Abstract
Aim:
This in vitro study evaluated the degree of dentinal tubule occlusion and depth of penetration of nano-hydroxyapatite (nHAp) derived from chicken eggshell powder with and without the addition of 2% sodium fluoride (NaF) using scanning electron microscope (SEM) and confocal laser scanning microscope (CLSM).
Materials and Methods:
nHAp was synthesized and characterized using X-ray diffraction and SEM-energy dispersive spectroscopy. Dentin discs were obtained from extracted teeth, pretreated with 17% ethylenediaminetetraacetic acid for 5 min and were divided into four groups based on the experimental agents as follows: Group 1: Untreated (control), Group 2: 2% NaF, Group 3: nHAp, Group 4: Combination of nHAp and 2% NaF. The treatment protocol was carried out for 7 days, after which the specimens were viewed under SEM and CLSM.
Statistical Analysis Used:
One-way ANOVA and Tukey's post hoc multiple comparison tests (P < 0.05).
Results:
All the experimental agents occluded the dentinal tubules, but to varying degrees and depths. Specimens treated with the combination of nHAp and 2% NaF showed complete dentinal tubular occlusion and significantly greater depth of penetration than those treated with nHAp and 2% NaF alone.
Conclusion:
The combination of nHAp and 2% NaF was the most effective in occluding dentinal tubules.
Keywords: Dentin hypersensitivity, desensitizing agents, eggshells, hydroxyapatite, sodium fluoride, tubule occlusion
INTRODUCTION
Dentin hypersensitivity is a commonly encountered, painful clinical condition for which no consistent remedy has been established.[1,2] Although various theories have been put forward over the years to explain the physiological mechanism behind this problem, hydrodynamic theory of dentinal sensitivity remains the most accepted theory till date.[3] Stimuli such as heat, cold, evaporation, osmotic, and pressure changes cause fluid movement within the dentinal tubules, thereby inducing sharp pain responses in the nerve fibers.[4] Furthermore, this condition is often associated with greater number of exposed and patent dentinal tubules.[5]
There are two modalities to treat hypersensitivity. These are desensitizing the nerve and decreasing dentin permeability by occluding the tubules.[3,6] Pashley et al. have shown that both dentin permeability and sensitivity are reduced when the dentinal tubules are occluded.[6,7] An ideal desensitizing agent should result in faster and long lasting tubule occlusion.[8] Tubule occlusion can be brought about either by deposition of mineral crystals on the surface and/or within the dentinal tubules. Superficial occlusion of tubules can provide only short-term relief as the precipitate can be removed either by daily tooth brushing or dissolved by saliva and/or consumption of acidic beverages. Effective treatment with long-term results has been related to intratubular deposition of mineral crystals.[9,10]
Recently, there has been a renewed interest in developing materials with a bioactive potential that could block the exposed dentinal tubules and subsequently reduce the fluid flow within the tubules.[1] Hydroxyapatite (HAp) is one such material that exhibits excellent bioactive properties and striking similarities to dental hard tissues.[11] Approximately 97% of tooth enamel and 70% of dentin are composed of HAp.[12] Recent studies have highlighted the ability of nano-HAp (nHAp) to occlude exposed dentinal tubules.[12,13] With the increase in demand for environmentally friendly techniques, synthesizing nHAp from calcium-rich natural sources remains a viable and a more economical option. One such biowaste, which is a rich source of calcium in the form of carbonates and oxides, is chicken eggshell.[14] It contains 94% calcium carbonate, 1% calcium phosphate, 1% magnesium carbonate, and 4% organic matter.[15]
Ever since the introduction of sodium fluoride (NaF) as a desensitizing agent by Lukomsky in 1941,[16] an innumerable number of studies have tested the efficacy of this material, as well as used it as a comparison to assess newer methods.[17,18] Fluorides such as sodium and stannous fluoride can reduce the permeability of dentin by precipitating calcium fluoride crystals in the inlet of dentinal tubules.[5,17] Therefore, this study aimed to incorporate 2% NaF to nHAp. The objectives of this study were
To confirm the presence of nHAp in the synthesized powder using X-ray diffraction (XRD) and to determine its particle size and composition using scanning electron microscopy-energy dispersive spectroscopy (SEM-EDS)
To evaluate the degree of dentinal tubule occlusion produced by NaF, nHAp and the combination of nHAp and NaF using SEM
To evaluate the depth of penetration of these experimental agents into the dentinal tubules using confocal laser scanning microscope (CLSM).
MATERIALS AND METHODS
Synthesis of nano-hydroxyapatite
A simple combustion method given by Sasikumar and Vijayaraghavan[19] was adopted to synthesize nanocrystalline HAp powder from the eggshells. Biowaste chicken eggshells were collected and cleaned in boiling water for 30 min. The shells were dried and then crushed to a fine powder. The powder was dissolved in concentrated nitric acid, followed by the addition of 1M citric acid. Ammonium hydroxide was added in a ratio of 1:1 to adjust the pH of the solution to 9.5. The addition of 1M diammonium hydrogen phosphate at the rate of 1 ml/min to this solution resulted in a white precipitate, which was dissolved with nitric acid. The solution was stirred at 70°C for 2 h until the formation of a transparent gel. Heating the gel in a preheated muffle furnace at 250°C yielded a black colored precursor. Sintering the precursor at 900°C for 2 h resulted in the formation of a white colored powder.
The powder was analyzed using XRD (Bruker D8 Advance, Bruker AXS GmbH, Karlsruhe, Germany) in reflection mode with CuKα radiation to confirm the presence of HAp. The data were analyzed in 2θ range from 10° to 80° with a scanning step of 2° per min. The powder was sputter coated with gold and viewed under SEM (FEI Quanta 200 FEG, Berlin, Germany) to determine the morphology at ×100,000 magnification and the particle size at ×200,000 magnification. Elemental analysis of the powder was performed using EDS.
Specimen preparation
The human teeth samples were collected in conformation with the provisions of the Declaration of Helsinki. Seventy caries-free recently extracted human maxillary and mandibular molars were collected and stored in distilled water containing 0.2% thymol. The teeth were decoronated at the cemento-enamel junction and the root portions were discarded. Using a diamond disc under copious water cooling, the occlusal surface of each crown portion was cut perpendicular to the long axis of the tooth so as to expose the dentin surface. Another cut was made 3 mm apical to the exposed dentin surface to obtain a dentin disc of 3 mm thickness. Each disc was marked on the pulpal side to aid in the identification of the experimental coronal surface. The exposed coronal dentin surfaces were polished with 600-grit silicon carbide paper and the specimens were ultrasonicated in distilled water for 10 min to remove the smear layer. To mimic the open dentinal tubules of hypersensitive dentin and to remove the smear plugs, all the dentin discs were pretreated with 17% ethylenediaminetetraacetic acid for 5 min and then rinsed thoroughly with distilled water. The dentin discs thus obtained were randomly divided into 4 groups as follows:
Group 1 (n = 10): Control, no surface treatment was done. The specimens were stored in artificial saliva until SEM evaluation
Group 2 (n = 20): 2% NaF solution
Group 3 (n = 20): nHAp, 1.8 g of nHAp was made into a slurry by mixing with 0.3 mL of distilled water
Group 4 (n = 20): nHAp and 2% NaF 1.8 g of nHAp was made into a slurry by mixing with 0.3 mL of 2% NaF solution.
The experimental agents in Groups 2-4 were applied on the dentin discs using a micro brush, left for 7 min and then rinsed with distilled water. After treatment, the specimens were stored in artificial saliva for the rest of the day at 37°C and the same procedure was carried out for 7 days.
Of the 20 samples in Groups 2-4, 10 samples were evaluated for the degree of tubule occlusion using SEM, whereas the remaining 10 samples were evaluated for the depth of penetration using CLSM. Few grains of the fluorescent dye rhodamine B were dissolved in the experimental solutions of these groups when used for CLSM evaluation.
Ten dentin discs from each group were dried in a desiccator for 24 h, mounted on aluminum stubs, sputter coated with gold and then subjected to SEM analysis at a magnification of ×1000 and ×2000 to evaluate dentinal tubule occlusion. Qualitative assessment of the micrographs was done based on their surface characteristics and patency or occlusion of the dentinal tubules. The following ranking criteria[20] were adopted:
All the dentinal tubules are patent; no surface debris.
Partially occluded dentinal tubules with narrowing of tubular orifices; little or no surface debris.
Dentinal tubules were mostly occluded; little or no surface debris.
Dentinal tubules were mostly occluded; the dentinal surface was partially covered with film or precipitate.
Complete occlusion of all the dentinal tubules; the dentinal surface was completely covered with film or precipitate.
The remaining ten discs of the experimental groups were sectioned longitudinally in the center using a microtome (Leica SP1600, Leica Biosystems Nussloch GmbH, Germany) to evaluate the depth of penetration using CLSM (LSM 510 META, Carl Zeiss MicroImaging GmbH, Jena, Germany). For reliable comparisons, only tubules from the central portions on either side of the fractured disc were evaluated. The depth of penetration along the dentinal tubules from the dentinal surface to the bottom of the precipitate was the average value for at least 20 tubules from each specimen. The results were tabulated and statistically analyzed using one-way ANOVA and Tukey's post hoc multiple comparison tests (P < 0.05).
RESULTS
XRD analysis of the synthesized powder showed a sharp and well-defined peak at 34.128° of 2θ values confirming the presence of pure HAp. The Ca/P ratio of the powder was calculated to be 1.67, which is characteristic of HAp. The SEM micrograph of the synthesized powder showed rod shaped nHAp particles present in the form of agglomerated clusters. The size of the HAp particles ranged from 18.3 nm to 32.2 nm. Evaluation of dentinal tubule occlusion using SEM showed that all the experimental groups produced occlusion of the dentinal tubules, but the extent of coverage and the degree of occlusion varied between the groups. Ranking criteria based on surface characteristics of SEM photomicrographs is given in Table 1. The SEM micrographs of Group 1 (control) showed open dentinal tubules [Figure 1a]. Most of the dentinal tubules in Group 2 exhibited narrowing of tubular aperture due to fine precipitates on the tubular walls (arrows). However, full tubular occlusion was rare [Figure 1b]. In Group 3, a predominantly higher number of dentinal tubules showed complete tubular occlusion [Figure 1c]. Micrographs of Group 4 showed complete dentinal tubular occlusion with a protective layer covering the dentin surface. No patent dentinal tubules were seen. Agglomerated precipitates (arrows) were also seen on the surface [Figure 1d].
Table 1.
Ranking based on surface characteristics of scanning electron microscope photomicrographs in Groups 1 through 4*
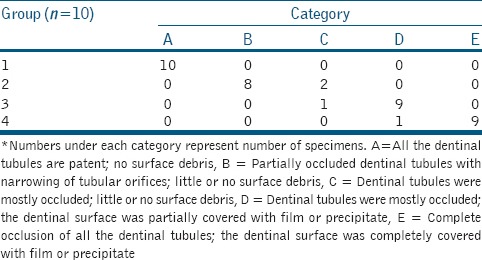
Figure 1.
Scanning electron micrographs of specimens treated with (a) 17% ethylenediaminetetraacetic acid showing open dentinal tubules, (b) 2% sodium fluoride showing partial occlusion of dentinal tubules (arrows), (c) nano-hydroxyapatite showing a predominantly higher number of tubular occlusion and partial coverage of the dentinal surface with film or precipitate, (d) combination of nano-hydroxyapatite and 2% sodium fluoride showing complete occlusion of all the dentinal tubules; presence of a protective film and agglomerated precipitates (arrows) on the surface
The mean depth of penetration of the experimental agents in Groups 2 through 4 is given in Table 2 and their respective CLSM images are given in Figure 2. Group 4 (356.66 ± 24.51 μm) showed the maximum depth of penetration, which was significantly higher when compared to Groups 2 (154.09 ± 25.16 μm) and 3 (260.21 ± 22.96 μm) (P < 0.05). Group 3 also showed a significantly higher depth of penetration than Group 2 (P < 0.05).
Table 2.
Mean and standard deviation of the depth of penetration of the experimental agents into the dentinal tubules (μm) (P<0.05)*
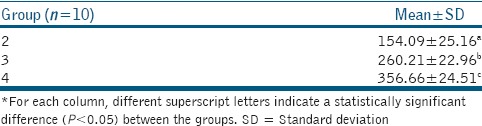
Figure 2.
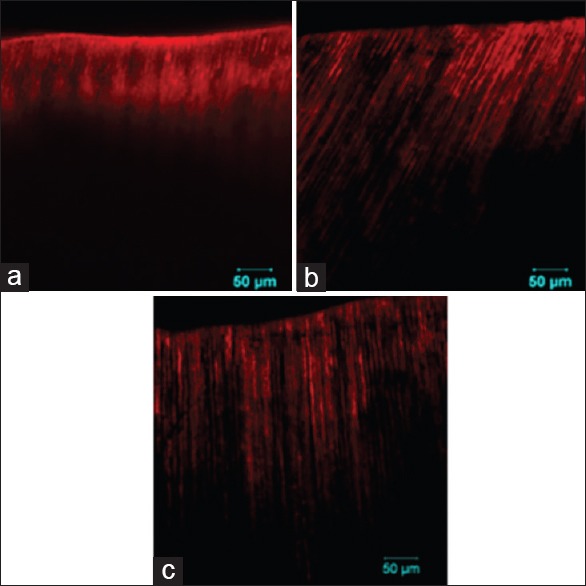
Confocal laser scanning microscope images of specimens treated with, (a) 2% sodium fluoride, (b) nano-hydroxyapatite and (c) combination of nano-hydroxyapatite and 2% sodium fluoride showing the penetration of the fluorescent labeled experimental agents into the dentinal tubules
DISCUSSION
Coronal dentin discs were used in this study to evaluate tubule occlusion and depth of penetration of the desensitizing agents into the tubules. Important variables such as dentin surface area, thickness, and surface characteristics can be controlled in coronal dentin discs compared to cervical dentin discs. This model was found appropriate for application of the experimental agents on a flat dentin surface and also enabled standardized comparisons of different treatment protocols with ease. This remains a time-tested screening model to study tubule occlusion by potential desensitizing agents.[21]
Although various sources are available for the synthesis of HAp, chicken eggshells were chosen in this study owing to its high calcium content and cost effectiveness. These eggshell waste help in reducing the cost of high-quality calcium source and at the same time promote recycle of material.[14,19] A combustion method was adopted in this study for synthesizing nHAp as this method provides an excellent control of parameters such as particle size, distribution, and morphology which in turn will influence the mechanical properties of the synthesized powder.[19]
SEM analysis of specimens treated with 2% NaF alone (Group 2) showed narrowing of the dentinal tubular lumen but failed to produce complete tubular occlusion. NaF reacts with the calcium of dentin resulting in the formation of calcium fluoride crystals, which are deposited onto the opening of the dentinal tubules.[5] However, these crystals are small in size and hence not effective in occluding the tubules.[2] This may be the reason for the significantly lesser depth of penetration of NaF (154.09 ± 25.16 μm) when compared to Groups 3 and 4. Similar results were obtained by Raafat Abdelaziz et al.,[2] who showed that fluoride treated specimens displayed significantly lower percentage of tubule occlusion compared to other experimental groups.
SEM micrographs of Group 3 (nHAp) showed a higher level of dentinal tubule occlusion with fewer partially occluded tubules when compared to Group 2 (2% NaF). The depth of penetration (260.21 ± 22.96 μm) was also significantly higher. Similar results were obtained by Ohta et al.[12] and Wang et al.[13] who showed that nHAp uniformly occluded the dentinal tubules.
The increased tubule occluding efficacy of nHAp could be attributed to its nanoscale particle size. Engineering HAp at the nano level confers superior functional properties to HAp such as high bioactivity and osteoconductivity due to its grain size, crystallinity and surface area to volume ratio in the body environment[22] compared to its microscale sized counterparts.[23] Since the surface area and proportion of surface atomicity increase with a decrease in particle size,[24] a greater penetration of ion constituents into the dentinal tubules would have occurred that might have resulted in better tubular occlusion.
Specimens treated with a combination of nHAp and 2% NaF (Group 4) showed complete obliteration of the dentinal tubules under SEM. A thick protective layer and large amounts of precipitates in the form of agglomerates were seen covering the entire dentinal surface. Among the groups tested, Group 4 showed the highest level of dentinal tubular occlusion and maximum depth of penetration (356.66 ± 24.51 μm), which were significantly higher than Groups 2 and 3. This significant increase could be attributed to the synergistic effect of both NaF and nHAp. This result is in accordance with previous studies by Reynolds et al.,[25] Kumar et al.[26] and Lan et al.,[27] which highlighted the synergistic effect of fluoride with other desensitizing agents.
The acidic pH of nHAp solution might also be a contributing factor for the deeper tubular penetration in Groups 3 and 4. Huang et al. in 2009[28] suggested that nHAp when used on demineralized enamel penetrates the enamel pores, acts as a template in the precipitation process and continuously attracts a large amount of calcium and phosphate ions from the remineralization solution to the enamel surface. Whether the same mechanism is possible in dentinal tubular occlusion is yet to be known.
Since HAp exhibits excellent bioactive properties,[11] this may be considered the material of choice for treating hypersensitivity in the near future. The vehicles used to carry nHAp into the dentinal tubules may also play a significant role in the depth of penetration of the later as shown in the case of NaF used in this study. Hence, further studies simulating intraoral conditions, including brushing and acidic challenges are necessary to investigate the longevity of this material in treating dentin hypersensitivity.
Since dentin hypersensitivity most commonly involves cervical areas,[29] the use of cervical dentin blocks would have more closely simulated the clinical scenario. This remains a limitation of this study. Variations associated with the source of dentin specimens and the morphology of dentin make interpretation of in vitro results difficult and extrapolation of such results to the clinical situation should be done with caution. The results of this in vitro study can serve as basis for further functional studies simulating dentinal fluid and pulpal pressure.
Since the synthesized nHAp is from animal origin, some patients may have religious and ethical concerns regarding the use of such products. The potential of disease transmission from animal products is also a concern. High calcination temperatures varying from 900°C are used in the synthesis of HAp from eggshells. At this high temperature, the disease causing pathogens are destroyed, thus alleviating all issues related to disease transmission.[30] In spite of the precautions taken, it is mandatory that the patients are aware of the origin of the product to enable them to make a fully informed decision.
Under the limitations of this in vitro study, it can be concluded that all the experimental agents occluded the dentinal tubules to varying degrees and also penetrated to varying depths into the dentinal tubules. The combination of nHAp and NaF showed complete tubular occlusion and maximum depth of penetration into the dentinal tubules. Although this combination was effective in occluding dentinal tubules, further clinical studies are needed to determine its effectiveness over time.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Acknowledgment
We sincerely thank Ms. Malini, Application Specialist, Central Research Facility, Sri Ramachandra University, Chennai, India for her expertise in CLSM imaging.
This research project was approved by the Institutional Ethical Committee, SRM Dental College (File No. SRMU/M and HS/SRMDC/2011/MDS-PG Student/301). The human teeth samples were collected in conformation with the provisions of the declaration of Helsinki.
REFERENCES
- 1.Chiang YC, Chen HJ, Liu HC, Kang SH, Lee BS, Lin FH, et al. A novel mesoporous biomaterial for treating dentin hypersensitivity. J Dent Res. 2010;89:236–40. doi: 10.1177/0022034509357148. [DOI] [PubMed] [Google Scholar]
- 2.Raafat Abdelaziz R, Mosallam RS, Yousry MM. Tubular occlusion of simulated hypersensitive dentin by the combined use of ozone and desensitizing agents. Acta Odontol Scand. 2011;69:395–400. doi: 10.3109/00016357.2011.572290. [DOI] [PubMed] [Google Scholar]
- 3.Pashley DH. Dentin-predentin complex and its permeability: Physiologic overview. J Dent Res. 1985;64:613–20. doi: 10.1177/002203458506400419. [DOI] [PubMed] [Google Scholar]
- 4.Brannstrom M. Dentin sensitivity and aspiration of odontoblasts. J Am Dent Assoc. 1963;66:366–70. doi: 10.14219/jada.archive.1963.0104. [DOI] [PubMed] [Google Scholar]
- 5.Orchardson R, Gillam DG. Managing dentin hypersensitivity. J Am Dent Assoc. 2006;137:990–8. doi: 10.14219/jada.archive.2006.0321. [DOI] [PubMed] [Google Scholar]
- 6.Pashley DH, Livingston MJ, Reeder OW, Horner J. Effects of the degree of tubule occlusion on the permeability of human dentine in vitro. Arch Oral Biol. 1978;23:1127–33. doi: 10.1016/0003-9969(78)90119-x. [DOI] [PubMed] [Google Scholar]
- 7.Pashley DH, Livingston MJ, Greenhill JD. Regional resistances to fluid flow in human dentine in vitro. Arch Oral Biol. 1978;23:807–10. doi: 10.1016/0003-9969(78)90159-0. [DOI] [PubMed] [Google Scholar]
- 8.Markowitz K, Pashley DH. Discovering new treatments for sensitive teeth: The long path from biology to therapy. J Oral Rehabil. 2008;35:300–15. doi: 10.1111/j.1365-2842.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 9.Kuroiwa M, Kodaka T, Kuroiwa M, Abe M. Dentin hypersensitivity. Occlusion of dentinal tubules by brushing with and without an abrasive dentifrice. J Periodontol. 1994;65:291–6. doi: 10.1902/jop.1994.65.4.291. [DOI] [PubMed] [Google Scholar]
- 10.Suge T, Ishikawa K, Kawasaki A, Yoshiyama M, Asaoka K, Ebisu S. Effects of fluoride on the calcium phosphate precipitation method for dentinal tubule occlusion. J Dent Res. 1995;74:1079–85. doi: 10.1177/00220345950740040801. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe J, Akashi M. Formation of hydroxyapatite provides a tunable protein reservoir within porous polyester membranes by an improved soaking process. Biomacromolecules. 2007;8:2288–93. doi: 10.1021/bm0702915. [DOI] [PubMed] [Google Scholar]
- 12.Ohta K, Kawamata H, Ishizaki T, Hayman R. Occlusion of dentinal tubules by nano-hydroxyapatite. J Dent Res. 2007 86(Spec Iss A):New Orleans Abstracts no.1759. [Google Scholar]
- 13.Wang ZJ, Sa Y, Ma X, Wang YN, Jiang T. The preparation of nano-hydroxyapatite and preliminary observation on its effects on the occlusion of dentinal tubule. Zhonghua Kou Qiang Yi Xue Za Zhi. 2009;44:297–300. [PubMed] [Google Scholar]
- 14.Sanosh KP, Chu MC, Balakrishnan A, Kim TN, Cho SJ. Utilization of biowaste eggshells to synthesize nanocrystalline hydroxyapatite powders. Mater Lett. 2009;63:2100–2. [Google Scholar]
- 15.Hui P, Meena SL, Singh G, Agarawal RD, Prakash S. Synthesis of hydroxyapatite bio-ceramic powder by hydrothermal method. J Miner Mater Charact Eng. 2010;9:683–94. [Google Scholar]
- 16.Lukomsky EH. Fluoride therapy for exposed dentin and alveolar atrophy. J Dent Res. 1941;20:649–59. [Google Scholar]
- 17.Morris MF, Davis RD, Richardson BW. Clinical efficacy of two dentin desensitizing agents. Am J Dent. 1999;12:72–6. [PubMed] [Google Scholar]
- 18.Kara C, Orbak R. Comparative evaluation of Nd: YAG laser and fluoride varnish for the treatment of dentinal hypersensitivity. J Endod. 2009;35:971–4. doi: 10.1016/j.joen.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Sasikumar S, Vijayaraghavan R. Low temperature synthesis of nanocrystalline hydroxyapatite from egg shells by combustion method. Trends Biomater Artif Organs. 2006;19:70–3. [Google Scholar]
- 20.Al-Saud LM, Al-Nahedh HN. Occluding effect of Nd: YAG laser and different dentin desensitizing agents on human dentinal tubules in vitro: A scanning electron microscopy investigation. Oper Dent. 2012;37:340–55. doi: 10.2341/10-188-L. [DOI] [PubMed] [Google Scholar]
- 21.Gillam DG, Mordan NJ, Newman HN. The dentin disc surface: A plausible model for dentin physiology and dentin sensitivity evaluation. Adv Dent Res. 1997;11:487–501. doi: 10.1177/08959374970110041701. [DOI] [PubMed] [Google Scholar]
- 22.LeGeros RZ. Calcium phosphates in oral biology and medicine. Monogr Oral Sci. 1991;15:1–201. [PubMed] [Google Scholar]
- 23.Murugan R, Ramakrishna S. Coupling of therapeutic molecules onto surface modified coralline hydroxyapatite. Biomaterials. 2004;25:3073–80. doi: 10.1016/j.biomaterials.2003.09.089. [DOI] [PubMed] [Google Scholar]
- 24.Kaehler T. Nanotechnology: Basic concepts and definitions. Clin Chem. 1994;40:1797–9. [PubMed] [Google Scholar]
- 25.Reynolds EC, Cai F, Cochrane NJ, Shen P, Walker GD, Morgan MV, et al. Fluoride and casein phosphopeptide-amorphous calcium phosphate. J Dent Res. 2008;87:344–8. doi: 10.1177/154405910808700420. [DOI] [PubMed] [Google Scholar]
- 26.Kumar VL, Itthagarun A, King NM. The effect of casein phosphopeptide-amorphous calcium phosphate on remineralization of artificial caries-like lesions: An in vitro study. Aust Dent J. 2008;53:34–40. doi: 10.1111/j.1834-7819.2007.00006.x. [DOI] [PubMed] [Google Scholar]
- 27.Lan WH, Liu HC, Lin CP. The combined occluding effect of sodium fluoride varnish and Nd: YAG laser irradiation on human dentinal tubules. J Endod. 1999;25:424–6. doi: 10.1016/S0099-2399(99)80271-4. [DOI] [PubMed] [Google Scholar]
- 28.Huang SB, Gao SS, Yu HY. Effect of nano-hydroxyapatite concentration on remineralization of initial enamel lesion in vitro. Biomed Mater. 2009;4:034104. doi: 10.1088/1748-6041/4/3/034104. [DOI] [PubMed] [Google Scholar]
- 29.Gillam DG, Aris A, Bulman JS, Newman HN, Ley F. Dentine hypersensitivity in subjects recruited for clinical trials: Clinical evaluation, prevalence and intra-oral distribution. J Oral Rehabil. 2002;29:226–31. doi: 10.1046/j.1365-2842.2002.00813.x. [DOI] [PubMed] [Google Scholar]
- 30.Han Y, Xu K, Montay G, Fu T, Lu J. Evaluation of nanostructured carbonated hydroxyapatite coatings formed by a hybrid process of plasma spraying and hydrothermal synthesis. J Biomed Mater Res. 2002;60:511–6. doi: 10.1002/jbm.10097. [DOI] [PubMed] [Google Scholar]


