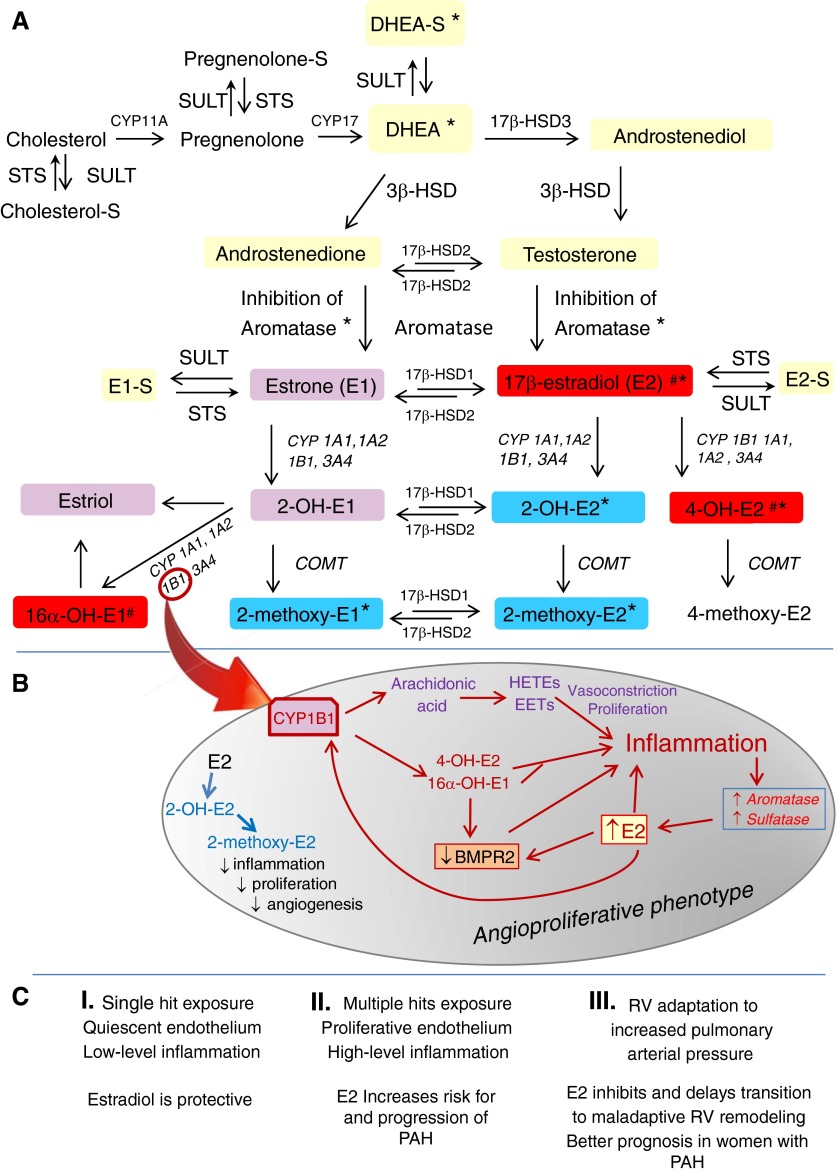
The fact that pulmonary arterial hypertension (PAH) is more common in women than in men (1) suggests that estrogens importantly contribute to the pathophysiology of PAH (2). Nonetheless, the primary human estrogen 17β-estradiol (estradiol) exerts beneficial, rather than detrimental, effects in classical models of PAH (i.e., hypoxia- and monocrotaline-induced PAH). This puzzling inconsistency is called the “estrogen paradox” (2). The apparent contradictions posed by the effects of female sex and estrogens in experimental PAH versus human PAH could be explained by the limitations of the experimental models used and the opposite roles estradiol may play in the pulmonary vasculature versus the right ventricle (Figure 1C). Although the estrogen paradox remains unresolved, mounting evidence (both in patients with PAH and in novel rodent models of angioproliferative PAH) suggests that precursors and metabolites of sex steroids influence the development and progression of PAH and could account for the estrogen paradox (2, 3).
Figure 1.
(A) Metabolism of sex steroids: steroid sulfatase (STS) and sulfotransferase (SULT) control the delicate balance between inactive sulfated sex steroids and sex steroids and their biologically active metabolites and metabolic precursors. *Beneficial effects in experimental pulmonary arterial hypertension (PAH). #Adverse effects in experimental PAH. Yellow boxes: precursors of estrogen synthesis. Red, violet, and blue boxes: high, intermediate, and no estrogenic activity, respectively. (B) Inflammation and estradiol (E2) feed-forward mechanisms. In PAH, CYP1B1 may be a “hub” for instigating and perpetuating an estradiol feed-forward mechanism that involves estradiol metabolites and BMPR2, resulting in stimulation of angioproliferation (red arrows: increases enzyme expression/activity, estradiol and arachidonic acid metabolites production, estradiol levels and inflammation, and BMPR2 down-regulation). (C) Three-tier effects of estradiol in PAH. The effect on vascular pathobiology, progression, and prognosis of PAH is shown. BMPR2 = bone morphogenetic protein receptor type 2; COMT = catechol-O-methyltransferase; CYP = cytochrome p450 enzymes; DHEA-S = dehydroepiandrosterone sulfate; EETs = epoxyeicosatrienoic acids; HETEs = hydroxyeicosatetraenoic acids; HSD = hydroxysteroid dehydrogenase; RV = right ventricle.
In this issue of the Journal, Ventetuolo and colleagues (pp. 1168–1175) report that in men, elevated levels of estradiol and lower levels of dehydroepiandrosterone sulfate (DHEA-S) are associated with a higher risk for PAH (4). Notably, elevated estradiol levels and greater ratios of estradiol:testosterone are correlated with shorter 6-minute-walk distances and higher right atrial pressures. In contrast, DHEA-S levels are inversely associated with right atrial pressures and pulmonary vascular resistances (4). However, testosterone levels are unchanged in men with PAH and do not correlate with markers of disease severity.
Previous reports by this same group suggest that in patients with advanced liver disease, independent of sex, genetic variation in aromatase activity (the rate-limiting enzyme in the conversion of androgens to estrogens; Figure 1A) and elevated plasma estradiol levels are correlated with an increased risk for portopulmonary hypertension (5). Very similar changes in estrone and DHEA-S levels occur in men with rheumatoid arthritis, a chronic inflammatory disease with female preponderance. In patients with rheumatoid arthritis compared with healthy controls, both plasma and synovial fluid DHEA-S concentrations are lower, and estradiol levels are higher (6). Importantly, measures of inflammation strongly correlate with plasma estradiol levels and increased aromatase activity (6). Also, irrespective of sex, inflammation in rheumatoid arthritis is a driving force in shifting estradiol metabolism toward the 16-hydroxylation pathway and producing changes in sex steroids and metabolites similar to those reported in PAH (7).
The biosynthesis and metabolism of estradiol is complex (Figure 1A). In brief, DHEA is the pivotal precursor for estradiol biosynthesis, with DHEA-S functioning as a reservoir of DHEA. The ratio of DHEA to DHEA-S is controlled by the balanced effects of steroid sulfotransferase (SULT; DHEA→DHEA-S) and sulfatase (STS; DHEA-S→DHEA) (8). Once formed, estradiol can be converted to biologically active metabolites via the sequential steps of hydroxylation (mediated by multiply CYP450 enzymes) followed by methylation of hydroxyl groups (catalyzed by catechol-O-methyltransferase).
Since the first report that 2-methoxyestradiol (major nonestrogenic metabolite of estradiol) attenuates the development and progression of monocrotaline-induced PAH (9), it has become increasingly clear that estradiol precursors, estradiol per se, and estradiol metabolites can exhibit both detrimental and protective effects in PAH (2, 3). In this regard, the 2-hydroxylation-catechol-O-methyltransferase pathway produces nonestrogenic, antiproliferative, antiangiogenic, and antiinflammatory metabolites (2-hydroxyestradiol and 2-methoxyestradiol), and these metabolites are protective in hypoxia-, monocrotaline-, sugene + hypoxia–, and bleomycin-induced PAH (2). In contrast, the 4-hydroxylation and 16-hydroxylation pathways produce 4-hydroxyestradiol and 16α-hydroxyestrone, which are estrogenic, very reactive, and exert significant mitogenic, angiogenic, and inflammatory actions (2, 3, 10).
The enzyme CYP1B1 is involved in both the metabolism of estradiol (Figure 1B) and arachidonic acid (produces epoxyeicosatrienoic acids and 15-hydroxyeicosatetraenoic acids). In the lungs, epoxyeicosatrienoic acids and 15-hydroxyeicosatetraenoic acids have inflammatory, mitogenic, and vasoconstrictive effects and are implicated in the development of hypoxic PAH (11). Notably, epoxyeicosatrienoic acids have opposite effects in the systemic circulation, where they induce vasodilation and antiinflammatory effects, which may explain the plausible divergent effects of estradiol in the systemic versus the pulmonary circulation.
Recent studies implicate CYP1B1-mediated estradiol metabolism to 16α-hydroxyestrone as a predisposing factor for the development of PAH. In this regard, CYP1B1 is overexpressed and 16α-hydroxyestrone levels are increased in experimental PAH. Also, lack of CYP1B1 or inhibition of its activity prevents the development of sugen + hypoxia–induced PAH, and 16α-hydroxyestrone induces PAH in mice (12). Furthermore, CYP1B1 activity increases the penetrance of disease in women who carry bone morphogenetic protein receptor type 2 (BMPR2) mutations, and this is associated with increased urinary 16α-hydroxyestrone levels (13). Finally, 16α-hydroxyestrone worsens penetrance of disease in BMPR2 mutant mice and suppresses BMPR2 protein levels in control mice (14).
Recent studies link BMPR2 deficiency in mice and humans to exaggerated inflammatory responses. Importantly, chronic inflammation instigates development of PAH in Bmrp2+/−, but not in wild-type, mice (15). Also, there are reports of significant cross-talk between estradiol and BMPR2, suggesting that estradiol-driven suppression of BMPR2 signaling may contribute to a proliferative phenotype and predispose women to PAH (16). Chronic inflammatory diseases are associated with increased estradiol levels and a shift in estradiol metabolism toward the 16-hydroxylation pathway (supra vide; 6, 7, 10). It remains unclear whether the pro-inflammatory status related to BMPR2 deficiency alters estradiol synthesis and metabolism or whether estradiol-driven suppression of BMPR2 signaling is enhanced in an inflammatory environment.
Estradiol is not only a substrate for CYP1B1 but also, via estrogen receptor alpha, induces the transcriptional activation of CYP1B1 (17). Thereby, in an inflammatory environment, elevated estradiol levels via a feed-forward mechanism may shift estradiol metabolism toward production of pro-inflammatory, angiogenic, and mitogenic estradiol metabolites and arachidonic acid metabolites, which may perpetuate the progression of disease.
The current study reports that total and free testosterone levels are unchanged, even though circulating estradiol levels are increased. This is somewhat surprising, as testosterone and androstenedione are major (if not only) substrates for estradiol and estrone synthesis in men. It is noteworthy that the levels of circulating testosterone in men are 100 times higher than the levels of estradiol. The excess of substrate, small sample size, much greater (two- to threefold) variations in sex steroid levels in men with PAH compared with healthy controls, and limitations of using radioimmunoassay for quantification of sex steroids (18) may render subtle changes in testosterone levels undetectable.
Reduced levels of DHEA-S in men with PAH are consistent with increased STS activity. Current evidence suggests that inflammatory cytokines increase STS activity. Also, STS expression is controlled via estrogen receptor alpha signaling, and at least in breast cancer, STS is up-regulated by local estradiol concentrations (8). In patients with chronic liver diseases, inflammation induces STS expression, and this is accompanied by increased circulating estradiol levels (19). Taken together, these findings suggest that in an inflammatory environment, estradiol, through feed-forward mechanisms, may increase the bioavailability of its metabolic precursors. This feed-forward mechanism may be reinforced by increased aromatase activity and subsequently elevated estradiol levels, as implicated in both men and women with portopulmonary hypertension (5). Notably, recent studies (some reported in form of an abstract) (20, 21), indicate that inhibition of aromatase and subsequent reduction in estradiol levels is associated with reduced occlusive and plexiform lesions in female rats with sugen + hypoxia PAH. The current study does not provide data regarding the inflammatory status or aromatase activity. As the aromatization of androgenic precursors is the only source for estradiol production in men, the elevated estradiol levels are highly indicative of increased aromatase activity in men with PAH. We view inflammation as a key perpetuating factor in the estradiol–BMPR2–angioproliferation axis; changes in estradiol metabolism through several feed-forward mechanisms may perpetuate the progression of disease and expose women (and men) to greater risk of developing PAH (Figure 1B).
Despite the discussed limitations, Ventetuolo and colleagues (4) should be commended for identifying dysregulated sex steroid metabolism as a potential pathogenic contributor to PAH in men. Their current study and prior work provide a strong impetus for further investigation of sex steroids in the pathophysiology of PAH. Further studies using highly selective and sensitive mass spectrometry-based methods should focus not only on simultaneous quantification of multiple estrogens and their metabolites and metabolic precursors but also on quantification of a multitude of markers of inflammation. In patients with PAH, accurate assessment of the activity of the numerous enzymes involved in metabolizing parent sex steroids and their metabolites may be very difficult. Animal and cell culture studies focused on intracellular disposition and intracellular/extracellular equilibrium (in particular in pulmonary vascular cell compartments) would augment our understanding of the role of inflammation on the estradiol–BMPR2–angioproliferation axis in PAH.
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137:376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 2.Tofovic SP. Estrogens and development of pulmonary hypertension: interaction of estradiol metabolism and pulmonary vascular disease. J Cardiovasc Pharmacol. 2010;56:696–708. doi: 10.1097/FJC.0b013e3181f9ea8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austin ED, Lahm T, West J, Tofovic SP, Johansen AK, Maclean MR, Alzoubi A, Oka M. Gender, sex hormones and pulmonary hypertension. Pulm Circ. 2013;3:294–314. doi: 10.4103/2045-8932.114756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ventetuolo CE, Baird GL, Barr RG, Bluemke DA, Fritz JS, Hill NS, Klinger JR, Lima JA, Ouyang P, Palevsky HI, et al. Higher estradiol and lower dehydroepiandrosterone-sulfate levels are associated with pulmonary arterial hypertension in men. Am J Respir Crit Care Med. 2016;193:1168–1175. doi: 10.1164/rccm.201509-1785OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts KE, Fallon MB, Krowka MJ, Brown RS, Trotter JF, Peter I, Tighiouart H, Knowles JA, Rabinowitz D, Benza RL, et al. Pulmonary Vascular Complications of Liver Disease Study Group. Genetic risk factors for portopulmonary hypertension in patients with advanced liver disease. Am J Respir Crit Care Med. 2009;179:835–842. doi: 10.1164/rccm.200809-1472OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tengstrand B, Carlström K, Felländer-Tsai L, Hafström I. Abnormal levels of serum dehydroepiandrosterone, estrone, and estradiol in men with rheumatoid arthritis: high correlation between serum estradiol and current degree of inflammation. J Rheumatol. 2003;30:2338–2343. [PubMed] [Google Scholar]
- 7.Capellino S, Straub RH, Cutolo M. Aromatase and regulation of the estrogen-to-androgen ratio in synovial tissue inflammation: common pathway in both sexes. Ann N Y Acad Sci. 2014;1317:24–31. doi: 10.1111/nyas.12398. [DOI] [PubMed] [Google Scholar]
- 8.Mueller JW, Gilligan LC, Idkowiak J, Arlt W, Foster PA. The regulation of steroid action by sulfation and desulfation. Endocr Rev. 2015;36:526–563. doi: 10.1210/er.2015-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tofovic SP, Salah EM, Mady HH, Jackson EK, Melhem MF. Estradiol metabolites attenuate monocrotaline-induced pulmonary hypertension in rats. J Cardiovasc Pharmacol. 2005;46:430–437. doi: 10.1097/01.fjc.0000175878.32920.17. [DOI] [PubMed] [Google Scholar]
- 10.Tofovic SP, Wenzel S, Stewart NA. Role of estradiol metabolism in asthma. Biosci Hypotheses. 2009;2:128–134. [Google Scholar]
- 11.Zhu D, Ran Y. Role of 15-lipoxygenase/15-hydroxyeicosatetraenoic acid in hypoxia-induced pulmonary hypertension. J Physiol Sci. 2012;62:163–172. doi: 10.1007/s12576-012-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White K, Johansen AK, Nilsen M, Ciuclan L, Wallace E, Paton L, Campbell A, Morecroft I, Loughlin L, McClure JD, et al. Activity of the estrogen-metabolizing enzyme cytochrome P450 1B1 influences the development of pulmonary arterial hypertension. Circulation. 2012;126:1087–1098. doi: 10.1161/CIRCULATIONAHA.111.062927. [DOI] [PubMed] [Google Scholar]
- 13.Austin ED, Cogan JD, West JD, Hedges LK, Hamid R, Dawson EP, Wheeler LA, Parl FF, Loyd JE, Phillips JA., III Alterations in oestrogen metabolism: implications for higher penetrance of familial pulmonary arterial hypertension in females. Eur Respir J. 2009;34:1093–1099. doi: 10.1183/09031936.00010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fessel JP, Chen X, Frump A, Gladson S, Blackwell T, Kang C, Johnson J, Loyd JE, Hemnes A, Austin E, et al. Interaction between bone morphogenetic protein receptor type 2 and estrogenic compounds in pulmonary arterial hypertension. Pulm Circ. 2013;3:564–577. doi: 10.1086/674312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soon E, Crosby A, Southwood M, Yang P, Tajsic T, Toshner M, Appleby S, Shanahan CM, Bloch KD, Pepke-Zaba J, et al. Bone morphogenetic protein receptor type II deficiency and increased inflammatory cytokine production. A gateway to pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192:859–872. doi: 10.1164/rccm.201408-1509OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mair KM, Yang XD, Long L, White K, Wallace E, Ewart MA, Docherty CK, Morrell NW, MacLean MR. Sex affects bone morphogenetic protein type II receptor signaling in pulmonary artery smooth muscle cells. Am J Respir Crit Care Med. 2015;191:693–703. doi: 10.1164/rccm.201410-1802OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuchiya Y, Nakajima M, Yokoi T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005;227:115–124. doi: 10.1016/j.canlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Handelsman DJ, Wartofsky L. Requirement for mass spectrometry sex steroid assays in the Journal of Clinical Endocrinology and Metabolism. J Clin Endocrinol Metab. 2013;98:3971–3973. doi: 10.1210/jc.2013-3375. [DOI] [PubMed] [Google Scholar]
- 19.Jiang M, Klein M, Zanger UM, Mohammad MK, Cave MC, Gaikwad NW, Dias NJ, Selcer KW, Guo Y, He J, et al. Inflammatory regulation of steroid sulfatase: a novel mechanism to control estrogen homeostasis and inflammation in chronic liver disease. J Hepatol. 2016;64:44–52. doi: 10.1016/j.jhep.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tofovic SP, Bilan V, Mi Z, Jackson EK, Schneider F. Aromatase inhibition attenuates and ovariectomy and 4-hydroxyestradiol have mixed effects on development of angioproliferative pulmonary hypertension in female rats. Am J Respir Crit Care Med. 2013;187:A6099. [Google Scholar]
- 21.Mair KM, Wright AF, Duggan N, Rowlands DJ, Hussey MJ, Roberts S, Fullerton J, Nilsen M, Loughlin L, Thomas M, et al. Sex-dependent influence of endogenous estrogen in pulmonary hypertension. Am J Respir Crit Care Med. 2014;190:456–467. doi: 10.1164/rccm.201403-0483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]