Abstract
Rationale: Modulation of breathing by hypoxia accommodates variations in oxygen demand and supply during, for example, sleep and ascent to altitude, but the precise molecular mechanisms of this phenomenon remain controversial. Among the genes influenced by natural selection in high-altitude populations is one for the adenosine monophosphate–activated protein kinase (AMPK) α1-catalytic subunit, which governs cell-autonomous adaptations during metabolic stress.
Objectives: We investigated whether AMPK-α1 and/or AMPK-α2 are required for the hypoxic ventilatory response and the mechanism of ventilatory dysfunctions arising from AMPK deficiency.
Methods: We used plethysmography, electrophysiology, functional magnetic resonance imaging, and immediate early gene (c-fos) expression to assess the hypoxic ventilatory response of mice with conditional deletion of the AMPK-α1 and/or AMPK-α2 genes in catecholaminergic cells, which compose the hypoxia-responsive respiratory network from carotid body to brainstem.
Measurements and Main Results: AMPK-α1 and AMPK-α2 deletion virtually abolished the hypoxic ventilatory response, and ventilatory depression during hypoxia was exacerbated under anesthesia. Rather than hyperventilating, mice lacking AMPK-α1 and AMPK-α2 exhibited hypoventilation and apnea during hypoxia, with the primary precipitant being loss of AMPK-α1 expression. However, the carotid bodies of AMPK-knockout mice remained exquisitely sensitive to hypoxia, contrary to the view that the hypoxic ventilatory response is determined solely by increased carotid body afferent input to the brainstem. Regardless, functional magnetic resonance imaging and c-fos expression revealed reduced activation by hypoxia of well-defined dorsal and ventral brainstem nuclei.
Conclusions: AMPK is required to coordinate the activation by hypoxia of brainstem respiratory networks, and deficiencies in AMPK expression precipitate hypoventilation and apnea, even when carotid body afferent input is normal.
Keywords: adenosine monophosphate–activated protein kinase, apnea, brainstem, hypoxia, ventilation
At a Glance Commentary
Scientific Knowledge on the Subject
Ventilatory adjustments are critical to the body’s capacity to accommodate deficits in oxygen availability during sleep and ascent to altitude. During hypoxia, increased afferent discharge from the carotid bodies to the brainstem delivers increased ventilatory drive, which restores oxygen supply and protects against apnea. The precise molecular mechanisms involved remain unclear. However, natural selection in high-altitude populations has acted on the gene for the α1-catalytic subunit of the adenosine monophosphate–activated protein kinase (AMPK), which governs cell-autonomous adaptations during metabolic stress.
What This Study Adds to the Field
We demonstrate, for the first time to our knowledge, that AMPK deficiency leads to respiratory depression during hypoxia characterized by hypoventilation and apnea rather than hyperventilation. Moreover, consequent deficits in the drive to breathe during hypoxia arise at the level of the brainstem, even when carotid body afferent input is normal. This identifies AMPK as a potential new target for therapeutic interventions for sleep-disordered breathing associated with metabolic syndrome–related disorders and ascent to altitude.
Adenosine monophosphate (AMP)–activated protein kinase (AMPK) is central to the cell-autonomous control of energy supply (1) and comprises a heterotrimer of catalytic α- and regulatory β- and γ-subunits, which are ubiquitously expressed throughout eukaryotes. Under metabolic stresses such as hypoxia, binding of AMP to one site on the γ-subunit and AMP or adenosine diphosphate (ADP) to another increases AMPK activity by, respectively, allosteric activation and promotion of increased phosphorylation at Thr172 of the α-subunit by liver kinase B1 (2, 3). Alternatively, calcium calmodulin–dependent kinase kinase-β may activate AMPK in response to increases in cytoplasmic calcium (4). AMPK switches off anabolic pathways and switches on catabolic pathways (1), thereby compensating for deficits in ATP supply via, for example, mitochondrial oxidative phosphorylation. Regulated O2 supply is key to the maintenance of oxidative phosphorylation and thus cellular energy status in mammals, not least because of the limited capacity for cellular O2 storage relative to the extensive reserves of other substrates. It was proposed, therefore, that natural selection may have employed AMPK to coordinate whole-body adjustments in response to O2 deficits in animals (5). Consistent with this view, recent studies on high-altitude Andean populations showed that the gene for the AMPK α1-subunit (PRKAA1) has been influenced by natural selection through single-nucleotide polymorphisms (6). Moreover tissue-specific changes in AMPK expression occur in metabolic syndrome–related disorders that are associated with sleep-disordered breathing (7, 8).
Ventilatory adjustments are critical to the body’s capacity to accommodate variations in O2 demand and supply during sleep and ascent to altitude. This phenomenon is exemplified by the fact that adaptation of mammals to hypoxia at altitude is initially characterized by progressive increases in ventilatory drive that partially restore arterial Po2 and protect against apnea (9). Ventilatory movements are delivered by respiratory central pattern generators (rCPGs) distributed bilaterally in the pons and ventral medulla. These semiautonomous neural networks comprise core circuits of excitatory and inhibitory interneurons that deliver rhythmic patterns of activity (10) and confer a set point about which respiratory rhythm is continuously modulated through the integration of inputs from those central (10, 11) and peripheral (12) chemosensors that monitor O2, CO2, and pH. It is generally accepted that the carotid bodies represent the primary arterial chemoreceptors (12) and that the acute hypoxic ventilatory response (HVR) is delivered by increased afferent discharge from the carotid bodies to the rCPGs via, in great part, catecholaminergic networks within the caudal brainstem (13, 14).
The present study demonstrates conclusively that AMPK deficiency attenuates the HVR, and thus precipitates hypoventilation and apnea, by blocking the activation of hypoxia-responsive nuclei within the caudal brainstem (15, 16), even where carotid body afferent inputs are normal. Some of these results were previously reported in the form of an abstract (17).
Methods
The experiments in this study were carried out in compliance with the regulations of the U.K. Animals (Scientific Procedures) Act of 1986.
Breeding of Mice, Genotyping, and Single-Cell Polymerase Chain Reactions
Standard approaches were used in the experiments (see Supplementary Methods in the online supplement). All mice studied were between 3 and 12 months of age.
Computational Video Analysis of Thoracic Activity
Thoracic movements were captured by digital camera, and the thoracic motion index was calculated for each successive frame (see Supplementary Methods).
Isolated Carotid Body
The methods used to study single-fiber chemoafferent activity were adapted from those described previously (18) (see Supplementary Methods). Plots of firing frequency versus superfusate Po2 were fitted by nonlinear regression using Prism software (GraphPad Software, La Jolla, CA).
Plethysmography
Following 10- to 20-minute acclimation under normoxia (air), mice were exposed to hypoxia (12% or 8% O2 with 0.05% CO2, balanced with N2) or hypoxia and hypercapnia (8% O2, 5% CO2, balanced with N2). Apnea was defined as cessations of breathing greater than the average duration, including an interval, of two successive breaths (500 ms) during normoxia (19), with a detection threshold of 0.25 mm Hg (SD of noise). Breathing variability was assessed using Poincaré plots and by calculating the SD of interbreath intervals (BBn) (19).
Functional Magnetic Resonance Imaging
Mice were anesthetized with 0.8–1.3% isoflurane in air, their body temperature was maintained at 37°C, and their breathing frequency was monitored using pressure pad sensors. A 7-Tesla horizontal bore magnetic resonance imaging scanner (Agilent Technologies, Yarnton, UK), equipped with a high-performance gradient insert (12-cm inner diameter, maximum gradient strength 400 mT/m) was used. A birdcage coil (72-mm diameter) delivered radiofrequency transmission with signal reception via a mouse two-channel phased array brain coil. All sequences were acquired with a field of view 19.2 × 19.2 mm with 30 contiguous coronal slices of 0.4-mm thickness.
Structural images were acquired by fast spin echo sequence (train length, 8; repetition time, 3,100 ms; effective echo time, 36 ms; 8 signal averages; acquisition matrix, 192 × 192; zero filled to 256 × 256). Functional images (230 volumes) were acquired during normoxia (21% O2) and hypoxia (8% O2) using three-shot echo planar imaging sequences (repetition time, 6,000 ms [2,000 ms/shot]; effective echo time, 7.08 ms; flip angle, 90 degrees; 1 signal average; acquisition matrix, 64 × 64).
Bias-corrected (www.slicer.org) structural images were coregistered to a template (www.spmmouse.org) and averaged using SPM8 imaging software (Wellcome Trust Centre for Neuroimaging, University College London, London, UK). Functional data were realigned to mean volumes of each series, spatially normalized (SPM8 coregistration procedures) using each animal’s structural scan, and smoothed using a 0.7 × 0.7 × 4–mm full width at half maximum Gaussian filter.
For first-level analysis, see the Supplementary Methods. In second-level (group) analysis, the t-map for contrast (knockout > control) was thresholded at a level of P < 0.005 with a four-voxel cluster threshold (SPM8 imaging software). Between-group differences were analyzed using a region-of-interest tool. For regions with significant group differences, all voxel signals were averaged at each time point (see also Supplementary Methods).
c-Fos Labeling
Mice were subjected to perfusion following a single exposure to hypoxia (8% O2), brain sections incubated in rabbit anti-c-Fos (1:20,000 or 1:100,000; Calbiochem, San Diego, CA) and rat anti–tyrosine hydroxylase (anti-TH; 1:1000; EMD Millipore, Billerica, MA) antibodies diluted in blocking buffer, then in biotinylated horse antirabbit IgG (1:500; Vector Laboratories, Burlingame, CA), and goat antirat IgG (1:750, A594; Invitrogen, Paisley, UK) secondary antibodies (see also Supplementary Methods).
Statistical Analysis
Statistical comparisons were made using GraphPad Prism 6 software for the following: for afferent discharge, single- or two-factor analysis of variance with Bonferroni–Dunn post hoc analysis; and for plethysmography, one-way analysis of variance with Bonferroni multiple-comparisons test. P < 0.05 was considered significant. For functional magnetic resonance imaging (fMRI), a two-sample t test (SPM8) was used with the level of significance set at P < 0.005.
Results
Loss of AMPK Leads to Ventilatory Dysfunction during Hypoxia
Global knockout of both AMPK-α1 and AMPK-α2 is embryonic lethal. We therefore employed conditional deletion of AMPK-α1 and AMPK-α2 subunits using mice in which these genes were flanked by loxP sequences (α1flx/α2flx) (20). To direct AMPK deletion to carotid body (12) and brainstem (21) catecholaminergic cells, which contribute to ventilatory control during hypoxia, these knockout mice were crossed with mice in which Cre recombinase was targeted via the tyrosine hydroxylase (TH) promoter (22). Transient developmental expression of TH occurs in disparate cell groups that do not express TH in the adult mouse (22), including dorsal root ganglion cells, pancreatic islets, a subset of heart wall cells, and a subset of gastrointestinal tract cells, none of which contribute to the acute HVR. Therefore, such conditional AMPK deletion overcomes embryonic lethality and provides greater discrimination with respect to circuit mechanisms. Cell-specific gene deletion was confirmed by single-cell endpoint reverse transcription–polymerase chain reaction and whole-brain quantitative polymerase chain reaction (Figures 1A and 1B), and restriction of Cre to TH-positive cells in the adult mouse was verified by viral transfection of a Cre-inducible vector carrying a reporter gene (see Figure E1 in the online supplement).
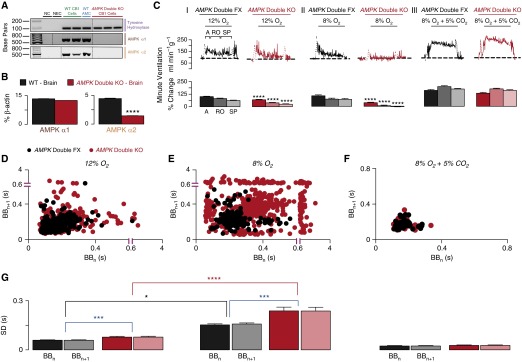
Figure 1.
Conditional deletion of adenosine monophosphate–activated protein kinase (AMPK) α-catalytic subunits in tyrosine hydroxylase–expressing cells markedly attenuates the hypoxic ventilatory response. (A) Single-cell endpoint reverse transcription–polymerase chain reaction amplicons for tyrosine hydroxylase and the catalytic α1- and α2-subunits of AMPK from acutely isolated carotid body type I (CB1) cells of wild-type (WT) and AMPK-α1/AMPK-α2 double-knockout (AMPK Double KO) mice. (B) Quantitative reverse transcription–polymerase chain reaction assays of AMPK-α1 and AMPK-α2 subunit expression of mouse brain as a percentage of β-actin from WT and AMPK-α1/AMPK-α2 double-knockout mice (n = 3). (C) Upper panels show example records of minute ventilation (in milliliters per minute per gram of body weight) versus time during (I) 12% O2, (II) 8% O2, and (III) 8% O2 with 5% CO2 for AMPK-α1/AMPK-α2 double-floxed (AMPK Double FX, black; n = 31) and AMPK-α1/AMPK-α2 double-knockout (AMPK Double KO, red; n = 22) mice. Lower panels show mean ± SEM for increase in minute ventilation for the peak of the augmenting phase (A), after 100 seconds of roll off (RO), and the plateau of the sustained phase (SP) of the response to hypoxia. ****P < 0.0001. (D–F) Exemplary Poincaré plots of the interbreath interval (BBn) versus subsequent interval (BBn+1) of AMPK-α1/AMPK-α2 double-floxed (AMPK Double FX, black) and AMPK-α1/AMPK-α2 double-knockout (AMPK Double KO, red) mice during (D) mild hypoxia (12% O2 + 0.05% CO2), (E) severe hypoxia (8% O2 + 0.05% CO2), and (F) hypoxia with hypercapnia (8% O2 + 5% CO2). (G) Corresponding mean ± SEM for the SD of BBn and BBn+1 for each genotype during 12% O2 (AMPK double FX, n = 31; AMPK double KO, n = 22), 8% O2 + 0.05% CO2 (AMPK Double FX, n = 12; AMPK Double KO, n = 19), and 8% O2 + 5% CO2 (AMPK Double FX, n = 20; AMPK Double KO, n = 29). *P < 0.05; ***P < 0.001; ****P < 0.0001. AMC = adrenomedullary chromaffin cells; NC = negative control (cell aspirant but no cell reverse transcriptase added); NEC = negative extracellular control (aspirant of extracellular medium).
We found no significant difference between AMPK-α1/AMPK-α2 double knockouts and controls under normoxic conditions with respect to arterial oxygen saturation as measured by pulse oximetry (SpO2) (see Figure E2), venous blood gases, venous blood pH (see Table E1), weight gain versus age (see Figure E3), breathing frequency, tidal volume, or minute ventilation (see Figure E4). Nevertheless, AMPK deletion profoundly affected the HVR.
In control mice, hypoxia evoked pronounced hyperventilation characterized by robust increases in minute ventilation (Figures 1C and 1D), breathing frequency, and tidal volume (see Figure E5). Dual deletion of AMPK-α1 and AMPK-α2 markedly attenuated the HVR in response to 12% (mild hypoxia) (Figure 1C) and 8% O2 (severe hypoxia) (Figure 1C), which conferred comparable stimuli in knockouts and controls in terms of the fall in arterial SpO2 (see Figure E2). Surprisingly, the degree to which the HVR was attenuated increased in a manner directly related to the severity of hypoxia (Figures 1C and 1D), suggesting that AMPK offsets respiratory depression during hypoxia.
Depression of the HVR was most severe upon exposure of AMPK-α1/AMPK-α2 double knockouts to 8% O2, which highlights the contribution of AMPK to all phases of the response. For controls, the initial augmenting phase, which is generally accepted to result from increased afferent input from the carotid body, peaked at 106 ± 10% (∼30 s) relative to normoxia. Following subsequent ventilatory depression (roll off, ∼100 s), minute ventilation declined to 72 ± 8% greater than that measured during normoxia, which was maintained for the latter 2–5 minutes of hypoxia (sustained phase). For AMPK-α1/AMPK-α2 double knockouts, minute ventilation during the HVR was markedly attenuated compared with controls, measuring only 36 ± 5% relative to normoxia (P < 0.0001) during the augmenting phase before declining to −4 ± 5% during the sustained phase (2–5 min; 8% O2) (P < 0.0001). Deficits in minute ventilation resulted from reductions in both breathing frequency and tidal volume (see Figure E5). By contrast, marked and comparable increases in minute ventilation were evoked upon exposure of controls and knockouts to hypoxia with hypercapnia (8% O2 and 5% CO2) (Figure 1C). Moreover, in analysis of BBn, we identified greater levels of disordered breathing in AMPK knockouts during hypoxia but not during hypercapnic hypoxia, and the severity of disordered breathing increased in a manner directly related to the degree of hypoxia (Figures 1D–1G).
Hypoxia-evoked hypoventilation in AMPK-α1/AMPK-α2 double knockouts was accompanied by frequent, prolonged apneas (≤6 s) (Figures 2A and 2B; see also Movies E1 and E2). Both spontaneous and post-sigh apneas were observed in AMPK-α1/AMPK-α2 double knockouts (Figure 2B). These resulted from failure of ventilatory drive, which was evident from the absence within ventilatory records of transitions between inspiration and expiration (personal communication, Buxco Research Company/Data Sciences International, St. Paul, MN) and confirmed by computational video analysis of thoracic activity (Figure 2C; see also Movies E1 and E2). At 8% O2, apnea frequency measured 14.3 ± 2.2 per minute, apnea duration was 953 ± 40 milliseconds, and apnea duration index (frequency × duration) was 13.8 ± 2.2, which were significantly greater (P < 0.0001) than measures of apnea frequency (1.8 ± 0.3/min), apnea duration (709 ± 17 ms), and apnea duration index (1.3 ± 0.2) during hyperventilation in controls (Figures 2DI–2DIII). As might be expected, given the outcomes for minute ventilation, apnea frequency and duration also increased in a manner directly related to the severity of hypoxia, and these increases were completely reversed during hypercapnic hypoxia (Figure 2D). Therefore, AMPK deletion selectively blocks the HVR and thus precipitates hypoventilation and apnea. Furthermore, because hypercapnic ventilatory drive is retained in these mice and is delivered by CO2- and/or pH-sensitive catecholaminergic neurons (14, 23), it is evident that AMPK deletion does not simply block neuronal activation or synaptic transmission per se (see also below, AMPK Is Not Required for Carotid Body Activation by Hypoxia).
Figure 2.
Deletion of adenosine monophosphate–activated protein kinase (AMPK) precipitates hypoventilation and apnea. (A) Records of ventilatory activity obtained using whole-body plethysmography from AMPK-α1/AMPK-α2 double-floxed (AMPK Double FX; n = 31) and AMPK-α1/AMPK-α2 double-knockout (AMPK Double KO; n = 22) mice during (I) normoxia (21% O2), (II) hypoxia (8% O2), and (III) hypoxia with hypercapnia (8% O2 + 5% CO2). (BI and BII) Typical ventilatory records on an expanded time scale during hypoxia (8% O2). (CI and CII) Computational video analysis of thoracic movement (upper panels) with corresponding ventilatory traces (lower panels). (D) Mean ± SEM for (I) apneic index (per minute), (II) apnea duration (in seconds), and (III) apnea duration index (ADI) (frequency × duration) (AMPK Double FX, n = 31; AMPK Double KO, n = 22). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
AMPK Is Not Required for Carotid Body Activation by Hypoxia
Surprisingly, reductions in superfusate Po2 increased chemoafferent discharge exponentially in all carotid bodies (Figures 3A and 3B), with peak discharge frequency (at Po2 ∼74 mm Hg) measuring 12.5 ± 1.6 Hz (n = 10) for controls and 13.9 ± 1.5 Hz (n = 8; P < 0.001) for AMPK-α1/AMPK-α2 double knockouts. Therefore, AMPK is not required for carotid body activation during hypoxia, and AMPK deletion does not compromise either exocytosis in or subsequent synaptic transmission by TH-expressing cells. Hence, AMPK deletion must block the HVR by attenuating activation of the hypoxia-responsive respiratory network at a point distal to the carotid body.
Figure 3.
Adenosine monophosphate–activated protein kinase (AMPK) deletion does not inhibit carotid body activation by hypoxia. Upper panels show extracellular recordings of single-fiber chemoafferent discharge versus Po2 for (A) AMPK-α1/AMPK-α2 double-floxed (AMPK Double FX) and (B) AMPK-α1/AMPK-α2 double-knockout (AMPK Double KO) mice. (Inset) Single-fiber discriminations. (Middle panels) Frequency × time histograms. (Lower panels) Frequency–Po2 response curves.
AMPK Deletion Markedly Attenuates Activation of Brainstem Nuclei by Hypoxia
Catecholaminergic and other neurons of the nucleus tractus solitarius (NTS) and ventrolateral medulla receive inputs from the carotid body (21, 24, 25), are activated by central nervous system hypoxia, and may independently regulate rCPGs in a manner dependent on continued basal (normoxic) afferent input from the carotid bodies (13, 16, 26). Brainstem activity during hypoxia was therefore assessed by fMRI in anesthetized mice in light of the fact that 9% or less O2 induces cerebral vasodilation sufficient to eliminate the blood oxygen–level dependent (BOLD) signal driven, under normoxia, by increases in cerebral blood flow in response to sensory stimulation (27). This allowed analysis of regional differences in signal change resulting from reductions in O2 consumption (decreased BOLD signal) in AMPK-α1/AMPK-α2 double knockouts relative to controls during acute exposure to 8% O2 (2 min; ∼40% SpO2).
In second-level (group) fMRI analysis during hypoxia, we identified significantly different BOLD signals in two well-defined dorsal active regions (DARs) and ventral active regions (VARs) of the caudal brainstem of all AMPK-α1/AMPK-α2 double knockouts compared with controls (Figures 4C and 4D). Signal change for the DAR measured −23.58 ± 1.17 in controls and −14.93 ± 0.91 in AMPK-α1/AMPK-α2 double knockouts (P < 0.01) (Figure 4E). Signal change for the VAR measured −11.78 ± 0.91 in controls and −8.3 ± 0.37 in AMPK-α1/AMPK-α2 double knockouts (P < 0.05). The DAR and VAR exhibited pronounced right–left asymmetry, although removing the four-voxel cluster threshold revealed limited evidence of right side–dominant bilateral symmetry for the VAR alone (see Figure E6). Hypoxia (8% O2) evoked concomitant increases in breathing frequency of 16.54 ± 12.92 breaths per minute in controls (n = 5) (Figures 4A and 4B). By contrast, all AMPK-α1/AMPK-α2 double knockouts exhibited concomitant decreases in breathing frequency of −59.80 ± 1.75 breaths per minute (n = 6; P < 0.001) (Figures 4A and 4B). Furthermore, anesthesia sufficient to decrease the breathing frequency of AMPK-α1/AMPK-α2 double knockouts less than 90 breaths per minute precipitated irreversible respiratory failure within 30 seconds of hypoxia (n = 4; excluded from fMRI analysis). Therefore, anesthesia exacerbates hypoxic respiratory depression in AMPK-α1/AMPK-α2 double knockouts.
Figure 4.
Functional magnetic resonance imaging demonstrates that adenosine monophosphate–activated protein kinase (AMPK) deletion inhibits activation by hypoxia of dorsal and ventral regions of the brainstem. (A) Example records of breathing frequency (breaths per minute) versus time during hypoxia (8% O2) for anesthetized AMPK-α1/AMPK-α2 double-floxed (AMPK Double FX, black; n = 6) and AMPK-α1/AMPK-α2 double-knockout (AMPK Double KO, red; n = 6) mice. (B) Mean ± SEM for change in breathing frequency during hypoxia. **P < 0.01. (C) Dorsal active region (DAR) and ventral active region (VAR) of brainstem that exhibited significantly lower signal change (P < 0.005) during hypoxia in AMPK Double KO than in Double FX control mice. (D) Variability of signal time courses for DAR and VAR are shown in blue for each individual mouse (same mice as in Figures 4A, lower panel) overlaid on mean signal for AMPK Double FX (black line) and AMPK Double KO (red line). AU = arbitrary units. (E) Mean ± SEM percentage signal changes for DAR and VAR during hypoxia (same mice as in Figure 4A, lower panel). *P < 0.05; **P < 0.01.
The caudal location relative to the bregma of the DAR (Figure 4C) is consistent with that of the NTS, within which poorly defined subgroups of A2 catecholaminergic neurons receive carotid body afferent inputs (24) and are activated during hypoxia (16, 21). The location of the VAR aligns well with the C1/A1 catecholaminergic neurons of the ventrolateral medulla, which are activated during hypoxia via afferent inputs from both carotid bodies and the NTS (21, 28, 29). We therefore used immediate early gene expression (c-fos), the only truly physiological approach available (13, 21), to identify the subpopulations of cells within the brainstem of AMPK-α1/AMPK-α2 double knockouts that exhibit deficits in activity during hypoxia in vivo. Cell counts revealed no deficit in TH-positive cells across any of the regions studied (not shown). Regardless, AMPK deletion selectively attenuated hypoxia-evoked (8% O2) increases in c-fos expression in cells adjacent to the area postrema (subpostrema [SubP]) and within the medial subnucleus (SolM) (Figure 5A, left panel). The SubP of controls contained 1.09 ± 0.56 TH-positive and c-Fos–positive cells per 100 μm2 and 0.79 ± 0.21 c-Fos–positive and TH-negative cells per 100 μm2, which decreased in AMPK-α1/AMPK-α2 double knockouts to, respectively, 0.27 ± 0.1 cells per 100 μm2 (P < 0.01) and 0.28 ± 0.1 cells per 100 μm2 (P < 0.01) (Figure 5B). In SolM, however, a reduction in c-fos expression was observed only in TH-negative cells, from 1.91 ± 0.3 cells per 100 μm2 in controls to 1.16 ± 0.27 cells per 100 μm2 (P < 0.05) in AMPK-α1/AMPK-α2 double knockouts (Figure 5B). By contrast, a small but significant increase in the number of c-Fos–positive cells was observed in TH-positive neurons within the commissural, ventral, and ventrolateral A2 regions (Figure 5B). Rostral to these A2 nuclei, a significant reduction in c-fos expression was also observed in all dorsal C2 neurons (Figures 5A and 5B), from 6.75 ± 0.62 to 5.4 ± 0.44 TH-positive cells per 100 μm2 (P < 0.05) and from 0.78 ± 0.20 to 0.23 ± 0.1 TH-negative cells per 100 μm2 (P < 0.05).
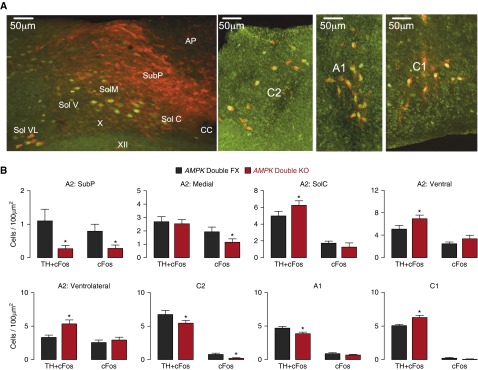
Figure 5.
Adenosine monophosphate–activated protein kinase (AMPK) deletion attenuates c-fos expression in discrete areas of the nucleus tractus solitarius and ventrolateral medulla. (A) Immunostaining of a brainstem section derived from an AMPK-α1/AMPK-α2 double-knockout mouse (AMPK Double KO) shows c-Fos (green) and tyrosine hydroxylase (TH, red) staining in the A2 and C2 regions of the nucleus of the solitary tract as well as in the ventrolateral C1/A1 region. Scale bar = 50 μm. (B) Bar charts show for each region shown in A the number of TH-positive and TH-negative cells per 100 μm2 in which c-Fos expression increased during hypoxia (8% O2) for AMPK-α1/AMPK-α2 double-floxed mice (AMPK Double FX, black; n = 19 sections, n = 6 mice) and AMPK Double KO mice (red; n = 17 sections, n = 6 mice). *P < 0.01. AP = area postrema; CC = central canal; SolC = commissural, SolM = medial, SolV = ventral, SolVL = ventrolateral, and SubP = subpostrema regions of the nucleus of the solitary tract; X = dorsal motor nucleus of the vagus; XII = hypoglossal nucleus.
Ventral A1 regions proximal to the VAR identified by fMRI of AMPK-α1/AMPK-α2 double knockouts exhibited selective reductions of c-fos expression in TH-positive but not TH-negative cells, from 4.66 ± 0.26 to 3.88 ± 0.23 cells per 100 μm2 (P < 0.05) (Figures 5A and B). By contrast, AMPK deletion increased the number c-fos–expressing, TH-positive cells within the C1 region after hypoxia, from 5.05 ± 0.21 to 6.29 ± 0.30 cells per 100 μm2 (P < 0.05) (Figures 5A and 5B).
These data suggest differential AMPK dependency of the activation during hypoxia of dorsal and ventral brainstem nuclei known to contribute to the HVR. Although we cannot rule out the possibility of confounding developmental abnormalities associated with AMPK deletion, we observed no deficits in ventilatory function during normoxia or hypercapnic hypoxia, no obvious structural anomaly by fMRI, and no deficit in TH-positive or TH-negative cell counts within the brainstem of AMPK-α1/AMPK-α2 double knockouts.
Loss of AMPK α1-Subunit Expression Is the Primary Cause of Ventilatory Dysfunction during Hypoxia
Figure 6 shows a comparison of exemplary Poincaré plots of BBn versus subsequent BBn (BBn+1) during normoxia (Figure 6AI) and hypoxia (Figure 6BI) for controls and knockouts of AMPK-α2, AMPK-α1, and AMPK-α1/AMPK-α2. During hypoxia, while BBn of AMPK-α2 knockouts were similar to those of controls, breathing irregularities resulted from AMPK-α1 deletion. These irregularities increased in severity with deletion of both AMPK-α1 and AMPK-α2. Confirmation was provided by comparison of the SD of BBn (Figures 6AII and 6BII), which increased from 167 ± 24 milliseconds during hypoxia with AMPK-α1 deletion to 238 ± 23 milliseconds with deletion of both AMPK-α1 and AMPK-α2 (P < 0.001). Likewise, comparison of apnea duration index (Figure 6C) and minute ventilation (Figure 6D) demonstrated that ventilatory dysfunction was driven primarily by loss of AMPK-α1 but was more severe for AMPK-α1/AMPK-α2 double knockouts. Hence AMPK-α2 containing heterotrimers confer only marginal compensation for loss of AMPK-α1.
Figure 6.
Respiratory dysfunction during hypoxia is mediated primarily by loss of the adenosine monophosphate–activated protein kinase (AMPK) α1-catalytic subunit. Exemplary Poincaré plots of the interbreath interval (BBn) versus subsequent interval (BBn+1) for mice with AMPK-α1/AMPK-α2 double floxed (AMPK Double FX; n = 31), AMPK-α2 knockout (AMPK-α2 KO; n = 16), AMPK-α1 knockout (AMPK-α1 KO; n = 19), and AMPK-α1/AMPK-α2 double knockout (AMPK Double KO; n = 22) during (AI) normoxia (21% O2) and (BI) hypoxia (8% O2). (AII and BII) Corresponding mean ± SEM for the SD of BBn and BBn+1 for each genotype under normoxia and 8% O2. (C) Mean ± SEM for (I) apneic index (per minute), (II) apnea duration (in seconds), and (III) apnea duration index (ADI). (D) Increases in minute ventilation relative to normoxia at the peak of the augmenting phase (A), after 100 seconds of roll off (RO), and the plateau of the sustained phase (SP) of the response to hypoxia. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Discussion
Deficiencies of AMPK expression precipitated ventilatory dysfunction during hypoxia characterized by marked hypoventilation rather than hyperventilation and frequent, prolonged apneas. Upon hypoxia at altitude or during sleep, AMPK activation may therefore support hyperventilation and thus protect against hypoxia-induced ventilatory instability (9), while AMPK deficiency may confer greater susceptibility to disordered breathing. That loss of AMPK-α1 was the primary precipitant is notable in this respect, given that natural selection in high-altitude Andean populations has led to single-nucleotide polymorphisms in PRKAA1, the gene for AMPK-α1 (6).
Against expectations, however, AMPK deletion failed to attenuate hypoxia-evoked afferent discharge from the carotid body. This is contrary to our previous pharmacological studies, which suggested that AMPK activation by hypoxia might elicit type I cell exocytosis and increase afferent discharge (18), but is consistent with more recent investigations (30). Previous conflicting observations may have arisen due to off-target effects of pharmacological tools, such as aminoimidazole-4-carboxamide-1-β-d-ribofuranoside–mediated reductions in the adenylate pool and ATP (20, 31). Regardless, AMPK is clearly not necessary for carotid body activation by hypoxia. Given that AMPK deletion blocked the HVR, this may appear contrary to the view that increased afferent discharge from the carotid body to the brainstem determines the ventilatory response to a fall in arterial Po2 (12). However, evidence suggests that the HVR is determined by the coordinated action of the carotid body and a hypoxia-responsive circuit within the brainstem (26) comprising, in great part, TH-expressing catecholaminergic cells (14, 15).
Consistent with this view, in fMRI analysis, we identified that AMPK deletion markedly attenuated activation by hypoxia of dorsal and ventral nuclei of the caudal brainstem, even though carotid body afferent discharge was retained, and this was corroborated by our analysis of c-fos expression. The caudal location relative to the bregma of the DAR is consistent with areas of the NTS that are activated by hypoxia and which represent the primary site of receipt of carotid body afferent input (13, 16, 24). Here AMPK deletion selectively attenuated c-fos expression during hypoxia by mixed subpopulations of C2 neurons and A2 neurons (SubP, SolM) within the SolM proximal to the midline and the SubP, which are activated during hypoxia (21). A2 neurons of the NTS provide afferent input to and determine, together with the carotid body, activation by hypoxia of A1/C1 neurons within the ventrolateral medulla (21, 28), the position of which (28) aligns well with the VAR identified by fMRI analysis. By contrast, projections of the NTS mostly avoid (28) the Bötzinger and pre-Bötzinger complexes (32). AMPK deletion selectively reduced c-fos expression by TH-positive A1 neurons during hypoxia. This may be significant, given that carotid body afferent input alone is sufficient to activate C1 neurons and thus elicits hypotension (29), which was likewise induced by hypoxia in AMPK knockouts and controls.
Our findings therefore suggest that respiratory input from the carotid chemoreflex may be blocked by loss of AMPK function within a neuronal circuit spanning the C2/A2 neurons of the NTS and the A1 neurons of the ventrolateral medulla, which opposes respiratory depression during hypoxia. This is consistent with optogenetic and pharmacological interventions at the NTS (16, 33) and the proposal that NTS neurons lie on the sensory side of the central respiratory network (34, 35). Observed right–left asymmetry of brainstem activation during hypoxia (Figure 4) may provide for specialization sufficient to prevent delays in respiratory responses to hypoxic stress by limiting conflicting outputs from each side of the brain (36) as for cognitive performance (37). Furthermore, and despite the fact that cell counts were not biased by considerations on right–left asymmetry of brainstem activation, the observed changes in c-fos expression suggest that AMPK deficiency attenuates the activation by hypoxia of a discrete subpopulation of respiratory neurons within the C2, A2, and A1 nuclei, which may also explain in part the modest deficits in c-fos expression of AMPK knockouts relative to the ventilatory dysfunction observed. In this respect, it is notable that C2 and A2 neurons are both catecholaminergic and glutamatergic (34, 38) and that 6–10% of TH-positive C2, A2, and A1 neurons also express neuronal nitric oxide synthase, which supports the HVR by synthesizing NO (39) and/or S-nitrosothiols (40) and in a manner that may be facilitated by AMPK (41). However, further investigation is required to determine how right–left asymmetry is orchestrated by AMPK and the complex interplay of those neurotransmitters deployed during hypoxia.
AMPK deletion in catecholaminergic cells may simply lead to the failure of central integration and transduction of peripheral chemoafferent input and consequent failure of the HVR due to the inability of affected neurons to respond appropriately to metabolic stress (42). However, given that carotid body afferent discharge remains exquisitely sensitive to a fall in Po2 and that ventilatory responses to hypercapnia remain unaffected even during severe (8%) hypoxia, our data demonstrate that AMPK deletion does not compromise, per se, the capacity during hypoxia for activation of chemosensory catecholaminergic neurons, exocytosis, or effective delivery of increased respiratory drive. This is consistent with the observation that neuronal integrity during hypoxia may be preserved, in part, by AMPK-independent mechanisms (43) that maintain ATP supply by accelerating glycolysis and in a manner supported by mobilization of astrocyte glycogen stores (44). That being said, we must also consider why (1) the degree of block by AMPK deletion of the HVR increased in a manner directly related to the severity of hypoxia; (2) the HVR can be triggered by central nervous system hypoxia alone, provided that there is continued receipt of basal (normoxic) afferent input from the carotid bodies (26); and (3) interference at any point within this circuit abolishes the HVR (e.g., carotid body resection [45] or AMPK deletion). We therefore hypothesize that AMPK signaling pathways support coincidence detection at a point of signal integration within and thus activation of a hypoxia-responsive circuit that encompasses C2/A2 neurons and ventrolateral A1 neurons. We envisage that coincidence detection is delivered by the capacity for AMPK activation by increases in AM(D)P/ATP ratio (1) determined by “local hypoxic stress” within the NTS (decreased ATP supply), which is coupled with “applied metabolic stress” (increased ATP usage) and/or increased cytoplasmic Ca2+ delivered via afferent input from the carotid bodies to the NTS and, in turn, to ventrolateral A1 neurons (Figure 7). Afferent input and brainstem hypoxia could thereby determine, each in part, the set point about which AMPK and thus the brainstem respiratory network are activated during hypoxia. Thereafter, AMPK-dependent modulation of cellular metabolism (1), ion channels, and thus neuronal firing frequency (46) and/or transmitter release (40, 41) may facilitate efferent output and thereby deliver increased drive to breathe in a manner that may be attenuated or augmented by appropriate regulation of AMPK expression.
Figure 7.
Schematic describing the new hypothesis on the integration by adenosine monophosphate–activated protein kinase (AMPK) of local and applied metabolic stresses. AP = area postrema; NTS = nucleus tractus solitarius.
It is also noteworthy that AMPK-knockout mice not only hypoventilate during hypoxia but also lie down and appear to enter a torpor-like state from which they recover rapidly when returned to normoxia (see Movies E1 and E2). These mice may therefore engage alternative strategies to reduce O2 consumption and maintain supply, perhaps by entering a hypometabolic state (47). This would explain the comparable arterial SpO2 for controls and AMPK knockouts during hypoxia, despite the attenuated HVR of the latter.
During anesthesia, loss of suprapontine (voluntary) control of ventilation (48) may have exacerbated hypoxic ventilatory depression in AMPK knockouts and perhaps revealed the true extent of deficiencies in brainstem respiratory drive during hypoxia, which in the extreme precipitated death by irreversible respiratory failure. Interestingly, such outcomes are consistent with the effect of anesthesia on patients with sleep apnea (49). Moreover, the observed respiratory failure could be triggered by exacerbation of the Cushing reflex (13, 50), although this is elicited only under anesthesia by ischemic hypoxia (∼1% O2) and is maintained or enhanced by hypercapnia (13, 50, 51). By contrast, hypoxic ventilatory depression was evident in conscious AMPK knockouts during mild and severe hypoxia, as were deficits in brainstem activity, and it was reversed rather than exacerbated by hypercapnia. Furthermore, and contrary to the marked increases in blood pressure associated with the Cushing reflex (13, 50), hypoxic ventilatory depression in AMPK knockouts was associated with a fall in blood pressure equivalent to that of controls (see Figure E7) and wild-type mice (52). Notwithstanding these facts, hypoxia triggers increased blood pressure in both rats (53) and humans (54), and we cannot rule out the possibility that outcomes for blood pressure may reflect species-dependent variations allied to the Cushing reflex (50).
We conclude that AMPK opposes central respiratory depression during hypoxia and resists hypoventilation and apnea by supporting increases in central respiratory drive, even where carotid body afferent input is normal. Consequently, AMPK deficiency precipitates ventilatory dysfunction during hypoxia. AMPK is therefore key to O2 and thus energy (ATP) supply to the body as a whole. Moreover, modulation of AMPK activity or expression may afford new therapeutic strategies against hypoventilation and apnea associated, for example, with metabolic syndrome–related disorders and ascent to altitude (7–9).
Footnotes
This work was funded primarily by the Wellcome Trust (WT081195MA [A.M.E.]) and was also supported by the British Heart Foundation (RG/12/14/29885).
Author Contributions: A.M.E. and A.D.M. wrote the manuscript; A.M.E., O.A.O., A.D.M., and S.H. developed the conditional AMPK-knockout mice and performed genotyping; M.F. and B.V. developed the AMPK floxed mice; A.D.M. designed and validated primers; A.D.M., O.A.O., S.L., and A.M.E. performed reverse transcription–polymerase chain reactions; A.D.M., S.H., and A.M.E. performed plethysmography; A.D.M., S.H., A.M.E., and M.B.D. analyzed respiratory data; P.P. and A.D.M. performed computational video analysis; A.M.E., L.J., I.M., M.A.J., S.L., and A.D.M. performed magnetic resonance imaging and analysis; A.P.H. and P.K. performed afferent discharge blind and under subcontract at the University of Birmingham; U.-A.U., J.N.-D., M.B.D., and A.M.E. performed immunocytochemistry; A.D.M., S.L., S.H., and A.M.E. performed blood gas and blood pressure analyses. All authors discussed the results and provided feedback.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as 10.1164/rccm.201508-1667OC on December 15, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hardie DG. AMPK—sensing energy while talking to other signaling pathways. Cell Metab. 2014;20:939–952. doi: 10.1016/j.cmet.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gowans GJ, Hawley SA, Ross FA, Hardie DG. AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metab. 2013;18:556–566. doi: 10.1016/j.cmet.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakamoto K, McCarthy A, Smith D, Green KA, Hardie DG, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Evans AM. AMP-activated protein kinase and the regulation of Ca2+ signalling in O2-sensing cells. J Physiol. 2006;574:113–123. doi: 10.1113/jphysiol.2006.108381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bigham AW, Julian CG, Wilson MJ, Vargas E, Browne VA, Shriver MD, Moore LG. Maternal PRKAA1 and EDNRA genotypes are associated with birth weight, and PRKAA1 with uterine artery diameter and metabolic homeostasis at high altitude. Physiol Genomics. 2014;46:687–697. doi: 10.1152/physiolgenomics.00063.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruderman NB, Carling D, Prentki M, Cacicedo JM. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest. 2013;123:2764–2772. doi: 10.1172/JCI67227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chau EH, Lam D, Wong J, Mokhlesi B, Chung F. Obesity hypoventilation syndrome: a review of epidemiology, pathophysiology, and perioperative considerations. Anesthesiology. 2012;117:188–205. doi: 10.1097/ALN.0b013e31825add60. [DOI] [PubMed] [Google Scholar]
- 9.Ainslie PN, Lucas SJ, Burgess KR. Breathing and sleep at high altitude. Respir Physiol Neurobiol. 2013;188:233–256. doi: 10.1016/j.resp.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 10.Smith JC, Abdala AP, Borgmann A, Rybak IA, Paton JF. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci. 2013;36:152–162. doi: 10.1016/j.tins.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Day TA, Wilson RJ. Brainstem PCO2 modulates phrenic responses to specific carotid body hypoxia in an in situ dual perfused rat preparation. J Physiol. 2007;578:843–857. doi: 10.1113/jphysiol.2006.119594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nurse CA. Synaptic and paracrine mechanisms at carotid body arterial chemoreceptors. J Physiol. 2014;592:3419–3426. doi: 10.1113/jphysiol.2013.269829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guyenet PG. Neural structures that mediate sympathoexcitation during hypoxia. Respir Physiol. 2000;121:147–162. doi: 10.1016/s0034-5687(00)00125-0. [DOI] [PubMed] [Google Scholar]
- 14.Guyenet PG. Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol. 2014;4:1511–1562. doi: 10.1002/cphy.c140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun MK, Reis DJ. Differential responses of barosensitive neurons of rostral ventrolateral medulla to hypoxia in rats. Brain Res. 1993;609:333–337. doi: 10.1016/0006-8993(93)90892-q. [DOI] [PubMed] [Google Scholar]
- 16.King TL, Heesch CM, Clark CG, Kline DD, Hasser EM. Hypoxia activates nucleus tractus solitarii neurons projecting to the paraventricular nucleus of the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1219–R1232. doi: 10.1152/ajpregu.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahmoud AD, Lewis S, Juričić L, Foretz M, Viollet B, Marshall I, Evans AM. AMPK couples oxygen to energy supply at the whole-body level by delivering increased drive to breathe during hypoxia and thus protects against apnoea. Proc Physiol Soc. 2015;34:PC041. [Google Scholar]
- 18.Wyatt CN, Mustard KJ, Pearson SA, Dallas ML, Atkinson L, Kumar P, Peers C, Hardie DG, Evans AM. AMP-activated protein kinase mediates carotid body excitation by hypoxia. J Biol Chem. 2007;282:8092–8098. doi: 10.1074/jbc.M608742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng YJ, Nanduri J, Khan SA, Yuan G, Wang N, Kinsman B, Vaddi DR, Kumar GK, Garcia JA, Semenza GL, et al. Hypoxia-inducible factor 2α (HIF-2α) heterozygous-null mice exhibit exaggerated carotid body sensitivity to hypoxia, breathing instability, and hypertension. Proc Natl Acad Sci USA. 2011;108:3065–3070. doi: 10.1073/pnas.1100064108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lantier L, Fentz J, Mounier R, Leclerc J, Treebak JT, Pehmøller C, Sanz N, Sakakibara I, Saint-Amand E, Rimbaud S, et al. AMPK controls exercise endurance, mitochondrial oxidative capacity, and skeletal muscle integrity. FASEB J. 2014;28:3211–3224. doi: 10.1096/fj.14-250449. [DOI] [PubMed] [Google Scholar]
- 21.Hirooka Y, Polson JW, Potts PD, Dampney RA. Hypoxia-induced Fos expression in neurons projecting to the pressor region in the rostral ventrolateral medulla. Neuroscience. 1997;80:1209–1224. doi: 10.1016/s0306-4522(97)00111-5. [DOI] [PubMed] [Google Scholar]
- 22.Lindeberg J, Usoskin D, Bengtsson H, Gustafsson A, Kylberg A, Söderström S, Ebendal T. Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis. 2004;40:67–73. doi: 10.1002/gene.20065. [DOI] [PubMed] [Google Scholar]
- 23.Kumar NN, Velic A, Soliz J, Shi Y, Li K, Wang S, Weaver JL, Sen J, Abbott SB, Lazarenko RM, et al. Regulation of breathing by CO2 requires the proton-activated receptor GPR4 in retrotrapezoid nucleus neurons. Science. 2015;348:1255–1260. doi: 10.1126/science.aaa0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koshiya N, Guyenet PG. NTS neurons with carotid chemoreceptor inputs arborize in the rostral ventrolateral medulla. Am J Physiol. 1996;270:R1273–R1278. doi: 10.1152/ajpregu.1996.270.6.R1273. [DOI] [PubMed] [Google Scholar]
- 25.Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- 26.Smith CA, Forster HV, Blain GM, Dempsey JA. An interdependent model of central/peripheral chemoreception: evidence and implications for ventilatory control. Respir Physiol Neurobiol. 2010;173:288–297. doi: 10.1016/j.resp.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sicard KM, Duong TQ. Effects of hypoxia, hyperoxia, and hypercapnia on baseline and stimulus-evoked BOLD, CBF, and CMRO2 in spontaneously breathing animals. Neuroimage. 2005;25:850–858. doi: 10.1016/j.neuroimage.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alheid GF, Jiao W, McCrimmon DR. Caudal nuclei of the rat nucleus of the solitary tract differentially innervate respiratory compartments within the ventrolateral medulla. Neuroscience. 2011;190:207–227. doi: 10.1016/j.neuroscience.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbott SB, DePuy SD, Nguyen T, Coates MB, Stornetta RL, Guyenet PG. Selective optogenetic activation of rostral ventrolateral medullary catecholaminergic neurons produces cardiorespiratory stimulation in conscious mice. J Neurosci. 2013;33:3164–3177. doi: 10.1523/JNEUROSCI.1046-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim D, Kang D, Martin EA, Kim I, Carroll JL. Effects of modulators of AMP-activated protein kinase on TASK-1/3 and intracellular Ca2+ concentration in rat carotid body glomus cells. Respir Physiol Neurobiol. 2014;195:19–26. doi: 10.1016/j.resp.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasenour CM, Ridley DE, Hughey CC, James FD, Donahue EP, Shearer J, Viollet B, Foretz M, Wasserman DH. 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR) effect on glucose production, but not energy metabolism, is independent of hepatic AMPK in vivo. J Biol Chem. 2014;289:5950–5959. doi: 10.1074/jbc.M113.528232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto K, Lalley P, Mifflin S. Acute intermittent optogenetic stimulation of nucleus tractus solitarius neurons induces sympathetic long-term facilitation. Am J Physiol Regul Integr Comp Physiol. 2015;308:R266–R275. doi: 10.1152/ajpregu.00381.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vardhan A, Kachroo A, Sapru HN. Excitatory amino acid receptors in commissural nucleus of the NTS mediate carotid chemoreceptor responses. Am J Physiol. 1993;264:R41–R50. doi: 10.1152/ajpregu.1993.264.1.R41. [DOI] [PubMed] [Google Scholar]
- 35.Aicher SA, Saravay RH, Cravo S, Jeske I, Morrison SF, Reis DJ, Milner TA. Monosynaptic projections from the nucleus tractus solitarii to C1 adrenergic neurons in the rostral ventrolateral medulla: comparison with input from the caudal ventrolateral medulla. J Comp Neurol. 1996;373:62–75. doi: 10.1002/(SICI)1096-9861(19960909)373:1<62::AID-CNE6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 36.Vallortigara G, Rogers LJ, Bisazza A. Possible evolutionary origins of cognitive brain lateralization. Brain Res Brain Res Rev. 1999;30:164–175. doi: 10.1016/s0165-0173(99)00012-0. [DOI] [PubMed] [Google Scholar]
- 37.Dadda M, Zandonà E, Agrillo C, Bisazza A. The costs of hemispheric specialization in a fish. Proc Biol Sci. 2009;276:4399–4407. doi: 10.1098/rspb.2009.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stornetta RL, Sevigny CP, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. J Comp Neurol. 2002;444:191–206. doi: 10.1002/cne.10141. [DOI] [PubMed] [Google Scholar]
- 39.Gozal D, Gozal E, Torres JE, Gozal YM, Nuckton TJ, Hornby PJ. Nitric oxide modulates ventilatory responses to hypoxia in the developing rat. Am J Respir Crit Care Med. 1997;155:1755–1762. doi: 10.1164/ajrccm.155.5.9154888. [DOI] [PubMed] [Google Scholar]
- 40.Lipton AJ, Johnson MA, Macdonald T, Lieberman MW, Gozal D, Gaston B. S-nitrosothiols signal the ventilatory response to hypoxia. Nature. 2001;413:171–174. doi: 10.1038/35093117. [DOI] [PubMed] [Google Scholar]
- 41.Murphy BA, Fakira KA, Song Z, Beuve A, Routh VH. AMP-activated protein kinase and nitric oxide regulate the glucose sensitivity of ventromedial hypothalamic glucose-inhibited neurons. Am J Physiol Cell Physiol. 2009;297:C750–C758. doi: 10.1152/ajpcell.00127.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Culmsee C, Monnig J, Kemp BE, Mattson MP. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001;17:45–58. doi: 10.1385/JMN:17:1:45. [DOI] [PubMed] [Google Scholar]
- 43.Cheng F, Xie S, Guo M, Fang H, Li X, Yin J, Lu G, Li Y, Ji X, Yu S. Altered glucose metabolism and preserved energy charge and neuronal structures in the brain of mouse intermittently exposed to hypoxia. J Chem Neuroanat. 2011;42:65–71. doi: 10.1016/j.jchemneu.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Almeida A, Moncada S, Bolaños JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol. 2004;6:45–51. doi: 10.1038/ncb1080. [DOI] [PubMed] [Google Scholar]
- 45.Wade JG, Larson CP, Jr, Hickey RF, Ehrenfeld WK, Severinghaus JW. Effect of carotid endarterectomy on carotid chemoreceptor and baroreceptor function in man. N Engl J Med. 1970;282:823–829. doi: 10.1056/NEJM197004092821501. [DOI] [PubMed] [Google Scholar]
- 46.Ikematsu N, Dallas ML, Ross FA, Lewis RW, Rafferty JN, David JA, Suman R, Peers C, Hardie DG, Evans AM. Phosphorylation of the voltage-gated potassium channel Kv2.1 by AMP-activated protein kinase regulates membrane excitability. Proc Natl Acad Sci USA. 2011;108:18132–18137. doi: 10.1073/pnas.1106201108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guppy M, Withers P. Metabolic depression in animals: physiological perspectives and biochemical generalizations. Biol Rev Camb Philos Soc. 1999;74:1–40. doi: 10.1017/s0006323198005258. [DOI] [PubMed] [Google Scholar]
- 48.Horn EM, Waldrop TG. Suprapontine control of respiration. Respir Physiol. 1998;114:201–211. doi: 10.1016/s0034-5687(98)00087-5. [DOI] [PubMed] [Google Scholar]
- 49.Boushra NN. Anaesthetic management of patients with sleep apnoea syndrome. Can J Anaesth. 1996;43:599–616. doi: 10.1007/BF03011774. [DOI] [PubMed] [Google Scholar]
- 50.Paton JF, Dickinson CJ, Mitchell G. Harvey Cushing and the regulation of blood pressure in giraffe, rat and man: introducing ‘Cushing’s mechanism’. Exp Physiol. 2009;94:11–17. doi: 10.1113/expphysiol.2008.043455. [DOI] [PubMed] [Google Scholar]
- 51.Harris AP, Helou S, Traystman RJ, Jones MD, Jr, Koehler RC. Efficacy of the Cushing response in maintaining cerebral blood flow in premature and near-term fetal sheep. Pediatr Res. 1998;43:50–56. doi: 10.1203/00006450-199801000-00008. [DOI] [PubMed] [Google Scholar]
- 52.Campen MJ, Shimoda LA, O’Donnell CP. Acute and chronic cardiovascular effects of intermittent hypoxia in C57BL/6J mice. J Appl Physiol (1985) 2005;99:2028–2035. doi: 10.1152/japplphysiol.00411.2005. [DOI] [PubMed] [Google Scholar]
- 53.Bakehe M, Hedner J, Dang T, Chambille B, Gaultier CL, Escourrou P. Role of the autonomic nervous system in the acute blood pressure elevation during repetitive hypoxic and hypercapnic breathing in rats. Blood Press. 1996;5:371–375. doi: 10.3109/08037059609078077. [DOI] [PubMed] [Google Scholar]
- 54.Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol (1985) 1989;67:2101–2106. doi: 10.1152/jappl.1989.67.5.2101. [DOI] [PubMed] [Google Scholar]