Abstract
Background—
Older recipients of implantable cardioverter-defibrillators (ICDs) are at increased risk for short-term mortality in comparison with younger patients. Although hospice use is common among decedents aged >65, its use among older ICD recipients is unknown.
Methods and Results—
Medicare patients aged >65 matched to data in the National Cardiovascular Data Registry – ICD Registry from January 1, 2006 to March 31, 2010 were eligible for analysis (N=194 969). The proportion of ICD recipients enrolled in hospice, cumulative incidence of hospice admission, and factors associated with time to hospice enrollment were evaluated. Five years after device implantation, 50.9% of patients were either deceased or in hospice. Among decedents, 36.8% received hospice services. The cumulative incidence of hospice enrollment, accounting for the competing risk of death, was 4.7% (95% confidence interval [CI], 4.6%–4.8%) within 1 year and 21.3% (95% CI, 20.7%–21.8%) at 5 years. Factors most strongly associated with shorter time to hospice enrollment were older age (adjusted hazard ratio, 1.77; 95% CI, 1.73–1.81), class IV heart failure (versus class I; adjusted hazard ratio, 1.79; 95% CI, 1.66–1.94); ejection fraction <20 (adjusted hazard ratio, 1.57; 95% CI, 1.48–1.67), and greater hospice use among decedents in the patients’ health referral region.
Conclusions—
More than one-third of older patients dying with ICDs receive hospice care. Five years after implantation, half of older ICD recipients are either dead or in hospice. Hospice providers should be prepared for ICD patients, whose clinical trajectories and broader palliative care needs require greater focus.
Keywords: defibrillators, implantable; health services research; heart failure; patient outcome assessment
More than 50 000 implantable cardioverter-defibrillators (ICDs) are inserted annually in patients aged ≥65,1,2 with >40% of ICDs placed in patients aged ≥70 years and >10% in patients ≥80.1 As guidelines and systematic reviews explicitly discourage use of age alone as an exclusion for ICD implantation,3,4 demographic trends and the growing burden of heart disease will make millions more older patients eligible for these devices in the coming years.5 Moreover, older patients receiving ICDs are at higher risk for short-term death, in comparison with younger patients,6 and face other important uncertainties regarding their clinical course and healthcare needs after implantation.7
Clinical Perspective on p 2037
One of the most challenging healthcare transitions for older patients is hospice enrollment, because this represents a shift in the primary goal of care from survival to comfort and generally signifies that the end of life is approaching. Although hospice is the main provider of end-of-life care in the United States,8 its use among patients with ICDs is unknown. Early work suggests that hospice providers may be ill prepared to address the unique end-of-life care needs of these patients. For example, 1 study found that only 10% of hospice providers have a device deactivation policy.9 At the same time, a limited number of small studies involving retrospective postdeath family interviews10 and patient and provider surveys11–13 suggest that unwanted shocks, particularly near the end of life, contribute to pain, decreased quality of life, and patient and family distress.14,15
Despite the high mortality rate of older ICD patients, very little is known about the extent to which they currently access hospice services. Such information may help hospice providers better understand and prepare for their role in caring for these patients, assist in targeting patients who have the greatest need for hospice care earlier in their clinical course, further inform patients and their families about their future care needs, and support shared decision making regarding initial implantation. To address these knowledge gaps, we leveraged data from National Cardiovascular Data Registry (NCDR) ICD Registry linked to Medicare claims to describe the incidence and features of hospice use in a large, nationally representative sample of older patients following ICD implantation, and to identify factors associated with hospice enrollment in this cohort.
Methods
Data Sources
This study analyzed data from the NCDR ICD Registry, the details of which have been previously published.16 In brief, this registry was established in 2005 in concert with the Centers for Medicare and Medicaid Services expansion of coverage for primary prevention ICD implantation, with the goal of prospectively enrolling all Medicare beneficiaries receiving ICDs for primary prevention of sudden cardiac death as a condition of payment. Participating sites are trained on data collection, including the use of standardized definitions, and submitted data are subject to audit for errors and completeness.17 In practice, the majority of the >1500 participating hospitals enter data for all ICD recipients regardless of insurance status or indication.2,18
To ascertain hospice enrollment among ICD recipients, ICD Registry data for patients receiving implants between January 1, 2006 and March 31, 2010 were combined with Medicare fee-for-service hospice claims from January 1, 2006 to December 31, 2010, ensuring at least 9 months follow-up after device insertion. Indirect patient linkage was accomplished via a previously established algorithm by using the following identifiers: age, sex, admission date or procedure date, and hospital Medicare provider number to achieve a 63% match rate.19 Death dates were derived from Centers for Medicare and Medicaid Services enrollment files and validated with data from the Social Security Administration. To assess regional variation in hospice referral after ICD placement, 2010 data from the Dartmouth Atlas were linked to the analytic file to characterize hospice use among decedents in the ICD patients’ hospital referral region (HRR). This year was selected because it was the only one corresponding to our study period for which these data were available.
The Yale University Human Investigation Committee and Institutional Review Boards at Beth Israel Deaconess Medical Center and Hebrew SeniorLife Institute for Aging Research approved the conduct of this study.
Study Population
Patients >65 years who had ICDs inserted between January 1, 2006 through March 31, 2010 were eligible (Figure 1), aligned with use of the ICD Registry Version 1.0 collection form. Patients who were not fee-for-service Medicare patients were excluded because claims data for health maintenance organization enrollees were not available in the data files. There was no requirement for continuous fee-for-service enrollment before the index procedure. Patients enrolled in hospice before device placement were also excluded. For patients with multiple procedures during the study period, the first entry into the registry was taken as the index procedure for analytic purposes. Thus, baseline characteristics and time-to-event analyses are anchored to the index procedure, which may have been either a new or replacement ICD insertion.
Figure 1.
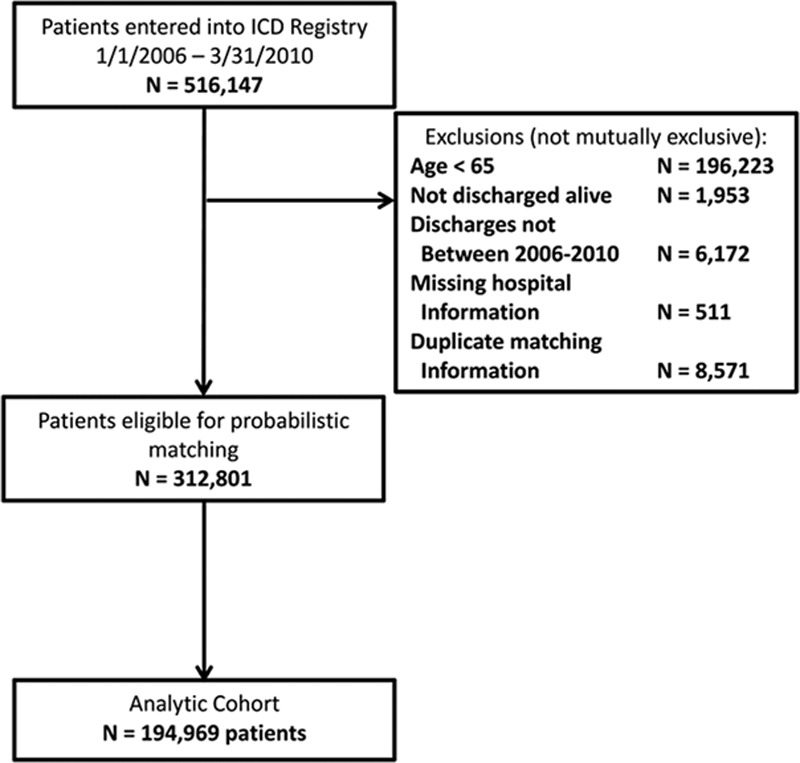
Derivation of study cohort. ICD indicates implantable cardioverter-defibrillator.
Patient Characteristics
Patient characteristics were derived from ICD Registry Data Collection Form v1.08 at the time of device placement unless otherwise stated. Demographic variables included age, sex, and race (white, black, or other). Clinical information included cardiac conditions, noncardiac conditions, diagnostic studies, and procedural details. Cardiac conditions, as documented in the standard ICD Registry form, included congestive heart failure, functional status (New York Heart Association class I–IV), atrial fibrillation/flutter, ventricular tachycardia, nonischemic cardiomyopathy, any ischemic heart disease, myocardial infarction, coronary artery bypass grafting, percutaneous coronary intervention, previous ICD implantation, and cerebrovascular disease. We also included type of device (single chamber, dual chamber, or biventricular) and whether the device was for primary or secondary prevention. In the ICD Registry, secondary prevention indicates patients who have previously experienced sustained ventricular arrhythmias or, for those with an ICD implant before their index procedure in this cohort, appropriate ICD therapy. We also ascertained the most recent estimates of left ventricular ejection fraction (%) and glomerular filtration rate (mL/min), calculated using the Modification of Diet in Renal Disease Study Group (MDRD) Study.20 Noncardiac comorbidities abstracted from the ICD registry form included chronic lung disease, diabetes mellitus, and hypertension.
We postulated that dementia and cancer would be associated with hospice referral. Because these diagnoses are not included in the ICD Registry, these were obtained from International Classification of Diseases, Ninth Revision codes on the Medicare claim associated with the index procedure. To construct the cancer and dementia variables, we used a modified version of the Hierarchical Condition Categories.21,22
Finally, Medicare claims data were used to determine whether subjects were admitted to a skilled nursing facility in the 6 months before the index ICD procedure.
Hospice Use
Whether or not a subject was enrolled in hospice following ICD placement was determined by using Medicare hospice claims. If the subject was enrolled, we determined the dates of admission and discharge, length of stay, site of care (hospital versus home-based), and primary admitting diagnosis.
Hospice Use in HRR
Previous research has shown that regional penetration of hospice is highly associated with whether or not individual patients are enrolled in hospice. To account for this observation, the percentage of decedents who were enrolled in hospice during the last 6 months of life in the HRRs in which the subjects had their ICD inserted was ascertained from the Dartmouth Atlas by using 2010 data. Based on its distribution, this variable was categorized into quartiles as follows: 19.3% to <41.0%, 41.0% to <47.8%, 47.8% to <54.3%, 54.3% to 70.3%.
Statistical Analysis
All baseline demographic data, clinical information, and procedural variables were described using frequencies for categorical variables and means (standard deviations) and medians (first to third quartiles) for continuous variables.
The proportion of subjects who enrolled in hospice and the median time from index procedure to hospice enrollment were calculated. For subjects admitted to hospice, length of stay was evaluated in days and other characteristics of the hospice admission were presented as proportions. Time from ICD implantation and time from hospice admission to death were calculated in years. Cumulative incidence was calculated accounting for the competing risk of death for the time to hospice admission outcome.
Our primary analysis used time to hospice enrollment as measured from the date of ICD placement to date of first hospice enrollment as the outcome. Subjects who were never enrolled in hospice or were disenrolled from Medicare fee-for-service were censored at the end of the follow-up period (December 31, 2010). Missing data for each independent variable was iteratively imputed by using the Fully Conditional Specification method of multiple imputation to create 10 imputed data sets.23 For each data set, a proportional subdistribution hazards model that accounted for the competing risk of death was used to examine the association between the independent variables and time to hospice enrollment.24 Hazard ratios and their associated standard errors were aggregated by taking the average of the estimates across 10 imputed data sets. Statistical inference was made based on the averaged aggregate estimates and standard errors using standard pooling rules for multiply imputed data.25
Independent variables were selected from the data set a priori as those that were presumed to be potentially associated with survival in ICD patients or hospice enrollment based on the literature26 and clinical judgment. Candidate independent variables included age (per 10 years), female sex, race (white versus nonwhite), congestive heart failure, New York Heart Association class (using class I as reference), history of atrial fibrillation/flutter, history of ventricular tachycardia (any), ischemic heart disease, cerebrovascular disease, replacement ICD, cardiac resynchronization therapy versus non–cardiac resynchronization therapy device, chronic lung disease, diabetes mellitus, dementia, history of cancer, skilled nursing facility claim in previous 6 months, left ventricular ejection fraction category (<20%, 20%–39%, ≥40); glomerular filtration rate (per 10 U increment), and the proportion of decedents who died in hospice in the patients’ HRR categorized in quartiles. Unadjusted analyses examined the association between each individual independent variable and time to hospice. Variables associated with time to hospice at a P value of <0.2 were entered in the multivariable proportional subdistribution hazards model. Hazard ratios and 95% confidence intervals (CIs) were generated from these analyses again using the average of the estimates across 10 imputed datasets.
Replacement ICD was considered a priori to be an independent variable that might introduce survivorship bias and interactions with other independent variables based on previous studies.27 Thus, a second model was fit evaluating replacement ICD combined with prespecified interaction terms including age, atrial fibrillation, ventricular tachycardia, congestive heart failure, and left ventricular ejection fraction. This second model did not impact the observed relationships, and thus we present only the results of the main model.
All analyses were performed using SAS v9.4 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
From the 516 147 patients entered into the ICD Registry during the study period, 312 801 were eligible for probabilistic matching after exclusions for age <65, discharge date outside of the study period, duplicates or missing hospital information (Figure 1). Only 624 (0.3%) patients were excluded because of previous hospice enrollment. Probabilistic matching to Medicare data yielded a final analytic cohort of 194 969. Comparisons between matched and nonmatched patients according to demographic and clinical characteristics showed no significant differences. The median follow-up time for the final analytic cohort was 1.92 years (first to third quartile, 1.13–2.97). During the 5-year follow-up period, a total of 52 990 (27.1%) patient deaths were observed. The 1-year and 5-year incidences of mortality were 12.1% and 48.8%, respectively.
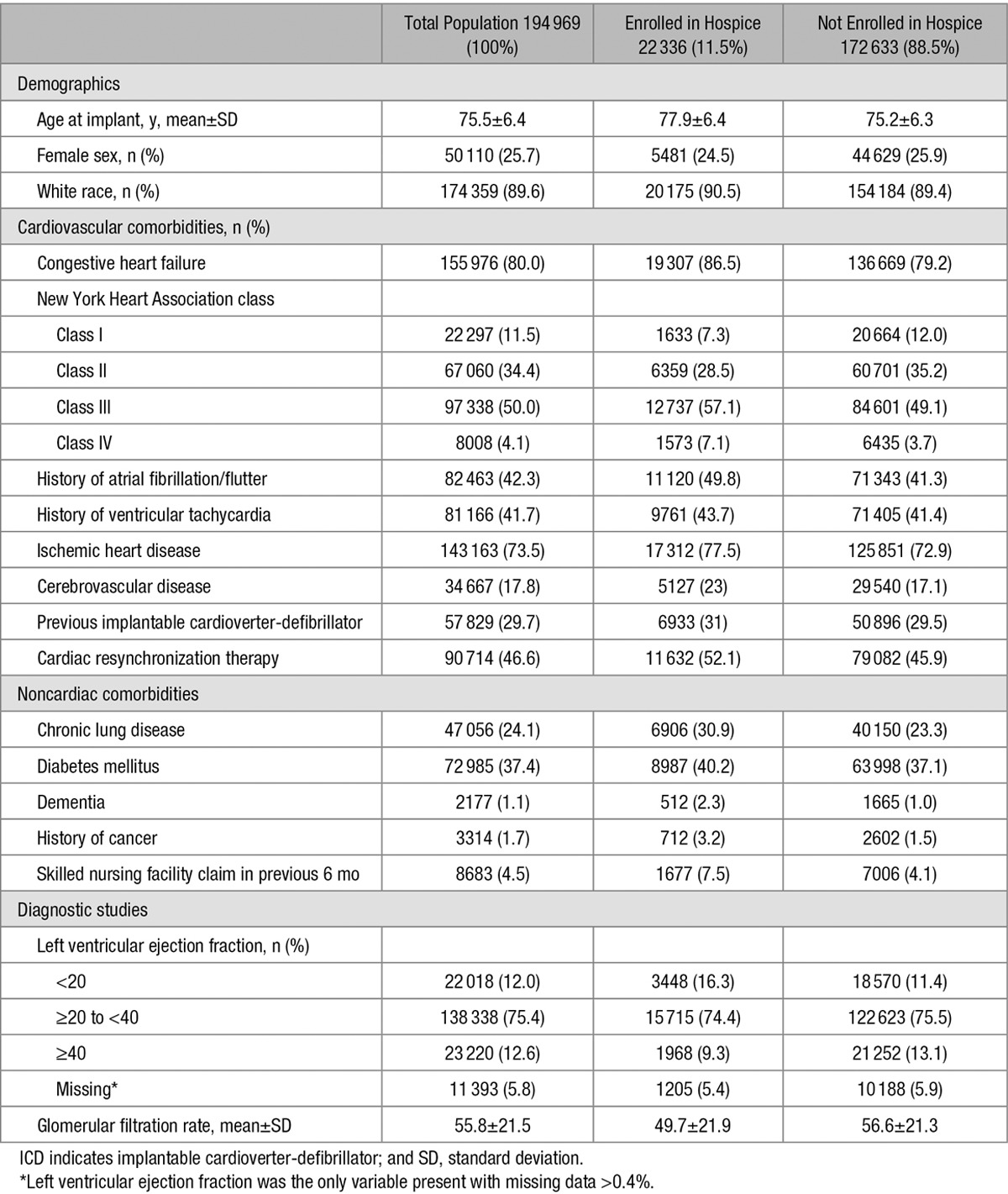
The demographic, cardiovascular, and noncardiovascular characteristics of the total cohort and stratified according to hospice enrollment are shown in Table 1. The overall cohort’s mean age was 75.5±6.4 years, 25.7% were female, and 89.6% were white. In terms of cardiovascular disease, the majority of patients had congestive heart failure (80.0%) and ischemic heart disease (73.5%), including previous coronary artery bypass grafting (42.6%) or percutaneous coronary intervention (34.5%). Clinical arrhythmias were common, including atrial fibrillation (42%) and ventricular tachycardia (41.7%). A total of 29.7% had received a previous ICD, 46.6% had cardiac resynchronization therapy devices, and 79.0% of all implant devices were placed for primary prevention. Among noncardiac conditions, only 1.1% of patients had a diagnosis of dementia, and only 1.7% had a previous diagnosis of cancer.
Table 1.
Observed Baseline Characteristics of Study Cohort at Time of ICD Implantation Overall and According to Enrollment in Hospice Services During Follow-up
Hospice Enrollment
A total of 22 336 patients (11.5%) were enrolled in hospice during the 5-year follow-up period. The cumulative incidence of hospice admission, accounting for the competing risk of death, was 4.7% (95% CI, 4.6%–4.8%) at 1 year and 21.3% (95% CI, 20.7%–21.8%) at 5 years (Figure 2). For those enrolled in hospice, the median time from ICD implantation to hospice enrollment was 1.3 years (first to third quartiles, 0.57–2.3 years). A total of 36.8% of decedents received hospice services. At 5 years follow-up, 50.9% patients had either died or been enrolled in hospice.
Figure 2.
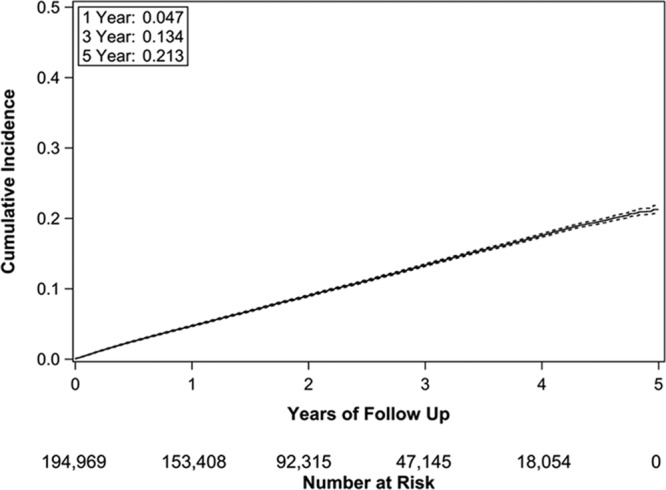
Cumulative incidence (solid line) with 95% confidence intervals (dashed lines) of hospice admission following implantable cardioverter-defibrillator implantation.
For subjects who were admitted to hospice, the median length of stay for their first hospice admission was 10 days (first to third quartiles, 4–36 days). Death was documented for 87.7% of hospice enrollees, and 84.3% of hospice services were provided at home (versus inpatient). The most common primary admitting diagnoses were congestive heart failure or cardiomyopathy (38.5%) and other heart disease (15.2%). Among noncardiac conditions, cancer diagnoses (17.5%), chronic kidney disease or renal failure (6.3%), chronic obstructive pulmonary disease and bronchiectasis (4.6%), and acute cerebrovascular disease (2.6%) were most common.
Factors Associated With Time to Hospice Enrollment
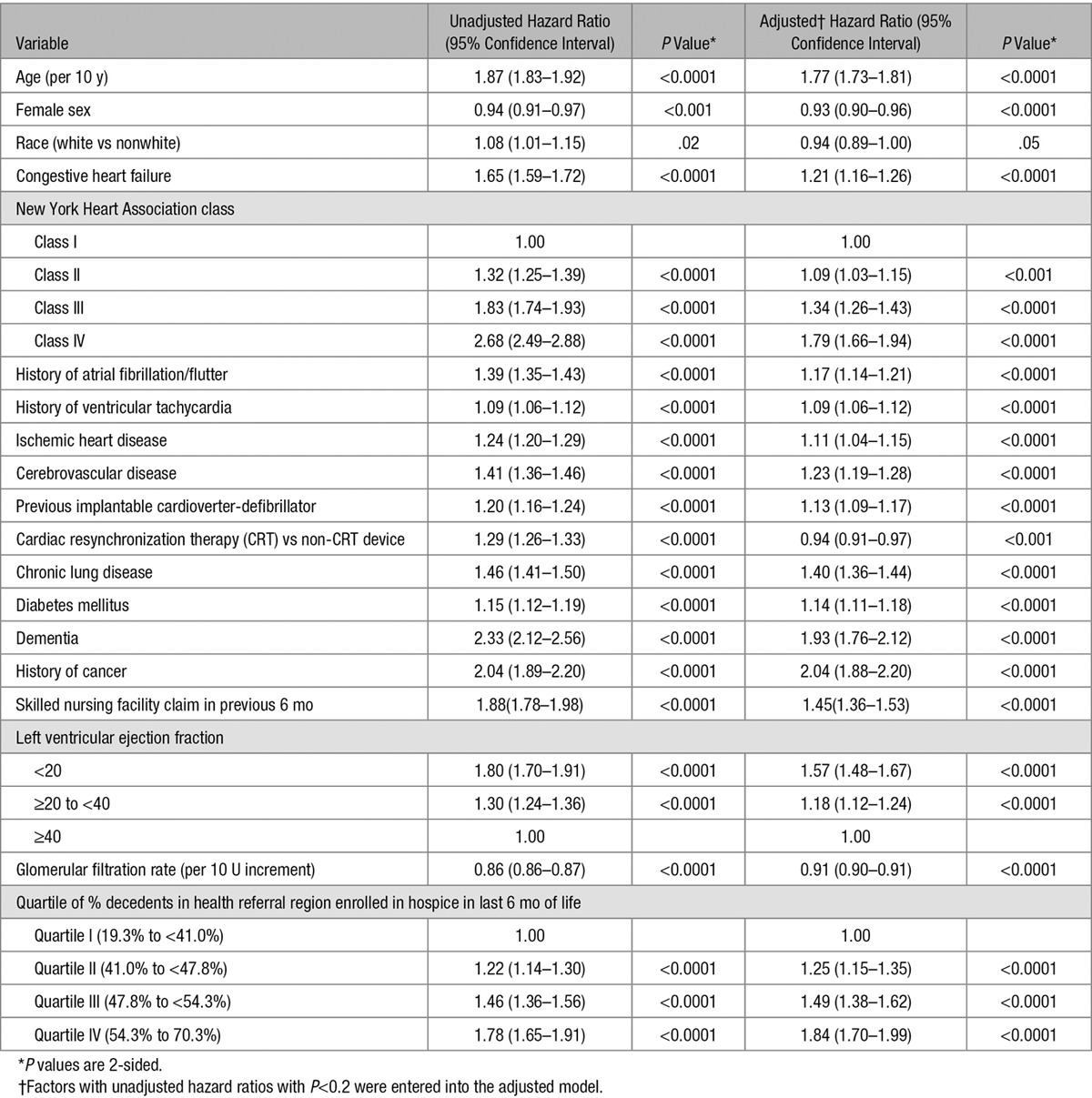
Unadjusted and adjusted associations between baseline characteristics and time to first hospice enrollment are shown in Table 2. In unadjusted analysis, all independent variables were associated with time to hospice enrollment and were entered into the multivariable model. All independent variables in the adjusted model remained significantly associated with the outcome, with the following factors being most strongly associated with a shorter time to hospice enrollment: cancer (adjusted hazard ratio [AHR], 2.04; 95% CI, 1.88–2.20), dementia (AHR, 1.93; 95% CI, 1.76–2.12), older age (AHR, 1.77; 95% CI, 1.73–1.81), class IV heart failure (versus class I [referent]; AHR, 1.79; 95% CI, 1.66–1.94); left ventricular ejection fraction <20 (AHR, 1.57; 95% CI, 1.48–1.67), chronic lung disease (AHR, 1.40; 95% CI, 1.36–1.44), and greater regional hospice penetration.
Table 2.
Unadjusted and Adjusted Associations Between Baseline Characteristics and Time to First Hospice Enrollment Following Implantable Cardioverter-Defibrillator Implantation
Discussion
This report from a nationwide sample provides the first comprehensive assessment of hospice use in older patients following ICD implantation. After accounting for the competing risk of death, the cumulative incidence rate of hospice admission in the 5 years following ICD placement was 21%. This finding underscores the need for hospice providers to prepare to care for dying ICD patients, including establishing protocols for turning off such devices and avoiding shocks at the end of life. At the same time, 63% of decedents did not receive hospice care. This finding, coupled with the fact that, at 5 years postimplantation, 51% of older ICD patients were either dead or in hospice, calls for a much greater understanding of the broader palliative care needs of older ICD patients and improved strategies to deliver that care.
Hospice is the main provider of end-of-life care for older Americans, with nearly half of all Medicare decedents making use of hospice services in 2012.28 In 2007, it was estimated that 43% of Medicare decedents with cancer and 34% of those with severe cognitive impairment received at least 3 days of hospice services.29 Thus, based on our findings, patients dying with ICDs are relatively common users of hospice, with 37% of decedents receiving such services and a cumulative incidence rate of enrollment over 5 years of 21% among those who had not already died. Nonetheless, hospices may be ill prepared to manage ICD patients, as indicated in a 2010 nationwide survey of 900 hospice providers indicating that, although 97% admitted patients with ICDs, only 10% had an ICD deactivation policy.9 Development and use of such policies, including routinely querying new hospice enrollees about the presence of an ICD, could be a potential quality metric for hospice providers.
The observation that half of older ICD recipients were either in hospice or deceased by 5 years postinsertion signals a critical need to better understand the palliative care needs of this vulnerable population. Very little is currently known about the clinical course of older ICD patients with respect to prognosis, communication, participation in advance care–planning discussions, sources of suffering, family member burden, and quality of end-of-life care. One concerning study found 65% of ICD patients (n=125, only 3% in hospice) had shock therapies active at the time of death, with 31% receiving shocks in the hours before death.15 Given that hospice has been shown to improve several key outcomes among dying patients,30–32 including those with cardiac disease,33 it is reasonable to assume that the 63% of ICD decedents who did not receive hospice may have benefited from such services. Moreover, given that the median length of stay was only 10 days, in comparison with 17 days for all hospice recipients nationwide in 2014,29 ICD patients who were enrolled in hospice may have benefited from earlier referral.
More broadly, the high mortality rate observed in our older ICD cohort, and the fact that the majority of decedents were never enrolled in hospice, suggests the need for greater integration of palliative care principles farther upstream in their clinical course. For example, general cardiologists and electrophysiologists should ensure that decision making at the time of device insertion is informed and aligned with patient preferences,34 and that such discussions are revisited in follow-up as the patient’s clinical status evolves.35 Primary care providers should also actively address such issues, and general symptom management, as well, in the broader context of the patient’s comorbid conditions and overall status. Finally, specialized palliative consultation should be sought when needed, even if ongoing, potentially life-prolonging treatment, such as ICD use, is still desired.36
Our multivariable analysis identified several patient-level factors associated with time to hospice enrollment, particularly previous diagnosis of cancer or dementia as well as age and severity of heart failure. Because these factors are also associated with greater mortality in these patients,26 their presence could help medical providers further refine patient selection for ICD implantation, and target those ICD patients who may particularly benefit from a more proactive palliative care approach or early hospice enrollment. Local market forces – as measured by the proportion of decedents in each patient’s HRR – also emerged as strongly associated with our primary outcome. These findings are consistent with other studies of hospice use suggesting that the availability of providers and local clinical practice play important roles in the provision of hospice care, independent of clinical necessity or patient preferences.37
Our study has several potential limitations. Only ICD recipients in the ICD Registry who were matched to fee-for-service Medicare claims were eligible for our analyses, and thus our findings may not extend simply to patients who are younger or those in managed care plans. Although our probabilistic match strategy showed similar patient characteristics between matched and unmatched subjects, it is possible that residual differences remain between these groups. Follow-up ended in December 2010, and it is possible that hospice use among ICD recipients has evolved in the intervening years, although patient and market factors associated with hospice use have remained generally stable over time.18,38 Notably, access to palliative care more broadly has expanded markedly in the years including and extending beyond our study period.39 Thus, it is possible that referral of ICD patients toward hospice care may in fact be even more common that what we identified. In evaluating factors associated with hospice use, we were limited to variables ascertained from the ICD Registry and Medicare claims. Factors potentially influencing hospice use, such as patient preference and severity of comorbid conditions (eg, dementia), were not available. In addition, we characterized variables at the time of ICD implantation, and were not able to account for interval development of conditions, such as cerebrovascular disease, progression of heart failure severity, or the incidence of ICD shocks. Characterizing these specific elements of patients’ evolution from healthy enough to receive an ICD to sick enough to merit hospice enrollment therefore remains a critical target of further study. This is particularly urgent given the influence of ICDs themselves on patients’ likelihood of dying either suddenly or from a more progressive process, outcomes that may not align well with many older patients’ preferences.40
In summary, substantial numbers of ICD recipients use hospice services, yet with relatively short lengths of stay. Hospice providers should be prepared to manage the unique needs of these patients. In addition, the large portion of ICD patients who either died or were in hospice within 5 years of device implantation argues strongly that these patients, particularly those with advanced heart failure, may benefit from a palliative care approach earlier in their clinical course, including earlier hospice referral. Finally, our study demonstrates a need for a clearer understanding of patients’ clinical trajectories following ICD implantation, including the ways in which these patients die, to provide the highest-quality care throughout the entire experience of living and dying with an ICD.
Sources of Funding
Dr Kramer is supported by a Paul B. Beeson Career Development Award in Aging Research (NIH-NIA K23AG045963). Dr Mitchell is supported by NIH-NIA K24AG033640. Dr Normand’s effort was funded by Grant U01FD004493, the Medical Device Epidemiology Network Methodology Center, from the Food and Drug Administration. This research was also supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR). The views expressed in this article represent those of the author(s), and do not necessarily represent the official views of the NCDR or its associated professional societies identified at www.ncdr.com. ICD Registry is an initiative of the American College of Cardiology Foundation and the Heart Rhythm Society.
Disclosures
Dr Reynolds reports a consulting contract with Medtronic. Dr Spertus reports a contract from the American College of Cardiology Foundation to provide analytic support for the National Cardiovascular Data Registry. The other authors report no conflicts.
Footnotes
Guest editor for this article was Mariann Piano, PhD.
CLINICAL PERSPECTIVE
Although hospice is the main provider of end-of-life care in the United States, its use among patients with implantable cardioverter-defibrillators (ICDs) is unknown. This report leveraged data from National Cardiovascular Data Registry-ICD Registry linked to Medicare claims to describe the incidence and features of hospice use in a large, nationally representative sample of older patients following ICD implantation. Among 194 969 ICD recipients, after accounting for the competing risk of death, the cumulative incidence rate of hospice admission in the 5 years following ICD placement was 21%, with factors including cancer, dementia, older age, heart failure severity, and regional penetration of hospice care most strongly associated with a shorter time to hospice enrollment. Overall, our finding that substantial numbers of ICD recipients use hospice services underscores the need for hospice providers to prepare to care for dying ICD patients, including establishing protocols for identifying and turning off such devices and avoiding shocks at the end of life. At the same time, 63% of decedents did not receive hospice care, and lengths of stay for hospice enrollment were relatively short. These findings, coupled with the fact that, at 5 years postimplantation, 51% of older ICD patients were either dead or in hospice, calls for a much greater understanding of the broader palliative care needs of older ICD patients and improved strategies to deliver that care.
References
- 1.Epstein AE, Kay GN, Plumb VJ, McElderry HT, Doppalapudi H, Yamada T, Shafiroff J, Syed ZA, Shkurovich S ACT Investigators. Implantable cardioverter-defibrillator prescription in the elderly. Heart Rhythm. 2009;6:1136–1143. doi: 10.1016/j.hrthm.2009.04.010. doi: 10.1016/j.hrthm.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Hammill SC, Kremers MS, Stevenson LW, Heidenreich PA, Lang CM, Curtis JP, Wang Y, Berul CI, Kadish AH, Al-Khatib SM, Pina IL, Walsh MN, Mirro MJ, Lindsay BD, Reynolds MR, Pontzer K, Blum L, Masoudi F, Rumsfeld J, Brindis RG. Review of the registry’s fourth year, incorporating lead data and pediatric ICD procedures, and use as a national performance measure. Heart Rhythm. 2010;7:1340–1345. doi: 10.1016/j.hrthm.2010.07.015. doi: 10.1016/j.hrthm.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 3.Epstein AE, Dimarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 Guidelines for device-based therapy of cardiac rhythm abnormalities. Heart Rhythm. 2008;5:e1–62. doi: 10.1016/j.hrthm.2008.04.014. [Google Scholar]
- 4.Barra S, Providência R, Paiva L, Heck P, Agarwal S. Implantable cardioverter-defibrillators in the elderly: rationale and specific age-related considerations. Europace. 2015;17:174–186. doi: 10.1093/europace/euu296. doi: 10.1093/europace/euu296. [DOI] [PubMed] [Google Scholar]
- 5.Forman DE, Rich MW, Alexander KP, Zieman S, Maurer MS, Najjar SS, Cleveland JC, Jr, Krumholz HM, Wenger NK. Cardiac care for older adults. Time for a new paradigm. J Am Coll Cardiol. 2011;57:1801–1810. doi: 10.1016/j.jacc.2011.02.014. doi: 10.1016/j.jacc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hess PL, Al-Khatib SM, Han JY, Edwards R, Bardy GH, Bigger JT, Buxton A, Cappato R, Dorian P, Hallstrom A, Kadish AH, Kudenchuk PJ, Lee KL, Mark DB, Moss AJ, Steinman R, Inoue LY, Sanders G. Survival benefit of the primary prevention implantable cardioverter-defibrillator among older patients: does age matter? An analysis of pooled data from 5 clinical trials. Circ Cardiovasc Qual Outcomes. 2015;8:179–186. doi: 10.1161/CIRCOUTCOMES.114.001306. doi: 10.1161/CIRCOUTCOMES.114.001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramer DB, Matlock DD, Buxton AE, Goldstein NE, Goodwin C, Green AR, Kirkpatrick JN, Knoepke C, Lampert R, Mueller PS, Reynolds MR, Spertus JA, Stevenson LW, Mitchell SL. Implantable cardioverter-defibrillator use in older adults: proceedings of a Hartford Change Agents Symposium. Circ Cardiovasc Qual Outcomes. 2015;8:437–446. doi: 10.1161/CIRCOUTCOMES.114.001660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teno JM, Gozalo PL, Bynum JP, Leland NE, Miller SC, Morden NE, Scupp T, Goodman DC, Mor V. Change in end-of-life care for Medicare beneficiaries: site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA. 2013;309:470–477. doi: 10.1001/jama.2012.207624. doi: 10.1001/jama.2012.207624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein N, Carlson M, Livote E, Kutner JS. Brief communication: Management of implantable cardioverter-defibrillators in hospice: a nationwide survey. Ann Intern Med. 2010;152:296–299. doi: 10.1059/0003-4819-152-5-201003020-00007. doi: 10.7326/0003-4819-152-5-201003020-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end-of-life care. Ann Intern Med. 2004;141:835–838. doi: 10.7326/0003-4819-141-11-200412070-00006. [DOI] [PubMed] [Google Scholar]
- 11.Mueller PS, Jenkins SM, Bramstedt KA, Hayes DL. Deactivating implanted cardiac devices in terminally ill patients: practices and attitudes. Pacing Clin Electrophysiol. 2008;31:560–568. doi: 10.1111/j.1540-8159.2008.01041.x. doi: 10.1111/j.1540-8159.2008.01041.x. [DOI] [PubMed] [Google Scholar]
- 12.Kelley AS, Reid MC, Miller DH, Fins JJ, Lachs MS. Implantable cardioverter-defibrillator deactivation at the end of life: a physician survey. Am Heart J. 2009;157:702–8.e1. doi: 10.1016/j.ahj.2008.12.011. doi: 10.1016/j.ahj.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Kapa S, Mueller PS, Hayes DL, Asirvatham SJ. Perspectives on withdrawing pacemaker and implantable cardioverter-defibrillator therapies at end of life: results of a survey of medical and legal professionals and patients. Mayo Clin Proc. 2010;85:981–990. doi: 10.4065/mcp.2010.0431. doi: 10.4065/mcp.2010.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lampert R, Hayes DL, Annas GJ, Farley MA, Goldstein NE, Hamilton RM, Kay GN, Kramer DB, Mueller PS, Padeletti L, Pozuelo L, Schoenfeld MH, Vardas PE, Wiegand DL, Zellner R American College of Cardiology; American Geriatrics Society; American Academy of Hospice and Palliative Medicine; American Heart Association; European Heart Rhythm Association; Hospice and Palliative Nurses Association. HRS Expert Consensus Statement on the Management of Cardiovascular Implantable Electronic Devices (CIEDs) in patients nearing end of life or requesting withdrawal of therapy. Heart Rhythm. 2010;7:1008–1026. doi: 10.1016/j.hrthm.2010.04.033. doi: 10.1016/j.hrthm.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 15.Kinch Westerdahl A, Sjöblom J, Mattiasson AC, Rosenqvist M, Frykman V. Implantable cardioverter-defibrillator therapy before death: high risk for painful shocks at end of life. Circulation. 2014;129:422–429. doi: 10.1161/CIRCULATIONAHA.113.002648. doi: 10.1161/CIRCULATIONAHA.113.002648. [DOI] [PubMed] [Google Scholar]
- 16.Hammill S, Phurrough S, Brindis R. The National ICD Registry: now and into the future. Heart Rhythm. 2006;3:470–473. doi: 10.1016/j.hrthm.2006.01.019. doi: 10.1016/j.hrthm.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Messenger JC, Ho KK, Young CH, Slattery LE, Draoui JC, Curtis JP, Dehmer GJ, Grover FL, Mirro MJ, Reynolds MR, Rokos IC, Spertus JA, Wang TY, Winston SA, Rumsfeld JS, Masoudi FA NCDR Science and Quality Oversight Committee Data Quality Workgroup. The National Cardiovascular Data Registry (NCDR) Data Quality Brief: the NCDR Data Quality Program in 2012. J Am Coll Cardiol. 2012;60:1484–1488. doi: 10.1016/j.jacc.2012.07.020. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Kremers MS, Hammill SC, Berul CI, Koutras C, Curtis JS, Wang Y, Beachy J, Blum Meisnere L, Conyers del M, Reynolds MR, Heidenreich PA, Al-Khatib SM, Pina IL, Blake K, Norine Walsh M, Wilkoff BL, Shalaby A, Masoudi FA, Rumsfeld J. The National ICD Registry Report: version 2.1 including leads and pediatrics for years 2010 and 2011. Heart Rhythm. 2013;10:e59–e65. doi: 10.1016/j.hrthm.2013.01.035. doi: 10.1016/j.hrthm.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 19.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. doi: 10.1016/j.ahj.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 21.Waltham, MA: Health Economics Research, Inc.; 2000. [Accessed October 20, 2015]. Diagnostic cost group hierarchical condition category models for Medicare risk adjustment. Report prepared for the Health Care Financing Administration. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Reports/downloads/pope_2000_2.pdf. [Google Scholar]
- 22.Center for Medicare and Medicaid Services, Qualitynet Resources. Clinical classification software (CCS)/ICD-9-CM crosswalks. http://www.qualitynet.org/dcs/ContentServer?cid=1182785083979&pagename=QnetPublic%2FPage%2FQnetTier3&c=Page. Accessed April 10, 2016.
- 23.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 24.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 25.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 26.Alba AC, Braga J, Gewarges M, Walter SD, Guyatt GH, Ross HJ. Predictors of mortality in patients with an implantable cardiac defibrillator: a systematic review and meta-analysis. Can J Cardiol. 2013;29:1729–1740. doi: 10.1016/j.cjca.2013.09.024. doi: 10.1016/j.cjca.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 27.Kramer DB, Kennedy KF, Noseworthy PA, Buxton AE, Josephson ME, Normand SL, Spertus JA, Zimetbaum PJ, Reynolds MR, Mitchell SL. Characteristics and outcomes of patients receiving new and replacement implantable cardioverter-defibrillators: results from the NCDR. Circ Cardiovasc Qual Outcomes. 2013;6:488–497. doi: 10.1161/CIRCOUTCOMES.111.000054. doi: 10.1161/CIRCOUTCOMES.111.000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Report to the Congress: Medicare Payment Policy. Washington, DC: Medicare Payment Advisory Commission; 2014. [Accessed September 1, 2015]. Http://medpac.Gov/documents/reports/mar14_entirereport.Pdf. [Google Scholar]
- 29.National Hospice and Palliative Care Organization. NHPCO’s Facts and Figures: Hospice Care in America. 2015 Edition. [Accessed October 14, 2015]. http://www.nhpco.org/sites/default/files/public/Statistics_Research/2015_Facts_Figures.pdf.
- 30.Obermeyer Z, Makar M, Abujaber S, Dominici F, Block S, Cutler DM. Association between the Medicare hospice benefit and health care utilization and costs for patients with poor-prognosis cancer. JAMA. 2014;312:1888–1896. doi: 10.1001/jama.2014.14950. doi: 10.1001/jama.2014.14950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baer WM, Hanson LC. Families’ perception of the added value of hospice in the nursing home. J Am Geriatr Soc. 2000;48:879–882. doi: 10.1111/j.1532-5415.2000.tb06883.x. [DOI] [PubMed] [Google Scholar]
- 32.Miller SC, Mor V, Wu N, Gozalo P, Lapane K. Does receipt of hospice care in nursing homes improve the management of pain at the end of life? J Am Geriatr Soc. 2002;50:507–515. doi: 10.1046/j.1532-5415.2002.50118.x. [DOI] [PubMed] [Google Scholar]
- 33.Blecker S, Anderson GF, Herbert R, Wang NY, Brancati FL. Hospice care and resource utilization in Medicare beneficiaries with heart failure. Med Care. 2011;49:985–991. doi: 10.1097/MLR.0b013e318235c221. doi: 10.1097/MLR.0b013e318235c221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis KB, Stacey D, Matlock DD. Making decisions about implantable cardioverter-defibrillators from implantation to end of life: an integrative review of patients’ perspectives. Patient. 2014;7:243–260. doi: 10.1007/s40271-014-0055-2. doi: 10.1007/s40271-014-0055-2. [DOI] [PubMed] [Google Scholar]
- 35.Kramer DB, Buxton AE, Zimetbaum PJ. Time for a change–a new approach to ICD replacement. N Engl J Med. 2012;366:291–293. doi: 10.1056/NEJMp1111467. doi: 10.1056/NEJMp1111467. [DOI] [PubMed] [Google Scholar]
- 36.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, Dahlin CM, Blinderman CD, Jacobsen J, Pirl WF, Billings JA, Lynch TJ. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 37.Givens JL, Tjia J, Zhou C, Emanuel E, Ash AS. Racial and ethnic differences in hospice use among patients with heart failure. Arch Intern Med. 2010;170:427–432. doi: 10.1001/archinternmed.2009.547. doi: 10.1001/archinternmed.2009.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borne RT, Peterson PN, Greenlee R, Heidenreich PA, Wang Y, Curtis JP, Tzou WS, Varosy PD, Kremers MS, Masoudi FA. Temporal trends in patient characteristics and outcomes among Medicare beneficiaries undergoing primary prevention implantable cardioverter-defibrillator placement in the United States, 2006-2010. Results from the National Cardiovascular Data Registry’s Implantable Cardioverter-Defibrillator Registry. Circulation. 2014;130:845–853. doi: 10.1161/CIRCULATIONAHA.114.008653. doi: 10.1161/CIRCULATIONAHA.114.008653. [DOI] [PubMed] [Google Scholar]
- 39.Kelley AS, Morrison RS. Palliative care for the seriously ill. N Engl J Med. 2015;373:747–755. doi: 10.1056/NEJMra1404684. doi: 10.1056/NEJMra1404684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaufman SR, Mueller PS, Ottenberg AL, Koenig BA. Ironic technology: old age and the implantable cardioverter defibrillator in US health care. Soc Sci Med. 2011;72:6–14. doi: 10.1016/j.socscimed.2010.09.052. doi: 10.1016/j.socscimed.2010.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]


