Abstract
Previously, we demonstrated that the endogenous glutamate receptor antagonist kynurenic acid dose-dependently and significantly affected rat heart mitochondria. Now we have investigated the effects of L-tryptophan, L-kynurenine, 3-hydroxykynurenine and kynurenic, anthranilic, 3-hydroxyanthranilic, xanthurenic and quinolinic acids on respiratory parameters (ie, state 2, state 3), respiratory control index (RC) and ADP/oxygen ratio in brain, liver and heart mitochondria of adult rats. Mitochondria were incubated with glutamate/malate (5 mM) or succinate (10 mM) and in the presence of L-tryptophan metabolites (1 mM) or in the absence, as control. Kynurenic and anthranilic acids significantly reduced RC values of heart mitochondria in the presence of glutamate/malate. Xanthurenic acid significantly reduced RC values of brain mitochondria in the presence of glutamate/malate. Furthermore, 3-hydroxykynurenine and 3-hydroxyanthranilic acid decreased RC values of brain, liver and heart mitochondria using glutamate/malate. In the presence of succinate, 3-hydroxykynurenine and 3-hydroxyanthranilic acid affected RC values of brain mitochondria, whereas in liver and heart mitochondria only 3-hydroxykynurenine lowered RC values significantly. Furthermore, lowered ADP/oxygen ratios were observed in brain mitochondria in the presence of succinate with 3-hydroxykynurenine and 3-hydroxyanthranilic acid, and to a lesser extent with glutamate/malate. In addition, 3-hydroxyanthranilic acid significantly lowered the ADP/oxygen ratio in heart mitochondria exposed to glutamate/malate, while in the liver mitochondria only a mild reduction was found. Tests of the influence of L-tryptophan and its metabolites on complex I in liver mitochondria showed that only 3-hydroxykynurenine, 3-hydroxyanthranilic acid and L-kynurenine led to a significant acceleration of NADH-driven complex I activities. The data indicate that L-tryptophan metabolites had different effects on brain, liver and heart mitochondria. Alterations of L-tryptophan metabolism might have an impact on the bioenergetic activities of brain, liver and/or heart mitochondria and might be involved in the development of clinical symptoms such as cardiomyopathy, hepatopathy and dementia.
Keywords: respiratory parameters of mitochondria, brain, heart, liver, kynurenine metabolites, xanthurenic acid, misfoldome, dementia
Introduction
L-tryptophan (L-TRP) degradation along the kynurenine pathway has a biologically notable function for the central nervous system (CNS) as well as for the periphery.1–4 It is linked with the synthesis of neuroreactive metabolites with various properties – kynurenic acid (KYNA) with inhibitory activities,3 quinolinic acid (QUIN) with excitatory,3 and 3-hydroxykynurenine (3-OH-KYN) with neurotoxic properties.5–7 KYNA is well known as an antagonist of ionotropic excitatory amino acid (EAA) receptors and of nicotinic cholinergic subtype α7 receptors.3,8 L-kynurenine (L-KYN), as a major intermediate in the oxidative metabolism of L-TRP,1–4 is actively accumulated in different tissues (brain, liver, heart and others) and taken up by various cells (astrocytes, neurons, macrophages) including their mitochondria.9,10 It can be converted to KYNA, 3-OH-KYN or anthranilic acid (ANA) in the brain, liver or heart.1–4 The enzymes responsible for their formation, kynurenine aminotransferases, kynurenine-3-monooxygenase or kynureninase, are present in mitochondria and/or in the cytosol.10–14
There is substantial evidence that in the rat brain, KYNA synthesis increases significantly under pathological conditions.3,15,16 Increased KYNA formation has also been found in the brain of human patients suffering from CNS diseases such as Huntington’s chorea,17 Alzheimer’s disease,18 or Down’s syndrome19 and also in patients infected with HIV-120–22 and stroke patients.23 Importantly, this augmentation of KYNA is in line with a marked increase of activity of the kynurenine aminotransferases, a group of mitochondrial enzymes.18,21,22 Therefore, alterations of L-TRP metabolism, such as changes of enzyme activities and/or substrate availability, may influence mitochondrial function in cells and may contribute to impairment of organs in the periphery and in the CNS. Metabolic disturbance accompanied by oxidative stress and dysfunction of mitochondria has been suggested in various neurodegenerative disorders.24–26 In most cases, measurement of mitochondrial respiratory parameters, mitochondrial respiratory control or the ability of mitochondria to produce ATP allowed us to measure mitochondrial function and dysfunction in different systems.27 Previously, we demonstrated that within L-TRP metabolites of the kynurenine pathway, KYNA, ANA, 3-OH-KYN and 3-hydroxyanthranilic acid (3-OH-ANA) influenced dose-dependently (between 125 µM and 5 mM) respiratory parameters of rat heart mitochondria.28,29 This suggested that alteration of L-KYN metabolism might influence heart cell function and cardiovascular processes.30 Furthermore, our previous data also suggested that complex I of heart mitochondria is more sensitive to the KYNA action.28,29 Importantly, a marked and long-lasting increase of L-TRP and L-TRP metabolites in the blood has been reported in stroke patients, even up to 6 months after stroke.23 Higher KYNA and/or ANA levels might impair heart function, likely causing congestive cardiac dysfunction.31,32 Furthermore, it seems that high KYNA levels might significantly affect glutamatergic and nicotine cholinergic neurotransmission, causing impairment of memory or cognition.3,4,8,18,22
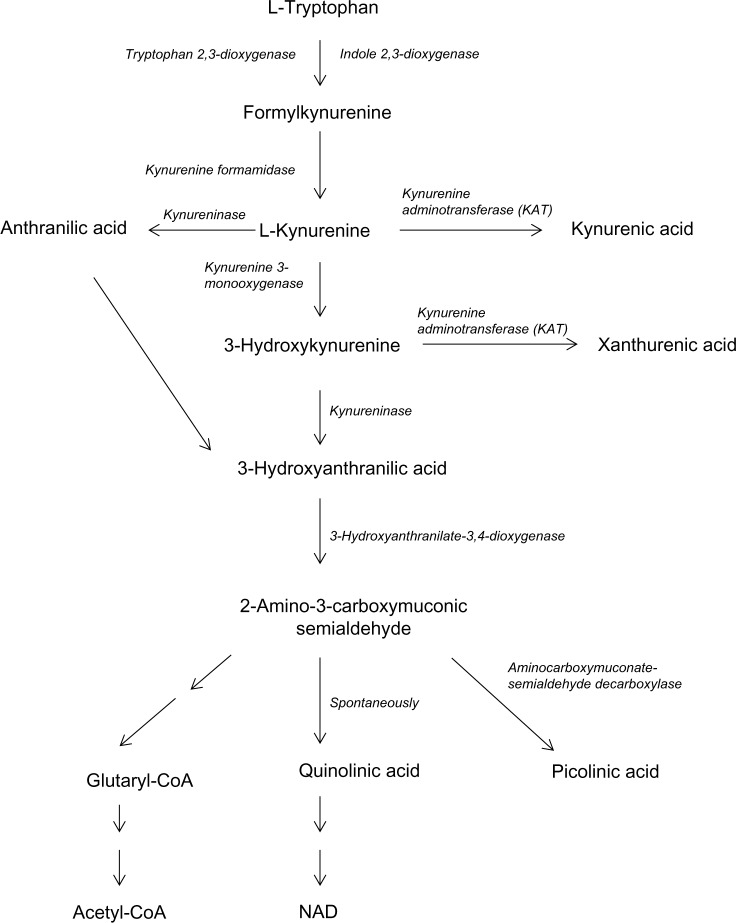
Therefore, the aim of the present study was to investigate if L-TRP and L-TRP metabolites via the kynurenine pathway (Fig. 1) such as L-KYN, KYNA, ANA, 3-OH-KYN, 3-OH-ANA, XAN or QUIN might affect brain and liver mitochondrial function of 3 month old rats, as we have observed them to affect heart mitochondria. Furthermore, the influence of L-TRP metabolites on enzymatic activities of complex I was studied in liver mitochondria.
Figure 1.
Tryptophan metabolism along kynurenine pathway.
Materials and Methods
Chemicals
ANA, 3-OH-ANA, L-KYN, 3-OH-KYN, KYNA, L-TRP, QUIN, xanthurenic acid (XAN), L-malic acid, rotenone, NADH (disodium salt), decylubiquinone (2,3-dimethoxy-5-methyl-6-decyl-1,4-benzoquinone) and fatty acid-free bovine serum albumin (BSA, fraction V) came from Sigma-Aldrich. Triethanolamine-HCl, KCN, succinic acid and L-glutamic acid were purchased from Fluka. ADP (potassium salt) was obtained from Boehringer. Other chemicals were from Merck.
Animals
Male, 3 month old, Sprague-Dawley rats (Him:OFA/SPF) were obtained from the breeding facility of the Medical University of Vienna and housed under standard laboratory conditions at a 12 h light/12 h dark cycle with free access to conventional animal food and water. For this study, 24 rats weighing 350 ± 31 g were used. Food was withdrawn overnight before animals were killed for organ harvesting (one rat per experimental day) by cervical dislocation followed by decapitation. The study was discussed and approved by the ethics and animal welfare committee of the University of Veterinary Medicine, Vienna, in accordance with good scientific practice guidelines and national legislation, and was carried out according to Austrian ethical regulations.
Isolation of mitochondria
Immediately after killing each rat, the heart and liver were quickly removed. In parallel, the brain was rapidly extracted from the skull and removed from the brainstem, cerebellum and olfactory bulb. The isolated organs were plunged into ice-cold isolation buffer containing sucrose (300 mM for heart and brain; 250 mM for liver), 20 mM triethanolamine and 1 mM ethylenedi-aminetetraacetic acid (pH 7.4, 4 °C). All isolation steps were performed at 2–4 °C. The tissues were minced with scissors and subsequently homogenised by an electrically driven Potter-Elvehjem homogeniser. Mitochondria were separated by differential centrifugation. Tissue homogenates were centrifuged for 10 min at 571 × g. The supernatants were filtered through cheesecloth and centrifuged for 10 min at 7410 × g to sediment the mitochondria. The resulting pellets were manually re-suspended and centrifuged for 10 min at 7410 × g. This latter step was repeated once more for heart and brain mitochondria and twice for liver mitochondria. Final mitochondrial pellets were carefully re-suspended in respective isolation buffer giving protein concentrations of approximately 30–40 mg/mL. Protein concentrations were measured with the biuret method using BSA for the calibration curve. Errors due to interfering pigments and turbidity caused by lipids were eliminated by the addition of KCN.33
Measurement of mitochondrial respiratory para meters
Mitochondrial oxygen consumption rates were measured in an air-saturated incubation buffer (pH 7.4, 25 °C) consisting of 300 mM sucrose, 20 mM triethanolamine and 1 mM diethylenetriaminepentaacetic acid (DETAPAC) supplemented with 1 mg/mL BSA (for heart mitochondria: 0.5 mg/mL BSA) within a DW1 oxygen electrode chamber connected to a CB1-D3 control unit (Hansatech Instruments).
State 2 respiration was stimulated in the presence of 4 mM potassium phosphate, either by 5 mM glutamate/5 mM malate (substrates for mitochondrial complex I) or 10 mM succinate (substrate for mitochondrial complex II) in the presence of 2 µg/mL rotenone to prevent electron transfer from complex I. Transition to state 3 respiration and thereby ATP synthesis was initiated by the addition of 250 µM ADP. The respiratory control (RC) value was calculated as the ratio of oxygen consumption rates during state 3 and state 2. The ADP/oxygen (ADP/O) ratio was calculated from the total amount of ADP added and the amount of oxygen consumed during state 3 respiration.
Treatment of isolated mitochondria with tryptophan (L-TRP) and its metabolites
Aliquot stock solutions of L-TRP and L-TRP metabolites (50 or 125 mM) were prepared while adding necessary amounts of KOH. Aliquots were frozen in liquid nitrogen (3-OH-ANA, 3-OH-KYN, XAN) or at −20 °C (ANA, L-KYN, KYNA, QUIN, L-TRP). During experiments, stock solutions were stored at 4 °C. In addition, 3-OH-ANA, 3-OH-KYN and XAN were protected from light.
Diluted mitochondrial suspensions were preincubated in the BSA-containing incubation buffer at 25 °C in an oxygen electrode chamber with L-TRP or L-TRP metabolites (1 mM final concentration) for 3 min before state 2 respiration was induced. Control mitochondria were preincubated for 3 min with 1 mM KCl (as a vehicle solution/ionic equivalent for L-TRP metabolites) under otherwise identical conditions.
Respiratory parameters of glutamate/malate and succinate- respiring brain, liver and heart mitochondria isolated from the same rat were measured on the same day. Each day two compounds, either L-TRP or L-TRP metabolites, and the respective controls were studied. These experiments were repeated with six mitochondrial suspensions prepared from six animals per treatment group and organ.
Determination of NADH oxidase, NADH-ferricyanide and NADH-decylubiquinone oxidoreductase activities
Due to a limited amount of brain and heart mitochondrial suspension, measurement of enzyme activity of complex I was performed only with liver mitochondria. For all enzymatic measurements, aliquots of rat liver mitochondria (10 mg protein per mL) were frozen at −20 °C (at least overnight), thawed at room temperature and stored at 4 °C during experiments. Enzyme activities were measured in an incubation buffer consisting of 300 mM sucrose, 20 mM triethanolamine and 1 mM DETAPAC (pH 7.4, 25 °C).
NADH oxidase
NADH oxidase activity was measured using a DW1 oxygen electrode. Mitochondria were suspended in the air-saturated incubation buffer and supplemented with tryptophan metabolites. After 1 min, 1 mM NADH was added and NADH oxidase activity was registered from oxygen consumption.
Control experiments were performed in the absence of L-TRP or its metabolites.
NADH-decylubiquinone and NADH-ferricyanide oxidoreductase
NADH-decylubiquinone and NADH- ferricyanide oxidoreductase activities were determined with a U3300 spectrophotometer (Hitachi) set at 2 nm slit width in 1 mL quartz cuvettes under magnetic stirring. Mitochondria were suspended in the incubation buffer and blocked with 1 mM KCN to avoid electron flow via cytochrome oxidase onto oxygen.
NADH-decylubiquinone oxidoreductase
In the case of NADH-decylubiquinone oxidoreductase assays, KCN-inhibited mitochondria were incubated with 150 µM NADH followed by the addition of L-TRP or its metabolites. After 1 min of incubation decylubiquinone (100 µM) was added and the NADH-decylubiquinone oxidoreductase activity was monitored for 3 min at 340 nm as the oxidation of NADH.
Activities were calculated using an extinction coefficient of 6.22 Mm−1 cm−1 for NADH.34
NADH-ferricyanide oxidoreductase
Mitochondria were supplemented with 0.5 mM K3[Fe(CN)6] followed by the addition of tryptophan or its metabolites. After 1 min of incubation, NADH (0.5 mM final concentration) was added and the NADH-ferricyanide oxidoreductase activity was monitored for 3 min at 420 nm as the reduction of K3[Fe(CN)6]. Activities were calculated using an extinction coefficient of 1 mM−1 cm−1 for ferricyanide.34 The effect of 3-OH-ANA and 3-OH-KYN on NADH-ferricyanide oxidoreductase activity could not be determined due to direct interaction of ferricyanide with these tryptophan metabolites.
Data analyses
All measurements were performed in duplicate; respective averaged values were used for further statistical analysis. Data are mean ± S.E.M of 6 separate experiments. Differences in respiratory parameters of control mitochondria and mitochondria treated with L-TRP or L-TRP metabolites were analyzed by a Student’s t-test and by one-way ANOVA, followed by post-hoc two-tailed Dunnett’s multiple comparison test. Differences in enzyme activities of control and treated mitochondria were analyzed by unpaired two-tailed Student’s t-test. The level for statistical significance was P < 0.05. Asterisks indicate significant differences in comparison to control mitochondria: *P < 0.05, **P < 0.01, ***P < 0.001.
Results
Respiratory parameters of control brain, liver and heart mitochondria
With regard to investigated control brain, liver and heart mitochondria, we observed that heart mitochondria exert the highest oxygen consumption rates (state 2: 22.0 ± 0.7 nmol O × min−1 × mg−1, state 3: 138.1 ± 5.3 nmol O × min−1 × mg−1), followed by liver mitochondria (state 2: 13.2 ± 0.5 nmol O × min−1 × mg−1, state 3: 54.5 ± 1.8 nmol O × min−1 × mg−1) and brain mitochondria (state 2: 4.9 ± 0.2 nmol O × min−1 × mg−1, state 3: 20.7 ± 1.1 nmol O × min−1 × mg−1) using glutamate/malate as substrates. Succinate-respiring mitochondria showed higher oxygen consumption rates in comparison to glutamate/malate-supplied mitochondria. However, the highest respiratory activities, as in the glutamate/malate data, were found in heart mitochondria (state 2: 85.7 ± 2.4 nmol O × min−1 × mg−1, state 3: 269.4 ± 6.7 nmol O × min−1 × mg−1), followed by liver mitochondria (state 2: 26.4 ± 1.0 nmol O × min−1 × mg−1, state 3: 101.0 ± 2.8 nmol O × min−1 × mg−1) and brain mitochondria (state 2: 7.7 ± 0.4 nmol O × min−1 × mg−1, state 3: 32.2 ± 1.3 nmol O × min−1 × mg−1). The highest RC values were detected in glutamate/malate-respiring heart mitochondria (6.3 ± 0.2), while all other RC values were in the range of 3.2–4.2. Data represent means ± S.E.M. of 24 mitochondrial preparations per organ. Control values of respiratory parameters of brain or heart mitochondria are in the line with previously published data.28,29,35
Influence of tryptophan and its metabolites on respiratory parameters of brain mitochondria
The most pronounced effects in brain mitochondria were detected in samples treated with 3-OH-ANA and 3-OH-KYN, which led to decreased RC values and ADP/O ratios in comparison to controls independently of whether mitochondria were supplemented with glutamate/malate (3-OH-ANA: 69.2% of CO, P < 0.001 for RC; 3-OH-KYN: 89.3% of CO, P < 0.05 for RC, 91.2% of CO, P < 0.01 for ADP/O; Table 1) or succinate (3-OH-ANA: 77.7% of CO, P < 0.001 for RC, 85.7% of CO, P < 0.01 for ADP/O; 3-OH-KYN: 74.0% of CO, P < 0.001 for RC and 79.1% of CO, P < 0.001 for ADP/O; Table 2) as respiratory substrates. Decreased RC values resulted mainly from increased oxygen consumption rates during state 2 respiration (with glutamate/malate: 3-OH-ANA: 134.9% of CO, P = 0.126; with succinate: 3-OH-KYN: 128.1% of CO; 3-OH-ANA: 127.1% of CO, P = 0.190), but these effects were not statistically significant.
Table 1.
Respiratory parameters of glutamate/malate-respiring rat brain mitochondria (2 mg protein per mL) in the absence (Control) or in the presence of 1 mM L-tryptophan (L-TRP) or its metabolites L-kynurenine (L-KYN), kynurenic acid (KYNA), anthranilic acid (ANA), 3-hydroxykynurenine (3-OH-KYN), 3-hydroxyanthranilic acid (3-OH-ANA), xanthurenic acid (XAN) and quinolinic acid (QUIN), respectively.
| SUBSTANCES | STATE 2 | STATE 3 | RC | ADP/O |
|---|---|---|---|---|
| Control | 4.97 ± 0.30 | 23.4 ± 1.3 | 4.76 ± 0.29 | 2.24 ± 0.15 |
| L-TRP | 4.67 ± 0.30 | 22.7 ± 1.1 | 4.94 ± 0.11 | 2.21 ± 0.15 |
| QUIN | 4.90 ± 0.36 | 22.8 ± 1.2 | 4.70 ± 0.15 | 2.29 ± 0.17 |
| ANOVA, F | 0.231 | 0.089 | 0.403 | 0.071 |
| P | 0.797 | 0.915 | 0.675 | 0.932 |
| Control | 5.28 ± 0.37 | 21.7 ± 1.9 | 4.11 ± 0.12 | 2.49 ± 0.04 |
| L-KYN | 5.34 ± 0.44 | 22.3 ± 1.8 | 4.19 ± 0.15 | 2.44 ± 0.04 |
| 3-OH-KYN | 5.64 ± 0.55 | 20.6 ± 1.9 | 3.67 ± 0.11* | 2.27 ± 0.05** |
| ANOVA, F | 0.169 | 0.209 | 4.763 | 6.831 |
| P | 0.846 | 0.814 | 0.025 | 0.0078 |
| Control | 4.42 ± 0.47 | 15.6 ± 1.9 | 3.55 ± 0.27 | 2.23 ± 0.06 |
| KYNA | 4.80 ± 0.30 | 16.3 ± 1.5 | 3.41 ± 0.25 | 2.21 ± 0.07 |
| ANA | 4.37 ± 0.27 | 13.1 ± 1.3 | 2.98 ± 0.22 | 2.19 ± 0.05 |
| ANOVA, F | 0.442 | 1.116 | 1.449 | 0.095 |
| P | 0.651 | 0.353 | 0.266 | 0.910 |
| Control | 4.84 ± 0.55 | 22.1 ± 2.8 | 4.54 ± 0.11 | 2.31 ± 0.07 |
| 3-OH-ANA | 6.53 ± 0.85 | 20.3 ± 2.6 | 3.14 ± 0.12*** | 2.12 ± 0.08 |
| XAN | 5.10 ± 0.83 | 20.2 ± 2.8 | 4.03 ± 0.16* | 2.36 ± 0.09 |
| ANOVA, F | 1.440 | 0.142 | 29.514 | 2.417 |
| P | 0.268 | 0.869 | <0.001 | 0.123 |
Notes: States are given in nmol O × min−1 × mg−1. Data are means ± S.E.M; n = 6;
P < 0.05,
P < 0.01;
P < 0.001 in comparison to the respective control.
Table 2.
Respiratory parameters of rotenone-inhibited succinate-respiring rat brain mitochondria (2 mg protein per mL) in the absence (Control) or in the presence of 1 mM L-tryptophan (L-TRP) or its metabolites L-kynurenine (L-KYN), kynurenic acid (KYNA), anthranilic acid (ANA), 3-hydroxykynurenine (3-OH-KYN), 3-hydroxyanthranilic acid (3-OH-ANA), xanthurenic acid (XAN) and quinolinic acid (QUIN), respectively.
| SUBSTANCES | STATE 2 | STATE 3 | RC | ADP/O |
|---|---|---|---|---|
| Control | 8.25 ± 0.38 | 33.7 ± 1.3 | 4.10 ± 0.11 | 1.56 ± 0.05 |
| L-TRP | 8.24 ± 0.50 | 33.2 ± 1.3 | 4.08 ± 0.13 | 1.51 ± 0.04 |
| QUIN | 8.22 ± 0.34 | 33.6 ± 1.4 | 4.09 ± 0.07 | 1.51 ± 0.06 |
| ANOVA, F | 0.0014 | 0.030 | 0.017 | 0.305 |
| P | 0.999 | 0.970 | 0.983 | 0.742 |
| Control | 7.20 ± 0.81 | 33.3 ± 3.0 | 4.70 ± 0.12 | 1.39 ± 0.02 |
| L-KYN | 7.44 ± 0.62 | 33.4 ± 2.1 | 4.54 ± 0.13 | 1.33 ± 0.01 |
| 3-OH-KYN | 9.22 ± 0.83 | 31.9 ± 3.2 | 3.48 ± 0.10*** | 1.10 ± 0.02*** |
| ANOVA, F | 2.123 | 0.090 | 32.786 | 56.241 |
| P | 0.154 | 0.914 | <0.001 | <0.001 |
| Control | 6.99 ± 0.36 | 28.6 ± 2.2 | 4.08 ± 0.22 | 1.35 ± 0.01 |
| KYNA | 6.54 ± 0.41 | 27.2 ± 1.9 | 4.17 ± 0.23 | 1.31 ± 0.02 |
| ANA | 6.57 ± 0.42 | 26.9 ± 2.0 | 4.13 ± 0.17 | 1.37 ± 0.02 |
| ANOVA, F | 0.397 | 0.197 | 0.048 | 2.872 |
| P | 0.679 | 0.823 | 0.953 | 0.088 |
| Control | 8.38 ± 1.12 | 33.3 ± 3.7 | 4.04 ± 0.14 | 1.47 ± 0.04 |
| 3-OH-ANA | 10.65 ± 1.16 | 33.4 ± 3.6 | 3.14 ± 0.08*** | 1.26 ± 0.03** |
| XAN | 8.36 ± 1.41 | 32.4 ± 3.7 | 4.04 ± 0.22 | 1.46 ± 0.05 |
| ANOVA, F | 1.131 | 0.020 | 10.847 | 8.051 |
| P | 0.349 | 0.980 | 0.0012 | 0.0042 |
Notes: States are given in nmol O × min−1 × mg−1. Values represent means ± S.E.M; n = 6;
P < 0.01;
P < 0.001 in comparison to the respective control.
While pre-treatment of brain mitochondria with XAN significantly reduced RC values in mitochondria respiring glutamate/malate (88.8% of CO, P < 0.05; Table 1), a moderate but not significant decrease of RC value was seen in the presence of ANA (83,9% of CO, P = 0.13). ANA and XAN lowered State 3, but not significantly (83.9% of CO, P = 0.30; 91.4% of CO, P = 0.65, respectively). No changes in respiratory parameters were found by XAN in succinate-consuming brain mitochondria (Table 2).
Brain mitochondria preincubated with 1 mM L-TRP, L-KYN, KYNA, ANA or QUIN, respectively, did not show any significant differences in comparison to control mitochondria, whether with glutamate/malate (one-way ANOVA, Table 1) or with succinate (one-way ANOVA, Table 2) as respiratory substrates.
Influence of tryptophan and its metabolites on respiratory parameters of liver mitochondria
L-TRP and most of its metabolites tested in this study were without significant impact on respiratory parameters of glutamate/malate-respiring (one-way ANOVA, Table 3) as well as succinate-respiring liver mitochondria (one-way ANOVA, Table 4). However, 3-OH-KYN and 3-OH-ANA significantly reduced RC values of glutamate/malate-consuming liver mitochondria (3-OH-KYN: 82.4% of CO, P < 0.01; 3-OH-ANA: 84.8% of CO, P < 0.05) mainly due to enhanced state 2 oxygen consumption rates (3-OH-ANA: 116.7% of CO, P < 0.05; 3-OH-KYN: 117.6% of CO, P = 0.07, Table 3). In the presence of 3-OH-ANA, the ADP/O ratios were mildly, but significantly decreased in comparison to control mitochondria (91.2% of CO, P < 0.05, Table 3). KYNA and ANA lowered RC values, but not to a statistically significant degree (86.9% of CO, P = 0.068 and 86.9% of CO, P = 0.071, respectively), mainly due to a tendency of increased state 2 oxygen consumption (111.0% of CO, P = 0.23; 107.9% of CO, P = 0.36).
Table 3.
Respiratory parameters of glutamate/malate-respiring rat liver mitochondria (1 mg protein per mL) in the absence (Control) or in the presence of 1 mM L-tryptophan (L-TRP) or its metabolites L-kynurenine (L-KYN), kynurenic acid (KYNA), anthranilic acid (ANA), 3-hydroxykynurenine (3-OH-KYN), 3-hydroxyanthranilic acid (3-OH-ANA), xanthurenic acid (XAN) and quinolinic acid (QUIN), respectively.
| SUBSTANCES | STATE 2 | STATE 3 | RC | ADP/O |
|---|---|---|---|---|
| Control | 15.7 ± 1.4 | 63.6 ± 3.2 | 4.14 ± 0.21 | 2.27 ± 0.19 |
| L-TRP | 15.7 ± 1.7 | 60.3 ± 4.3 | 3.94 ± 0.18 | 2.26 ± 0.19 |
| QUIN | 14.5 ± 1.3 | 60.8 ± 4.3 | 4.23 ± 0.13 | 2.29 ± 0.18 |
| ANOVA, F | 0.212 | 0.207 | 0.725 | 0.0058 |
| P | 0.811 | 0.815 | 0.500 | 0.994 |
| Control | 13.1 ± 0.7 | 52.1 ± 2.7 | 3.98 ± 0.16 | 2.57 ± 0.07 |
| L-KYN | 13.3 ± 0.7 | 52.6 ± 3.2 | 3.96 ± 0.14 | 2.60 ± 0.05 |
| 3-OH-KYN | 15.4 ± 0.9 | 50.5 ± 3.9 | 3.28 ± 0.15** | 2.53 ± 0.06 |
| ANOVA, F | 2.601 | 0.106 | 7.114 | 0.397 |
| P | 0.107 | 0.900 | 0.0067 | 0.679 |
| Control | 12.7 ± 0.8 | 53.3 ± 3.6 | 4.22 ± 0.20 | 2.51 ± 0.08 |
| KYNA | 14.1 ± 0.7 | 51.6 ± 3.6 | 3.67 ± 0.17 | 2.44 ± 0.10 |
| ANA | 13.7 ± 0.6 | 50.3 ± 3.9 | 3.67 ± 0.18 | 2.42 ± 0.07 |
| ANOVA, F | 0.957 | 0.157 | 2.877 | 0.369 |
| P | 0.406 | 0.856 | 0.088 | 0.698 |
| Control | 11.4 ± 0.5 | 49.0 ± 1.7 | 4.35 ± 0.18 | 2.51 ± 0.05 |
| 3-OH-ANA | 13.3 ± 0.6* | 48.7 ± 2.5 | 3.69 ± 0.11* | 2.29 ± 0.07* |
| XAN | 11.2 ± 0.7 | 45.3 ± 2.5 | 4.07 ± 0.25 | 2.52 ± 0.11 |
| ANOVA, F | 3.646 | 0.835 | 3.167 | 2.826 |
| P | 0.051 | 0.453 | 0.071 | 0.091 |
Notes: States are given in nmol O × min−1 × mg−1. Values represent means ± S.E.M; n = 6;
P < 0.05,
P < 0.01 in comparison to the respective control.
Table 4.
Respiratory parameters of rotenone-inhibited succinate-respiring rat liver mitochondria (0.5 mg protein per mL) in the absence (Control) or in the presence of 1 mM L-tryptophan (L-TRP) or its metabolites L-kynurenine (L-KYN), kynurenic acid (KYNA), anthranilic acid (ANA), 3-hydroxykynurenine (3-OH-KYN), 3-hydroxyanthranilic acid (3-OH-ANA), xanthurenic acid (XAN) and quinolinic acid (QUIN), respectively.
| SUBSTANCES | STATE 2 | STATE 3 | RC | ADP/O |
|---|---|---|---|---|
| Control | 28.4 ± 2.4 | 99.1 ± 5.1 | 3.53 ± 0.11 | 1.61 ± 0.05 |
| L-TRP | 29.6 ± 1.9 | 100.5 ± 4.6 | 3.42 ± 0.11 | 1.59 ± 0.03 |
| QUIN | 27.8 ± 2.2 | 99.4 ± 4.7 | 3.62 ± 0.13 | 1.62 ± 0.04 |
| ANOVA, F | 0.167 | 0.021 | 0.741 | 0.134 |
| P | 0.848 | 0.979 | 0.494 | 0.875 |
| Control | 26.4 ± 1.9 | 98.7 ± 5.6 | 3.76 ± 0.14 | 1.51 ± 0.03 |
| L-KYN | 24.9 ± 1.8 | 93.2 ± 8.1 | 3.72 ± 0.09 | 1.55 ± 0.05 |
| 3-OH-KYN | 29.0 ± 1.3 | 95.6 ± 3.9 | 3.31 ± 0.13* | 1.48 ± 0.06 |
| ANOVA, F | 1.494 | 0.203 | 4.180 | 0.519 |
| P | 0.256 | 0.819 | 0.036 | 0.605 |
| Control | 23.1 ± 1.2 | 98.1 ± 4.6 | 4.30 ± 0.12 | 1.57 ± 0.05 |
| KYNA | 22.7 ± 1.1 | 94.6 ± 3.0 | 4.20 ± 0.13 | 1.51 ± 0.03 |
| ANA | 21.7 ± 1.0 | 89.5 ± 3.5 | 4.17 ± 0.19 | 1.53 ± 0.04 |
| ANOVA, F | 0.464 | 1.302 | 0.198 | 0.545 |
| P | 0.638 | 0.301 | 0.822 | 0.591 |
| Control | 27.7 ± 2.0 | 108.3 ± 7.4 | 3.93 ± 0.06 | 1.65 ± 0.04 |
| 3-OH-ANA | 29.0 ± 2.3 | 103.3 ± 5.4 | 3.62 ± 0.17 | 1.55 ± 0.04 |
| XAN | 25.9 ± 2.0 | 102.3 ± 5.7 | 4.01 ± 0.19 | 1.62 ± 0.03 |
| ANOVA, F | 0.524 | 0.260 | 1.890 | 2.190 |
| P | 0.603 | 0.775 | 0.185 | 0.146 |
Notes: States are given in nmol O × min−1 × mg−1. Values represent means ± S.E.M; n = 6;
P < 0.05 in comparison to the respective control.
While the RC values of succinate-consuming liver mitochondria were significantly reduced by treatment with 3-OH-KYN (88.0% of CO, P < 0.05), with 3-OH-ANA only a tendency of decrease was observed (92.1% P = 0.11; Table 4).
Influence of tryptophan and its metabolites on respiratory parameters of heart mitochondria
3-OH-ANA, 3-OH-KYN, ANA and KYNA, (1 mM final concentration) significantly reduced the RC values of glutamate/malate-respiring heart mitochondria (3-OH-ANA: 62.7% of CO, P < 0.001; 3-OH-KYN: 64.4% of CO, P < 0.001; ANA: 68.5% of CO, P < 0.001; KYNA: 79.6% of CO, P < 0.01; Table 5) due to a decreased oxygen consumption during state 3 (3-OH-KYN: 63.1% of CO, P < 0.01; ANA: 71.5% of CO, P < 0.001; 3-OH-ANA: 75.3% of CO, P < 0.01; Table 5) and/or an increased state 2 respiration (3-OH-ANA: 120.9% of CO, P < 0.01; KYNA: 118.7% of CO, P < 0.05, Table. 5). A significantly lower ADP/O ratio, in comparison to the respective control mitochondria, was only observed in heart mitochondria treated with 3-OH-ANA (87.1% of CO, P < 0.01; Table 5).
Table 5.
Respiratory parameters of glutamate/malate-respiring rat heart mitochondria (0.5 mg protein per mL) in the absence (Control) or in the presence of 1 mM L-tryptophan (L-TRP) or its metabolites L-kynurenine (L-KYN), kynurenic acid (KYNA), anthranilic acid (ANA), 3-hydroxykynurenine (3-OH-KYN), 3-hydroxyanthranilic acid (3-OH-ANA), xanthurenic acid (XAN) and quinolinic acid (QUIN), respectively.
| SUBSTANCES | STATE 2 | STATE 3 | RC | ADP/O |
|---|---|---|---|---|
| Control | 22.4 ± 1.8 | 151 ± 11 | 6.81 ± 0.32 | 2.45 ± 0.09 |
| L-TRP | 22.5 ± 2.0 | 142 ± 11 | 6.34 ± 0.29 | 2.49 ± 0.11 |
| QUIN | 22.3 ± 2.2 | 143 ± 11 | 6.56 ± 0.38 | 2.47 ± 0.10 |
| ANOVA, F | 0.0034 | 0.219 | 0.506 | 0.046 |
| P | 0.997 | 0.806 | 0.613 | 0.955 |
| Control | 23.7 ± 1.7 | 150 ± 13 | 6.32 ± 0.29 | 2.63 ± 0.07 |
| L-KYN | 23.3 ± 1.5 | 145 ± 15 | 6.18 ± 0.31 | 2.63 ± 0.07 |
| 3-OH-KYN | 23.2 ± 1.6 | 94.7 ± 8.6** | 4.07 ± 0.21*** | 2.48 ± 0.07 |
| ANOVA, F | 0.022 | 6.117 | 21.191 | 1.393 |
| P | 0.978 | 0.011 | <0.001 | 0.279 |
| Control | 20.9 ± 1.0 | 123.7 ± 5.5 | 5.97 ± 0.28 | 2.51 ± 0.04 |
| KYNA | 24.8 ± 0.9* | 117.4 ± 4.1 | 4.75 ± 0.15** | 2.43 ± 0.05 |
| ANA | 21.7 ± 0.8 | 88.6 ± 1.7*** | 4.09 ± 0.12*** | 2.51 ± 0.07 |
| ANOVA, F | 5.418 | 20.955 | 24.161 | 0.798 |
| P | 0.017 | <0.001 | <0.001 | 0.469 |
| Control | 21.0 ± 0.6 | 127.8 ± 7.8 | 6.09 ± 0.29 | 2.64 ± 0.05 |
| 3-OH-ANA | 25.4 ± 1.0** | 96.4 ± 3.8** | 3.82 ± 0.12*** | 2.30 ± 0.08** |
| XAN | 22.6 ± 0.6 | 126.8 ± 8.8 | 5.61 ± 0.31 | 2.56 ± 0.08 |
| ANOVA, F | 9.341 | 6.213 | 21.843 | 6.530 |
| P | 0.0023 | 0.011 | <0.001 | 0.0091 |
Notes: States are given in nmol O × min−1 × mg−1. Values represent means ± S.E.M; n = 6;
P < 0.05,
P < 0.01
P < 0.001; in comparison to the respective control.
By contrast, L-TRP, QUIN, L-KYN and XAN did not significantly influence the bioenergetic function of glutamate/malate-respiring heart mitochondria (one-way ANOVA, Table 5).
In contrast to heart mitochondria consuming glutamate/malate, respiratory parameters of heart mitochondria supplemented with succinate were marginally affected by L-TRP and its metabolites (one-way ANOVA, Table 6) suggesting a selective interference of tryptophan metabolites KYNA, ANA or 3-OH-ANA in the activity of mitochondrial complex I. Only 3-OH-KYN significantly reduced RC values (88.1% of CO, P < 0.01), and XAN mildly but significantly decreased state 3 respiration (92.5% of CO, P < 0.05) of succinate-consuming heart mitochondria (Table 6).
Table 6.
Respiratory parameters of rotenone-inhibited succinate-respiring rat heart mitochondria (0.25 mg protein per mL) in the absence (Control) or in the presence of 1 mM L-tryptophan (L-TRP) or its metabolites L-kynurenine (L-KYN), kynurenic acid (KYNA), anthranilic acid (ANA), 3-hydroxykynurenine (3-OH-KYN), 3-hydroxyanthranilic acid (3-OH-ANA), xanthurenic acid (XAN) and quinolinic acid (QUIN), respectively.
| SUBSTANCES | STATE 2 | STATE 3 | RC | ADP/O |
|---|---|---|---|---|
| Control | 81.3 ± 2.3 | 262 ± 10 | 3.25 ± 0.10 | 1.34 ± 0.04 |
| L-TRP | 84.5 ± 3.1 | 268 ± 11 | 3.18 ± 0.07 | 1.34 ± 0.04 |
| QUIN | 82.5 ± 2.9 | 254 ± 11 | 3.07 ± 0.05 | 1.30 ± 0.04 |
| ANOVA, F | 0.342 | 0.476 | 1.301 | 0.404 |
| P | 0.715 | 0.630 | 0.301 | 0.675 |
| Control | 77.8 ± 6.2 | 269 ± 23 | 3.46 ± 0.08 | 1.31 ± 0.03 |
| L-KYN | 80.9 ± 7.6 | 270 ± 26 | 3.33 ± 0.03 | 1.27 ± 0.03 |
| 3-OH-KYN | 79.6 ± 7.3 | 242 ± 24 | 3.05 ± 0.05** | 1.23 ± 0.04 |
| ANOVA, F | 0.048 | 0.400 | 13.050 | 1.673 |
| P | 0.9538 | 0.677 | <0.001 | 0.221 |
| Control | 88.0 ± 3.0 | 265 ± 10 | 3.03 ± 0.04 | 1.18 ± 0.04 |
| KYNA | 95.2 ± 6.2 | 260 ± 11 | 2.78 ± 0.12 | 1.19 ± 0.02 |
| ANA | 90.1 ± 6.7 | 262 ± 13 | 2.96 ± 0.12 | 1.21 ± 0.04 |
| ANOVA, F | 0.446 | 0.046 | 1.702 | 0.235 |
| P | 0.648 | 0.955 | 0.216 | 0.793 |
| Control | 95.7 ± 4.1 | 281.4 ± 7.3 | 2.97 ± 0.11 | 1.21 ± 0.05 |
| 3-OH-ANA | 96.0 ± 5.0 | 267.2 ± 4.2 | 2.84 ± 0.15 | 1.21 ± 0.05 |
| XAN | 91.7 ± 4.1 | 260.4 ± 3.5* | 2.87 ± 0.13 | 1.23 ± 0.05 |
| ANOVA, F | 0.299 | 4.148 | 0.311 | 0.054 |
| P | 0.746 | 0.037 | 0.738 | 0.947 |
Notes: States are given in nmol O × min−1 × mg−1. Values represent means ± S.E.M; n = 6;
P < 0.05,
P < 0.01 in comparison to the respective control.
Influence of tryptophan and its metabolites on enzyme activities of liver mitochondrial complex I
Since the most pronounced effects on respiratory parameters were found in glutamate/malate-respiring mitochondria, it was of interest to research whether mitochondrial complex I was directly targeted by tryptophan metabolites.
NADH oxidase
An indirect measure of complex I activity is the NADH oxidation by oxygen involving the whole mitochondrial electron transfer chain, which can be detected from oxygen consumption. NADH oxidase was monitored at saturating substrate concentration (1 mM NADH). Significant acceleration of NADH-driven oxygen consumption (Table 7) was observed in the presence of 3-OH-ANA (143.2% of CO, P < 0.001), followed by 3-OH-KYN (125.64% of CO, P < 0.001) and L-KYN (112.1%, P < 0.05), while KYNA and XAN were without significant influence on NADH oxidase activity.
Table 7.
NADH oxidase activity of freeze-thawed rat liver mitochondria (1 mg protein per mL) in the presence or absence of tryptophan metabolites, respectively, given in nmol O × min−1 × mg−1.
| SUBSTANCES | 0 µM | 1000 µM |
|---|---|---|
| L-KYN | 74.9 ± 3.0 | 84.0 ± 1.9* |
| KYNA | 77.5 ± 4.5 | 81.8 ± 2.0 |
| 3-OH-KYN | 69.8 ± 1.5 | 87.7 ± 2.3*** |
| XAN | 70.8 ± 1.0 | 73.4 ± 2.0 |
| 3-OH-ANA | 40.5 ± 1.8 | 58.0 ± 1.3*** |
Notes: Data are means ± S.E.M; n = 4;
P < 0.05,
P < 0.001 in comparison to the respective control (0 µM).
NADH-decylubiquinone oxidoreductase
Among the tryptophan metabolites tested, only 3-OH-KYN significantly enhanced NADH-decylubiquinone oxidoreductase activity (130.15% of CO, P < 0.001; Table 8). The effect of 3-OH-ANA was mild but significant (107.23% of CO, P < 0.05, Table 8).
Table 8.
NADH-decylubiquinone oxidoreductase activity of cyanide-inhibited freeze-thawed rat liver mitochondria (0.2 mg protein per mL) in the presence or absence of tryptophan or its metabolites, respectively, given in nmol NADH × min−1 × mg−1.
| SUBSTANCES | 0 µM | 125 µM | 1000 µM |
|---|---|---|---|
| L-TRP | 41.7 ± 1.2 (12) | 41.9 ± 1.9 (8) | 39.9 ± 1.2 (12) |
| L-KYN | 41.7 ± 0.7 (8) | 42.3 ± 0.9 (8) | – |
| KYNA | 43.3 ± 1.4 (8) | 42.8 ± 1.3 (8) | – |
| ANA | 41.3 ± 1.5 (12) | 41.2 ± 1.4 (12) | 40.5 ± 1.2 (12) |
| 3-OH-KYN | 39.8 ± 1.2 (12) | 51.8 ± 1.2 (12)*** | – |
| XAN | 40.6 ± 1.1 (12) | 39.4± 1.3 (12) | – |
| 3-OH-ANA | 40.1 ± 0.8 (12) | 43.0 ± 0.7 (12)* | – |
| QUIN | 40.9 ± 1.4 (12) | 41.3 ± 1.2 (12) | 39.8 ± 1.0 (12) |
Notes: Data are means ± S.E.M. (n);
P < 0.05,
P < 0.001 in comparison to the respective control (0 µM).
NADH-ferricyanide oxidoreductase
NADH-ferricyanide oxidoreductase was not significantly changed by L-TRP, ANA, KYNA or QUIN (Table 9). Treatment of liver mitochondria with L-KYN led to a moderate but significant increase of NADH–ferricyanide oxidoreductase (108.9% of CO, P < 0.05, Table 9).
Table 9.
NADH-ferricyanide oxidoreductase activity of cyanide-inhibited freeze-thawed rat liver mitochondria (0.02 mg protein per mL) in the presence or absence of tryptophan or its metabolites, respectively, given in nmol K3[Fe(CN)6] × min−1 × mg−1.
| SUBSTANCES | 0 µM | 1000 µM |
|---|---|---|
| L-TRP | 2212 ± 81 | 2210 ± 63 |
| L-KYN | 2108 ± 53 | 2296 ± 49* |
| KYNA | 2304 ± 74 | 2303 ± 80 |
| ANA | 2119 ± 19 | 2133 ± 17 |
| QUIN | 2025 ± 50 | 2048 ± 47 |
Notes: Data are means ± S.E.M; n = 13;
P < 0.05 in comparison to the respective control (0 µM).
Discussion
With this investigation we demonstrated for the first time that respiratory parameters of brain or liver mitochondria were not affected by KYNA, independent of whether glutamate/malate or succinate was used as substrate for respiration. In contrast, in heart mitochondria KYNA significantly impaired RC values of glutamate/malate-respiring mitochondria and moderately lowered ADP/O ratio. The latter findings are in line with our previously published observations.28,29 Furthermore, in heart mitochondria ANA was also found to lower selectively state 3 respiration and consequently RC values. This finding is also in line with our previously published data.28,29
In this study, the data indicate that enhanced KYNA synthesis due to distinct pathological conditions might lower ATP synthesis and cause impairment of heart mitochondria function, but not of the brain or liver mitochondria function. Previously we have demonstrated that in the asphyctic brain, KYNA levels are significantly increased,16 and other investigators have demonstrated lowering of ATP synthesis in the brain during asphyxia.36 These observations allowed us to suggest that lowering of ATP synthesis in the brain during asphyxia might be due to the effect of KYNA on brain mitochondria respiratory parameters, similarly to what we had observed in heart mitochondria.28,29 However, the present data do not support this assumption, since respiratory parameters of brain and liver mitochondria were not affected by KYNA, indicating profound differences between heart and brain or liver mitochondria. Importantly, ANA also did not significantly alter the respiratory parameters of brain or liver mitochondria, with glutamate/malate or with succinate. A moderate effect of ANA, namely lowering of RC, could be seen on brain mitochondria in the presence of glutamate/malate.
Thus, in contrast to glutamate/malate-consuming heart mitochondria, the complex I-selective effect of KYNA seen in heart mitochondria was not observed in liver or brain mitochondria. Although ANA is generally accepted as biologically inactive, it can form an anti-inflammatory complex with copper.37,38 Interestingly, a neurodegenerative effect of ANA was shown in organotypic cultures of rat hippocampus in vitro,39 but the mechanism of ANA-induced neurodegeneration has not been elicited yet. We cannot exclude that increased ANA levels might play a role indirectly in the impairment of mitochondria function, through the action of 3-OH-ANA synthesized from ANA.40 Increased levels of ANA were observed in the periphery and CNS in various pathological conditions.23,31
Another metabolite of L-TRP, 3-OH-KYN, which has a role in toxic processes,5 markedly affected the mitochondrial activities of all three investigated organs. RC values were reduced significantly by 3-OH-KYN in brain, liver and heart mitochondria, independently of whether glutamate/malate or succinate was used. The ADP/O ratio was most affected by 3-OH-KYN in succinate-respiring brain mitochondria, followed by glutamate/malate-respiring brain mitochondria. Increase of 3-OH-KYN levels in the CNS has been documented in some disorders,41–43 and can be involved in the impairment of the bioenergetic activity of brain mitochondria and brain function, respectively. Intracellular generation of reactive oxygen species (ROS), mainly superoxide radicals and H2O2, has been observed at low micromolar concentration of 3-OH-KYN, a process that strongly depends on 3-OH-KYN uptake ability, which is variable in different brain regions.44,45 Our finding on lowering ADP/O ratio predominantly in brain mitochondria strongly evidences the neurotoxic activity of 3-OH-KYN. This mechanism could also be a possible cause of neurodegeneration, in addition to the ROS-generating activity of 3-OH-KYN.45 On the other hand, 3-OH-KYN has been proposed as a peroxyl radical scavenger in inflammatory diseases44 and under special conditions of cellular redox status can act as an anti-oxidant.46 It seems that 3-OH-KYN has a role in anti-oxidant and pro-oxidant activities.
Alterations of L-TRP metabolism, eg, changes of enzyme activities and/or substrate availability, may influence mitochondrial function in cells and may contribute to impairment of organs in the periphery and in the CNS.24 Markedly increased kynurenine aminotransferase activity in the brain has been reported in patients with bronchopneumonia or AIDS.22 Another important factor is the aging process, since kynurenine aminotransferase activity is increased during the aging process in the rat brain and kidney, but not in the liver.47,48 Increased KYNA levels were reported in aged rat brains too.48,49 However, there are also data indicating reduction of L-TRP metabolism during the aging process.50 Importantly, enhanced KYNA levels were also observed in human cerebrospinal fluid,51 and accumulated data strongly suggest a correlation between increased KYNA levels and age-related neurodegenerative disorders.17–23 Although the present study doesn’t support the assumption of a correlation between neurodegeneration and the impairment of mitochondria caused by KYNA, it is important to note that an enhancement of kynurenine aminotransferase activities in the brain may increase the formation of XAN from 3-OH-KYN, as well. In the presence of XAN, the RC value of brain mitochondria was significantly lowered, since oxygen consumption during the passive state was slightly increased, whereas oxygen consumption during the active state was moderately lowered in the presence of glutamate/malate. Although the biological function of XAN is still obscure, accumulated data suggest its involvement in neurodegeneration. XAN possesses antioxidant activity, has been found to scavenge peroxyl radicals in vitro,44 and was recently shown to inhibit haem- and iron-induced lipid peroxidation mainly through chelation of haem and iron.52 Notably, XAN was found to act as an apoptosis-inducing metabolite in vascular smooth muscle and lens epithelial cells.53,54 Malina and Hess55 demonstrated that accumulation of XAN in the human aortic smooth muscle cell causes proapoptotic conformation of the mitochondrial proteins (Bax, Bak, Bcl-xs and Bad). Furthermore, the authors stated that Bax-driven apoptosis may be important in the development of diabetes, atherosclerosis and neurological disorders. Interestingly, they also showed that in isolated rat liver mitochondrial fraction XAN leads to accumulation of Ca2+, while low micromolar concentrations of XAN increase oxygen consumption in the presence of succinate. Gobaille et al provided interesting data indicating a role for XAN in neurotransmission/neuromodulation.56 XAN synthesis has been found to occur in the rat brain and is thought to be the main preventer of the accumulation of toxic 3-OH-KYN,56 but XAN affects mitochondria as well. Importantly, XAN can modify the regulatory sequences of protein, leading to stable interactions between proteins and formation of a new pathological network, called misfoldome.57 With respect to that, we can expect increased kynurenine aminotransferase activities, observed in the brain of Alzheimer’s patients or HIV-1 infected patients or in patients with pneumonia, to enhance not only KYNA,18,22 but probably XAN, too. This could impair mitochondria function and play a role in the induction of a new pathological network and formation of plaques. It is believable that lowering of kynurenine aminotransferase activities by inhibitors might represent an excellent pharmacological and therapeutic approach for both processes; lowering of XAN might reduce plaque formation and lowering of KYNA might improve brain activities. Interestingly, Cerebrolysin, an anti-dementia drug, blocked kynurenine aminotransferase activities in the rat and human brain in an in vitro study,58 and the drug is effective in preventing cognitive impairment in different experimental animal models.59,60 Interestingly, in a transgenic model of Alzheimer’s disease Cerebrolysin significantly ameliorated cerebrovascular amyloidosis.61
Similarly to 3-OH-KYN, 3-OH-ANA also significantly affected the bioenergetic activities of brain mitochondria with glutamate/malate and succinate as respiratory substrates, whereas liver and heart mitochondria were affected only in the presence of glutamate/malate, suggesting organ- and complex-dependent differences. This is in line with Quagliariello et al.62, who demonstrated an inhibition of oxygen uptake and oxidative phosphorylation induced by 1 mM 3-OH-ANA in rat liver and rat heart mitochondria respiring α-oxoglutarate or other complex I-dependent substrates, while succinate-driven complex II-dependent respiration was not inhibited by 3-OH-ANA. A further similarity to 3-OH-KYN is that 3-OH-ANA is a potent antioxidant63 and a pro-oxidant46 depending on its concentration, at least in vitro.45 Both 3-OH-ANA and 3-OH-KYN, having an amino and a hydroxyl group in orthoposition in their aromatic rings, are good electron donors able to reduce cytochrome c as well as to autoxidize under aerobic conditions. Oxidation of these amino-phenols gives rise to quinoneimines and can concomitantly lead to ROS production.46,64 Within investigated metabolites L-TRP, L-KYN and QUIN had no effect on the bioenergetic parameters of rat brain, liver or heart mitochondria independent of whether glutamate/malate or succinate were used. Lack of effect may be related to the chemical structure of these compounds. L-TRP contains a ring structure that stabilizes radicals,65 and it was found to be the amino acid with the highest antiradical activities in the ferrous/cumene hydroperoxide system.66 In similarity to L-TRP, L-KYN is also considered as a potential endogenous antioxidant.67–69 Bordelon et al reported a significant reduction of RC values and state 3 respiration rates in brain mitochondria isolated 6 and 12 h after intrastriatal injection of QUIN, in comparison to saline-treated controls.70 But this different outcome in the aforementioned in vivo model is the neurotoxic effect of QUIN in the striatum, involving increased Ca2+ influx and accumulation of Ca2+ in mitochondria leading to membrane potential collapse.
Guillemin and Brew pointed out significant correlations between the kynurenine pathway and the neuropathogenesis of Alzheimer's disease, particularly the aspects of QUIN neurotoxicity and its roles in lipid peroxidation and the amplification of local inflammation.25
Based on mitochondrial respiratory parameters determined in the absence and presence of L-TRP and its metabolites, we suggested that complex I of the respiratory chain, NADH-ubiquinone oxidoreductase, could be the target involved in the action of several L-TRP metabolites. Studies on enzymatic activities of complex I revealed that complex I of liver mitochondria was more sensitive to 3-OH-KYN and 3-OH-ANA, but not to KYNA or ANA, which specifically affected heart mitochondria. Glutamate/malate-respiring liver mitochondria treated with 3-OH-KYN or 3-OH-ANA showed significantly increased NADH-decylubiquinone oxidoreductase activities corresponding to the accelerated oxygen consumption observed during state 2. The activity of NADH oxidase involving electron transfer from complex I to complex IV was likewise enhanced by 3-OH-ANA and 3-OH-KYN. L-TRP and its metabolites, except 3-OH-KYN and 3-OH-ANA, influenced neither NADH-decylubiquinone oxidoreductase nor respiratory activities of liver mitochondria. Interestingly, NADH oxidase and NADH-ferricyanide oxidoreductase were moderately but significantly increased by L-KYN while oxygen consumption rates of glutamate/malate-respiring liver mitochondria were not affected by L-KYN. This difference might be due to a partial conversion of L-KYN to 3-OH-KYN by kynurenine 3-monooxygenase located in the outer mitochondrial membrane.50 Nicotinamide nucleotide transhydrogenase, an enzyme of the inner mitochondrial membrane, could deliver the co-substrate NADPH required for this conversion, from NADH, which was present in saturating concentrations (1 mM) in NADH oxidase and NADH-ferricyanide oxidoreductase assays with freeze-thawed liver mitochondria.
The effect of L-TRP and its metabolites on respiratory parameters of brain, liver and heart mitochondria was tested at high concentration (1 mM). Therefore, one could argue that the physiological significance of these in vitro model experiments for the in vivo situation could be questionable. It is important to mention that submicromolar endogenous levels of L-TRP metabolite, as reported by our studies18,21–23,51 and several others3,17,20,25,43 do not take into account that concentration found in homogenate of whole tissue could be significantly lower than in vivo concentrations in compartments of their synthesis, eg, mitochondria.
The metabolism of L-TRP via kynurenine is over- activated under various pathological conditions as can be seen in the CNS or serum of HIV-1 infected patients,20–22 stroke patients,23,31 picorna virus encephalomyocarditis,71 and particularly in neuroborreliosis (personal observation).
In addition, several metabolites might have synergistic effects contributing to the impairments of mitochondria and to pathological processes.72 Therefore, the combined effects of the neuroactive kynurenine pathway metabolites, ie 3-OH-KYN and ANA or 3-OH-KYN and QUIN etc, and also dose response, will be of particular significance. In our previous study, we tested only the combinatorial effect of KYNA and ANA on heart mitochondria,29 but the effect of combinations of L-TRP metabolites on brain, liver or heart mitochondria needs to be the topic of further investigation.
In summary, our data revealed significant differences between respiratory parameters of brain, liver and heart mitochondria of adult male Sprague-Dawley rats regarding their response to L-TRP, L-KYN, KYNA, ANA, 3-OH-KYN, XAN, 3-OH-ANA or QUIN in the presence of glutamate/malate or succinate. Brain and liver mitochondria were less affected by kynurenines compared to heart mitochondria. Notably, differences in the effect of KYNA on heart mitochondria and the effect of XAN on brain mitochondria might be of particular significance, taking into consideration the aging process and inflammatory and/or degenerative processes.
Acknowledgments
The technical assistance of Brigitte Holnsteiner is gratefully acknowledged. This work was supported by the Austrian National Bank (Jubiläumsfond, Grant 12316 to H. Baran).
Abbreviations
- ANA
anthranilic acid
- ADP/O
adenosine diphosphate/oxygen ratio
- BSA
bovine serum albumin
- CNS
central nervous system
- CO
control
- DETAPAC
diethylenetriaminepentaacetic acid
- EAA
excitatory amino acid
- KYNA
kynurenic acid
- L-KYN
L-kynurenine
- L-TRP
L-tryptophan
- 3-OH-ANA
3-hydroxyanthranilic acid
- 3-OH-KYN
3-hydroxykynurenine
- XAN
xanthurenic acid
- QUIN
quinolinic acid
- RC
respiratory control
- ROS
reactive oxygen species
Footnotes
ACADEMIC EDITOR: Gilles Guillemin, Editor in Chief
PEER REVIEW: Eight peer reviewers contributed to the peer review report. Reviewers’ reports totaled 3310 words, excluding any confidential comments to the academic editor.
FUNDING: This work was supported by the Austrian National Bank (Jubiläumsfond, Grant 12316 to H. Baran). The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: HB. Analyzed the data: KS, MB-S, MA, HB, BK, CK. Wrote the first draft of the manuscript: HB. Contributed to the writing of the manuscript: KS, BK. Agree with manuscript results and conclusions: HB, KS, MB-S, MA, CK, BK. Jointly developed the structure and arguments for the paper: HB, KS, BK. Made critical revisions and approved final version: KS, BK, HB. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Brown RR. Biochemistry and pathology of tryptophan metabolism and its regulation by amino acids, vitamin B6, and steroid hormones. Am J Clin Nutr. 1971;24:243–7. doi: 10.1093/ajcn/24.2.243. [DOI] [PubMed] [Google Scholar]
- 2.Bender DA, McCreanor GM. The preferred route of kynurenine metabolism in the rat. Biochim Biophys Acta. 1982;717:56–60. doi: 10.1016/0304-4165(82)90379-8. [DOI] [PubMed] [Google Scholar]
- 3.Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45:309–79. [PubMed] [Google Scholar]
- 4.Schwarcz R, Bruno JP, Muchowski PJ, Wu H-Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat Rev Neurosci. 2012;13(7):465–77. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eastman CL, Guilarte TR. The role of hydrogen peroxide in the in vitro cytotoxicity of 3-hydroxykynurenine. Neurochem Res. 1990;15:1101–7. doi: 10.1007/BF01101711. [DOI] [PubMed] [Google Scholar]
- 6.Iwahashi H, Ishii T, Sugata R, Kido R. The effects of caffeic acid and its related catechols on hydroxyl radical formation by 3-hydroxyanthranilic acid, ferric chloride, and hydrogen peroxide. Arch Biochem Biophys. 1990;276:242–7. doi: 10.1016/0003-9861(90)90033-u. [DOI] [PubMed] [Google Scholar]
- 7.Ishii T, Iwahashi H, Sugata R, Kido R. Formation of hydroxanthommatin-derived radical in the oxidation of 3-hydroxykynurenine. Arch Biochem Biophys. 1992;294:616–22. doi: 10.1016/0003-9861(92)90733-d. [DOI] [PubMed] [Google Scholar]
- 8.Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits α7 nicotinic receptor activity and increases non-α7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–73. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Speciale C, Schwarcz R. Uptake of kynurenine into rat brain slices. J Neurochem. 1990;54:156–63. doi: 10.1111/j.1471-4159.1990.tb13296.x. [DOI] [PubMed] [Google Scholar]
- 10.Guillemin GJ, Smith DG, Smythe GA, Armati PJ, Brew BJ. Expression of the kynurenine pathway enzymes in human microglia and macrophages. Adv Exp Med Biol. 2003;527:105–12. doi: 10.1007/978-1-4615-0135-0_12. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto H, Hayaishi O. Solubilization and partial purification of kynurenine hydroxylase from mitochondrial outer membrane and its electron donors. Arch Biochem Biophys. 1969;131:603–8. doi: 10.1016/0003-9861(69)90435-4. [DOI] [PubMed] [Google Scholar]
- 12.Noguchi T, Minatogawa Y, Okuno E, Nakatani M, Morimoto M. Purification and characterization of kynurenine-2-oxoglutarate aminotransferase from the liver, brain and small intestine of rats. Biochem J. 1975;151:399–406. doi: 10.1042/bj1510399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inada J, Okuno E, Kimura M, Kido R. Intracellular localization and characterization of 3-hydroxykynureninase in human liver. Int J Biochem. 1984;16:623–8. doi: 10.1016/0020-711x(84)90031-4. [DOI] [PubMed] [Google Scholar]
- 14.Kawai J, Okuno E, Kido R. Organ distribution of rat kynureninase and changes of its activity during development. Enzyme. 1988;39:181–9. doi: 10.1159/000469117. [DOI] [PubMed] [Google Scholar]
- 15.Baran H, Gramer M, Hönack D, Löscher W. Systemic administration of kainate induces marked increases of endogenous kynurenic acid in various brain regions and plasma of rats. Eur J Pharmacol. 1995;286:167–75. doi: 10.1016/0014-2999(95)00443-o. [DOI] [PubMed] [Google Scholar]
- 16.Baran H, Kepplinger B, Herrera-Marschitz M, Stolze K, Lubec G, Nohl H. Increased kynurenic acid in the brain after neonatal asphyxia. Life Sci. 2001;69:1249–56. doi: 10.1016/s0024-3205(01)01215-2. [DOI] [PubMed] [Google Scholar]
- 17.Connick JH, Carla V, Moroni F, Stone TW. Increase in kynurenic acid in Huntington’s disease motor cortex. J Neurochem. 1989;52:985–7. doi: 10.1111/j.1471-4159.1989.tb02552.x. [DOI] [PubMed] [Google Scholar]
- 18.Baran H, Jellinger K, Deecke L. Kynurenine metabolism in Alzheimer’s disease. J Neural Transm. 1999;106:165–81. doi: 10.1007/s007020050149. [DOI] [PubMed] [Google Scholar]
- 19.Baran H, Cairns N, Lubec B, Lubec G. Increased kynurenic acid levels and decreased brain kynurenine aminotransferase I in patients with Down syndrome. Life Sci. 1996;58:1891–9. doi: 10.1016/0024-3205(96)00173-7. [DOI] [PubMed] [Google Scholar]
- 20.Heyes MP, Saito K, Crowley JS, et al. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992;115:1249–73. doi: 10.1093/brain/115.5.1249. [DOI] [PubMed] [Google Scholar]
- 21.Baran H, Hainfellner JA, Kepplinger B, Mazal PR, Schmid H, Budka H. Kynurenic acid metabolism in the brain of HIV-1 infected patients. J Neural Transm. 2000;107:1127–38. doi: 10.1007/s007020070026. [DOI] [PubMed] [Google Scholar]
- 22.Baran H, Hainfellner JA, Kepplinger B. Kynurenic acid metabolism in various types of brain pathology in HIV-1 infected patients. Int J Tryptophan Res. 2012;5:49–64. doi: 10.4137/IJTR.S10627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kepplinger B, Sedlnitzky-Semler B, Eigner S, Kalina P, Berger P, Baran H. Stroke patients after repetitive transcranial magnetic stimulation (rTMS) – Alterations of tryptophan metabolites in the serum. Int J Neurorehabilitation. 2014;1:128. doi: 10.4172/2376-0281.1000128. [DOI] [Google Scholar]
- 24.Beal MF, Howell N, Bodis-Wollner I. Mitochondria and free radicals in neurodegenerative diseases. John Wiley & Sons Inc; New York: 1997. p. 609. [Google Scholar]
- 25.Guillemin GJ, Brew BJ. Implications of the kynurenine pathway and quinolinic acid in Alzheimer’s disease. Redox Rep. 2002;7:199–206. doi: 10.1179/135100002125000550. [DOI] [PubMed] [Google Scholar]
- 26.Sas K, Robotka H, Toldi J, Vécsei L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J Neurol Sci. 2007;257:221–39. doi: 10.1016/j.jns.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 27.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baran H, Staniek K, Kepplinger B, Gille L, Stolze K, Nohl H. Kynurenic acid influences the respiratory parameters of rat heart mitochondria. Pharmacology. 2001;62:119–23. doi: 10.1159/000056082. [DOI] [PubMed] [Google Scholar]
- 29.Baran H, Staniek K, Kepplinger B, Stur J, Draxler M, Nohl H. Kynurenines and the respiratory parameters on rat heart mitochondria. Life Sci. 2003;72:1103–15. doi: 10.1016/s0024-3205(02)02365-2. [DOI] [PubMed] [Google Scholar]
- 30.Rudzite V, Skards JI, Fuchs D, Reibnegger G, Wachter H. Serum kynurenine and neopterin concentrations in patients with cardiomyopathy. Immunol Lett. 1992;32:125–9. doi: 10.1016/0165-2478(92)90104-v. [DOI] [PubMed] [Google Scholar]
- 31.Darlington LG, Forrest CM, Mackay GM, et al. On the biological importance of the 3-hydroxyanthranilic acid: anthranilic acid ratio. Int J Tryptophan Res. 2010;3:51–9. doi: 10.4137/ijtr.s4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ristagno G, Fries M, Brunelli L, et al. Early kynurenine pathway activation following cardiac arrest in rats, pigs, and humans. Resuscitation. 2013;84:1604–10. doi: 10.1016/j.resuscitation.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Bode C, Goebell H, Stähler E. Elimination of errors caused by turbidity in the determination of protein by the biuret method. Z Klin Chem Klin Biochem. 1968;6:418–22. [PubMed] [Google Scholar]
- 34.Sled VD, Vinogradov AD. Reductive inactivation of the mitochondrial three subunit NADH dehydrogenase. Biochim Biophys Acta. 1993;1143:199–203. doi: 10.1016/0005-2728(93)90143-4. [DOI] [PubMed] [Google Scholar]
- 35.Stahl WL, Smith JC, Napolitano LM, Basford RE. Brain mitochondria. I. isolation of bovine brain mitochondria. J Cell Biol. 1963;19:293–307. doi: 10.1083/jcb.19.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lubec B, Dell’Anna E, Fang-Kircher S, Marx M, Herrera-Marschitz M, Lubec G. Decrease of brain protein kinase C, protein kinase A, and cyclin-dependent kinase correlating with pH precedes neuronal death in neonatal asphyxia. J Investig Med. 1997;45:284–94. [PubMed] [Google Scholar]
- 37.Miche H, Brumas V, Berthon G. Copper(II) interactions with nonsteroidal anti-inflammatory agents. II. Anthranilic acid as a potential •OH-inactivating ligand. J Inorg Biochem. 1997;68:27–38. doi: 10.1016/s0162-0134(97)00005-6. [DOI] [PubMed] [Google Scholar]
- 38.Gaubert S, Bouchaut M, Brumas V, Berthon G. Copper-ligand interactions and the physiological free radical processes. Part 3. Influence of histidine, salicylic acid and anthranilic acid on copper-driven Fenton chemistry in vitro. Free Radic Res. 2000;32:451–61. doi: 10.1080/10715760000300451. [DOI] [PubMed] [Google Scholar]
- 39.Whetsell WO, Jr, Shapira NA. Neuroexcitation, excitotoxicity and human neurological disease. Lab Invest. 1993;68:372–87. [PubMed] [Google Scholar]
- 40.Baran H, Schwarcz R. Presence of 3-hydroxyanthranilic acid in rat tissues and evidence for its production from anthranilic acid in the brain. J Neurochem. 1990;55:738–44. doi: 10.1111/j.1471-4159.1990.tb04553.x. [DOI] [PubMed] [Google Scholar]
- 41.Pearson SJ, Reynolds GP. Increased brain concentrations of a neurotoxin, 3-hydroxykynurenine, in Huntington’s disease. Neurosci Lett. 1992;14:199–201. doi: 10.1016/0304-3940(92)90749-w. [DOI] [PubMed] [Google Scholar]
- 42.Colin-Gonzalez AL, Maldonado PD, Santamaria A. 3-Hydroxykynurenine: an intriguing molecule exerting dual actions in the central nervous system. Neurotoxicology. 2013;34:189–204. doi: 10.1016/j.neuro.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Lewitt PA, Li J, Lu M, Beach TG, Adler CH, Guo L. 3-hydroxykynurenine and other Parkinson’s disease biomarkers discovered by metabolomic analysis. Mov Disord. 2013;28:1653–60. doi: 10.1002/mds.25555. [DOI] [PubMed] [Google Scholar]
- 44.Christen S, Peterhans E, Stocker R. Antioxidant activities of some tryptophan metabolites: possible implication for inflammatory diseases. Proc Natl Acad Sci U S A. 1990;87:2506–10. doi: 10.1073/pnas.87.7.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reyes Ocampo J, Lugo Huitron R, Gonzalez-Esquivel D, et al. Kynurenines with neuroactive and redox properties: relevance to aging and brain diseases. Oxid Med Cell Longev. 2014 doi: 10.1155/2014/646909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giles GI, Collins CA, Stone TW, Jacob C. Electrochemical and in vitro evaluation of the redox-properties of kynurenine species. Biochem Biophys Res Commun. 2003;300:719–24. doi: 10.1016/s0006-291x(02)02917-0. [DOI] [PubMed] [Google Scholar]
- 47.Gramsbergen JB, Schmidt W, Turski WA, Schwarcz R. Age-related changes in kynurenic acid production in rat brain. Brain Res. 1992;588:1–5. doi: 10.1016/0006-8993(92)91337-e. [DOI] [PubMed] [Google Scholar]
- 48.Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Grant R. Changes in kynurenine pathway metabolism in the brain, liver and kidney of aged female Wistar rats. FEBS J. 2011;278:4425–34. doi: 10.1111/j.1742-4658.2011.08366.x. [DOI] [PubMed] [Google Scholar]
- 49.Moroni F, Russi P, Carla V, Lombardi G. Kynurenic acid is present in the rat brain and its content increases during development and aging processes. Neurosci Lett. 1988;94:145–50. doi: 10.1016/0304-3940(88)90285-6. [DOI] [PubMed] [Google Scholar]
- 50.Comai S, Costa CV, Ragazzi E, Bertazzo A, Allegri G. The effect of age on the enzyme activities of tryptophan metabolism along the kynurenine pathway in rats. Clin Chim Acta. 2005;360:67–80. doi: 10.1016/j.cccn.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 51.Kepplinger B, Baran H, Kainz A, Ferraz-Leite H, Newcombe J, Kalina P. Age-related increase of kynurenic acid in human cerebrospinal fluid–IgG and ß2-microglobulin changes. Neurosignals. 2005;14:126–35. doi: 10.1159/000086295. [DOI] [PubMed] [Google Scholar]
- 52.Lima VL, Dias F, Nunes RD, et al. The antioxidant role of xanthurenic acid in the Aedes aegypti midgut during digestion of a blood meal. PLoS One. 2012 doi: 10.1371/journal.pone.0038349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malina H, Richter C, Frueh B, Hess OM. Lens epithelial cell apoptosis and intracellular Ca2+ increase in the presence of xanthurenic acid. BMC Ophthalmol. 2002 doi: 10.1186/1471-2415-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malina HZ, Richter C, Mehl M, Hess OM. Pathological apoptosis by xanthurenic acid, a tryptophan metabolite: activation of cell caspases but not cytoskeleton breakdown. BMC Physiol. 2001 doi: 10.1186/1472-6793-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malina HZ, Hess OM. Xanthurenic acid translocates proapoptotic Bcl-2 family proteins into mitochondria and impairs mitochondrial function. BMC Cell Biology. 2004 doi: 10.1186/1471-2121-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gobaille S, Kemmel V, Brumaru D, Dugave C, Aunis D, Maitre M. Xanthurenic acid distribution, transport, accumulation and release in the rat brain. J Neurochem. 2008;105:982–93. doi: 10.1111/j.1471-4159.2008.05219.x. [DOI] [PubMed] [Google Scholar]
- 57.Malina HZ. Xanthurenic acid provokes formation of unfolded proteins in endoplasmic reticulum of the lens epithelial cells. Biochem Biophys Res Commun. 1999;265:600–5. doi: 10.1006/bbrc.1999.1716. [DOI] [PubMed] [Google Scholar]
- 58.Baran H, Kepplinger B. Cerebrolysin lowers kynurenic acid formation- an in vitro study. Eur Neuropsychopharmacol. 2009;19:161–8. doi: 10.1016/j.euroneuro.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Valousková V, Gschanes A. Effects of NGF, b-FGF, and cerebrolysin on water maze performance and on motor activity of rats: short- and long-term study. Neurobiol Learn Mem. 1999;71:132–49. doi: 10.1006/nlme.1998.3877. [DOI] [PubMed] [Google Scholar]
- 60.Ren J, Sietsma D, Qiu S, Moessler H, Finklestein SP. Cerebrolysin enhances functional recovery following focal cerebral infarction in rats. Restor Neurol Neurosci. 2007;25:25–31. [PubMed] [Google Scholar]
- 61.Rockenstein E, Adame A, Mante M, et al. Amelioration of the cerebrovascular amyloidosis in a transgenic model of Alzheimer’s disease with the neurotrophic compound Cerebrolysin™. J Neural Transm. 2005;112:269–82. doi: 10.1007/s00702-004-0181-4. [DOI] [PubMed] [Google Scholar]
- 62.Quagliariello E, Papa S, Saccone C, Alifano A. Effect of 3-hydroxyanthranilic acid on the mitochondrial respiratory system. Biochem J. 1964;91:137–46. doi: 10.1042/bj0910137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomas SR, Witting PK, Stocker R. 3-Hydroxyanthranilic acid is an efficient, cell-derived co-antioxidant for α-tocopherol, inhibiting human low density lipoprotein and plasma lipid peroxidation. J Biol Chem. 1996;271:32714–21. doi: 10.1074/jbc.271.51.32714. [DOI] [PubMed] [Google Scholar]
- 64.Hiramatsu R, Hara T, Akimoto H, et al. Cinnabarinic acid generated from 3-hydroxyanthranilic acid strongly induces apoptosis in thymocytes through the generation of reactive oxygen species and the induction of caspase. J Cell Biochem. 2008;103:42–53. doi: 10.1002/jcb.21384. [DOI] [PubMed] [Google Scholar]
- 65.Tsopmo A, Diehl-Jones BW, Aluko RE, Kitts DD, Elisia I, Friel JK. Tryptophan released from mother’s milk has antioxidant properties. Pediatr Res. 2009;66:614–8. doi: 10.1203/PDR.0b013e3181be9e7e. [DOI] [PubMed] [Google Scholar]
- 66.Pazos M, Andersen ML, Skibsted LH. Amino acid and protein scavenging of radicals generated by iron/hydroperoxide system: an electron spin resonance spin trapping study. J Agric Food Chem. 2006;54:10215–21. doi: 10.1021/jf062134n. [DOI] [PubMed] [Google Scholar]
- 67.Matuszak Z, Reszka K, Chignell CF. Reaction of melatonin and related indoles with hydroxyl radicals: EPR and spin trapping investigations. Free Radic Biol Med. 1997;23:367–72. doi: 10.1016/s0891-5849(96)00614-4. [DOI] [PubMed] [Google Scholar]
- 68.Peyrot F, Ducrocq C. Potential role of tryptophan derivatives in stress responses characterized by the generation of reactive oxygen and nitrogen species. J Pineal Res. 2008;45:235–46. doi: 10.1111/j.1600-079X.2008.00580.x. [DOI] [PubMed] [Google Scholar]
- 69.Bitzer-Quintero OK, Davalos-Marin AJ, Ortiz GG, et al. Antioxidant activity of tryptophan in rats under experimental endotoxic shock. Biomed Pharmacother. 2010;64:77–81. doi: 10.1016/j.biopha.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 70.Bordelon YM, Chesselet MF, Nelson D, Welsh F, Erecinska M. Energetic dysfunction in quinolinic acid-lesioned rat striatum. J Neurochem. 1997;69:1629–39. doi: 10.1046/j.1471-4159.1997.69041629.x. [DOI] [PubMed] [Google Scholar]
- 71.Baran H, Kepplinger B, Lang C, et al. Enhancement of kynurenic acid (KYNA) and neopterin levels in serum of piglets after encephalomyocarditis virus (EMCV) infection; Proceedings of the 18th Congress of the International Pig Veterinary Society; 2004. p. 364. [Google Scholar]
- 72.Guidetti P, Schwarcz R. 3-Hydroxykynurenine and quinolinate: pathogenic synergism in early grade Huntington’s disease? Adv Exp Med Biol. 2003;527:137–45. doi: 10.1007/978-1-4615-0135-0_16. [DOI] [PubMed] [Google Scholar]