Abstract
Magnetic resonance–based assessment of quadriceps muscle fat has been proposed as surrogate marker in sarcopenia, osteoarthritis, and neuromuscular disorders. We presently investigated the association of quadriceps muscle fat with isometric strength measurements in healthy males using chemical shift encoding-based water-fat magnetic resonance imaging. Intermuscular adipose tissue fraction and intramuscular proton density fat fraction correlated significantly (P < 0.05) with isometric strength (up to r = −0.83 and −0.87, respectively). Reproducibility of intermuscular adipose tissue fraction and intramuscular proton density fat fraction was 1.5% and 5.7%, respectively.
Key Words: muscle fat, muscle strength, magnetic resonance imaging, proton density fat fraction
Quantitative magnetic resonance imaging (MRI) allows for noninvasive assessment of skeletal muscle fat. Single-voxel magnetic resonance spectroscopy has been widely used for the quantification of intramyocellular and extramyocellular lipids but is not able to assess the spatial distribution of intermuscular adipose tissue (IMAT).1 High-resolution T1-weighted imaging has been traditionally used to determine the distribution and the total area/volume of IMAT. Intermuscular adipose tissue has been shown to be inversely related to quadriceps muscle strength in subjects with sarcopenia, knee osteoarthritis, and neuromuscular disorders.2–4 However, T1-weighted imaging requires effective segmentation procedures to account for partial volume effects in IMAT quantification.5 In addition, IMAT includes the intermuscular fat (fat between muscles) and the intramuscular fat (fat within muscles), and these 2 components cannot be easily distinguished using T1-weighted imaging. However, the determination of the intramuscular fat fraction is particularly important in neuromuscular disorders because disease severity is characterized by increasing intramuscular fat infiltration. Chemical shift encoding-based water-fat MRI is an emerging technique, which is advantageous, compared with T1-weighted imaging, because it allows the separate assessment and quantification of intermuscular and intramuscular fat.6–8
By using chemical shift encoding-based water-fat MRI, Kumar et al9 reported that quadriceps intramuscular fat fraction rather than muscle size was associated with knee osteoarthritis. Furthermore, water-fat MRI-based proton density fat fraction (PDFF) has been obtained in subjects with neuromuscular disorders, for example, Pompe disease, facioscapulohumeral muscular dystrophy, and limb-girdle muscular dystrophy 2I, showing an inverse relationship between intramuscular fat fraction and muscle strength.10–13 The authors concluded that PDFF may provide a sensitive measurement of intramuscular fat, which can be used as imaging biomarker to assess disease progression and monitor therapy. Despite the previous studies on investigating the relationship between IMAT, intramuscular PDFF, and muscle strength in patients with knee osteoarthritis and neuromuscular diseases, little is known about the relationship between IMAT, intramuscular PDFF, and muscle strength in healthy volunteers. Detecting minor changes of intramuscular fat that correlate with muscle strength may particularly help initiate early, individualized therapy protocols to maintain or improve muscle function. Therefore, the aim of the study was to investigate the association of quadriceps muscle fat with isometric strength measurements in healthy males using chemical shift encoding-based water-fat MRI.
METHODS
Subjects
Nine, healthy men (mean [SD] age, 28 [8] years; mean [SD] body mass index, 28.1 [3.9] kg/m2) were recruited for this study. Inclusion criteria were no history of diabetes, neuromuscular disorders, and previous quadriceps muscle injuries. The study was approved by the Institutional Committee for Human Research. All subjects gave written informed consent before participation in the study.
Physical Strength Measurements
Right quadriceps muscle maximum isometric torque (Nm) produced by knee extension at 60- and 90-degree knee flexion angle was obtained by using a rotational dynamometer (Isomed 2000; D&R Fertsl GmbH, Hemau, Germany). The subjects were seated in upright position (90-degree hip flexion) and carefully fastened with safety belts to avoid any kind of additional movement. The aim was to generate the individual maximum isometric torque in the quadriceps muscle at 60- and 90-degree knee flexion angle. The subjects performed 3 repetitions with maximum isometric muscle activity by full recovery in between and the highest value in each angle was used for data analysis. Based on the lever principle, 60-degree knee flexion angle is the peak of isometric torque (point of maximum achievable isometric torque) in strength measurements of the quadriceps muscle.14 The 90-degree knee flexion angle served as a reference value.
Magnetic Resonance Imaging
The whole thigh musculature of the subjects was scanned on a 3T whole-body scanner (Ingenia; Philips Healthcare, Best, Netherlands) using the built-in-the-table posterior coil (12-channel array) and the anterior coil (16-channel array). The MR examination consisted of 2 axial imaging stacks in foot-to-head direction to achieve whole thigh coverage.
A 6-echo 3-dimensional spoiled gradient echo sequence was used for chemical shift-based water-fat separation. The sequence acquired the 6 echoes in a single time relaxation using nonflyback (bipolar) read-out gradients and the following imaging parameters: time relaxation/time echomin/Δtime echo = 10/1.04/0.8 milliseconds, field of view = 300 × 525 mm2, acquisition matrix = 96 × 263, slice thickness = 4 mm, slice locations = 65, receiver bandwidth = 2345 Hz per pixel, frequency direction = anterior/posterior (to minimize breathing artifacts), SENSE in left/right direction with reduction factor R = 2, number of averages = 1, scan time = 1 minute and 48 seconds per stack. A flip angle of 3 degrees was used to minimize T1-bias effects.15–17
Imaging-Based Fat Quantification
The gradient echo imaging data were processed online using the mDIXON Quant method provided by the manufacturer. mDIXON Quant is not labeled for the use under discussion. It performs a complex-based water-fat decomposition using a precalibrated 7-peak fat spectrum and a single T2* to model the signal variation with echo time.15,18,19 Proton density fat fraction maps were then computed as the ratio of the fat signal over the sum of fat and water signals.
Segmentation of the right quadriceps muscle was performed on the PDFF maps by using the free open-source software Medical Imaging Interaction Toolkit (developed by the Division of Medical and Biological Informatics, German Cancer Research Center, Heidelberg, Germany; www.mitk.org). By using a 2-dimensional region growing algorithm, the quadriceps muscle region of interest (ROI) including all muscle components (ie, rectus femoris, vastus medialis, vastus intermedius, and vastus lateralis), muscular fasciae, and the intermuscular fat were semiautomatically segmented from the insertion at the patella tendon upward in 40 consecutive slices in all subjects (ROI I in Fig. 1). The ROI I including all quadriceps muscle components was used to measure the quadriceps muscle volume. The mean PDFF value over ROI I was defined as the IMAT fraction (ie, IMAT = intermuscular fat + intramuscular fat). Quadriceps lean tissue volume was calculated by the equation: (100 – IMAT fraction) × quadriceps muscle volume. Furthermore, the 4 muscle components were separately segmented in the 10 most proximal of the 40 previously mentioned slices.20 The segmentation was manually performed by placing ROIs in each muscle component with a margin of approximately 3 mm to the outer contour of the rectus femoris and a margin of approximately 5 mm to the outer contour of the vastus medialis, intermedius, and lateralis to avoid the accidental inclusion of muscular fascia and intermuscular fat (ROIs II in Fig. 1). Quadriceps intramuscular PDFF was determined by averaging the PDFF over all manually placed ROIs II. All segmentation steps were performed by 1 operator.
FIGURE 1.
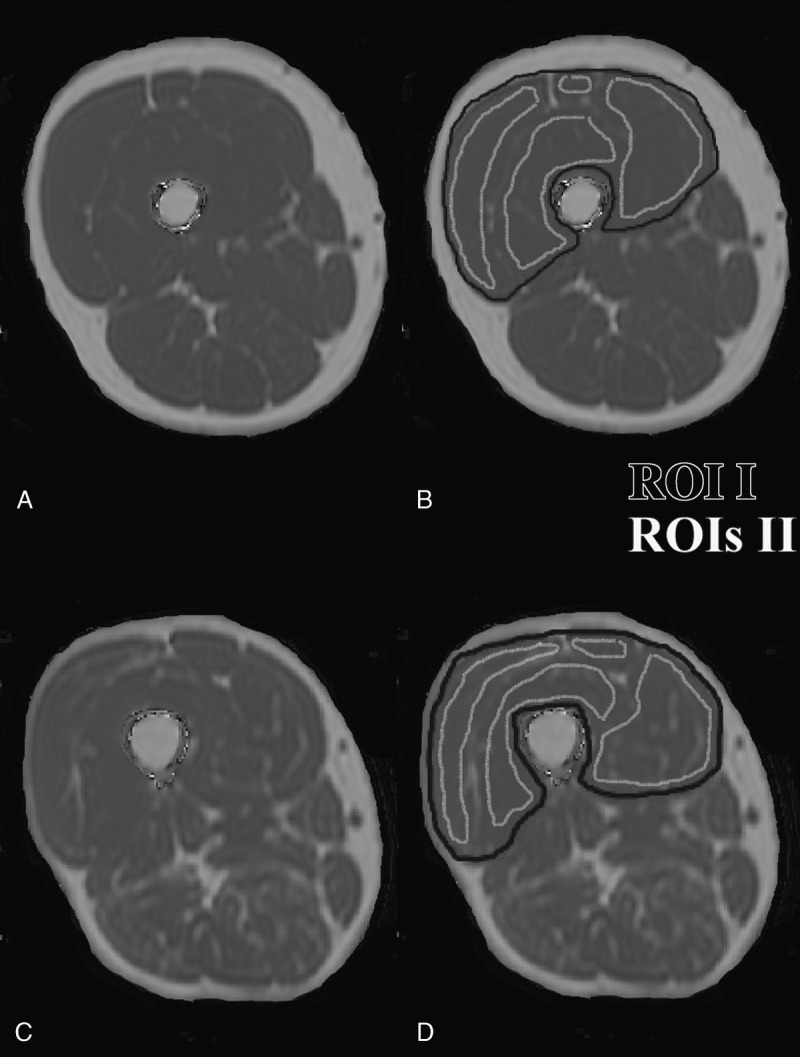
Representative fat fraction maps of the right thigh of a subject with low (A) and high (C) IMAT fraction/intramuscular PDFF. B and D display the segmentation of the quadriceps muscle (ROI I, color coded in black) used for quadriceps muscle volume and IMAT fraction calculation and the 4 muscle components with a margin to the outer contour of each muscle component (ROIs II, color coded in white) used for quadriceps intramuscular PDFF determination.
Reproducibility
Three subjects were scanned 3 times with repositioning to assess the reproducibility error of the MRI measurements. The previously mentioned segmentation procedure was performed in all images 1 time by 1 operator. Reproducibility errors of the IMAT fraction and intramuscular PDFF were expressed as root mean square absolute precision error in percent (absolute units) and as root mean square coefficients of variation in percent (relative units), according to Gluer et al.21
Statistical Analysis
The statistical analyses were performed with SPSS (SPSS, Chicago, Ill). All tests were performed using a 2-sided 0.05 level of significance. Parameters were presented as mean (SD). The Kolmogorov-Smirnov test showed for most parameters a significant difference from a normal distribution (P < 0.05). Therefore, correlations between MRI-derived parameters and measured physical strength were evaluated with the Spearman correlation coefficient r.
RESULTS
Quadriceps muscle volume and IMAT fraction averaged over all subjects amounted to 872.6 (150.1) cm3 and 5.24% (1.30%), respectively. Mean (SD) quadriceps lean tissue volume was 827.6 (148) cm3 and intramuscular PDFF was 4.02% (1.11%). Maximal isometric torque of the quadriceps muscle assessed at 60- and 90-degree knee flexion had mean (SD) values of 304 (53) and 230 (43) Nm, respectively.
Quadriceps IMAT fraction and intramuscular PDFF correlated significantly (P < 0.05) with physical strength (up to r = −0.83 and −0.87, respectively; Table 1; Fig. 2). A statistical trend (P < 0.01) was observed for the association of quadriceps muscle volume and lean tissue volume with physical strength (up to r = 0.65; Table 1). Furthermore, a significant correlation was found between quadriceps IMAT fraction and intramuscular PDFF (r = 0.98; P < 0.05).
TABLE 1.
Spearman Correlation Coefficients r (P Value) of MRI-Derived Parameters Versus Measured Physical Strength (Maximal Isometric Torque at 60- and 90-Degree Knee Flexion)
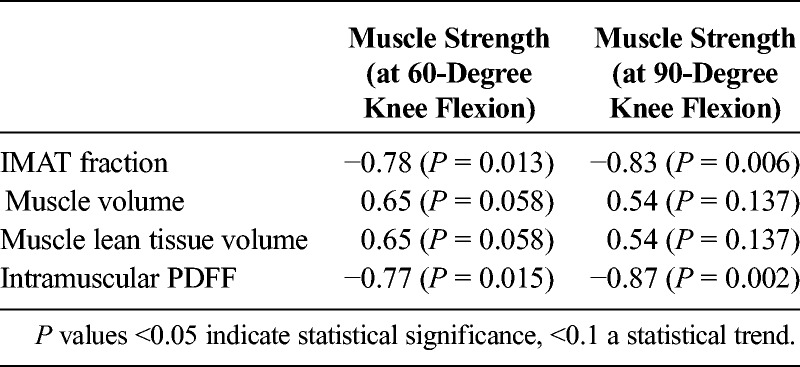
FIGURE 2.
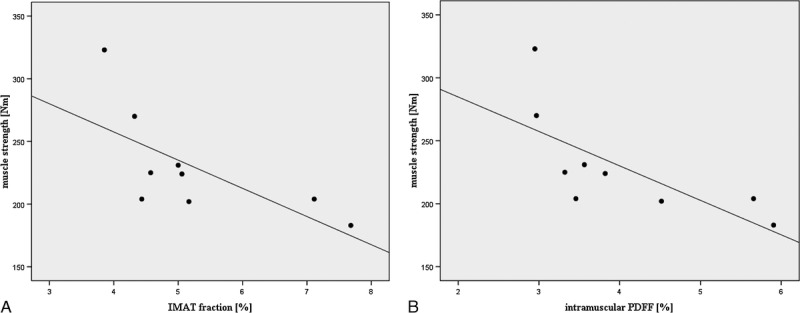
Correlation of quadriceps IMAT fraction and intramuscular PDFF fraction with muscle strength (maximal isometric torque measured at 90-degree knee flexion). Spearman correlation coefficients amounted to r value of −0.83 and −0.87 (P < 0.05), respectively.
The root mean square absolute precision error of the IMAT fraction and intramuscular PDFF amounted to 0.07% and 0.17% (absolute units), respectively. The root mean square coefficients of variation of the IMAT fraction and intramuscular PDFF was 1.5% and 5.7% (relative units), respectively.
DISCUSSION
The present study demonstrated that quadriceps intermascular and intramuscular fat could be reliably quantified using chemical shift encoding-based water-fat MRI. Strong associations were observed between water-fat MRI-derived quadriceps muscle fat parameters and corresponding physical strength measurements in healthy males.
Dual-energy x-ray absorptiometry, computed tomography, and MRI have been used for the assessment of quadriceps muscle cross-sectional area and volume, which are important parameters in the context of neuromuscular diseases, knee osteoarthritis, anterior cruciate ligament injury, and sarcopenia associated with aging and chronic diseases including chronic obstructive pulmonary disease.22–26 Only MRI allows deeper insight beyond muscle morphology because this technique provides additional parameters of muscle composition. Interestingly, T1-weighted imaging-based IMAT explained a small amount of variance in knee extensor strength in subjects with knee osteoarthritis, whereas quadriceps muscle volume was unrelated to strength measurements.3 T1-Weighted imaging is limited to the determination of total area/volume of IMAT, whereas chemical shift encoding-based water-fat MRI is able to separately quantify intermuscular and intramuscular fat, which constitutes the IMAT. It is particularly important in subjects with neuromuscular disorders, where intramuscular fat infiltration occurs, to separately measure the intramuscular fat fraction and differentiate it from IMAT.11
Studies in patients with neuromuscular disorders revealed an inverse association of PDFF and strength at the thigh.12,13 However, the strength measurements were performed with a handheld myometer. Little is known about the association between IMAT, intramuscular PDFF, and muscle strength in healthy volunteers. Specifically, the range of intramuscular PDFF in healthy volunteers is much lower than the range of PDFF in muscles affected by neuromuscular diseases and it is unknown whether PDFF correlates with muscle strength in muscles that are not severely fatty infiltrated. Therefore, we investigated the muscle fat/muscle strength relationship in the quadriceps muscle in healthy males by using a water-fat separation methodology that accounts for known confounding factors on the PDFF estimation and by using a more accurate and reliable rotational dynamometer than in previous studies. We observed strong correlations (up to r = −0.87) between intramuscular PDFF (as well as IMAT fraction) and quadriceps muscle strength. Furthermore, we found statistical trends for the association of quadriceps muscle volume and lean tissue volume with physical strength (up to r = 0.65). This finding is consistent with a study by Kumar et al9 who reported that quadriceps intramuscular fat fraction rather than muscle size was associated with knee osteoarthritis. Thus, chemical shift encoding-based water-fat MRI can provide clinically important information beyond quadriceps muscle morphology and potentially track early changes in muscle biomechanics in muscles that are not severely atrophied or fatty infiltrated in the beginning of a disease process.
The present study had some limitations. Manual and (semi)automatic algorithms have been proposed for the segmentation of the quadriceps muscle.7,27,28 We presently performed a mixture of a partly semiautomatic and partly manual segmentation of the quadriceps muscle and obtained acceptable reproducibility errors. Because we investigated young, healthy males, the fat distribution of the quadriceps muscle was relatively homogeneous. Therefore, the segmentation of the entire muscle compartments was not required.27 We decided to perform the segmentation with a clear anatomical landmark, that is, from the insertion at the patella tendon upward in 40 consecutive slices (to obtain IMAT fraction) and in the 10 most proximal of the 40 previously mentioned slices (to obtain intramuscular PDFF). In the future, a fast and technically robust segmentation tool has to be developed to allow the application of chemical shift encoding-based water-fat MRI in clinical routine. A further limitation of our study was the relatively small sample size. Because of the technical character of this study and the dependency of muscular fat on age and sex,29 we decided to include only men with a limited age range.
In conclusion, quadriceps muscle composition could be reliably assessed using chemical shift encoding-based water-fat MRI. Strength of the quadriceps muscle was strongly associated with water-fat MRI-derived muscle fat and less related to quadriceps muscle volume and lean tissue volume as parameters of muscle morphology. Thus, chemical shift encoding-based water-fat MRI may provide additional, clinically important information in tracking muscle strength early changes in muscles that do not show visual pathological fatty infiltration.
Footnotes
The study was funded by Philips Healthcare (to D.C.K.) and European Research Council ERC-StG-2014 637164 (to J.S.K.).
The authors declare no conflict of interest.
REFERENCES
- 1. Boesch C, Machann J, Vermathen P, et al. Role of proton MR for the study of muscle lipid metabolism. NMR Biomed. 2006; 19: 968– 988. [DOI] [PubMed] [Google Scholar]
- 2. Janssen BH, Voet NB, Nabuurs CI, et al. Distinct disease phases in muscles of facioscapulohumeral dystrophy patients identified by MR detected fat infiltration. PLoS One. 2014; 9: e85416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maly MR, Calder KM, Macintyre NJ, et al. Relationship of intermuscular fat volume in the thigh with knee extensor strength and physical performance in women at risk of or with knee osteoarthritis. Arthritis Care Res (Hoboken). 2013; 65: 44– 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heymsfield SB, Gonzalez MC, Lu J, et al. Skeletal muscle mass and quality: evolution of modern measurement concepts in the context of sarcopenia. Proc Nutr Soc. 2015; 74: 355– 366. [DOI] [PubMed] [Google Scholar]
- 5. Positano V, Christiansen T, Santarelli MF, et al. Accurate segmentation of subcutaneous and intermuscular adipose tissue from MR images of the thigh. J Magn Reson Imaging. 2009; 29: 677– 684. [DOI] [PubMed] [Google Scholar]
- 6. Karampinos DC, Baum T, Nardo L, et al. Characterization of the regional distribution of skeletal muscle adipose tissue in type 2 diabetes using chemical shift-based water/fat separation. J Magn Reson Imaging. 2012; 35: 899– 907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morrow JM, Sinclair CD, Fischmann A, et al. Reproducibility, and age, body-weight and gender dependency of candidate skeletal muscle MRI outcome measures in healthy volunteers. Eur Radiol. 2014; 24: 1610– 1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Noble JJ, Keevil SF, Totman J, et al. In vitro and in vivo comparison of two-, three- and four-point Dixon techniques for clinical intramuscular fat quantification at 3T. Br J Radiol. 2014; 87: 20130761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumar D, Karampinos DC, MacLeod TD, et al. Quadriceps intramuscular fat fraction rather than muscle size is associated with knee osteoarthritis. Osteoarthritis Cartilage. 2014; 22: 226– 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Horvath JJ, Austin SL, Case LE, et al. Correlation between quantitative whole-body muscle magnetic resonance imaging and clinical muscle weakness in Pompe disease. Muscle Nerve. 2015; 51: 722– 730. [DOI] [PubMed] [Google Scholar]
- 11. Willis TA, Hollingsworth KG, Coombs A, et al. Quantitative muscle MRI as an assessment tool for monitoring disease progression in LGMD2I: a multicentre longitudinal study. PLoS One. 2013; 8: e70993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Willis TA, Hollingsworth KG, Coombs A, et al. Quantitative magnetic resonance imaging in limb-girdle muscular dystrophy 2I: a multinational cross-sectional study. PLoS One. 2014; 9: e90377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dahlqvist JR, Vissing CR, Thomsen C, et al. Severe paraspinal muscle involvement in facioscapulohumeral muscular dystrophy. Neurology. 2014; 83: 1178– 1183. [DOI] [PubMed] [Google Scholar]
- 14. Knapik JJ, Wright JE, Mawdsley RH, et al. Isometric, isotonic, and isokinetic torque variations in four muscle groups through a range of joint motion. Phys Ther. 1983; 63: 938– 947. [DOI] [PubMed] [Google Scholar]
- 15. Bydder M, Yokoo T, Hamilton G, et al. Relaxation effects in the quantification of fat using gradient echo imaging. Magn Reson Imaging. 2008; 26: 347– 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karampinos DC, Yu H, Shimakawa A, et al. T1-corrected fat quantification using chemical shift-based water/fat separation: application to skeletal muscle. Magn Reson Med. 2011; 66: 1312– 1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu CY, McKenzie CA, Yu H, et al. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med. 2007; 58: 354– 364. [DOI] [PubMed] [Google Scholar]
- 18. Ren J, Dimitrov I, Sherry AD, et al. Composition of adipose tissue and marrow fat in humans by 1H NMR at 7 Tesla. J Lipid Res. 2008; 49: 2055– 2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu H, Shimakawa A, McKenzie CA, et al. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med. 2008; 60: 1122– 1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marcon M, Ciritsis B, Laux C, et al. Cross-sectional area measurements versus volumetric assessment of the quadriceps femoris muscle in patients with anterior cruciate ligament reconstructions. Eur Radiol. 2015; 25: 290– 298. [DOI] [PubMed] [Google Scholar]
- 21. Gluer CC, Blake G, Lu Y, et al. Accurate assessment of precision errors: how to measure the reproducibility of bone densitometry techniques. Osteoporos Int. 1995; 5: 262– 270. [DOI] [PubMed] [Google Scholar]
- 22. Hansen RD, Williamson DA, Finnegan TP, et al. Estimation of thigh muscle cross-sectional area by dual-energy X-ray absorptiometry in frail elderly patients. Am J Clin Nutr. 2007; 86: 952– 958. [DOI] [PubMed] [Google Scholar]
- 23. Mathur S, Takai KP, Macintyre DL, et al. Estimation of thigh muscle mass with magnetic resonance imaging in older adults and people with chronic obstructive pulmonary disease. Phys Ther. 2008; 88: 219– 230. [DOI] [PubMed] [Google Scholar]
- 24. Strandberg S, Lindström M, Wretling ML, et al. Muscle morphometric effect of anterior cruciate ligament injury measured by computed tomography: aspects on using non-injured leg as control. BMC Musculoskelet Disord. 2013; 14: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Øiestad BE, Juhl CB, Eitzen I, et al. Knee extensor muscle weakness is a risk factor for development of knee osteoarthritis. A systematic review and meta-analysis. Osteoarthritis Cartilage. 2015; 23: 171– 177. [DOI] [PubMed] [Google Scholar]
- 26. Nakayama T, Kuru S, Okura M, et al. Estimation of net muscle volume in patients with muscular dystrophy using muscle CT for prospective muscle volume analysis: an observational study. BMJ Open. 2013; 3: e003603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barnouin Y, Butler-Browne G, Voit T, et al. Manual segmentation of individual muscles of the quadriceps femoris using MRI: a reappraisal. J Magn Reson Imaging. 2014; 40: 239– 247. [DOI] [PubMed] [Google Scholar]
- 28. Karlsson A, Rosander J, Romu T, et al. Automatic and quantitative assessment of regional muscle volume by multi-atlas segmentation using whole-body water-fat MRI. J Magn Reson Imaging. 2015; 41: 1558– 1569. [DOI] [PubMed] [Google Scholar]
- 29. Hogrel JY, Barnouin Y, Azzabou N, et al. NMR imaging estimates of muscle volume and intramuscular fat infiltration in the thigh: variations with muscle, gender, and age. Age (Dordr). 2015; 37: 9798. [DOI] [PMC free article] [PubMed] [Google Scholar]


