Abstract
N-methyl D-aspartate receptor (NMDA) subunit 2B (NR2B)-containing NMDA receptors and mitochondrial protein cyclophilin D (CypD) are well characterized in mediating neuronal death after ischemia, respectively. However, whether and how NR2B and CypD work together in mediating synaptic injury after ischemia remains elusive. Using a de novo ischemia model of oxygen-glucose deprivation (OGD) in hippocampal slices, we identified a NR2B-dependent mechanism for CypD translocation onto the mitochondrial inner membrane. CypD depletion (CypD null mice) prevented OGD-induced impairment in synaptic transmission recovery. Overexpression of neuronal CypD mice (CypD+) exacerbated OGD-induced loss of synaptic transmission. Inhibition of CypD-dependent mitochondrial permeability transition pore (mPTP) opening by cyclosporine A (CSA) attenuated ischemia-induced synaptic perturbation in CypD+ and non-transgenic (nonTg) mice. The treatment of antioxidant EUK134 to suppress mitochondrial oxidative stress rescued CypD-mediated synaptic dysfunction following OGD in CypD+ slices. Furthermore, OGD provoked the interaction of CypD with P53, which was enhanced in slices overexpressing CypD but was diminished in CypD-null slices Inhibition of p53 using a specific inhibitor of p53 (pifithrin-μ) attenuated the CypD/p53 interaction following OGD, along with a restored synaptic transmission in both nonTg and CypD+ hippocampal slices. Our results indicate that OGD-induced CypD translocation potentiates CypD/P53 interaction in a NR2B dependent manner, promoting oxidative stress and loss of synaptic transmission. We also evaluate a new ex-vivo chronic OGD-induced ischemia model for studying the effect of oxidative stress on synaptic damage.
Keywords: NR2B, mitochondria, OGD, synaptic transmission, CypD, p53
1. Introduction
Many in vivo, in vitro, and human studies have established a role for glutamate excitotoxicity in neuronal damage after ischemia. Excessive release of glutamate and overactivation of glutamate receptors [predominantly N-methyl-D-aspartate (NMDA) receptor] are primarily responsible for the neuronal death that occurs during ischemic stroke (Lipton and Rosenberg, 1994, Mattson, 1997, Lee et al., 1999, Arundine and Tymianski, 2004, Tu et al., 2010). More specifically, overactivation of the N-methyl D- aspartate receptor subunit 2B (NR2B) causes intracellular Ca2+ overload and recruits several molecules, such as phosphatase and tensin homologs deleted on chromosome TEN (PTEN) (Zhang et al., 2013) and death-associated protein kinase 1 (DAPK1) (Tu et al., 2010) that directly bind to the NMDA receptors, and nNOS that indirectly couples to the NMDA receptors via PSD95 (Cao et al., 2005), leading to neuronal loss (Taghibiglou et al., 2009a, Tu et al., 2010, Lai et al., 2011, Zhang et al., 2013, Lai et al., 2014). Studies on the majority of NMDA receptor antagonists showed no beneficial efficacy in clinical trials (Ikonomidou and Turski, 2002, Kemp and McKernan, 2002, Albensi et al., 2004). It is therefore necessary to study the intracellular players connecting NMDA receptor activation to neuronal death after ischemia.
In addition to NR2B-containing NMDA receptors and the associated intracellular signaling pathways, mitochondria play a critical role in ischemia-mediated neuronal necrosis. It is known that ATP production within mitochondria is ceased upon the onset of ischemia, thus neurons are initially insufficiently supply/ or depleted with energy, leading to ion hemostasis interruption, massive glutamate release, NR2B overactivation (Siesjo, 1992, Martin et al., 1994, Taylor et al., 1999). When mitochondria are reintroduced with glucose and oxygen during reperfusion, further mitochondria damage with elevated oxidative stress is elicited (Choi, 1995, Chan, 2004, Honda et al., 2005), which is harmful for the recovery of neuronal function after ischemia. Therefore, suppressing mitochondrial oxidative stress and damage is anticipated to promote neuronal function recovery.
Cyclophilin D (CypD), a key component of mitochondrial permeability transition pore (MPTP), is located in matrix under physiology condition. CypD can be translocated onto mitochondrial inner membrane during pathological insults such as oxidative stress, which triggers the opening of mPTP and elevates reactive oxygen free radicals (ROS) accumulation in cells (Baines et al., 2005, Schinzel et al., 2005, Du et al., 2008). In ischemia condition, either genetic deletion of CypD or pharmacological inhibition of CypD-dependent mPTP opening suppresses oxidative stress in parallel, reduces necrosis cell death (Shiga et al., 1992, Uchino et al., 1998, Khaspekov et al., 1999, Baines et al., 2005, Schinzel et al., 2005). The most recent studies demonstrated that in response to oxidative stress induced by brain ischemia/reperfusion injury, p53, a tumor suppressor protein, accumulates in the mitochondrial matrix and triggers mPTP opening and necrosis by physical interaction with CypD (Karch and Molkentin, 2012, Vaseva et al., 2012, Zhao et al., 2013, Pei et al., 2014). However, whether and how CypD is translocated onto mitochondrial membrane and contributes to functional recovery when neurons reintroduced with glucose and oxygen remains unknown.
In the present study, using a de novo ischemia model, OGD, in hippocampal slices lacking and gaining neuronal expression of CypD, we identified an NR2B-dependent mechanism to trigger CypD translocation onto the mitochondrial inner membrane, enhancing CypD/p53 interaction and oxidative stress, leading to inhibition of the recovery of synaptic transmission following OGD. Our results offer new insight into the role of NR2B-containing NMDA receptors and mitochondrial CypD translocation in OGD-induced synaptic dysfunction. Disassociation of NMDA receptor with mitochondrial CypD translocation could be a new target for restoring synaptic function upon ischemic stroke.
2. Materials and methods
2.1 Animals
Protocols involving animal use were approved by the Animal Care and Use Committee of the University of Kansas-Lawrence in accordance with the National Institutes of Health guidelines for animal care. CypD homozygous null mice (CypD−, mice with Ppif−/− depletion) were kind gifts from Dr. Jeffery D. Molkentin (Baines et. al., 2005). The offspring of CypD null mice (termed CypD− mice) mice were identified by PCR using primers [IMR5115: TTCTCACCAGTGCATAGGGCTCTG and IMR5116: GCTTTGTTATCCCAGCTGGCGC (reverse)]. To generate transgenic mice overexpressing CypD in neurons (termed CypD+ mice) driven by the Thy1-promoter, we subcloned full length human CypD coding sequences into a Thy1 transgenic construct. The construct was verified by sequencing. Transgenic founders were backcrossed 10 times into C57BL6/J mice for analysis of expression patterns. Tg mice were identified by PCR using primers [5'-GCTTTCCCCACCACAGA-3' (forward) and 5'-TGTTAGGACCAGCATTAG -3' (backward)]. Male mice at 3-5 month-old were used for this study.
2.2 Pharmacological treatment
Drugs were prepared as stock solutions and diluted to the desired final concentration in artificial cerebral spinal fluid (ACSF) containing: 124 mM NaCl, 4.4 mM KCl, 2 mM CaCl2, 2 mM MgSO4, 1mM Na2HPO4, 25 mM NaHCO3, 10mM D-(+)-glucose or sucrose (for glucose-free ACSF), immediately before application. Hippocampal slices with drugs were incubated in recovery or recording sub-immerse chambers as needed. The final concentrations and sources of the drugs were as follows: Cyclosporin A (CSA; 1 μM, Sigma), Ro 25-6981 (Ro25; 1 μM, Sigma), PPPA (0.5 μM; Tocris), Pifithrin-μ (PFT; 5 μM, Sigma), EUK 134 (EUK; 500 nM, Cayman Chemical). The final concentration of vehicle control ethanol was less than 0.5% in all experiments, which is the same concentration of ethanol in the solution containing drugs for the treatment. All reagents were purchased from Sigma (St. Louis, MO) instead of otherwise stated.
2.3 Slice preparation and oxygen glucose deprivation (OGD)
Animals were decapitated according to the approved protocol and hippocampi were rapidly removed. Transverse hippocampal slices (400 μm in thickness) were sectioned as we previously described (Huang et al., 2014, Zhang et al., 2014). All steps were performed in ice-cold oxygenated ACSF solution. Before recording, hippocampal slices were recovered at 30°C for 1.5 h in ACSF continuously bubbled with 95% O2 and 5% CO2. During recording, we transferred hippocampal slices into the recording immerse chamber that was maintained at 22 ± 0.5 °C and perfused with oxygenated normal ACSF (or 95% N2 and 5% CO2 bubbled glucose-free ACSF for inducing OGD) at a rate of 2.5–3 ml/min. The slice was incubated for another 15-20 minutes followed by recordings of a stable baseline of synaptic transmission for 20 minutes. Field-excitatory post-synaptic potentials (fEPSPs) were recorded for the CA1 region of the hippocampus by placing both the stimulating and the recording electrodes in the CA1 stratum radiatum. We assayed the basal synaptic transmission (input-output curve) by plotting stimulus voltage (V) against slopes of fEPSPs to generate input-output relations; we then established a 20 min baseline recording using low-frequency stimulation (0.033 Hz; 0.1 ms impulse duration) and the adjusted intensity that induced fEPSPs with ~50% of the maximal fEPSP amplitude. In experiments using pharmacological reagents, drugs were continuously perfused over slices starting 5 minutes before OGD induction and administered for the entire period of OGD. Values of fEPSP amplitude are expressed as mean ± SEM percentage change relative to mean baseline amplitude. Reported effects on synaptic function are equal to synaptic transmission recovery of fEPSPs calculated as the averaged relative amplitude of fEPSPs with respect to baseline values after re-introduction of oxygenated normal ACSF (35 to 40 min after the end of OGD).
2.4 Mitochondrial isolation and inner membrane fraction preparation
Brain slices were prepared and subjected to OGD with drug treatment as indicated above, and then mitochondria were isolated from brain slices as described previously (Messier, 2005, Huang et al., 2014, Wang et al., 2014). Samples were placed in 9 ml of ice-cold mitochondria isolation buffer [225 mM mannitol, 75mM sucrose, 2mM K2HPO4 (pH 7.2)], and homogenized (10 strokes) using a Douce homogenizer (Kontes Glass Co.). Homogenate was centrifuged at 1300g for 5min at 4°C. The resultant supernatant was then centrifuged at 34,000g for 10 min after layering on 15% Percoll. After centrifugation, the homogenate was resuspended and incubated for 5 min on ice in 20ml of mitochondria isolation buffer with 0.02% digitonin (Sigma), centrifuged at 8,000g for 10min. The pellet was washed twice in 1.5ml mitochondria isolation buffer and centrifuged again at 8,000g for 10min and the final pellets were resuspended in 200 μl mitochondria isolation buffer. The mitochondrial inner membrane fraction was prepared as described previously (Du et al., 2008) and the total protein concentration of isolated mitochondria fraction was determined by protein assay (Bio-Rad Lab).
2.5 Co-immunoprecipitation and immunoblotting assays
For immunoprecipitation, hippocampal slices were homogenized in non-denaturing lysis buffer containing 50 mM Tris HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 0.5% NP-40 and protease inhibitors (EMD Millipore, #539137). After centrifugation at 12,000 × g for 10 min at 4°C, the supernatant of homogenate or mitochondrial inner membrane fractions (500 μg) was incubated with an antibody to p53 (Santa Cruz, #) for 16 h at 4°C and then incubated with protein A/G-agarose beads (Thermo scientific, #20421) for 2 h at 4°C. Precipitated complexes were washed in lysis buffer and bound proteins were analyzed by immunoblotting. For western blot experiments, samples were separated in SDS-PAGE gels, transferred onto membranes, and then incubated with polyclonal rabbit anti-CypD (generated in our lab (Du et al., 2008), mouse anti-CypD (Abcam, #ab110324), mouse anti-actin (Sigma, #2228) or mouse anti-Hsp 60 (Enzo, #ADI-ESP-741) antibody, followed by anti-rabbit IgG (Life Technologies, #G-21234) or anti-mouse IgG (Life Technologies, #M-30107) incubation. The density of immunoreactive bands relative to Hsp60 was determined using NIH Image J software.
2.6 CypD histological assay
Tg CypD+, CypD−, and nonTg mouse brains were fixed by perfusion with 4% paraformaldehyde and coronal sections (30 μm) were cut with a Vibratome (Leica VTS1000, Wetzlar Germany). Sections were collected and immersed in wash buffer (0.1 M sodium phosphate, 0.5 M sodium chloride, 0.1% Triton X-100, pH 7.4) for 1 hour. After preincubation with blocking solution (10% normal goat serum, 0.3% Triton X-100 in PBS , pH 7.4) for one hour at room temperature, sections were co-immunostained with primary antibodies [mouse anti-CypD (Ab110324, 1:500; Abcam) and rabbit anti-SODII (1:5000, ADI-SOD-111, Enzo life Sciences, USA) or rabbit anti-MAP2 (1 : 500, A16657, life technologies, USA) at 4 °C for overnight. Sections were then incubated with Alexa Fluor 594-conjugated goat anti-rabbit IgG and 488 goat anti-mouse IgG secondary antibodies (1 : 1000, Invitrogen) for 1 h at room temperature. Nuclei were stained by DRAQ5 (5 μM, Cell Signaling) for 10 min at room temperature. The staining images were taken under confocal microscopy. Brain sections incubated with non-immune IgG or 2nd antibody alone were used as negative controls. An investigator who was blinded to experimental groups analyzed all images. For quantification of human CypD staining, brain sections were randomly selected and measured using MetaMorph software (Molecular Devices, CA).
2.7 Statistical analysis
All data are reported as the mean ± SEM. Statistical comparisons between experimental groups or between fEPSP amplitudes measured during baseline and after the OGD protocol were performed by applying one-way ANOVA followed by individual post hoc Fisher test. Differences were considered significant when p < 0.05.
3. Results
3.1 Generation and characterization of transgenic mice overexpressing neuronal CypD
To determine the effect of neuronal CypD, we generated transgenic (Tg) CypD mice with overexpression of human CypD in neurons under the control of Thy-1 promoter (Tg CypD+ mice). Tg CypD+ mice were identified as bearing the transgene from analysis PCR amplification of tail DNA (Fig. 1A). CypD levels were increased by 3-4 fold in brains of Tg CypD+ mice compared to the brains of nonTg littermates (Fig. 1B-C) as shown by immunoblotting with specific antibody to CypD (Du et al., 2008). Immunostaining of brain section with CypD antibody verified that CypD was increased in cortical and hippocampal neurons of Tg CypD+ mice but was absent in CypD null mice (CypD−) (Fig. 1D-E). Consistent with the immunoblotting results, CypD expression levels were increased by 3-4 fold in neurons of cortex and hippocampus (Fig. F). CypD-positive staining signals were extensively overlaid with MAP-2, a neuronal marker, verifying neuronal expression of CypD in Tg CypD+ mice. No CypD staining signals were found in CypD null mice (Fig. 1D-E). Using the double immunofluorescent staining with CypD and mitochondrial protein SODII, increased expression of neuronal CypD in hippocampus and cortex of CypD+ mice was identically co-localized with SODII (Fig. 1G), indicating mitochondrial localization of CypD in CypD+ mice. CypD null mice were also used for the study to determine whether blockade of CypD affords a protective effect on ischemia-induced synaptic injury.
Figure 1. Identification and characterization of transgenic (Tg) CypD+ mice.
A) Cyclophilin D (CypD) transgenic mice (+) and nontransgenic (nonTg, −) control mice were identified by PCR results. B-C) Immunoblotting of brain homogenates from Tg CypD (lane 2, +) mice and nonTg littermate controls (lane 1, −) for CypD, using anti-human CypD antibody. C) Quantification of CypD immunoreactive bands normalized to β-actin. Data are presented as fold increase relative to nonTg mice. N = 5-6 mice/group. D-F). The double immunofluorescent staining of brain sections for CypD (red) and MAP2 (green) in hippocampus (D) and cortex (E) from the indicated Tg mice. Nuclei were stained by DRAQ5 as shown in blue. F) Quantification of CypD staining intensity in hippocampus and cortex regions of the indicated Tg mice. G) Representative immunostaining images for CypD (green) and SODII (red, mitochondrial marker) and nuclei (blue) in hippocampal and cortical neurons. Scale bar = 25 μm.
3.2 CypD is a critical molecular target for synaptic transmission recovery following ischemia
We first characterized loss of synaptic transmission during OGD and synaptic transmission recovery following reintroduction of glucose and oxygen in hippocampal slices. Depending on the incubation temperature, hippocampal slices showed diminished and irreversible fEPSPs within minutes at body temperature, but could be a longer time at hypothermia condition (Taylor et al., 1999). In the present study, we aimed to evaluate both protective and deleterious effects on synaptic transmission recovery after OGD, we thereby first explored a condition in which synaptic transmission can be relatively tolerant to a longer time of OGD insult and partially recovered after OGD. We recorded fEPSPs from the CA1 region of hippocampal slices of nonTg mice at different temperature. Consistent with the previous report (Taylor et al., 1999), performing OGD experiment at 22 ± 0.5 °C resulted in tolerance to 30 min of OGD and that fEPSP amplitude partially recovered and reached a stable level of depression after glucose and oxygen reintroduced (34.5 ± 6 % of baseline, n = 7 slices). In contrast, we observed a rapid decrease in fEPSP amplitude during 15 min of OGD at 33 ± 0.5°C (mean amplitude of the last 3min of OGD was 1.3 ± 2 % of baseline, n=9), and fEPSPs amplitude did not recover after 40 min following reintroduction of oxygenated ACSF (mean relative amplitude of fEPSPs was 9.2 ± 5 % of baseline, n = 9). Therefore, we assessed the effect of OGD insult at 22 ± 0.5°C for 30 minutes duration to assess the effect of CypD on synaptic functional recovery following OGD.
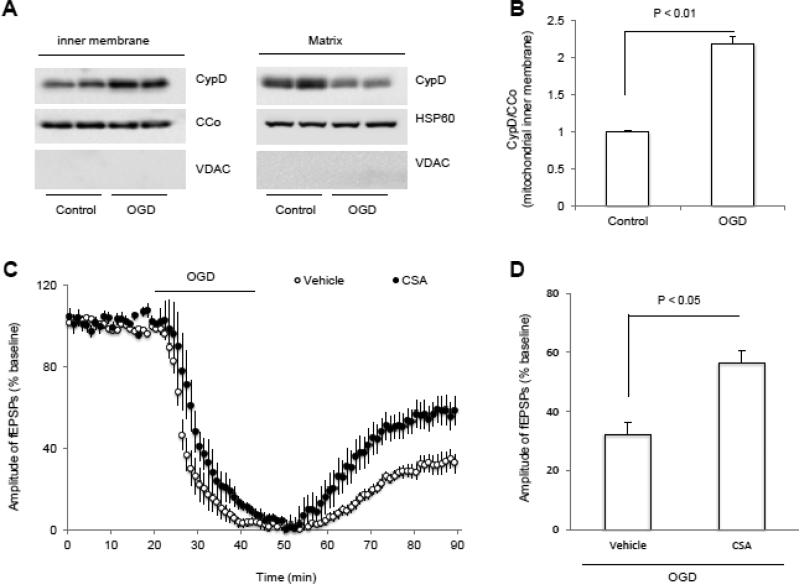
Given that CypD-mediated mPTP opening causes cell death after ischemic insult (Karch and Molkentin, 2012, Vaseva et al., 2012, Zhao et al., 2013, Pei et al., 2014) and that CypD translocation onto the mitochondrial inner membrane is a critical step for mPTP channel formation and mitochondrial permeable transition (Du et al., 2008, Du et al., 2011), we investigated if OGD triggers CypD translocation from mitochondrial matrix to the inner membrane, a key step for formation of MPTP, in the non Tg control animals. Immunoblotting of isolated mitochondrial inner membrane fractions revealed that CypD levels were significantly elevated after OGD when compared to non-OGD control mitochondrial inner membrane fractions (p < 0.05, Fig. 2A, B). To determine if CypD-mediated mPTP opening is involved in OGD-induced synaptic transmission deficit, hippocampal slices were treated with cyclosporine A (CSA, 1 μM), an inhibitor of CypD-dependent mPTP opening. Treatment with CSA enhanced synaptic transmission recovery after OGD followed by glucose and oxygen reperfusion (57.6 ± 7 % of baseline, n = 9 slices, p < 0.05 vs vehicle-treated slices; Fig. 2C, D), suggesting a role for CypD-mediated mPTP in the recovery of synaptic transmission after OGD.
Figure 2. OGD triggers CypD translocation and inhibition of mPTP by cyclosporin A (CSA) promotes synaptic transmission recovery after OGD.
A) Representative immunoblotting bands show CypD levels in mitochondrial inner membrane in the indicated groups of slices. VDAC, CCo and HSP60 were used as out membrane of mitochondria, inner membrane of mitochondria and mitochondrion matrix marker, respectively. B) Quantification of CypD immunoactive bands relative to CCo in the indicated groups. N = 4 mice per group. C) Changes of the amplitude of field-excitatory post-synaptic potentials (fEPSPs) in indicated groups. CSA (1 μM) treatment started 5 min before OGD (bar) and presented during entire OGD period. D) Synaptic transmission recovery of fEPSPs calculated as the averaged relative amplitude of fEPSPs compared to baseline values after re-introduction of oxygenated normal ACSF (from 35 to 40 min after the end of OGD). N =9 slices from 4-5 male mice (3-4 month-old age) per group.
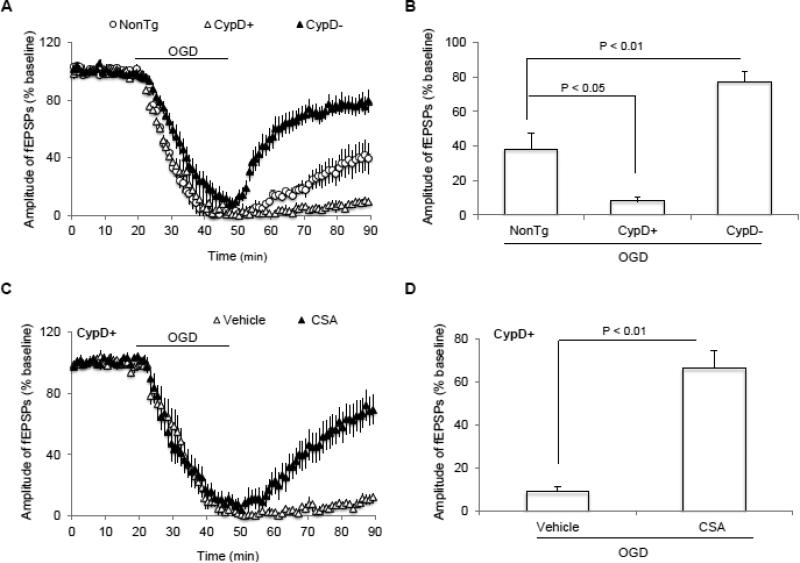
To evaluate the role of CypD in regulation of synaptic transmission recovery after OGD, CypD-deficient (CypD−) or CypD-overexpressed (CypD+) hippocampal slices were subjected to OGD. We found that loss of synaptic transmission was significantly prevented in CypD-deficient slices (77.9 ± 7 %, n = 14 slices) compared to nonTg slices insulted by OGD (40.2 ± 10 %, n = 9; p < 0.05; Fig. 3A, B). There was a greater suppression of synaptic transmission recovery (9.3 ± 2 %, n = 10 slices) in CypD-overexpressed slices than nonTg slices in the present of OGD (40.2 ± 10 %, n = 9; p < 0.05; Fig. 3A, B). Additionally, CSA treatment abrogated the effect of CypD overexpression on synaptic transmission recovery after OGD (66.2 ± 8 %, n = 11; p < 0.05; Fig. 3C, D). These data demonstrate the involvement of CypD in OGD-mediated impairment of synaptic transmission.
Figure 3. Effect of CypD on synaptic dysfunction after ischemia.
A) Cyclophilin D (CypD) overexpression suppresses and CypD deficiency restores synaptic transmission after oxygen and glucose deprivation (OGD), respectively. B) Summary of the field-excitatory post-synaptic potentials (fEPSPs) recovery during the last 5 min of OGD in indicated groups. C) Inhibition of CypD− mPTP by cyclosporin A (CSA) during OGD significantly preserved synaptic transmission in CypD overexpressed animals. D) Synaptic transmission recovery of fEPSPs calculated as the averaged relative amplitude of fEPSPs compared to baseline values after re-introduction of oxygenated normal artificial cerebrospinal fluid (ACSF, see methods section) (from 35 to 40 min after the end of OGD) in indicated groups. N = 8-14 slices from 4-5 male mice per group.
3.3 NR2B activation potentiates OGD-induced CypD translocation
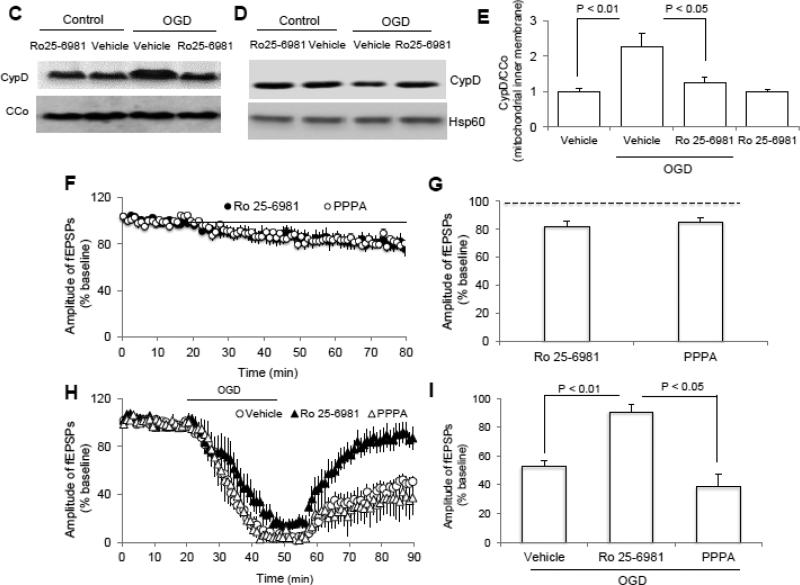
Since NR2B activation exacerbates neuronal injury, we sought to determine whether OGD-induced CypD translocation required NR2B activation in nonTg control animals. Consistent with previous reports, the phosphorylation level of NR2B at ser1303 was significantly increased upon OGD (Fig. 4A, B), suggesting that the overactivation of NR2B-containing NMDA receptors exists in our experimental conditions. Blockade of NR2B but not NR2A inactivation (Fig. 4C, E) significantly prevented OGD-induced CypD translocation. Accordingly, less CypD protein was found in OGD-induced mitochondrial matrix fraction compared to vehicle-treated control (Fig. 4D), indicating that more CypD protein was translocated from matrix to the inner membrane. Recordings of fEPSPs from non-OGD control slices treated with NR2B inhibitor, Ro 25-6981 (1 μM), or the NR2A inhibitor PPPA (0.5 μM), showed non-significant reduction in fEPSP amplitude (Fig. 4F, G). However, NR2B inactivation preserved synaptic transmission but not NR2A inhibitor treatment after OGD (Fig. 4H, I), suggesting the specific effect of NR2B inhibition on OGD-induced synaptic impairment. Our data indicate the CypD translocation via OGD-induced NR2B activation is, at least in part, responsible for the damage of synaptic transmission recovery after OGD.
Figure 4. N-methyl D-aspartate receptor subunit 2B (NR2B) activation is involved in OGD-induced cyclophilin D (CypD) translocation and synaptic injury.
A-B) Representative immunoblotting bands (A) and Quantification of (B) show the phosphorylation level of NR2B at ser1303 in indicated groups. C-D) Representative immunoblotting bands show CypD levels in mitochondrial inner membrane fraction and matrix (D) in indicated groups. E) Quantification of CypD immunoreactive bands normalized to CCo in indicated groups shown in panel C. N =4 mice per group. F) Inhibition of either NR2A (PPPA, 0.5 μM) or NR2B (Ro 25-6981, 1 μM) by its inhibitor perfusion (bar) suppressed synaptic transmission under normal condition. G) Average of the last 5 min of reperfusion fEPSPs amplitude in the indicated groups. H) Inhibition of NR2B but not NR2A significantly ameliorated synaptic injury after OGD. I) Synaptic transmission recovery of field-excitatory post-synaptic potentials (fEPSPs) calculated as the averaged relative amplitude of fEPSPs compared to baseline values after re-introduction of oxygenated normal artificial cerebrospinal fluid (ACSF, see methods section) (from 35 to 40 min after the end of OGD). N = 6-10 slices from 4-5 male mice (3-4 month-old age) per group.
3.4 CypD/p53 interaction and related oxidative stress are required for synaptic dysfunction in ischemia
Given that CypD/p53 interaction elicits mPTP opening and oxidative stress, leading to ischemia-induced cell death (Karch and Molkentin, 2012, Vaseva et al., 2012, Zhao et al., 2013, Pei et al., 2014), we next sought to investigate whether CypD/p53 interaction is responsible for OGD-induced deficit in synaptic transmission recovery among nonTg, CypD− and CypD+ mice. We determined the effect of NR2B on CypD/p53 interaction among these mice using co-immunoprecipitation procedure for hippocampal homogenates. As previously reported, CypD formed a complex with p53 in OGD-induced slices compared to vehicle-treated nonTg slices (Fig. 5A). CypD+ slices significantly increased OGD-induced CypD/p53 complex formation compared to nonTg slices (Fig. 5A). No CypD/p53 complex was found in CypD− slices either before or after OGD (Fig. 5A). These data indicate a protective effect of CypD depletion on OGD-induced synaptic dysfunction. Accordingly, increased CypD expression exacerbates synaptic perturbation induced by OGD-mediated ischemia, possibly via OGD-induced elevation of CypD/p53 complex formation.
Figure 5. The effect of p53 and NR2B activation on OGD-induced CypD/p53 complex formation in nonTg mice.
A) Immunoprecipitation of hippocampal homogenates with p53 antibody followed by immunoblotting with CypD antibody revealed CypD immunoreactive bands at 53kD in OGD-exposed non-transgenic (nonTg) hippocampal tissue. CypD/p53 complex was elevated in CypD-overexpressed mice and absent in CypD-deficient animals. β-actin bands show the equal amounts of protein used for Co-immunoprecipitation experiments. B). Pifithrin-μ (PFT, 5μM) treatment significantly suppressed OGD-induced CypD/p53 interaction in mitochondrial fractions. C) Inactivation of NR2B subunit instead of NR2A prevented CypD/p53 interaction from the isolated brain mitochondria after OGD. Experiments repeated at least 3 times; 5-6 mice per group. PFT: pifithrin-μ; Ro25: Ro25-6981
We next tested if inhibition of p53 by perfusion of nonTg slices with pifithrin-μ (PFT), an inhibitor of p53, affects interaction of CypD with p53. Administration of p53 inhibitor pifithrin-μ to nonTg hippocampal slice blocked CypD/p53 complex formation as seen in co-immunoprecipitation assay for brain mitochondria (Fig. 5B), suggesting the involvement of p53 activation in OGD-induced CypD/p53 interaction.
Given that NR2B activation was necessary for CypD translocation and was critical for OGD-induced synaptic dysfunction, we next investigated if inactivation of NR2B suppresses mitochondrial CypD/p53 interaction after OGD. As shown in Fig. 5C, treatment with NR2B inhibitor Ro 25-6981 but not NR2A inhibitor PPPA blocked OGD-induced CypD/p53 complex formation in nonTg mice. There were no significant effects of both inhibitors (R0 25-6981 and PPPA) on the CypD/p53 interaction in nonTg slices without OGD. These results suggest that activation of NR2B-containing glutamate receptors is required for OGD-induced CypD/p53 interaction, which in turn regulates synaptic transmission recovery after OGD.
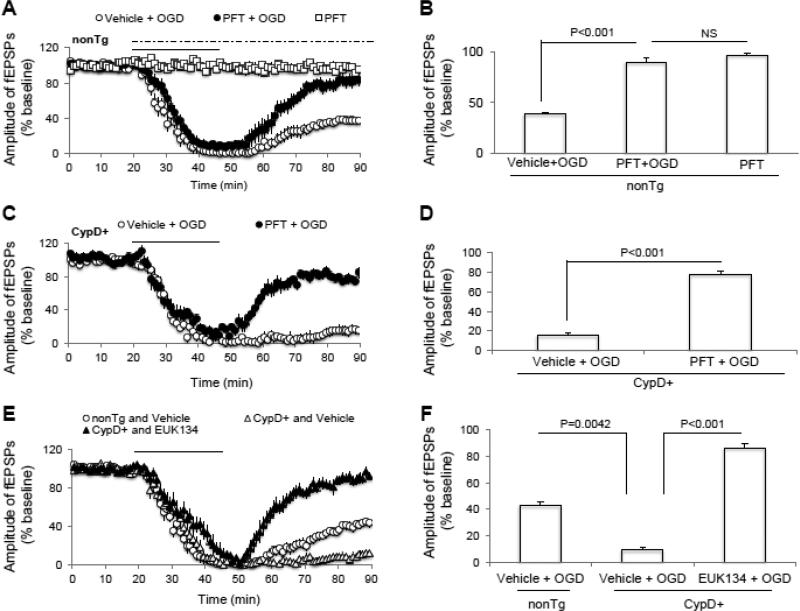
To further evaluate the direct role of p53 in synaptic transmission following OGD, we measured fEPSPs recovery from nonTg and CypD-overexpressed hippocampus with or without treatment of p53 inhibitor pifithrin-μ (PFT). The perfusion of PFT (1 μM) in nonTg slices did not affect synaptic transmission in the absence of OGD (p > 0.05; Fig. 6A, B), whereas treatment with PFT maintained synaptic transmission after OGD compared to vehicle-treated slices (p < 0.05; Fig. 6A, B). We further demonstrated that synaptic depression was significantly restored in CypD+ slices with the continuous perfusion of PFT compared to CypD+ vehicle treated slices after OGD/reperfusion (p < 0.05; Fig. 6C, D). Treatment of PFT to CypD− hippocampal slice failed to show further beneficial effects on synaptic transmission after OGD (data not show). These data indicate that blockade of p53 activity and CypD/p53 interaction protects against synaptic depression after OGD insult even under conditions of increased CypD expression. Taken together with the protective effect of lacking CypD in OGD-mediated perturbation, these results suggest that CypD/p53 interaction followed by NR2B activation contributes to OGD-impaired synaptic transmission recovery.
Figure 6. Suppression of CypD/p53 complex formation via blockade of p53 or antioxidant EUK134 perfusion maintained synaptic function after oxygen and glucose deprivation (OGD).
A) p53 inhibitor, pifithrin-μ (PFT, 5 μM), perfusion (dash line) of non-transgenic (nonTg) slices did not change field-excitatory post-synaptic potentials (fEPSPs) under baseline conditions. However, it significantly promoted synaptic transmission recovery after OGD (solid bar). B) Synaptic transmission recovery of fEPSPs calculated as the averaged relative amplitude of fEPSPs compared to baseline values after re-introduction of oxygenated normal artificial cerebrospinal fluid (from 35 to 40 min after the end of OGD). C-D) CypD-overexpressed slices pretreated with p53 inhibitor preserved synaptic transmission after OGD. E-F) CypD/p53 complex formation increases due to oxidative stress. The antioxidant EUK134 pretreatment (0.5 μM) abolishes the deleterious effect of OGD on synaptic transmission in CypD+ slices. N = 6-10 slices from 4-5 male mice (3-4 month-old age) per group.
CypD/p53 complex formation is involved in generation of mitochondrial oxidative stress (Karch and Molkentin, 2012, Vaseva et al., 2012, Zhao et al., 2013, Pei et al., 2014). We next examined if antioxidants treatment would promote synaptic transmission recovery after OGD. We tested the effect of antioxidant EUK134 on fEPSPs recovery after OGD (Fig. 6E, F). When slices overexpressing CypD were continuously perfused with antioxidant EUK 134 (500 nM), we observed significant rescue of synaptic transmission after OGD (86.3 ± 4 %, n = 8 slices; p < 0.05 vs. vehicle treated slices after OGD; Fig. 6E, F). The protective effect of EUK134 perfusion was not due to modulation of NR2B-containing receptor activation since it didn't affect pSer1303 NR2B after OGD. Thus, CypD/p53-mediated oxidative stress is involved in synaptic depression in ischemia.
4. Discussion
A large body of studies has established that activation of NR2B and the associated intracellular signaling pathways are responsible for ischemic stroke-induced synaptic injury and neuronal loss. An increasing number of evidence also suggests that mitochondria are important for ischemia-induced neuronal death. Here we provide the first piece of evidence, showing that NR2B activation actually works together with mitochondrial CypD and suppresses the recovery of synaptic transmission upon OGD. Our results suggest OGD-induced CypD translocation onto mitochondrial membrane dependent on NR2B activation. Additionally, OGD-induced CypD translocation facilitates CypD/p53 complex formation and enhances oxidative stress, which is responsible for the loss of synaptic transmission.
Our current data illustrated a new finding that OGD induced CypD translocation onto membrane via an NR2B-dependent mechanism. CypD is one of the key regulatory components of mPTP and plays a vital role in a number of disorders, including Alzheimer's disease (AD) (Du et al., 2008, Du et al., 2009, Du et al., 2010, Du et al., 2011), diabetes (Lumini-Oliveira et al., 2011, Itoh et al., 2012, Wang et al., 2014) and ischemia (Nakagawa et al., 2005, Schinzel et al., 2005, Vaseva et al., 2012, Pei et al., 2014). Inhibition of CypD activity and blockade of mPTP opening reduces infarct size after ischemia/reperfusion (Shiga et al., 1992). Studies in genetically CypD-deficient mice provided direct evidence for the role of CypD in mediating mPTP opening, oxidative stress and subsequent necrotic cell death after cerebral or cardiac ischemia/reperfusion (Nakagawa et al., 2005, Schinzel et al., 2005, Vaseva et al., 2012). However, whether and how CypD translocate onto mitochondrial membrane under OGD insults are not well understood. In the present study, we first detected an increased CypD level in the inner membrane of mitochondria subjected to OGD, suggesting that OGD-induced CypD translocation may underline the enhanced mPTP opening leading to mitochondrial oxidative stress and synaptic dysfunction. Indeed, inhibition of mPTP by perfusion of pharmacological inhibitor CSA ameliorated loss of synaptic transmission and increased synaptic recovery. Similar protective effects on OGD-induced synaptic failure were obtained from genetic depletion of CypD. Furthermore, overexpression of neuronal CypD exacerbated the synaptic transmission loss after OGD. We also demonstrated that OGD-induced NR2B activation is necessary for CypD translocation based on the observation that blockade of NR2B instead of NR2A suppressed CypD translocation onto the inner membrane of mitochondria. In parallel, blockade of NR2B but not NR2A activation in OGD protects against OGD-induced insufficient recovery of synaptic transmission.
While it is well known that glutamate mediates excitatory synaptic transmission in the brain and accelerates synaptic degeneration in a number of neuronal disorders including ischemic stroke (Lipton and Rosenberg, 1994, Mattson, 1997, Lee et al., 1999, Arundine and Tymianski, 2004), the mechanisms underlying NR2B-driven CypD translocation onto membrane have not been elucidated yet. As to our knowledge, extracellular glutamate levels rise abruptly in ischemia, thereby resulting in over-activation of NR2B-containing NMDAR at extrasynaptic sites. Activated NR2B subunits directly interact with PTEN (Zhang et al., 2013) or DAPK1 (Tu et al., 2010), or indirectly associate with nNOS via PSD95 (Cao et al., 2005), subsequently modulate downstream signaling pathways such as calpain, p25, striatal-enriched protein tyrosine phosphatase (STEP), p38, JNK, PKC and sterol regulatory element binding protein-1 (SREBP1) for excitotoxic neuronal death (Cheung et al., 2003, Taghibiglou et al., 2009a, Taghibiglou et al., 2009b, Xu et al., 2009, Li et al., 2013). Activation of NR2A-containing NMDA receptors via synaptic transmission leads to activation of the prosurvival signaling proteins Akt, ERK, and CREB (Xu et al., 2009, Lai et al., 2014). Similar to what was previously reported, blockade of NR2B instead of NR2A has a protective effect on synaptic transmission recovery after ischemia. More interestingly, our results suggest that activation of NR2B underlies OGD-induced CypD translocation onto the inner membrane of mitochondria, oxidative stress and deficits in synaptic transmission recovery. This study certainly adds new insights into the body of glutamate toxicity relevant to ischemia, which is a connection of NR2B activation to CypD-mediated synaptic injury upon OGD. Thus, blockade of NR2B activation by interception of NR2B with CypD interaction could hold a potential therapeutic strategy for ischemia-induced synaptic and neuronal damage.
Our results also indicated that CypD/p53 complex might not only cause cell death but also mediate the functional deficit of synaptic transmission recovery after OGD. P53 was recently reported to be rapidly translocated to mitochondria and to form CypD/p53 complex following ischemic insult, which results in mPTP opening and oxidative stress, leading to cell death in vivo and in vitro (Vaseva et al., 2012, Pei et al., 2014). Indeed, we observed NR2B activation-dependent mitochondrial translocation of CypD, which promoted CypD/p53 complex formation after OGD. The CypD/p53 interaction was modulated by CypD expression level, and in parallel the synaptic transmission recovery was associated with CypD expression. Similarly, inhibition of p53 by its inhibitor PFT during OGD significantly suppressed CypD/p53 interaction as well as promoted synaptic transmission recovery. These results suggest that CypD/p53 interaction contributes to synaptic transmission deficit in OGD. Furthermore, inhibition of CypD-dependent mPTP opening by CSA, or suppression of mitochondrial oxidative stress by administration of antioxidant EUK134 abolished impairment in OGD-insulted synaptic transmission in CypD-overexpressed slices in which CypD/p53 interaction was augmented after OGD.
It is noted that our experiments were performed in ex vivo conditions, and therefore the further studies using in vivo ischemic stroke model would be favorable. In addition, some of the OGD studies, including the present one, were carried out at room temperature condition in which hippocampal slice is more tolerant to a long time OGD insult (Taylor et al., 1999). The observed events might not fully stand in in vivo conditions. Given that hypothermia is the most beneficial and operative method in clinic for neuronal protection, the results generated in hypothermia condition are more closed to clinical implication. In this study, we performed our in vitro ischemic experiments at room temperature condition. We demonstrated that inhibiting oxidative stress either by CAS or EUK134 treatment was beneficial for synaptic transmission after OGD. Therefore, this novel ex vivo ischemia mouse model may be more appropriate to study oxidative damage rather than excitotoxic damage in ischemic condition. Additionally, NR2B-mediated CypD translocation might be only part of the mechanisms for the deficits of synaptic transmission recovery after OGD. Our current investigation did not distinguish the effects of NR2B-mediated CypD translocation on the severity of OGD damage during glucose and oxygen deprivation period from the direct effects on recovery processes when glucose and oxygen reintroduced to hippocampal slices.
In summary, using an ex vivo ischemia model on slices of CypD null mice, mice overexpressing neuronal CypD and nonTg littermates, we clearly demonstrate that NR2B activation promotes CypD translocation along with CypD/p53 interaction following OGD-induced ischemia. The absence of CypD or inhibition of p53 completely abolishes CypD/p53 interaction and improves synaptic transmission recovery after OGD. The possible underlying mechanism of OGD-induced synaptic injury is that NR2B activation drives CypD translocation to the mitochondrial inner membrane where CypD interacts with P53, promoting oxidative stress, eventually leading to synaptic dysfunction after OGD. Thus, disassociating NR2B activation and mitochondrial CypD membrane translocation might promote synaptic transmission recovery after OGD. Advanced understanding of the mitochondrial mechanism for ischemia-induced synaptic injury such as those shown here will help to develop new potential therapeutic strategies to prevent brain damage after brain ischemia.
Highlights.
-
1)
OGD induces CypD translocation in a NR2B-dependent manner
-
2)
NR2B-dependent CypD translocation facilitates CypD/p53 complex formation
-
3)
Pharmacological blockade of CypD translocation or CypD/p53 interaction rescues synaptic transmission
-
4)
Genetic modulation of CypD expression regulates CypD-mediated membrane transition pore, CypD/p53 interaction, and synaptic injury
-
5)
Evaluation of a new ex-vivo chronic OGD-induced ischemia model for studying the effect of oxidative stress-induced synaptic injury
Acknowledgments
This work was supported by grants from the National Institute of Aging and Neurological Disorder and Stroke.
Abbreviations
- CypD
Cyclophilin D
- NMDA
N-methyl D-aspartate
- NR2B
N-methyl D-aspartate receptor subunit 2B
- OGD
oxygen-glucose deprivation
- mPTP
mitochondrial permeability transition pore (mPTP)
- CSA
cyclosporine A
- PFT
Pifithrin-μ (; 5 μM, Sigma)
- fEPSPs
Field-excitatory post-synaptic potentials
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
We have no conflicts of interest to disclose.
Reference
- Albensi BC, Igoechi C, Janigro D, Ilkanich E. Why do many NMDA antagonists fail, while others are safe and effective at blocking excitotoxicity associated with dementia and acute injury? Am J Alzheimers Dis Other Demen. 2004;19:269–274. doi: 10.1177/153331750401900502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci. 2004;61:657–668. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- Cao J, Viholainen JI, Dart C, Warwick HK, Leyland ML, Courtney MJ. The PSD95-nNOS interface: a target for inhibition of excitotoxic p38 stress-activated protein kinase activation and cell death. The Journal of cell biology. 2005;168:117–126. doi: 10.1083/jcb.200407024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PH. Mitochondria and neuronal death/survival signaling pathways in cerebral ischemia. Neurochemical research. 2004;29:1943–1949. doi: 10.1007/s11064-004-6869-x. [DOI] [PubMed] [Google Scholar]
- Cheung HH, Teves L, Wallace MC, Gurd JW. Inhibition of protein kinase C reduces ischemia- induced tyrosine phosphorylation of the N-methyl-d-aspartate receptor. Journal of neurochemistry. 2003;86:1441–1449. doi: 10.1046/j.1471-4159.2003.01951.x. [DOI] [PubMed] [Google Scholar]
- Choi DW. Calcium: still center-stage in hypoxic-ischemic neuronal death. Trends in neurosciences. 1995;18:58–60. [PubMed] [Google Scholar]
- Du H, Guo L, Fang F, Chen D, Sosunov AA, McKhann GM, Yan Y, Wang C, Zhang H, Molkentin JD, Gunn- Moore FJ, Vonsattel JP, Arancio O, Chen JX, Yan SD. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Guo L, Yan S, Sosunov AA, McKhann GM, Yan SS. Early deficits in synaptic mitochondria in an Alzheimer's disease mouse model. Proc Natl Acad Sci U S A. 2010;107:18670–18675. doi: 10.1073/pnas.1006586107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Guo L, Zhang W, Rydzewska M, Yan S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiology of aging. 2009 doi: 10.1016/j.neurobiolaging.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Guo L, Zhang W, Rydzewska M, Yan S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiology of aging. 2011;32:398–406. doi: 10.1016/j.neurobiolaging.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Annals of the New York Academy of Sciences. 2005;1047:248–258. doi: 10.1196/annals.1341.022. [DOI] [PubMed] [Google Scholar]
- Huang S, Wang Y, Gan X, Fang D, Zhong C, Wu L, Hu G, Sosunov AA, McKhann GM, Yu H, ShiDu Yan S. Drp1-mediated mitochondrial abnormalities link to synaptic injury in diabetes model. Diabetes. 2014 doi: 10.2337/db14-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1:383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- Itoh T, Kouzu H, Miki T, Tanno M, Kuno A, Sato T, Sunaga D, Murase H, Miura T. Cytoprotective regulation of the mitochondrial permeability transition pore is impaired in type 2 diabetic Goto- Kakizaki rat hearts. Journal of molecular and cellular cardiology. 2012;53:870–879. doi: 10.1016/j.yjmcc.2012.10.001. [DOI] [PubMed] [Google Scholar]
- Karch J, Molkentin JD. Is p53 the long-sought molecular trigger for cyclophilin D-regulated mitochondrial permeability transition pore formation and necrosis? Circulation research. 2012;111:1258–1260. doi: 10.1161/CIRCRESAHA.112.280990. [DOI] [PubMed] [Google Scholar]
- Kemp JA, McKernan RM. NMDA receptor pathways as drug targets. Nat Neurosci. 2002;5(Suppl):1039–1042. doi: 10.1038/nn936. [DOI] [PubMed] [Google Scholar]
- Khaspekov L, Friberg H, Halestrap A, Viktorov I, Wieloch T. Cyclosporin A and its nonimmunosuppressive analogue N-Me-Val-4-cyclosporin A mitigate glucose/oxygen deprivation-induced damage to rat cultured hippocampal neurons. The European journal of neuroscience. 1999;11:3194–3198. doi: 10.1046/j.1460-9568.1999.00743.x. [DOI] [PubMed] [Google Scholar]
- Lai TW, Shyu WC, Wang YT. Stroke intervention pathways: NMDA receptors and beyond. Trends in molecular medicine. 2011;17:266–275. doi: 10.1016/j.molmed.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Lai TW, Zhang S, Wang YT. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Progress in neurobiology. 2014;115:157–188. doi: 10.1016/j.pneurobio.2013.11.006. [DOI] [PubMed] [Google Scholar]
- Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischaemic brain injury mechanisms. Nature. 1999;399:A7–14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- Li LL, Ginet V, Liu X, Vergun O, Tuittila M, Mathieu M, Bonny C, Puyal J, Truttmann AC, Courtney MJ. The nNOS-p38MAPK pathway is mediated by NOS1AP during neuronal death. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:8185–8201. doi: 10.1523/JNEUROSCI.4578-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- Lumini-Oliveira J, Magalhaes J, Pereira CV, Moreira AC, Oliveira PJ, Ascensao A. Endurance training reverts heart mitochondrial dysfunction, permeability transition and apoptotic signaling in long-term severe hyperglycemia. Mitochondrion. 2011;11:54–63. doi: 10.1016/j.mito.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Martin RL, Lloyd HG, Cowan AI. The early events of oxygen and glucose deprivation: setting the scene for neuronal death? Trends in neurosciences. 1994;17:251–257. doi: 10.1016/0166-2236(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Neuroprotective signal transduction: relevance to stroke. Neurosci Biobehav Rev. 1997;21:193–206. doi: 10.1016/s0149-7634(96)00010-3. [DOI] [PubMed] [Google Scholar]
- Messier C. Impact of impaired glucose tolerance and type 2 diabetes on cognitive aging. Neurobiol Aging 26 Suppl. 2005;1:26–30. doi: 10.1016/j.neurobiolaging.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- Pei L, Shang Y, Jin H, Wang S, Wei N, Yan H, Wu Y, Yao C, Wang X, Zhu LQ, Lu Y. DAPK1-p53 Interaction Converges Necrotic and Apoptotic Pathways of Ischemic Neuronal Death. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:6546–6556. doi: 10.1523/JNEUROSCI.5119-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinzel AC, Takeuchi O, Huang Z, Fisher JK, Zhou Z, Rubens J, Hetz C, Danial NN, Moskowitz MA, Korsmeyer SJ. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiga Y, Onodera H, Matsuo Y, Kogure K. Cyclosporin A protects against ischemia-reperfusion injury in the brain. Brain research. 1992;595:145–148. doi: 10.1016/0006-8993(92)91465-q. [DOI] [PubMed] [Google Scholar]
- Siesjo BK. Pathophysiology and treatment of focal cerebral ischemia. Part I: Pathophysiology. Journal of neurosurgery. 1992;77:169–184. doi: 10.3171/jns.1992.77.2.0169. [DOI] [PubMed] [Google Scholar]
- Taghibiglou C, Martin HG, Lai TW, Cho T, Prasad S, Kojic L, Lu J, Liu Y, Lo E, Zhang S, Wu JZ, Li YP, Wen YH, Imm JH, Cynader MS, Wang YT. Role of NMDA receptor-dependent activation of SREBP1 in excitotoxic and ischemic neuronal injuries. Nature medicine. 2009a;15:1399–1406. doi: 10.1038/nm.2064. [DOI] [PubMed] [Google Scholar]
- Taghibiglou C, Martin HG, Rose JK, Ivanova N, Lin CH, Lau HL, Rai S, Wang YT, Rankin CH. Essential role of SBP-1 activation in oxygen deprivation induced lipid accumulation and increase in body width/length ratio in Caenorhabditis elegans. FEBS letters. 2009b;583:831–834. doi: 10.1016/j.febslet.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Taylor CP, Weber ML, Gaughan CL, Lehning EJ, LoPachin RM. Oxygen/glucose deprivation in hippocampal slices: altered intraneuronal elemental composition predicts structural and functional damage. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:619–629. doi: 10.1523/JNEUROSCI.19-02-00619.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu W, Xu X, Peng L, Zhong X, Zhang W, Soundarapandian MM, Balel C, Wang M, Jia N, Zhang W, Lew F, Chan SL, Chen Y, Lu Y. DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell. 2010;140:222–234. doi: 10.1016/j.cell.2009.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino H, Elmer E, Uchino K, Li PA, He QP, Smith ML, Siesjo BK. Amelioration by cyclosporin A of brain damage in transient forebrain ischemia in the rat. Brain research. 1998;812:216–226. doi: 10.1016/s0006-8993(98)00902-0. [DOI] [PubMed] [Google Scholar]
- Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell. 2012;149:1536–1548. doi: 10.1016/j.cell.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu L, Fang D, Zhong C, Chen JX, Yan SS. Synergistic Exacerbation of Mitochondrial and Synaptic Dysfunction and Resultant Learning and Memory Deficit in a Mouse Model of Diabetic Alzheimer's Disease. J Alzheimers Dis. 2014 doi: 10.3233/JAD-140972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kurup P, Zhang Y, Goebel-Goody SM, Wu PH, Hawasli AH, Baum ML, Bibb JA, Lombroso PJ. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:9330–9343. doi: 10.1523/JNEUROSCI.2212-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wang Y, Yan S, Du F, Wu L, Yan S, Yan SS. Genetic deficiency of neuronal RAGE protects against AGE-induced synaptic injury. Cell death & disease. 2014;5:e1288. doi: 10.1038/cddis.2014.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Taghibiglou C, Girling K, Dong Z, Lin SZ, Lee W, Shyu WC, Wang YT. Critical role of increased PTEN nuclear translocation in excitotoxic and ischemic neuronal injuries. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:7997–8008. doi: 10.1523/JNEUROSCI.5661-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LP, Ji C, Lu PH, Li C, Xu B, Gao H. Oxygen glucose deprivation (OGD)/re-oxygenation-induced in vitro neuronal cell death involves mitochondrial cyclophilin-D/P53 signaling axis. Neurochemical research. 2013;38:705–713. doi: 10.1007/s11064-013-0968-5. [DOI] [PubMed] [Google Scholar]