Leukocyte adhesion plays key roles in immune responses and inflammation. Leukocyte adhesion requires an orchestrated set of coordinated events, starting with selectin-mediated rolling, followed by β2 integrin–dependent arrest (1). Leukocyte adhesion deficiency (LAD) syndromes are genetic disorders of adhesion molecules that affect leukocyte adhesion (2). In this issue of the Journal, Sorio and colleagues (pp. 1123–1133) show that gene mutations of cystic fibrosis transmembrane conductance regulator (CFTR) found in patients with cystic fibrosis (CF) can lead to a monocyte-specific adhesion deficiency (3).
More than 30 years ago, the first LAD was discovered (4) and later called LAD-I. Patients with LAD-I have widespread bacterial infections as a result of defective leukocyte adhesion functions caused by defective expression of β2 integrins (CD18) (5), which are key molecules in leukocyte arrest and migration. A second LAD syndrome, LAD-II, is caused by inherited defects in the gene encoding guanosine diphosphate (GDP)-fucose transporter 1 (6), which is a key player in fucose metabolism. The GDP-fucose transporter 1 defect results in the absence of sialyl Lewis X (7) and other structurally related fucosylated glycans, which make up the carbohydrate ligands for selectins (8). During the last decade, a rare, autosomal recessive LAD syndrome distinct from LAD-I and LAD-II has been reported (originally called LAD-I variant, now called LAD-III) (9). Similar to LAD-I and LAD-II, patients with LAD-III exhibit severe recurrent infections and marked leukocytosis, accompanied by a bleeding tendency, which is not seen in LAD-I and LAD-II. The expression of β2 integrins is normal, and leukocyte rolling is also intact in these patients, but the activation of leukocyte (and platelet) integrins is severely impaired, and lymphocytes fail to arrest. LAD-III is caused by mutations in the Kindlin-3 gene (10).
Similar to LAD-I, LAD-II, and LAD-III, CF is also a genetic disease. CF is a life-threatening disease that causes persistent lung infections and progressively limits the ability to breathe. In people with CF, a defective CFTR gene (11) causes a buildup of thick mucus in the lungs and intestines. The most common mutation is F508del, which is present in 90% of patients with CF. This mutation and several other mutations (N1303K, G85E, and G91R) lead to a misfolded CFTR protein that is prematurely degraded. About 5–10% of CFTR mutations (e.g., G542X) are a result of premature truncation or nonsense alleles. Other CFTR mutations (e.g., G551D and A455E) encode properly processed, full-length CFTR protein that lacks normal ion channel activity (12). Before the current paper, disrupted mucociliary transport resulting from defective CFTR in epithelial cells was thought to be the main mechanism of CF and cause abnormal mucociliary clearance, and dehydration of periciliary liquid. CF lung disease shows exaggerated neutrophil infiltration even before bacterial infection. The present study (3) suggests that a severe monocyte adhesion defect may play an important role in CF.
This is the first study that links the CFTR defect (probably all genotypes) to integrin function on leukocytes. Monocytes from patients with CF show an impressive defect (∼80%) in adhesion to intercellular adhesion molecule-1 (a ligand for the β2 integrins lymphocyte function-associated antigen-1 [LFA-1] and macrophage-1 antigen [Mac-1]), fibrinogen (a ligand for Mac-1), and vascular cell adhesion molecule-1 (a ligand for α4β1 integrin). A minimal defect is seen in lymphocyte adhesion, and no defect in neutrophil adhesion, suggesting that the CFTR-dependent leukocyte adhesion deficiency (LAD-IV) is monocyte specific. Unlike LAD-I, LAD-IV appears to affect both β2 and α4β1 integrins, whereas integrin expression on the monocyte surface is normal. Formyl methionyl leucyl phenylalanine–triggered chemotaxis was also impaired in CF monocytes assessed by in vitro migration assay. CFTR-correcting drugs VRT325 and VX809, which can recover the CFTR expression on monocytes from patients with F508del mutation CF, reconstituted monocyte adhesion. Conversely, CFTR inhibitors reduced adhesion of monocytes from healthy donors. In vivo evidence for this adhesion defect is provided by defective CFTR F508 del monocyte accumulation in mice. The mutant monocytes accumulate in the lung, but not the bronchoalveolar lavage.
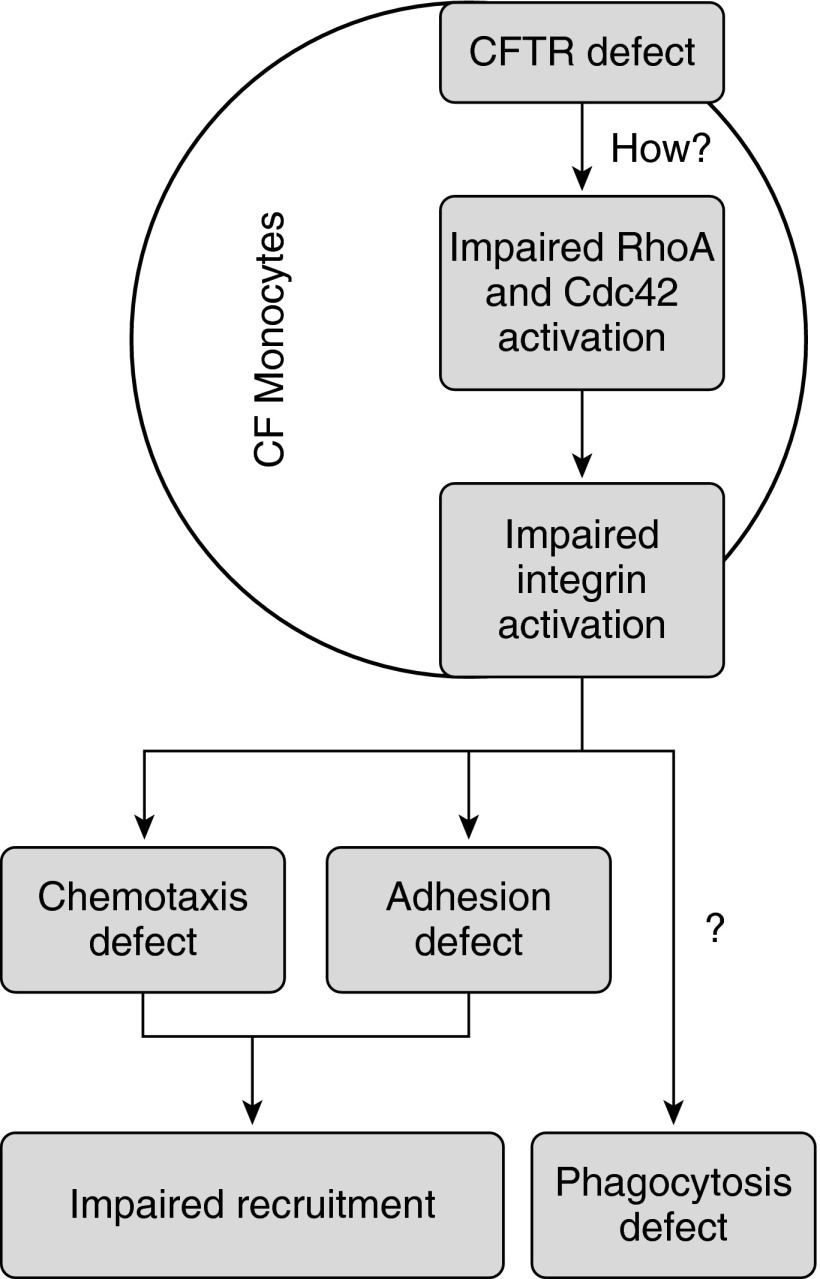
The activation of Ras homolog gene family member A (RhoA) and cell division control protein 42 (CDC42), which are both small rho GTPases and involved in integrin inside-out activation (13), are impaired in CF monocytes. This suggests that integrin activation may be defective in LAD-IV, but the details of the mechanism remain to be investigated (Figure 1). Consistent with an integrin activation defect, monoclonal antibodies KIM127 and 327C, which report β2 integrin activated conformations, fail to bind CF monocytes. Thus, similar to kindlin-3 deficiency in patients with LAD-III, CFTR deficiency appears to impair the activation, but not expression, of integrin. Different from LAD-III, only monocytes showed the adhesion defect in patients with CF.
Figure 1.
Effects of cystic fibrosis transmembrane conductance regulator (CFTR) defect on integrin activation. Absence or mutations in CFTR cause reduced Ras homolog gene family member A (RhoA) and cell division control protein 42 (Cdc42) activation, resulting in impaired α4 and β2 integrin activation and monocyte function. CF = cystic fibrosis.
Taken together, these findings show a severe monocyte adhesion defect in patients with CF. This may explain the excessive neutrophil infiltration. In addition to the mucociliary transport defect, patients with CF clearly suffer from a new leukocyte adhesion deficiency, tentatively called LAD-IV. CFTR is upstream of small rho GTPases in integrin inside-out activation. At this time, it is not known how CFTR influences Rho and Cdc42 activation, and thus integrin inside-out activation. LAD-IV is the first LAD that affects only monocytes (Table 1). It is also the first LAD that affects both β2 and α4 integrins. The β2 defect was shown for LFA-1 and Mac-1, but not the other two family members, αXβ2 and αDβ2. The α4 defect was shown for α4β1, but not α4β7, which may also be affected.
Table 1.
Leukocyte Adhesion Deficiencies Syndrome
| Discovered | Frequency | Mutated Molecules | Molecular Defect | Major Defect in | |
|---|---|---|---|---|---|
| LAD-I (3) | 1980 | Rare (∼300 cases) | CD18 (integrin β2) | Reduced or altered expression of CD18 | Neutrophils |
| LAD-II (6) | 1992 | Very rare (11 cases) | GFTP | No functional selectin ligands | Neutrophils |
| LAD-III (8) | 1997 | Rare (27 cases) | Kindlin-3 | Impaired β2 and β3 integrin activation | Neutrophils, platelets |
| LAD-IV | 2015 | Common (∼70,000 cases; ∼1,000 cases/yr) | CFTR | Impaired α4 and β2 integrin activation | Monocytes |
Definition of abbreviations: CFTR = cystic fibrosis transmembrane conductance regulator; GFTP = GDP-fucose transporter 1; LAD = leukocyte adhesion deficiencies.
Monocytes play an essential role in inflammation, resolution of inflammation, and protective immunity (14). Thus, the recruitment defect of monocytes in the lung of patients with CF may cause abnormal cytokine/chemokine homeostasis, impaired resolution of inflammation, and impaired pathogen capture. Beyond adhesion, integrins play vital roles in most other monocyte functions, including phagocytosis, which is also important in pathogen clearance, antigen presentation, and neutrophil clearance during inflammation resolution. All these may contribute to the pathogenesis of CF. The most exciting aspect of this paper is that it not only identifies LAD-IV but also introduces a new aspect of pathophysiology that may affect the care for and improve the lives of patients with CF.
Footnotes
Supported by National Institutes of Health grant P01 HL078784.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt S, Moser M, Sperandio M. The molecular basis of leukocyte recruitment and its deficiencies. Mol Immunol. 2013;55:49–58. doi: 10.1016/j.molimm.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Sorio C, Montresor A, Bolomini-Vittori M, Caldrer S, Rossi B, Dusi S, Angiari S, Johansson JE, Vezzalini M, Leal T, et al. Mutations of cystic fibrosis transmembrane conductance regulator gene cause a monocyte-selective adhesion deficiency. Am J Respir Crit Care Med. 2016;193:1123–1133. doi: 10.1164/rccm.201510-1922OC. [DOI] [PubMed] [Google Scholar]
- 4.Crowley CA, Curnutte JT, Rosin RE, André-Schwartz J, Gallin JI, Klempner M, Snyderman R, Southwick FS, Stossel TP, Babior BM. An inherited abnormality of neutrophil adhesion: its genetic transmission and its association with a missing protein. N Engl J Med. 1980;302:1163–1168. doi: 10.1056/NEJM198005223022102. [DOI] [PubMed] [Google Scholar]
- 5.Springer TA, Thompson WS, Miller LJ, Schmalstieg FC, Anderson DC. Inherited deficiency of the Mac-1, LFA-1, p150,95 glycoprotein family and its molecular basis. J Exp Med. 1984;160:1901–1918. doi: 10.1084/jem.160.6.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lübke T, Marquardt T, Etzioni A, Hartmann E, von Figura K, Körner C. Complementation cloning identifies CDG-IIc, a new type of congenital disorders of glycosylation, as a GDP-fucose transporter deficiency. Nat Genet. 2001;28:73–76. doi: 10.1038/ng0501-73. [DOI] [PubMed] [Google Scholar]
- 7.Etzioni A, Harlan JM, Pollack S, Phillips LM, Gershoni-Baruch R, Paulson JC. Leukocyte adhesion deficiency (LAD) II: a new adhesion defect due to absence of sialyl Lewis X, the ligand for selectins. Immunodeficiency. 1993;4:307–308. [PubMed] [Google Scholar]
- 8.Etzioni A, Frydman M, Pollack S, Avidor I, Phillips ML, Paulson JC, Gershoni-Baruch R. Brief report: recurrent severe infections caused by a novel leukocyte adhesion deficiency. N Engl J Med. 1992;327:1789–1792. doi: 10.1056/NEJM199212173272505. [DOI] [PubMed] [Google Scholar]
- 9.Kuijpers TW, Van Lier RA, Hamann D, de Boer M, Thung LY, Weening RS, Verhoeven AJ, Roos D. Leukocyte adhesion deficiency type 1 (LAD-1)/variant: a novel immunodeficiency syndrome characterized by dysfunctional β2 integrins. J Clin Invest. 1997;100:1725–1733. doi: 10.1172/JCI119697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moser M, Bauer M, Schmid S, Ruppert R, Schmidt S, Sixt M, Wang HV, Sperandio M, Fässler R. Kindlin-3 is required for β2 integrin-mediated leukocyte adhesion to endothelial cells. Nat Med. 2009;15:300–305. doi: 10.1038/nm.1921. [DOI] [PubMed] [Google Scholar]
- 11.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 12.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 13.Fan Z, Ley K. Leukocyte arrest: biomechanics and molecular mechanisms of β2 integrin activation. Biorheology. doi: 10.3233/BIR-15085. [online ahead of print] 16 Dec 2015; DOI: 10.3233/BIR-15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]