Abstract
BACKGROUND
The biological changes that lead to autism likely occur during prenatal life. Although earlier identification of the disorder has occurred within the past decade, the mean age of diagnosis is still not made before a mean age of 3 years. This is because autism remains a behaviorally defined disorder, placing limits on the age at which a confident diagnosis can be made. The study of the biological basis of autism prior to age 3 is essential and can most directly be achieved with prospective research designs.
METHODS
The literature on the early identification of autism is discussed, including the timescale for the onset of social symptoms. Also discussed is a new method for the prospective study of autism called the “1-Year Well-Baby Check-Up Approach,” which allows for the prospective study of the disorder in simplex families with infants as young as 12 months of age.
RESULTS
Although likely present at subtle, subclinical levels, early social abnormalities are not clearly detectable prior to 12 months in age in infants later diagnosed as having autism spectrum disorder.
CONCLUSIONS
Using the 1-Year Well-Baby Check-Up Approach or other prospective design, examining early biomarkers related to early brain overgrowth, cerebellar development, gene expression patterns and immune system function may be key to early diagnosis efforts under 3 years. We also note the importance of comparing and contrasting the early “signature” of autism in children from singleton versus multiplex families, which may be etiologically distinct.
Keywords: autism, early identification, biomarkers, screening, brain overgrowth, gene expression
INTRODUCTION
Detecting autism at the earliest possible age is of the utmost importance to optimize outcomes for children with the disorder.1 Mechanisms of developmental plasticity provide a clear rationale for providing an enriched environment, such as that afforded by careful early treatment, to significantly improve brain structure and function.2,3 Despite the fact that early intensive behavioral intervention (EIBI) became mainstream for young children with autism in the late 1980s following a report by Lovaas of a 47% “recovery” rate for children receiving 40 hours of treatment or more,4 rigorous scientific studies on EIBI are sparse. Challenges include the high cost of research and a heterogeneous subject population that varies considerably in symptom severity and in the etiologic mechanisms that contribute to those symptoms. The most problematic issue, however, relates to ethical restrictions that prevent the use of a no-treatment control group. As such, most studies either have no control group at all, or compare 2 groups of children receiving different types of treatments.
Since the initial 1987 report by Lovaas, at least one study replicated the original finding,5 but most studies have reported more modest gains. For example, Sheinkopf and Siegel6 reported that children receiving EIBI experienced significant gains in IQ following treatment, yet these children still met criteria for autistic disorder or pervasive developmental disorder (PDD-NOS). Magiati and colleagues7 compared the outcomes of 28 children enrolled in EIBI home-based programs and 16 children enrolled in autism-specific nursery school programs. Results indicated that although both groups showed improvements in age-equivalent scores related to language and cognitive ability, standard scores changed little over time. Like most EIBI studies, the mean age of participants was over 3 years at intake and over 5 years at the conclusion of the study, and age effects were not specifically analyzed. EIBI may prove more efficacious consistently across studies if it is initiated before symptoms become severe. This can be achieved only if diagnoses are made during the infancy or toddler periods.
A major impediment to the goal of achieving an early diagnosis of autism is the fact that the mean age of diagnosis for children with autism spectrum disorder (ASD) is usually over 3 years of age8 and much later in many places in the world. This is in stark contrast to reports from many parents that they first noted something amiss in their infant within the first year of life.9 Indeed, in a recent prospective study of autism, clinical abnormalities were detected in children as young as 12 months of age, although a definitive diagnosis of autism for most of these children was not given until age 3 years.10
Why does a definitive diagnosis of autism during the first years of life remain elusive? Impediments to earlier diagnosis include the gradual onset and heterogeneity of symptoms, as well as the virtual absence of prospective empirical studies of the disorder during the first years of life. It is the proverbial chicken-and-egg conundrum: In general, autism cannot be studied until it is reliably diagnosed, and it is difficult to diagnose earlier than current practice because there is scant research from that age period from which to generate hypotheses and new ideas. Despite some barriers, early identification of ASD as a whole has made great strides, including development of more refined diagnostic tools, broader public awareness, and greater success in lowering the age of first diagnosis. Consider, for example, that less than 20 years ago, the mean age of diagnosis in Denmark was 7 years.11
The greatest steps forward are yet to come as the field moves toward integrating biological data with traditional clinical symptoms. New findings in functional and structural brain imaging, immunology, and genetics, when combined with traditional clinical information, bring power and possibility for even earlier identification. As such, we envision the future of the early identification of autism as translational, with direct attempts to bridge the gap between clinical and biological research.
When does autism begin?
Both biological and behavioral evidence indicate that something is going awry within the first year of life (or earlier) for the majority of children eventually diagnosed with autism, although the disorder is almost always clinically undetected at that age. Behavioral evidence of early abnormalities comes almost exclusively from studies that use retrospective home videotape data. Maestro and colleagues,12 for example, retrospectively reviewed home videos from 30 children with ASD and found that 87.5% of the infants displayed symptoms within the first year, such as poor social relatedness, hypoactivity, and a lack of emotional modulation. This finding is consistent with similar analyses performed in the 1990s13-15 as well as more recent interviews with parents16 that implicate early emerging deficits in the majority of cases (but see discussion of regression, below). On the other hand, the first prospective study of autism failed to detect statistically significant differences in social and language behavior between typically developing infants and those at risk for autism prior to the first birthday.10 This provides compelling evidence that early clinical symptoms are subtle, and behavioral observation alone may be insufficient to diagnose autism during the first year of life.
Biological data provide a clearer picture that autism begins very early in development. For example, using pediatric records of head circumference, Courchesne and colleagues17 discovered that early brain overgrowth is a common feature of the disorder. Over 90% of the children with autism in that study had head circumference values above the 85th percentile, and many showed evidence of abnormal brain growth as early as 6 to 8 months of age. The essential point as it pertains to autism is not the role of macrocephaly per se—as several other disorders such as Sotos syndrome18 and Canavan disease19 are associated with large brain volumes—but rather the rate of change in brain size.20 In the Courchesne study, infants who eventually developed autism had normal or slightly below normal head circumference values at birth, but for many of these infants, these values shot up above the 85th percentile before their first birthday.17 Thus, a key factor may be the relative change from an average brain size to one that far exceeds normal size in a short span of time. The general finding of early brain overgrowth in autism as indexed by head circumference has been replicated by several independent research groups21-24 and confirmed with slightly older children using magnetic resonance imaging (MRI).23,25-27
Just hours after birth, indices of neurodevelopmental differences, such as abnormally high levels of brain-derived neurotrophic factor (BDNF), have been found in the blood spots of neonates eventually diagnosed with autism.28 Prior to birth, the balance of prenatal brain development has been called into question because the difference between biparietal diameter and head circumference as measured via ultrasound was abnormal in fetuses later diagnosed as having ASD.29 Abnormalities in prenatal brain development have also been suggested by neuropathological studies that report smaller and immature neurons as well as heterotopias and cases of disorganization of cortical lamina in the brain.30-32
Although scarce in number, existing biological studies suggest that for the majority of children who develop ASD, abnormal development is under way before 12 months, likely even during prenatal life. If this is true, why does a clear diagnosis elude the clinician until the patient’s early childhood?
Eluding early diagnosis: Gradual and inconsistent onset, heterogeneity, and comorbid symptoms
Diagnosing autism prior to age 3 years is a formidable challenge. Symptoms emerge subtly and with inconsistent patterns, are heterogeneous, and may coexist with symptoms that result from comorbid diseases or disorders. Furthermore, attempts at early diagnosis of autism are superimposed upon and interact with the rapid and profound changes that one would expect as part of typical development.
The first major issue for early identification efforts is the gradual and inconsistent patterns of onset of clinical symptoms. In general, symptoms seem to manifest via 1 of 3 patterns: early onset (ie, at or around the first birthday), late onset (ie, after the first birthday), or regression (ie, a period of normal development followed by a loss of previously mastered skills). In a recent prospective study, Landa and colleagues provided compelling evidence of both early- and late-onset patterns: At age 14 months, approximately half of the children with ASD who were followed from an early age showed no differences from typically developing infants in social behavior such as shared positive affect and initiation of joint attention. Such late-onset cases were “essentially indistinguishable from those without ASD on the social and communication variables examined.”33 By 24 months, late-onset cases exhibited a more typical ASD profile that included less frequent and muted social and communication behaviors.33
Although a symptom-onset pattern defined by regression was not a focus of the Landa study, several studies have provided evidence that approximately one-third of children with ASD develop typically during the first 1 to 2 years of life and then regress in communication and social domains.16,34 Gradual and variable symptom-onset patterns, then, provide clear evidence of the need for multiple screens for autism across the first 3 years of life, as is now recommended by the American Academy of Pediatrics (AAP).35
Although deficits in social relatedness and the presence of restricted, repetitive, and stereotyped patterns of behavior and interests are seen in all 3 disorders comprising the autism spectrum (ie, autistic disorder, PDD-NOS, and Asperger’s disorder36), symptoms can vary considerably, leaving the phenotypic end points of ASD ill-defined.35 For example, some individuals may be verbal but may not use language in socially meaningful ways, whereas others may be completely nonverbal. Some children with autism may have frequent and intense routines and patterns of repetitive behavior, yet others may exhibit only minimal levels of such behavior.
In addition to heterogeneity of the core symptoms of autism (social, communication, and repetitive behavior), individuals with ASD may have a range of related conditions. For example, 1% to 4% of children with autism have tuberous sclerosis37-39 and 1% to 2% have fragile X syndrome,40 although the association with fragile X is not found in all cohorts.41,42 Conversely, the prevalence of autism in tuberous sclerosis is approximately 16%,39 whereas rates of autism in fragile X disorder are slightly higher, at 33%.40 Furthermore, it has been historically estimated that up to 75% of individuals with autistic disorder have co-occurring mental retardation.43 New epidemiological studies, however, report that rates of mental retardation may be as low as 30%,44 and possibly even lower.45
Given the variability of symptoms, differences in age of onset, severity of symptomatology, and the murky nature of possible regression of symptoms for some children, ASDs may be especially difficult to diagnose early and accurately based on behavior alone. Furthermore, there is undeniable overlap between normal and abnormal early development; some children who are in fact typical may exhibit slight delays in language and/or social behavior, whereas some children truly at risk for ASD may exhibit some proficiency in language and social behavior during early development. Given the relatively unknown biological profile of the first years of life, however, these challenges remain, given that the state-of-the-art of early identification is based entirely on clinically observable behavioral signs.
Early identification state of the art: Diagnosis and screening based on behavior alone
Currently, there is no standardized mechanism for the early identification of autism. Although parents of children who are later identified as having ASD typically become concerned about their child’s speech and other behaviors between 15 and 18 months of age,46 they often delay discussing their concerns with their child’s physicians for several months. Delays in social skills often are not noticed until the child is over 2 years of age. In one study, a delay of 20 to 60 months was found between parental suspicion and diagnosis by a medical professional.47 Furthermore, many parents—particularly those who have not had prior children—may miss subtle signs of abnormal early development.
In order to promote and improve the early identification of autism, the AAP has recently published practice guidelines for the identification and evaluation of children with ASD.35 These guidelines recommend universal developmental surveillance during preventive, well-child visits by screening with standardized developmental tools during the 9-, 18-, and 24-month well-child visits, and ASD-specific screening at the 18- and/or 24-month well-child visits.35
Screening instruments may allow primary care physicians to detect developmental concerns earlier and more efficiently. Although a definitive diagnosis of autism is usually not given via early identification screening tools alone, they serve as an excellent platform to detect infants who are in need of additional evaluation. Despite their benefits, a recent survey of 1600 primary care physicians (800 primary care pediatricians and 800 family physicians) suggests that fewer than half used regular screenings to detect developmental delay.48 In a sample of 255 primary care pediatricians, Dosreis et al49 reported that only 8% regularly screened for ASD. Yet there are a number of available ASD-specific screening instruments that have adequate psychometric properties, including sensitivity and specificity, in samples of young children.
One of the first screening tools developed was the Checklist for Autism in Toddlers (CHAT),50 published almost 20 years ago. As a first-tier screening tool, the CHAT has demonstrated excellent specificity (ie, a test’s ability to detect true negatives) but very poor sensitivity (ie, a test’s ability to detect true positives). In the original version of the CHAT, parents are asked to complete 9 yes/no items about their toddler’s social interactions, communication, and behavior. Additionally, a clinician completes another 5 items based on observation of the toddler during the clinic visit. Because of the poor sensitivity (ie, .38), of the original CHAT, a revised version called the Modified Checklist for Autism in Toddlers (M-CHAT)51 was developed. This version relies solely on parental reporting of 23 yes/no items and has sensitivity and specificity values of .85 and .93 respectively.51 Such high sensitivity and specificity rates have been reported in samples of children outside the United States as well.52,53 Somewhat newer tools have been developed, such as the Early Screening for Autistic Traits Questionnaire54,55 and the Pervasive Developmental Disorders Screening Test-II,56 but replicated sensitivity and specificity values in large samples have not been established. (For a complete review of screening instruments, see the AAP practice guidelines for the identification and evaluation of children with ASD.35)
Screening, however, merely identifies a child who is at risk, and a thorough diagnostic evaluation by a trained professional is needed. In the field of research as well as in clinical settings, the Autism Diagnostic Observation Schedule (ADOS) is usually the instrument of choice to assist in diagnosing autism. The ADOS has been shown to have both good sensitivity and specificity.57 Furthermore, this tool provides not only diagnostic information, but also quantitative values associated with behavioral features of the disorder, such as the severity of social deficits. In this way it is possible to track the ebb and flow of symptom severity in affected children over time. Although this tool has limited clinical value in diagnosing children with a mental age below 16 months,58 a new modified version, referred to as ADOS: Toddler Module, has demonstrated validity with children as young as 12 months of age.59 Despite the promise of the Toddler Module, implementation of the ADOS requires considerable expertise and clinical skill. Although a few new diagnostic tools are being developed, such as the Autism Observation Scale for Infants,60 very few standardized tools have been extensively validated to assist in the early diagnosis of autism.
The most salient “red fiags” indicating risk are those related to social behavior
The American Academy of Neurology and Child Neurology Society Practice Parameter on Screening and Diagnosis of Autism61 suggests that the following “red flags” are absolute indications for immediate evaluation of a child: (1) no babbling or pointing or other gesture by 12 months, (2) no single words by 16 months, (3) no 2-word spontaneous (not echolalic) phrases by 24 months, and (4) any loss of any language or social skills at any age. However, because early symptoms of other childhood disorders, such as general language delay and global developmental delay, also include abnormalities in early language, abnormalities in social relatedness are more likely to suggest autism to clinicians. Social abnormalities can take many forms but generally fall into 1 of 4 domains: socio-emotional responding, such as a failure to engage in shared positive affect; social attention, such as a failure to orient to social signals, such a child’s own name being called; social interaction, such as failing to maintain interactions with other children; and social gestures, such as a failure to wave hello or engage in other greeting responses. Similar to typically developing infants, those at risk for developing ASD will show an uneven profile across these social domains: Some will exhibit joyful expression but have considerably poor eye contact, whereas others may display the exact opposite profile. From a clinical standpoint, it is important to consider that a child need not exhibit sweeping deficits in all categories of social behavior to be considered at risk.
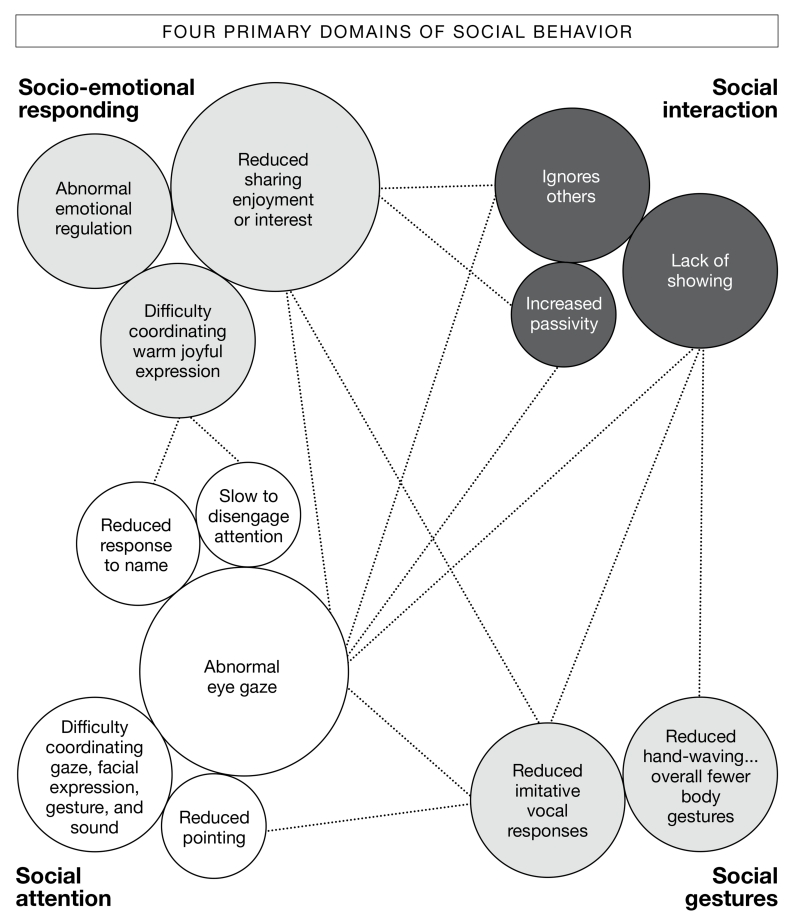
Although clear overlap exists between the 4 categories of social behavior described above, it may be scientifically useful to consider them separately because of potential differences in neuroanatomic systems that mediate their function. For example, difficulties in regulation of emotion might strongly involve defects in the amygdala and orbitofrontal cortex,62 whereas defects in eye gaze might strongly involve the superior temporal sulcus and parietal lobes.63 Considering social behavior as an integrated unit, but one that operates based on a distinct set of neural substrates, may facilitate genetic searches and novel conceptualization/compartmentalization of the present ASD diagnostic hierarchy, as well as guide treatment approaches. The most common “red flags” in these 4 social domains that emerge before 24 months based on retrospective, case, and prospective studies appear in FIGURE 1.
FIGURE 1. Major areas of weakness in social behavior for toddlers at risk for autism.
Social behavior is divided into 4 interrelated categories: socio-emotional responding, social interaction, social gestures, and social attention, which are shown in 4 different shades of gray. The size of the bubble reThects how frequently the behavior is reported in research studies, with larger bubbles representing the most commonly reported findings.
A strong social drive during the early months in autism?
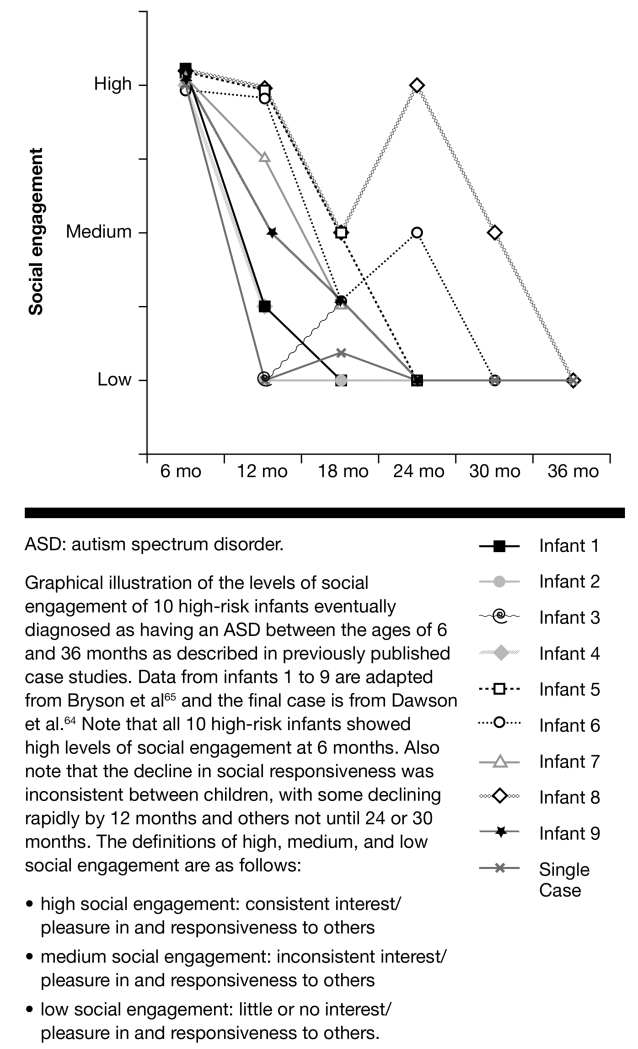
Contrary to what might be expected based on the clinical description of autism, controlled prospective group and single-case studies of infants later diagnosed with autism generate a very different picture: The vast majority of infants later diagnosed with autism show seemingly normal levels of social engagement during the first year of life; they smile, coo, laugh, and engage in back-and-forth social interactions with their caregivers and others. Consider that one infant at risk for autism studied from birth was described by Dawson and colleagues64 as making “a lot of vocalizations during play and responding to social interaction from adults by smiling and cooing” at 6 months. Similarly, a detailed prospective case report of 9 high-risk infants eventually diagnosed with ASD reported that 9 out of 9 showed significant social responsivity at 6 months, as shown in FIGURE 2. Forms of social responsivity included showing interest and pleasure in others, such as joyful giggling and consistent eye contact.65 By 12 months of age, however, the infant in the single-case study by Dawson and colleagues,64 as well as the majority of infants in the study by Bryson and colleagues65 began to exhibit deviances in social behavior that included poor eye contact and prolonged social distress. Although prospective data on autism are exceptionally rare, these few examples seem to suggest that one of the reasons autism is so difficult to identify early in life is that the core behavioral features of the disorder, namely social deficits, are not in clear evidence until the first birthday and may not peak in severity until many months later. In fact, 2 of the 9 infants studied by Bryson and colleagues65 tested negative for autism at 24 months and did not manifest substantial social abnormalities warranting a failure on the ADOS66 until 30 months of age (see FIGURE 2).
FIGURE 2. Levels of social engagement in infants at high risk for an ASD.
Retrospective videotape research has also examined social behaviors in ASD at 12 months of age, and results are identical to the illustration above: By age 1 year, a percentage of babies at risk for ASD exhibit social abnormalities.13,15,67,68 Less retrospective video research exists that examines the period of high social engagement illustrated above, namely the birth to 6-month period. Some,69,70 but not all,14 studies of this age have shown a reduction in the quantity of social behavior (eg, reduced attention to faces). Although this is consistent with the notion that something is going awry prior to or by 1 year of life (as discussed earlier), the prospective studies illustrated above clearly show that the quality of social behaviors in infants at risk for ASD is still quite high at age 6 months and perhaps until around the first birthday. If infants at risk for autism have the capability to display warm, positive social engagement during the first year of life, why and how does social development become more obviously derailed after the first birthday?
Is the emergence of atypical social behavior in autism linked to early brain overgrowth?
One of the central issues in neurodevelopmental science concerns the origins of social behavior and how it unfolds and expands during development. Typically developing infants begin life ready to socialize: They imitate actions such as tongue protrusion shortly after birth,71 prefer to track faces over non-face objects as early as 9 minutes after birth,72 and smile spontaneously by 2 weeks of age73 and in response to a caregiver by 6 weeks of age.74 Whereas social attention mechanisms in very young infants are thought to be mediated mainly by subcortical structures,75 cortical mechanisms become increasingly more functional throughout the first months. According to Johnson,76 the role of the cortex changes in key ways over time; as the baby develops, cortical function becomes more refined and focal. This “interactive specialization” (IS) view states that during development, activity-dependent interactions between brain regions sharpen the functions of particular regions.76 This speculation is supported by neuroimaging evidence that demonstrated more restricted and focal patterns of functional brain activity in 3- to 4-year-olds than in 13- to 24-month-olds in response to language sounds.77
How and why the systems supporting normal social behavior become derailed in autism is currently shrouded in mystery, but it has been recently proposed that the normal refinement that occurs across the first few years of development is complicated by the onset of early brain overgrowth in autism that peaks at about 12 to 24 months for many children with autism, as illustrated in FIGURE 3. This overlaps in time with the plummet in social behavior among infants who are eventually diagnosed with autism, as illustrated in FIGURE 2. Abnormal brain growth and size may be due to a reduction in synaptic pruning and consolidation resulting in an overabundance of neurites, or it may be due to an increase in neurogenesis and/or gliogenesis. In either case, faulty connections among centers in the brain are hypothesized to be linked to atypical social behaviors.78 Although direct evidence of a relationship between early brain overgrowth and the onset of autism symptoms has yet to be demonstrated, induced brain overgrowth in rodents during development has been shown to result in abnormal social behavior.79
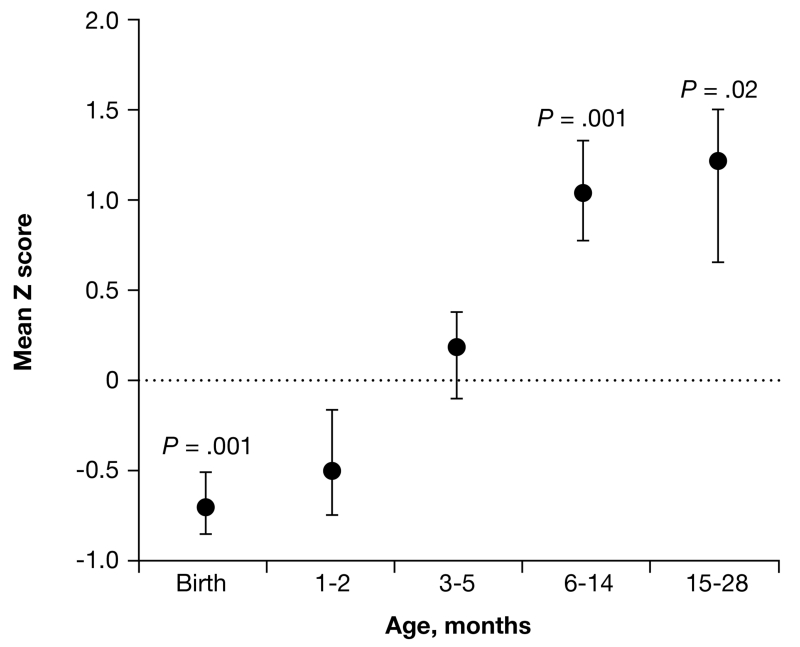
FIGURE 3. Early brain overgrowth in autism.
ASD: autism spectrum disorder; CDC: Centers for Disease Control; HC: head circumference.
At birth and at 1 to 2 months of age, HC in the longitudinal ASD group was statistically significantly below the CDC mean for healthy infants, but by 6 to 14 months it was more than 1 SD (84th percentile) above the mean for healthy infants. The CDC mean of healthy infants at each age is 0. Error bars are SEM.
Source: Reprinted with permission from Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290:337-344. Copyright 2003, American Medical Association.
Breaking the chicken-and-egg cycle: Methods for studying autism prospectively
As discussed above, a major impediment to the refinement and rapid evolution of early identification efforts resides in the fact that research on the disorder generally does not begin until a child has been identified as having the disorder, which may not be until age 3 years or later. Knowledge about the infancy and toddler periods in autism is rare and almost exclusively based on retrospective sources of information gathered well after a diagnosis is made (eg, home videotapes during infancy taken prediagnosis).13-15,67-70,80 Although such studies provide vital information regarding the early behavioral phenotype of autism, and may have even contributed to the development of early screening and diagnostic procedures, retrospective video methodology has limited experimental control and cannot assist in the search for early biomarkers.
Recently, however, 2 new approaches have been adopted to study autism prospectively that increase the chances of capturing symptoms significantly earlier in development and thus studying early biomarkers. The first is the infant-sibling approach, which is currently being used by several laboratories nationally. The second is a population-based screening approach developed by the first author and is termed the “1-Year Check-Up Approach” (1-YR CUA).
The infant-sibling approach: Studying autism as it occurs in multiplex families
The study of infant siblings of children with autism, as exemplified by Zwaigenbaum et al,10 is the predominant prospective approach currently in use. It has many of the major advantages of prospective studies, such as the ability to study young ages and to test hypotheses regarding early development in autism. The approach is based on the fact that mothers of children with autism have a 4% to 5% chance of giving birth to a second child who will develop ASD.81,82 As such, it has significant potential to reveal early emerging but subclinical biological and behavioral markers of autism and, in turn, guide the refinement of early diagnostic tools.
Despite the promise of the infant-sibling approach, it has some limitations. For example, given the sibling recurrence rate of about 4% to 5%, a study would have to recruit 100 pregnant mothers who already have a child with autism and follow their offspring in order to ultimately study 4 or 5 infants in that sample who will eventually develop ASD. Furthermore, because this design relies on identifying an already existing cohort of children with autism and following mothers from that cohort who get pregnant, only large and established laboratories—or collaborations among centers—are able to use this design to study infants at risk for autism.
It may also be important to consider that the infant-sibling approach researches autism only as it occurs in multiplex families. A recent study in Science suggests that the genetic mechanisms for autism as it occurs in multiplex families may be different from the mechanisms responsible for singleton cases.83 For example, Sebat and colleagues83 found that 10% of children with autism from singleton families had de novo gene copy number variations, in contrast to only 3% of children with autism from multiplex families and 1% of normal controls. Despite some limitations, the infant-sibling approach has the unique and major advantage of being able to gather data on infants before the first signs of autistic behavior appear. It also has the advantage of being able to gather data during the first year of life and has tremendous methodological control. (For a thorough review of the strengths and weaknesses of the infant-sibling approach, see Zwaigenbaum et al.84)
In the first study of this type, Zwaigenbaum and colleagues identified a cohort of 150 newborns who were born into a family with an existing child with ASD.10 Infants participated in assessments at 6, 12, and 24 months in age, although not all infants were tested at all ages. Of the 150 infants, 65 were followed to age 24 months; these infants were the focus of the original study. Assessments included: the Autism Observation Scale for Infants, an experimental measure designed to evaluate symptom severity in infants,60 a computer-based visual orienting task, a parent-report questionnaire that evaluates infant temperament,85 the Mullen Scales of Early Learning,86 and the MacArthur-Bates Communicative Development Inventories.87 The ADOS was used to assist in provisional diagnoses assigned at age 24 months.
Results from the Zwaigenbuam study, as well as a later baby-sibling study by Landa and colleagues,88 had one strong commonality: infants later diagnosed with ASD showed no statistically significant difference from typical infants prior to 1 year in age in assessment performance. The only exception was the temperament questionnaire, wherein parents reported increased passivity by as early as 6 months of age.10,88 Findings at age 6 months should be considered in light of the very small overall sample size and the fact that data were available for only 6 infants with ASD who eventually received a provisional diagnosis at age 24 months. Nonetheless, in contrast to findings at age 6 months, by age 1 year, infants later diagnosed as having ASD showed myriad atypicalities, in areas including eye contact, visual tracking, disengagement of visual attention, orienting to name, social smiling, reactivity, social interest, and affect. A subset of infants from this group study were described above as case studies65 and illustrated in FIGURE 2.
Population-based screening approaches: Studying autism as it occurs in simplex families
An alternative to the infant-sibling approach is one that attempts to capture and follow high-risk cases of ASD as they occur naturally in the population in order to reveal an early symptom profile. This method has the advantage of revealing autism as it presents itself to primary care physicians in the natural clinical setting. Moreover, in the event that there are differences in etiology and symptom onset in singleton vs multiplex cases, population-based screening approaches are essential. Similar to the infant-sibling approach, a population-based screening approach is labor intensive because it requires screening of thousands of infants. However, given the AAP’s recent recommendation for systematic screening at pediatric offices,76 the evaluation process can occur as part of the natural clinical procedure and need not introduce considerable additional effort for scientists or pediatricians.
The study of early potential markers of autism based on a population-screening approach has generally been used in the past to study autism between the ages of 18 and 24 months. Charman and colleagues,89 for example, studied a high-risk group based on failures of the M-CHAT at 18 months and found reduced social attention behaviors at 20 months in children later diagnosed as having ASD. Wetherby and colleagues90 followed 18 children younger than age 24 months from a pool of more than 3000 children screened who failed the Communication and Symbolic Behavior Scales Developmental Profile Infant-Toddler Checklist (CSBS-DP IT).90 This study revealed 9 “red flags” that differentiated children in the ASD group from both the developmentally delayed (DD) and typically developing groups, and included the following: (1) lack of appropriate gaze, (2) lack of warm, joyful expression with gaze, (3) lack of sharing enjoyment or interest, (4) lack of response to name, (5) lack of coordination of gaze, facial expression, gesture, and sound, (6) lack of showing toys or objects, (7) unusual prosody, (8) repetitive body movement, and (9) repetitive movement with objects.91 Although these early studies were certainly pioneering, biomarker information was not collected on affected infants.
The 1-Year Check-Up Approach
Systematic study of the youngest ages possible is required for the progression of early identification efforts, particularly if early biomarkers are to be discovered. Because clear signs of autism may not be present prior to 1 year of age but may appear to be emerging around that time, a variant of a population-based screening approach was developed by the first author and is currently in use at the Autism Center of Excellence at the University of California in San Diego (www.autismsandiego.org), where more than 10,000 infants have been screened to date. As suggested by the its name, this method is based on the fact that most babies see their pediatrician for a 1-year well-baby checkup. A first-pass developmental screen can be performed at that time and can be easily integrated into a standard 1-year check-up routine. Although a number of screening forms could potentially be used for this purpose, the CSBS-DP-IT90 was selected for the 1-YR CUA method in use at the ACE Center at UCSD. This tool was selected because it takes only 5 minutes for a caregiver to complete and it is a broad-spectrum instrument designed to detect communication and language delay rather than autism per se. Given that many children with autism have a history of early language difficulties, it is likely that this screen would also detect a significant number of infants who will eventually develop an ASD. It also has the added advantage of allowing scientists to study an ideal contrast group, namely, those who fail the screen because of a language or communication delay but do not have autism. Infants whose scores fall outside the normal range, regardless of underlying pathology, can be immediately referred for follow-up testing by trained specialists. Those babies who are judged by specialists to be at risk for developing ASD or other disorders can then participate in clinical, behavioral, biological, or treatment research studies and be followed longitudinally. Pediatricians are ideally suited for this role because of the close and important relationship they have with families during the first years of a baby’s life. Preliminary results of early efforts of the 1-YR CUA estimate that approximately 15% of true positives at age 12 months will receive an ASD diagnosis by the time they turn 3 years old.92
Although the 1-Yr CUA advocates screening all infants at 1 year of age to discover early behavioral and biomarkers, in clinical practice it is essential to rescreen infants at later ages such as at 18, 24, and 30 months. That is, because of the heterogeneity of symptoms and symptom onset patterns in this disorder, a percentage of infants will not test positive at 12 months and will be missed by the early screen. Infants who test positive at later ages due to a slow symptom onset or regression pattern represent important and distinct subgroups. Such subgroups may have unique etiologies and/or genetic underpinnings, and it will be important to compare and contrast these subgroups with infants who display an early symptom onset pattern.
As noted above, according to the first prospective infant sibling study of autism, the first statistically clear behavioral signs of autism appear at 12 months of age.10 Prior to 12 months, clear differences from typically developing infants have not yet been found.10 Thus, by training pediatricians to use the necessary screening tools at the 1-year check-up, it may be possible to catch the first signs of autism before the onset of glaring symptoms. Again, additional screening can also take place at later visits to identify late-onset cases that were missed by the 1-YR CUA.
The next frontier: Biomarkers as indicators of risk for autism
One promising approach that has yet to make headway in the field of autism is the supplementation of behavioral criteria with biological markers (or simply, biomarkers). Biomarkers have vast potential for improving diagnostic efficiency, as their more proximal placement relative to risk genes may allow them to more realistically reflect the translational consequences of these genes in an easily quantifiable, reliable, and objective manner. The identification of a reliable biomarker for ASD could be used to supplement and validate existing clinical methods, or to construct a more efficient diagnostic tool altogether that overcomes the limitations of clinical behavioral methods. For example, an understanding of an infant’s or toddler’s risk status based on a biomarker that is expressed at or even before the onset of symptoms might obviate the need to wait for behavioral criteria to be met before beginning treatment. This, in turn, would likely improve the prognosis of affected individuals.
Given the relatively late age of diagnosis of autism, past biological studies have necessarily examined abnormalities in older autistic children and in adults using MRI, functional magnetic resonance imaging (fMRI), gene expression, and other biological measures. Although it is essential to conduct such studies, most biological findings to date reflect developmental outcome stages 10 to 20 years after symptom onset and may not necessarily reflect early biological abnormalities.20 In fact, it is almost certain that gene expression patterns, concentrations of proteins, brain volume, etc, change across time with development and maturation in children with and without autism. However, at the same time, studies with older children and adults with autism can point toward key biological assays that can assist in early identification studies. Studies of early brain overgrowth in autism during the infancy and toddler periods, for example, were based on findings of large head size in older individuals with autism.
The pursuit of a biomarker for ASD dates back at least 20 years to the measurement of monoamine neurotransmitter metabolites in the urine, an effort that produced mixed results.93 Neurochemical profiling of blood, especially assessment of hyperserotonemia, received moderate support for a diagnostic role in autism. Overall, research concludes that serotonin levels are elevated in one-fourth of autistic individuals,94,95 and that brain serotonin synthesis is abnormal.96,97 Neurochemical profiling of cerebrospinal fluid similarly produced a strong initial candidate biomarker (ie, elevated levels of norepinephrine); however, this putative diagnostic indicator came to be considered more reflective of the effects of stressful clinical procedures rather than of the disorder itself.94 On the premise of autoimmune compromise in a subset of children with autism, other work has sought and found elevated urinary neopterin and biopterin levels in autistic children compared with age-matched control children. Of note, the unaffected biological relatives of these autistic children had urinary neopterin and biopterin levels that were intermediate to those found in the autistic and control children, further supporting the concept that these measures have potential utility as biomarkers for the disorder.98 Subsequently, elevated neopterin levels were observed in plasma from a small sample of children with autism (n=31), compared with age- and gender-matched controls (n=28).99 Despite this promise, neither urinary nor plasma neopterin levels are widely accepted as biomarkers for ASD; further exploration is required.
Each of these isolated approaches has failed to find a biomarker of universal relevance to all or even most individuals with ASD; indeed, none of these putative biomarkers serves nearly as well as ADOS or DSM-IV-TR criteria for accurately classifying patients with ASD and controls. The reasons for this are numerous and diverse. Most of the proposed measures are neither highly sensitive nor specific. Many of these procedures are also cumbersome, costly, and time intensive, thus precluding their use in everyday diagnostic scenarios for the foreseeable future. However, one of the most critical limitations shared by all of these approaches is their reliance on a single marker (or a very limited set of markers) to solve an extremely complex classification problem. Because no single biological factor may be either a necessary or sufficient index of ASD, no single marker should be expected to effectively differentiate all patients with ASD from all controls. This complexity has led our group and others to adopt a strategy of examining multiple characteristics simultaneously as one multivariate endophenotype of ASD.
One example of this comes from the examination of patterns of brain structure, which can be considered a putative biomarker that may represent the joint actions of a number of genes and environmental factors. Prior work from our group and others has demonstrated temporally divergent changes in overall brain volume in ASD, such that early brain overgrowth around the first year of life is later counteracted by an arrest of growth,20,100-104 as described above. The reasons for overgrowth are still unknown but could be linked to excessive numbers of neurons or a disproportional enlargement of white matter,25,105 particularly in the outer radiate portion.106 Two new studies that used diffusion tensor imaging (DTI) with children with ASD reported diverse white matter abnormalities, including an increased length in long-range association fibers107 and increased fractional anisotropy values108 in children with ASD. Structural brain imaging, however, is not yet considered a feasible biomarker technology for diagnostic purposes, largely due to pragmatic difficulties and the fact that not all babies at risk for ASD will exhibit this precise pattern of brain growth. As brain-imaging technologies become more efficient and less expensive, they may enter routine use during diagnostic procedures for ASD in combination with other biomarkers and/or behavioral characteristics.109
Recent evidence, both within the CNS as well as in peripheral blood samples, suggests that autism may involve immune system dysfunction.110 A recent example is the presence of neuroglial and innate neuroimmune system activation in brain tissue and cerebrospinal fluid of patients with autism.111 Several studies have also identified abnormalities in T-lymphocyte number and function, including abnormal accumulation of T lymphocytes in tissues such as the gastrointestinal tract,112 reduced numbers of circulating CD4+ T cells,113-115 and abnormal lymphocyte responsiveness.116-121 In particular, pro-inflammatory Th1 cytokines, such as tumor necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-1β, IL-6, and IL-12, have been found to be altered in ASD. The relative ease of pediatric phlebotomy and the relative accessibility of new bioassay technologies that can quickly and reliably assay plasma samples make the study of immune system function feasible.
Another promising area of biomarker research focuses on genes and their products. The specific factors that influence the development of ASD are unknown, but it is clear that genes play a large role in its causation122; thus, the pursuit of gene-based biomarkers is a quite reasonable approach to this research (TABLE). About 5% of autistic patients with autism have chromosomal abnormalities that may mediate monogenic or single majorgene effects. Up to 80% of patients with such cytogenetic abnormalities have duplications in chromosomal band 15q11-q13, where maternal transmission of the defect is implicated. Others have been found to have recessive genes (associated with synaptic pruning).123 However, the majority of molecular genetic research on ASD suggests that the disorder is not inherited in a Mendelian fashion; instead, multiple interacting genes as well as nongenetic (ie, environmental or epigenetic) factors are likely to be involved in up to 95% of cases of ASD.
TABLE. Replicated candidate gene biomarkers for autism.
| Locus | Marker |
|---|---|
| 15q11-q13 | Duplication |
| EN2 | rs1861972, A allele rs1861973, C allele |
| GABRB3 | 155CA-2, 87-bp allele |
| HOX1A | A218G, A allele |
| SLC25A12 | rs2056202, G(C) allele |
| MET | rs1858830 C allele |
In light of this complexity, methods such as linkage and association analysis have been used in an attempt to identify multiple interacting loci throughout the genome, each of which may have a small effect on an individual’s overall susceptibility to ASD. Consistent evidence for linkage has emerged on chromosome 7q,124,125 and association studies of functional and positional candidate genes for ASD also have identified several putative risk genes for the disorder. For example, EN2, which is the human homolog of the Drosophila melanogaster engrailed gene, was considered a functional candidate gene for ASD because it encodes a protein involved in cerebellar developmental patterning. Cerebellar defects have been long associated with autism.126 The gene was found to be associated with ASD in 3 separate samples studied by one group and was estimated to be involved in up to 40% of cases.127 Also, genes such as UBE3A (which codes for ubiquitin protein ligase E3A) and GABRB3 (which codes for gamma-aminobutyric acid [GABA] receptor γ 3) have been identified as positional candidate genes because they map to the common duplication site on chromosome 15q11-q13, and each gene has been found to be associated with ASD. Finally, genetic association of a common C allele in the promoter region of the pleiotropic MET gene was found in 204 autism families and replicated in a larger sample of 539 autism families.128 MET signaling has been shown to be involved in cortical and cerebellar growth as well as immune function and gastrointestinal repair.
Genetic linkage and association analyses are thus making great headway in unraveling etiologic contributions to ASD and have even begun to expand to include environmentally responsive genes in traditional linkage searches.129 Yet, despite all of the promise and potential of DNA variants to explain the substantial portion of risk for ASD that is attributable to hereditary factors, one aspect of associated genetic polymorphisms that limits their utility as true biomarkers is their time constancy. Thus, whether inherited or acquired, genetic polymorphisms are static factors that do not change throughout the life course of an individual. In contrast, gene expression is highly flexible and can vary over time within the same individual. These qualities afford gene expression greater potential to serve as a useful biomarker for various stages of ASD, such as the aymptomatic atrisk period and early periods of subtle deficit, and for aspects of ASD such as chronic disorder, remission, and treatment responsiveness or nonresponsiveness.
The advent of microarrays that can survey the entire expressed genome (ie, the transcriptome) has made it possible to simultaneously investigate the roles of several thousand genes in a disorder. Gene expression can reflect genetic or environmental influences or both. As such, it may be particularly useful for identifying risk factors for a complex disorder such as ASD that, in many cases, is thought to have a multifactorial polygenic etiology in which many genes and environmental factors interact. However, there may also be cases in which strictly genetic or environmental causes could be identified, and it is by no means a requirement that the effects of causal genetic or environmental influences be reflected in gene expression. Nevertheless, preliminary studies in this domain suggest that there are reproducible gene expression changes in ASD.
By studying monozygotic twins who were genetically identical but discordant for ASD, Hu et al130 identified potential biomarkers in blood cells based on differential gene expression levels. Some of the strongest candidate biomarkers to emerge included neurologically relevant genes such as argininosuccinate synthetase 1 (ASS1) and N-acetylglucosaminidase alpha (NAGLU), each of which was increased in the twin with ASD relative to the unaffected twin. Subsequent blood-based gene expression studies of children with ASD and their unaffected family members identified broader changes in families of genes involved in signal transduction, metabolism, cell growth and maintenance, response to external stimuli, and development.131 Recently, case-control analyses of children without an ASD and children with an ASD due to a fragile X mutation (FMR1-FM) or a 15q11-q13 duplication identified 68 genes that were dysregulated in cultured blood cells of both autism with FMR1-FM and autism with dup(15q).19 Of note, one of the most upregulated genes in patients with ASD with dup(15q) was the cytoplasmic FMR1 interacting protein 1 (CYFIP1), which may represent a potential molecular link between FMR1-FM and dup(15q).
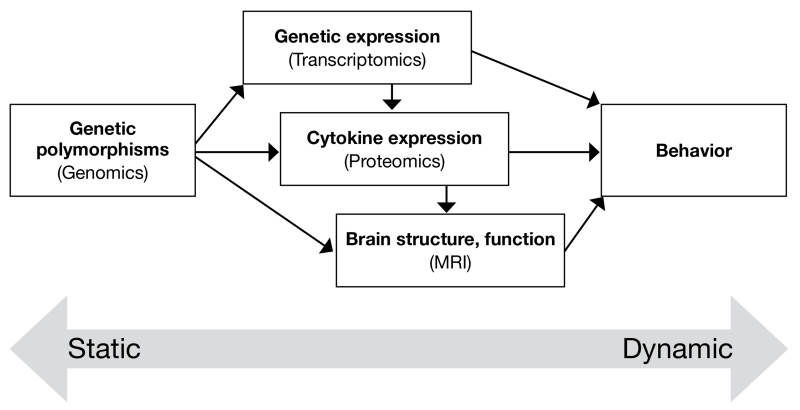
The pursuit of brain- and blood-based biomarkers for ASD is still in its infancy, but the early successes highlighted above provide the impetus for further investigation in this area. Critically, longitudinal and prospective studies of children with ASD, and those at risk for the disorder, should be undertaken to identify the most sensitive and specific biomarker profiles possible. For example, in our own work, we are surveying the expression of potential biomarkers (including DNA polymorphisms, cytokines, mRNA gene expression levels, and brain structural and functional indices) in children as young as 12 months old who are identified as being at risk for ASD, and performing annual reassessments of these markers throughout the crucial first years of life. In this manner, we can retrospectively identify those biomarkers that were dysregulated very early in children who ultimately went on to develop ASD or other developmental disorder. An important lesson learned from prior efforts to develop biomarkers for psychiatric disorders is that no single tissue, molecule, or marker is likely to yield sufficient power for improving existing behavioral classification schemes; however, integration of the best markers across modalities (schematically illustrated in FIGURE 4) may lead to highly reliable risk profiles, which then can be used to begin appropriate, group-tailored (ultimately, personalized) interventions. Beyond this working model, it may also be necessary to introduce an iterative component to address the phenotypic heterogeneity of the ASDs, which potentially may map onto heterogeneity in etiologic and biomarker profiles. Thus, as influential etiologic factors and their associated biomarkers are identified, segments of the larger ASD phenotype may be “carved out,” some of which are influenced by that factor and some of which are not (eg, regressive vs typical-onset ASD, or passive and aloof vs active and odd behavior). The subsequent detection of both environmental and biological influences on ASD and biomarkers of ASD within these subgroups will be facilitated by their relative homogeneity; however, additional phenotypic cleavage points should be anticipated until groups with a highly similar etiologic and biomarker profile are obtained.
FIGURE 4. Model for prediction of risk for ASD.
ASD: autism spectrum disorder.
This drawing represents different types of predictors of the risk for ASD as well as the relationship between them. These predictors, which range from the static (such as DNA) to the dynamic (such as behavior) must be integrated into a multidimensional model of risk prediction, as no single type of predictor is likely to account for all of the variance in which individuals do or do not become affected with ASD. When considered together, diverse characteristics, such as those illustrated in this model, may provide the best “fit” (or “fits”) for the observed patterns of ASD in families and in the population.
Source: Modified with permission from Ozdemir V, Williams-Jones B, Glatt SJ, et al. Shifting emphasis from pharmacogenomics to theragnostics. Nat Biotechnology. 2006;24:942-946.
CONCLUSION
Early recognition and diagnosis of children with ASD is essential because expeditious behavioral and educational interventions, including referral to a formal early intervention program, can improve the prognosis, especially for cognition, peer interactions, and the development of language.132 Knowing that a child has a specific diagnosis and is receiving therapy also can help a family to cope better; not knowing the child’s diagnosis increases parental anxiety and delays the introduction of interventions that can reduce behavioral problems and optimize outcomes in the child. In addition, since having one child with autism increases the risk that siblings also will have a disorder on the autism spectrum or with the broader phenotype, early identification could prompt parents to receive genetic counseling and allow them to plan for future children.
Analyses of case studies of infants later diagnosed with ASD show that abnormalities in social behavior are not clearly present within the first 6 months of life and, for some children, not until 2 years of age. This highlights the need for early identification efforts to go beyond the clinical domain, including, at some level, the addition of biomarkers.
Studies that attempt to join biology with behavior need to target the earliest ages if they are to be helpful for early identification. Prospective studies such as those that use the Baby Sibling or 1-YR CUA are imperative for this mission. Additionally, scientific evidence suggests that genetic and/or environmental factors affecting children that come from multiplex vs simplex cases might be different,128,133 and this should be taken into consideration for future research.
The progression of biomarker research in ASD mirrors that of other neurologic disorders in that it is currently in its infancy and marked largely by discovery rather than validation. As such, estimates of specificity, sensitivity, likelihood ratios, and predictive values of the reviewed biomarkers have not been routinely evaluated or reported. This represents an area of desperate need for future studies, especially replication studies predicated on the results reported here and elsewhere.
ACKNOWLEDGEMENTS
Support for this paper was provided by grants from Cure Autism Now and the National Institutes of Health (NIH) (1 R01 MH080134-01A1) awarded to Karen Pierce and an Autism Center of Excellence Grant from the NIH (P 50 MH081755-01).
Footnotes
DISCLOSURES:The authors report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Contributor Information
Karen Pierce, Department of Neurosciences University of California, San Diego San Diego, CA, USA.
Stephen J. Glatt, Department of Psychiatry and Behavioral Sciences Medical Genetics Research Center SUNY Upstate Medical University Syracuse, NY, USA.
Gregory S. Liptak, Department of Pediatrics SUNY Upstate Medical University Syracuse, NY, USA.
Laura Lee McIntyre, Department of Psychology Syracuse University Syracuse, NY, USA.
REFERENCES
- 1.Dawson G. Early behavioral intervention, brain plasticity, and the prevention of autism spectrum disorder. Dev Psychopathol. 2008;20:775–803. doi: 10.1017/S0954579408000370. [DOI] [PubMed] [Google Scholar]
- 2.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- 3.Spires TL, Grote HE, Varshney NK, et al. Environmental enrichment rescues protein deficits in a mouse model of Huntington’s disease, indicating a possible disease mechanism. J Neurosci. 2004;24:2270–2276. doi: 10.1523/JNEUROSCI.1658-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovaas OI. Behavioral treatment and normal educational and intellectual functioning in young autistic children. J Consult Clin Psychol. 1987;55:3–9. doi: 10.1037//0022-006x.55.1.3. [DOI] [PubMed] [Google Scholar]
- 5.Sallows GO, Graupner TD. Intensive behavioral treatment for children with autism: four-year outcome and predictors. Am J Ment Retard. 2005;110:417–438. doi: 10.1352/0895-8017(2005)110[417:IBTFCW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Sheinkopf SJ, Siegel B. Home-based behavioral treatment of young children with autism. J Autism Dev Disord. 1998;28:15–23. doi: 10.1023/a:1026054701472. [DOI] [PubMed] [Google Scholar]
- 7.Magiati I, Charman T, Howlin P. A two-year prospective follow-up study of community-based early intensive behavioural intervention and specialist nursery provision for children with autism spectrum disorders. J Child Psychol Psychiatry. 2007;48:803–812. doi: 10.1111/j.1469-7610.2007.01756.x. [DOI] [PubMed] [Google Scholar]
- 8.Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children: confirmation of high prevalence. Am J Psychiatry. 2005;162:1133–1141. doi: 10.1176/appi.ajp.162.6.1133. [DOI] [PubMed] [Google Scholar]
- 9.Baghdadli A, Picot MC, Pascal C, et al. Relationship between age of recognition of first disturbances and severity in young children with autism. Eur Child Adolesc Psychiatry. 2003;12:122–127. doi: 10.1007/s00787-003-0314-6. [DOI] [PubMed] [Google Scholar]
- 10.Zwaigenbaum L, Bryson S, Rogers T, et al. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Larsson HJ, Eaton WW, Madsen KM, et al. Risk factors for autism: perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiol. 2005;161:916–925. doi: 10.1093/aje/kwi123. discussion 926-918. [DOI] [PubMed] [Google Scholar]
- 12.Maestro S, Muratori F, Cesari A, et al. A view to regressive autism through home movies. Is early development really normal? Acta Psychiatr Scand. 2006;113:68–72. doi: 10.1111/j.1600-0447.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 13.Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. J Autism Dev Disord. 1994;24:247–257. doi: 10.1007/BF02172225. [DOI] [PubMed] [Google Scholar]
- 14.Maestro S, Casella C, Milone A, et al. Study of the onset of autism through home movies. Psychopathology. 1999;32:292–300. doi: 10.1159/000029102. [DOI] [PubMed] [Google Scholar]
- 15.Baranek GT. Autism during infancy: a retrospective video analysis of sensory-motor and social behaviors at 9-12 months of age. J Autism Dev Disord. 1999;29:213–224. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- 16.Werner E, Dawson G, Munson J, et al. Variation in early developmental course in autism and its relation with behavioral outcome at 3-4 years of age. J Autism Dev Disord. 2005;35:337–350. doi: 10.1007/s10803-005-3301-6. [DOI] [PubMed] [Google Scholar]
- 17.Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- 18.Cohen MM., Jr. Mental deficiency, alterations in performance, and CNS abnormalities in overgrowth syndromes. Am J Med Genet C Semin Med Genet. 2003;117:49–56. doi: 10.1002/ajmg.c.10013. [DOI] [PubMed] [Google Scholar]
- 19.Gordon N. Canavan disease: a review of recent developments. Eur J Paediatr Neurol. 2001;5:65–69. doi: 10.1053/ejpn.2001.0467. [DOI] [PubMed] [Google Scholar]
- 20.Courchesne E, Pierce K, Schumann CM, et al. Mapping early brain development in autism. Neuron. 2007;56:399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Dementieva YA, Vance DD, Donnelly SL, et al. Accelerated head growth in early development of individuals with autism. Pediatr Neurol. 2005;32:102–108. doi: 10.1016/j.pediatrneurol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Dissanayake C, Bui QM, Huggins R, et al. Growth in stature and head circumference in high-functioning autism and Asperger disorder during the first 3 years of life. Dev Psychopathol. 2006;18:381–393. doi: 10.1017/S0954579406060202. [DOI] [PubMed] [Google Scholar]
- 23.Hazlett HC, Poe M, Gerig G, et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- 24.Dawson G, Munson J, Webb SJ, et al. Rate of head growth decelerates and symptoms worsen in the second year of life in autism. Biol Psychiatry. 2007;61:458–464. doi: 10.1016/j.biopsych.2006.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Courchesne E, Karns C, Davis HR, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 26.Carper RA, Moses P, Tigue ZD, et al. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- 27.Sparks BF, Friedman SD, Shaw DW, et al. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- 28.Nelson KB, Grether JK, Croen LA, et al. Neuropeptides and neurotrophins in neonatal blood of children with autism or mental retardation. Ann Neurol. 2001;49:597–606. [PubMed] [Google Scholar]
- 29.Hobbs K, Kennedy A, Dubray M, et al. A retrospective fetal ultrasound study of brain size in autism. Biol Psychiatry. 2007;62:1048–1055. doi: 10.1016/j.biopsych.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 30.Bauman M. Brief report: neuroanatomic observations of the brain in pervasive developmental disorders. J Autism Dev Disord. 1996;26:199–203. doi: 10.1007/BF02172012. [DOI] [PubMed] [Google Scholar]
- 31.Bauman M, Kemper TL. Histoanatomic observations of the brain in early infantile autism. Neurology. 1985;35:866–875. doi: 10.1212/wnl.35.6.866. [DOI] [PubMed] [Google Scholar]
- 32.Bailey A, Luthert P, Dean A, et al. A clinicopathological study of autism. Brain. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- 33.Landa RJ, Holman KC, Garrett-Mayer E. Social and communication development in toddlers with early and later diagnosis of autism spectrum disorders. Arch Gen Psychiatry. 2007;64:853–864. doi: 10.1001/archpsyc.64.7.853. [DOI] [PubMed] [Google Scholar]
- 34.Niehus R, Lord C. Early medical history of children with autism spectrum disorders. J Dev Behav Pediatr. 2006;27:S120–S127. doi: 10.1097/00004703-200604002-00010. [DOI] [PubMed] [Google Scholar]
- 35.Johnson CP, Myers SM, the Council on Children with Disabilities Identification and evaluation of children with autism spectrum disorders. Pediatrics. 2007;120:1183–1215. doi: 10.1542/peds.2007-2361. [DOI] [PubMed] [Google Scholar]
- 36.Diagnostic and statistical manual of mental disorders. 4th edition. American Psychiatric Association; Washington, DC: 2000. text revision. [Google Scholar]
- 37.Gutierrez GC, Smalley SL, Tanguay PE. Autism in tuberous sclerosis complex. J Autism Dev Disord. 1998;28:97–103. doi: 10.1023/a:1026032413811. [DOI] [PubMed] [Google Scholar]
- 38.Curatolo P. Tuberous sclerosis: genes, brain, and behaviour. Dev Med Child Neurol. 2006;48:404. doi: 10.1017/S0012162206000880. [DOI] [PubMed] [Google Scholar]
- 39.Wong V. Study of the relationship between tuberous sclerosis complex and autistic disorder. J Child Neurol. 2006;21:199–204. doi: 10.2310/7010.2006.00046. [DOI] [PubMed] [Google Scholar]
- 40.Bailey A, Bolton P, Butler L, et al. Prevalence of the fragile X anomaly amongst autistic twins and singletons. J Child Psychol Psychiatry. 1993;34:673–688. doi: 10.1111/j.1469-7610.1993.tb01064.x. [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto O, Shimizu Y, Kawasaki Y. Brief report: low frequency of the fragile X syndrome among Japanese autistic subjects. J Autism Dev Disord. 1993;23:201–209. doi: 10.1007/BF01066429. [DOI] [PubMed] [Google Scholar]
- 42.Klauck SM, Münstermann E, Bieber-Martig B, et al. Molecular genetic analysis of the FMR-1 gene in a large collection of autistic patients. Hum Genet. 1997;100:224–229. doi: 10.1007/s004390050495. [DOI] [PubMed] [Google Scholar]
- 43.Hyman SL, Towbin KE. Autism spectrum disorders. In: Batshaw ML, Pellegrino L, Roizen NJ, editors. Children with disabilities. 6th ed. Brookes; Baltimore, MD: 2007. pp. 325–343. [Google Scholar]
- 44.Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children. JAMA. 2001;285:3093–3099. doi: 10.1001/jama.285.24.3093. [DOI] [PubMed] [Google Scholar]
- 45.Edelson MG. Are the majority of children with autism mentally retarded?: a systematic evaluation of the data. Focus on Autism and Other Developmental Disabilities. 2006;21:66–83. [Google Scholar]
- 46.Wiggins LD, Baio J, Rice C. Examination of the time between first evaluation and first autism spectrum diagnosis in a population based sample. J Dev Behav Pediatr. 2006;27:S79–S87. doi: 10.1097/00004703-200604002-00005. [DOI] [PubMed] [Google Scholar]
- 47.Sivberg B. Parents’ detection of early signs in their children having an autism spectrum disorder. J Pediatar Nurs. 2003;18:433–439. doi: 10.1016/s0882-5963(03)00139-8. [DOI] [PubMed] [Google Scholar]
- 48.Sices L, Feudtner C, McLaughlin J, et al. How do primary care physicians identify young children with developmental delays? A national survey. J Dev Behav Pediatr. 2003;24:409–417. doi: 10.1097/00004703-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 49.Dosreis S, Weiner CL, Johnson L, et al. Autism spectrum disorder screening and management practices among general pediatric providers. J Dev Behav Pediatr. 2006;27:S88–S94. doi: 10.1097/00004703-200604002-00006. [DOI] [PubMed] [Google Scholar]
- 50.Baron-Cohen S, Allen J, Gillberg C. Can autism be detected at 18 months? The needle, the haystack, and the CHAT. Br J Psychiatry. 1992;161:839–843. doi: 10.1192/bjp.161.6.839. [DOI] [PubMed] [Google Scholar]
- 51.Robins DL, Fein D, Barton ML, et al. The Modified Checklist for Autism in Toddlers: an initial study investigating the early detection of autism and pervasive developmental disorders. J Autism Dev Disord. 2001;31:131–144. doi: 10.1023/a:1010738829569. [DOI] [PubMed] [Google Scholar]
- 52.Seif Eldin A, Habib D, Noufal A, et al. Use of M-CHAT for a multinational screening of young children with autism in the Arab countries. Int Rev Psychiatry. 2008;20:281–289. doi: 10.1080/09540260801990324. [DOI] [PubMed] [Google Scholar]
- 53.Wong V, Hui LH, Lee WC, et al. A modified screening tool for autism (Checklist for Autism in Toddlers [CHAT-23]) for Chinese children. Pediatrics. 2004;114:e166–176. doi: 10.1542/peds.114.2.e166. [DOI] [PubMed] [Google Scholar]
- 54.Dietz C, Swinkels S, van Daalen E, et al. Screening for autistic spectrum disorder in children aged 14 to 15 months. II: population screening with the Early Screening of Autistic Traits Questionnaire (ESAT). Design and general findings. J Autism Dev Disord. 2006;36:713–722. doi: 10.1007/s10803-006-0114-1. [DOI] [PubMed] [Google Scholar]
- 55.Swinkels SH, Dietz C, van Daalen E, et al. Screening for autistic spectrum in children aged 14 to 15 months. I: the development of the Early Screening of Autistic Traits Questionnaire (ESAT) J Autism Dev Disord. 2006;36:723–732. doi: 10.1007/s10803-006-0115-0. [DOI] [PubMed] [Google Scholar]
- 56.Siegel B. The pervasive developmental disorders screening test-II. Harcourt Assessment; San Antonio, TX: 2004. [Google Scholar]
- 57.Gotham K, Risi S, Dawson G, et al. A replication of the Autism Diagnostic Observation Schedule (ADOS) revised algorithms. J Am Acad Child Adolesc Psychiatry. 2008;47:642–651. doi: 10.1097/CHI.0b013e31816bffb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gotham K, Risi S, Pickles A, et al. The Autism Diagnostic Observation Schedule: revised algorithms for improved diagnostic validity. J Autism Dev Disord. 2007;37:613–627. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- 59.Luyster R, Gotham K, Guthrie D, et al. The autism diagnostic observation schedule—toddler module: a new module of a standardized diagnostic measure for autism spectrum disorder. J Autism Dev Disord. doi: 10.1007/s10803-009-0746-z. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bryson SE, Zwaigenbaum L, McDermott C, et al. The Autism Observation Scale for Infants: scale development and reliability data. J Autism Dev Disord. 2008;38:731–738. doi: 10.1007/s10803-007-0440-y. [DOI] [PubMed] [Google Scholar]
- 61.Filipek PA, Accardo PJ, Ashwal S, et al. Practice parameter: screening and diagnosis of autism: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Child Neurology Society. Neurology. 2000;55:468–479. doi: 10.1212/wnl.55.4.468. [DOI] [PubMed] [Google Scholar]
- 62.Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4:165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- 63.Calder AJ, Beaver JD, Winston JS, et al. Separate coding of different gaze directions in the superior temporal sulcus and inferior parietal lobule. Curr Biol. 2007;17:20–25. doi: 10.1016/j.cub.2006.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dawson G, Osterling J, Meltzoff AN, et al. Case study of the development of an infant with autism from birth to two years of age. J Appl Dev Psychol. 2000;21:299–313. doi: 10.1016/S0193-3973(99)00042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bryson SE, Zwaigenbaum L, Brian J, et al. A prospective case series of high-risk infants who developed autism. J Autism Dev Disord. 2007;37:12–24. doi: 10.1007/s10803-006-0328-2. [DOI] [PubMed] [Google Scholar]
- 66.Lord C, Risi S, Lambrecht L, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 67.Osterling JA, Dawson G, Munson JA. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Dev Psychopathol. 2002;14:239–251. doi: 10.1017/s0954579402002031. [DOI] [PubMed] [Google Scholar]
- 68.Colgan SE, Lanter E, McComish C, et al. Analysis of social interaction gestures in infants with autism. Child Neuropsychol. 2006;12:307–319. doi: 10.1080/09297040600701360. [DOI] [PubMed] [Google Scholar]
- 69.Maestro S, Muratori F, Cavallaro MC, et al. Attentional skills during the first 6 months of age in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2002;41:1239–1245. doi: 10.1097/00004583-200210000-00014. [DOI] [PubMed] [Google Scholar]
- 70.Maestro S, Muratori F, Barbieri F, et al. Early behavioral development in autistic children: the first 2 years of life through home movies. Psychopathology. 2001;34:147–152. doi: 10.1159/000049298. [DOI] [PubMed] [Google Scholar]
- 71.Anisfeld M. Only tongue protrusion modeling is matched by neonates. Developmental Review. 1996;16:149–161. [Google Scholar]
- 72.Johnson MH, Dziurawiec S, Ellis H, et al. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40:1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- 73.Kawakamia K, Takai-Kawakami K, Tomonaga M, et al. Spontaneous smile and spontaneous laugh: an intensive longitudinal case study. Infant Behav Dev. 2007;30:146–152. doi: 10.1016/j.infbeh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 74.Jones SS, Hong HW. Onset of voluntary communication: smiling looks to mother. Infancy. 2001;2:353–370. doi: 10.1207/S15327078IN0203_4. [DOI] [PubMed] [Google Scholar]
- 75.Hunnius S. The early development of visual attention and its implications for social and cognitive development. Prog Brain Res. 2007;164:187–209. doi: 10.1016/S0079-6123(07)64010-2. [DOI] [PubMed] [Google Scholar]
- 76.Johnson MH, Griffin R, Csibra G, et al. The emergence of the social brain network: evidence from typical and atypical development. Dev Psychopathol. 2005;17:599–619. doi: 10.1017/S0954579405050297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Redcay E, Haist F, Courchesne E. Functional neuroimaging of speech perception during a pivotal period of language acquisition. Developmental Science. doi: 10.1111/j.1467-7687.2008.00674.x. In press. [DOI] [PubMed] [Google Scholar]
- 78.McCaffery P, Deutsch CK. Macrocephaly and the control of brain growth in autistic disorders. Prog Neurobiol. 2005;77:38–56. doi: 10.1016/j.pneurobio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 79.Fatemi SH, Earle J, Kanodia R, et al. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol. 2002;22:25–33. doi: 10.1023/A:1015337611258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Werner E, Dawson G. Validation of the phenomenon of autistic regression using home videotapes. Arch Gen Psychiatry. 2005;62:889–895. doi: 10.1001/archpsyc.62.8.889. [DOI] [PubMed] [Google Scholar]
- 81.Jorde LB, Hasstedt SJ, Ritvo ER, et al. Complex segregation analysis of autism. Am J Hum Genet. 1991;49:932–938. [PMC free article] [PubMed] [Google Scholar]
- 82.Szatmari P, Jones MB, Zwaigenbaum L, et al. Genetics of autism: overview and new directions. J Autism Dev Disord. 1998;28:351–368. doi: 10.1023/a:1026096203946. [DOI] [PubMed] [Google Scholar]
- 83.Sebat J, Lakshmi B, Malhotra D, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zwaigenbaum L, Thurm A, Stone W, et al. Studying the emergence of autism spectrum disorders in high-risk infants: methodological and practical issues. J Autism Dev Disord. 2007;37:466–480. doi: 10.1007/s10803-006-0179-x. [DOI] [PubMed] [Google Scholar]
- 85.Rothbart M. Measurement of temperament in infancy. Child Development. 1981;52:569–578. [Google Scholar]
- 86.Mullen EM. In: Mullen scales of early learning. AGS, editor. American Guidance Service Inc.; Circle Pines, MN: 1995. [Google Scholar]
- 87.Fenson L. MacArthur Communicative Development Inventories (Cdis): user’s guide and technical manual. Singular Publishing Group; San Diego, CA: 1993. [Google Scholar]
- 88.Landa R, Garrett-Mayer E. Development in infants with autism spectrum disorders: a prospective study. J Child Psychol Psychiatry. 2006;47:629–638. doi: 10.1111/j.1469-7610.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- 89.Charman T, Swettenham J, Baron-Cohen S, et al. Infants with autism: an investigation of empathy, pretend play, joint attention, and imitation. Dev Psychol. 1997;33:781–789. doi: 10.1037//0012-1649.33.5.781. [DOI] [PubMed] [Google Scholar]
- 90.Wetherby AM, Prizant BM. Communication and symbolic behavior scales developmental profile: first normed edition. Paul H. Brookes; Baltimore, MD: 2002. [Google Scholar]
- 91.Wetherby AM, Woods J, Allen L, et al. Early indicators of autism spectrum disorders in the second year of life. J Autism Dev Disord. 2004;34:473–493. doi: 10.1007/s10803-004-2544-y. [DOI] [PubMed] [Google Scholar]
- 92.Pierce K, Carter C, Weinfeld M, et al. Identifying and studying autism between 12-17 months: results from the 1-Year Well-Baby Check-Up approach; Paper presented at: International Meeting for Autism Research; San Diego, CA. May 7-9, 2009. [Google Scholar]
- 93.Martineau J, Hérault J, Petit E, et al. Catecholaminergic metabolism and autism. Dev Med Child Neurol. 1994;36:688–697. doi: 10.1111/j.1469-8749.1994.tb11911.x. [DOI] [PubMed] [Google Scholar]
- 94.Lam KS, Aman MG, Arnold LE. Neurochemical correlates of autistic disorder: a review of the literature. Res Dev Disabil. 2006;27:254–289. doi: 10.1016/j.ridd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 95.Cook EH. Autism: review of neurochemical investigation. Synapse. 1990;6:292–308. doi: 10.1002/syn.890060309. [DOI] [PubMed] [Google Scholar]
- 96.Chugani DC, Muzik O, Rothermel R, et al. Altered serotonin synthesis in the dentatothalamocortical pathway in autistic boys. Ann Neurol. 1997;42:666–669. doi: 10.1002/ana.410420420. [DOI] [PubMed] [Google Scholar]
- 97.Chugani DC, Muzik O, Behen M, et al. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Ann Neurol. 1999;45:287–295. doi: 10.1002/1531-8249(199903)45:3<287::aid-ana3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 98.Messahel S, Pheasant AE, Pall H, et al. Urinary levels of neopterin and biopterin in autism. Neurosci Lett. 1998;241:17–20. doi: 10.1016/s0304-3940(97)00976-2. [DOI] [PubMed] [Google Scholar]
- 99.Sweeten TL, Posey DJ, McDougle CJ. High blood monocyte counts and neopterin levels in children with autistic disorder. Am J Psychiatry. 2003;160:1691–1693. doi: 10.1176/appi.ajp.160.9.1691. [DOI] [PubMed] [Google Scholar]
- 100.Lainhart JE. Advances in autism neuroimaging research for the clinician and geneticist. Am J Med Genet C Semin Med Genet. 2006;142:33–39. doi: 10.1002/ajmg.c.30080. [DOI] [PubMed] [Google Scholar]
- 101.Carper RA, Courchesne E. Localized enlargement of the frontal cortex in early autism. Biol Psychiatry. 2005;57:126–133. doi: 10.1016/j.biopsych.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 102.Courchesne E. Brain development in autism: early overgrowth followed by premature arrest of growth. Ment Retard Dev Disabil Res Rev. 2004;10:106–111. doi: 10.1002/mrdd.20020. [DOI] [PubMed] [Google Scholar]
- 103.Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- 104.Courchesne E, Pierce K. Brain overgrowth in autism during a critical time in development: implications for frontal pyramidal neuron and interneuron development and connectivity. Int J Dev Neurosci. 2005;23:153–170. doi: 10.1016/j.ijdevneu.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 105.Herbert MR, Ziegler DA, Deutsch CK, et al. Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain. 2003;126:1182–1192. doi: 10.1093/brain/awg110. [DOI] [PubMed] [Google Scholar]
- 106.Herbert MR, Ziegler DA, Makris N, et al. Localization of white matter volume increase in autism and developmental language disorder. Ann Neurol. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- 107.Sundaram SK, Kumar A, Makki MI, et al. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb Cortex. 2008;18:2659–2665. doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ben Bashat D, Kronfeld-Duenias V, Zachor DA, et al. Accelerated maturation of white matter in young children with autism: a high b value DWI study. Neuroimage. 2007;37:40–47. doi: 10.1016/j.neuroimage.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 109.Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 110.Pardo CA, Vargas DL, Zimmerman AW. Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry. 2005;17:485–495. doi: 10.1080/02646830500381930. [DOI] [PubMed] [Google Scholar]
- 111.Vargas DL, Nascimbene C, Krishnan C, et al. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 112.Ashwood P, Anthony A, Pellicer AA, et al. Intestinal lymphocyte populations in children with regressive autism: evidence for extensive mucosal immunopathology. J Clin Immunol. 2003;23:504–517. doi: 10.1023/b:joci.0000010427.05143.bb. [DOI] [PubMed] [Google Scholar]
- 113.Fiumara A, Sciotto A, Barone R, et al. Peripheral lymphocyte subsets and other immune aspects in Rett syndrome. Pediatr Neurol. 1999;21:619–621. doi: 10.1016/s0887-8994(99)00053-3. [DOI] [PubMed] [Google Scholar]
- 114.Warren RP, Cole P, Odell JD, et al. Detection of maternal antibodies in infantile autism. J Am Acad Child Adolesc Psychiatry. 1990;29:873–877. doi: 10.1097/00004583-199011000-00005. [DOI] [PubMed] [Google Scholar]
- 115.Yonk LJ, Warren RP, Burger RA, et al. CD4+ helper T cell depression in autism. Immunol Lett. 1990;25:341–345. doi: 10.1016/0165-2478(90)90205-5. [DOI] [PubMed] [Google Scholar]
- 116.Ashwood P, Wakefield AJ. Immune activation of peripheral blood and mucosal CD3(+) lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms. J Neuroimmunol. 2006;173:126–134. doi: 10.1016/j.jneuroim.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 117.Plioplys AV, Greaves A, Kazemi K, et al. Lymphocyte function in autism and Rett syndrome. Neuropsychobiology. 1994;29:12–16. doi: 10.1159/000119056. [DOI] [PubMed] [Google Scholar]
- 118.Stubbs EG, Crawford ML. Depressed lymphocyte responsiveness in autistic children. J Autism Child Schizophr. 1977;7:49–55. doi: 10.1007/BF01531114. [DOI] [PubMed] [Google Scholar]
- 119.Warren RP, Margaretten NC, Pace NC, et al. Immune abnormalities in patients with autism. J Autism Dev Disord. 1986;16:189–197. doi: 10.1007/BF01531729. [DOI] [PubMed] [Google Scholar]
- 120.Molloy CA, Morrow AL, Meinzen-Derr J, et al. Familial autoimmune thyroid disease as a risk factor for regression in children with autism spectrum disorder: A CPEA Study. J Autism Dev Disord. 2006;36:317–324. doi: 10.1007/s10803-005-0071-0. [DOI] [PubMed] [Google Scholar]
- 121.Zimmerman AW, Jyonouchi H, Comi AM, et al. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr Neurol. 2005;33:195–201. doi: 10.1016/j.pediatrneurol.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 122.Bailey A, Le Couteur A, Gottesman I, et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- 123.Morrow EM, Yoo SY, Flavell SW, et al. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321:218–223. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Badner JA, Gershon ES. Regional meta-analysis of published data supports linkage of autism with markers on chromosome 7. Mol Psychiatry. 2002;7:56–66. doi: 10.1038/sj.mp.4000922. [DOI] [PubMed] [Google Scholar]
- 125.Trikalinos TA, Karvouni A, Zintzaras E, et al. A heterogeneity-based genome search meta-analysis for autism-spectrum disorders. Mol Psychiatry. 2006;11:29–36. doi: 10.1038/sj.mp.4001750. [DOI] [PubMed] [Google Scholar]
- 126.Courchesne E, Yeung-Courchesne R, Press GA, et al. Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med. 1988;318:1349–1354. doi: 10.1056/NEJM198805263182102. [DOI] [PubMed] [Google Scholar]
- 127.Benayed R, Gharani N, Rossman I, et al. Support for the homeobox transcription factor gene ENGRAILED 2 as an autism spectrum disorder susceptibility locus. Am J Hum Genet. 2005;77:851–868. doi: 10.1086/497705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Campbell DB, Sutcliffe JS, Ebert PJ, et al. A genetic variant that disrupts MET transcription is associated with autism. Proc Natl Acad Sci U S A. 2006;103:16834–16839. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Herbert MR, Russo JP, Yang S, et al. Autism and environmental genomics. Neurotoxicology. 2006;27:671–684. doi: 10.1016/j.neuro.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 130.Hu VW, Frank BC, Heine S, et al. Gene expression profiling of lymphoblastoid cell lines from monozygotic twins discordant in severity of autism reveals differential regulation of neurologically relevant genes. BMC Genomics. 2006;7:118. doi: 10.1186/1471-2164-7-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Baron CA, Liu SY, Hicks C, et al. Utilization of lymphoblastoid cell lines as a system for the molecular modeling of autism. J Autism Dev Disord. 2006;36:973–982. doi: 10.1007/s10803-006-0134-x. [DOI] [PubMed] [Google Scholar]
- 132.McEachin JJ, Smith T, Lovaas OI. Long-term outcome for children with autism who received early intensive behavioral treatment. Am J Ment Retard. 1993;97:359–372. discussion 373-391. [PubMed] [Google Scholar]
- 133.Sebat J, Lakshmi B, Malhotra D, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]