the results of the previous studies.[3,14]
The progress of a cement setting could dictate the hydration rate.[25] Two common methods for evaluating the setting time of calcium-silicate cements are Vicat and Gillmore, either of which are in accordance with the specific standards. Gillmore apparatus was used in this study to record the initial setting time of the materials based on ISO6876:2002. The setting time of CEM was reported to be lower than MTA in our study, consistent with the previous studies,[5] which can be attributed to the smaller particle size of the CEM, enhancing the surface area of the powder in contact with water. This can be advantageous in clinical conditions due to the less possibility of washout/dislodgement after placement of the material.[25] The setting time of CEM decreased even more by ultrasonication in samples exposed to acidic/alkaline environment and deionized water, which supports its aid in better hydration process. This is true, especially in acidic environment that was shown to have an adverse effect on hydration of the tricalcium-based cements.[3] Maximum compaction obtained through ultrasonication could lead to less porosity and omit the macroscopic voids of the material, thus helping the improvement of the structure of the material.[8]
CONCLUSION
With the limitation of this in vitro study, surface topography and elemental constituents of MTA and CEM cement were altered in different acidic/neutral/alkaline solutions. Setting times of MTA were statistically higher than CEM. While neutral and alkaline solutions may enhance biologic properties of the biomaterials, acidic solution negatively influenced them.
Financial support and sponsorship
This study was supported by the Iranian Center for Endodontic Research, Research Institute of Dental Research, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Parirokh M, Torabinejad M. Mineral trioxide aggregate: A comprehensive literature review - Part I: Chemical, physical, and antibacterial properties. J Endod. 2010;36:16–27. doi: 10.1016/j.joen.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Parirokh M, Torabinejad M. Mineral trioxide aggregate: A comprehensive literature review - Part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36:400–13. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Ma J, Shen Y, Haapasalo M. Acidic pH weakens the microhardness and microstructure of three tricalcium silicate materials. Int Endod J. 2015;48:323–32. doi: 10.1111/iej.12318. [DOI] [PubMed] [Google Scholar]
- 4.Saghiri MA, Lotfi M, Saghiri AM, Vosoughhosseini S, Fatemi A, Shiezadeh V, et al. Effect of pH on sealing ability of white mineral trioxide aggregate as a root-end filling material. J Endod. 2008;34:1226–9. doi: 10.1016/j.joen.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Asgary S, Shahabi S, Jafarzadeh T, Amini S, Kheirieh S. The properties of a new endodontic material. J Endod. 2008;34:990–3. doi: 10.1016/j.joen.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Asgary S, Eghbal MJ, Parirokh M, Ghoddusi J, Kheirieh S, Brink F. Comparison of mineral trioxide aggregate's composition with Portland cements and a new endodontic cement. J Endod. 2009;35:243–50. doi: 10.1016/j.joen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 7.Nekoofar MH, Aseeley Z, Dummer PM. The effect of various mixing techniques on the surface microhardness of mineral trioxide aggregate. Int Endod J. 2010;43:312–20. doi: 10.1111/j.1365-2591.2010.01683.x. [DOI] [PubMed] [Google Scholar]
- 8.Basturk FB, Nekoofar MH, Günday M, Dummer PM. The effect of various mixing and placement techniques on the compressive strength of mineral trioxide aggregate. J Endod. 2013;39:111–4. doi: 10.1016/j.joen.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Aminoshariae A, Hartwell GR, Moon PC. Placement of mineral trioxide aggregate using two different techniques. J Endod. 2003;29:679–82. doi: 10.1097/00004770-200310000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Yeung P, Liewehr FR, Moon PC. A quantitative comparison of the fill density of MTA produced by two placement techniques. J Endod. 2006;32:456–9. doi: 10.1016/j.joen.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005;31:97–100. doi: 10.1097/01.don.0000133155.04468.41. [DOI] [PubMed] [Google Scholar]
- 12.Han L, Okiji T. Bioactivity evaluation of three calcium silicate-based endodontic materials. Int Endod J. 2013;46:808–14. doi: 10.1111/iej.12062. [DOI] [PubMed] [Google Scholar]
- 13.Gandolfi MG, Taddei P, Tinti A, Prati C. Apatite-forming ability (bioactivity) of ProRoot MTA. Int Endod J. 2010;43:917–29. doi: 10.1111/j.1365-2591.2010.01768.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee YL, Lee BS, Lin FH, Yun Lin A, Lan WH, Lin CP. Effects of physiological environments on the hydration behavior of mineral trioxide aggregate. Biomaterials. 2004;25:787–93. doi: 10.1016/s0142-9612(03)00591-x. [DOI] [PubMed] [Google Scholar]
- 15.Saghiri MA, Lotfi M, Saghiri AM, Vosoughhosseini S, Aeinehchi M, Ranjkesh B. Scanning electron micrograph and surface hardness of mineral trioxide aggregate in the presence of alkaline pH. J Endod. 2009;35:706–10. doi: 10.1016/j.joen.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Shokouhinejad N, Yazdi KA, Nekoofar MH, Matmir S, Khoshkhounejad M. Effect of acidic environment on dislocation resistance of endosequence root repair material and mineral trioxide aggregate. J Dent (Tehran) 2014;11:161–6. [PMC free article] [PubMed] [Google Scholar]
- 17.Saghiri MA, Shokouhinejad N, Lotfi M, Aminsobhani M, Saghiri AM. Push-out bond strength of mineral trioxide aggregate in the presence of alkaline pH. J Endod. 2010;36:1856–9. doi: 10.1016/j.joen.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Shokouhinejad N, Nekoofar MH, Iravani A, Kharrazifard MJ, Dummer PM. Effect of acidic environment on the push-out bond strength of mineral trioxide aggregate. J Endod. 2010;36:871–4. doi: 10.1016/j.joen.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 19.Asgary S, Eghbal MJ, Parirokh M, Ghoddusi J. Effect of two storage solutions on surface topography of two root-end fillings. Aust Endod J. 2009;35:147–52. doi: 10.1111/j.1747-4477.2008.00137.x. [DOI] [PubMed] [Google Scholar]
- 20.Nekoofar MH, Namazikhah MS, Sheykhrezae MS, Mohammadi MM, Kazemi A, Aseeley Z, et al. pH of pus collected from periapical abscesses. Int Endod J. 2009;42:534–8. doi: 10.1111/j.1365-2591.2009.01550.x. [DOI] [PubMed] [Google Scholar]
- 21.Namazikhah MS, Nekoofar MH, Sheykhrezae MS, Salariyeh S, Hayes SJ, Bryant ST, et al. The effect of pH on surface hardness and microstructure of mineral trioxide aggregate. Int Endod J. 2008;41:108–16. doi: 10.1111/j.1365-2591.2007.01325.x. [DOI] [PubMed] [Google Scholar]
- 22.Camilleri J. Modification of mineral trioxide aggregate. Physical and mechanical properties. Int Endod J. 2008;41:843–9. doi: 10.1111/j.1365-2591.2008.01435.x. [DOI] [PubMed] [Google Scholar]
- 23.Nekoofar MH, Oloomi K, Sheykhrezae MS, Tabor R, Stone DF, Dummer PM. An evaluation of the effect of blood and human serum on the surface microhardness and surface microstructure of mineral trioxide aggregate. Int Endod J. 2010;43:849–58. doi: 10.1111/j.1365-2591.2010.01750.x. [DOI] [PubMed] [Google Scholar]
- 24.Camilleri J. Characterization of hydration products of mineral trioxide aggregate. Int Endod J. 2008;41:408–17. doi: 10.1111/j.1365-2591.2007.01370.x. [DOI] [PubMed] [Google Scholar]
- 25.Sharma SM, Gurtu A, Singhal A, Guha C, Dixit K, Aggarwal A, et al. Effect of dentin powder on setting time of mineral trioxide aggregate: An in vitro study. Minerva Stomatol. 2014;63:211–5. [PubMed] [Google Scholar]