Abstract
Recent quantum mechanical calculations of the interaction energy of pairs of helium atoms are accurate and some include reliable estimates of their uncertainty. We combined these ab initio results with earlier published results to obtain a helium-helium interatomic potential that includes relativistic retardation effects over all ranges of interaction. From this potential, we calculated the thermophysical properties of helium, i.e., the second virial coefficients, the dilute-gas viscosities, and the dilute-gas thermal conductivities of 3He, 4He, and their equimolar mixture from 1 K to 104 K. We also calculated the diffusion and thermal diffusion coefficients of mixtures of 3He and 4He. For the pure fluids, the uncertainties of the calculated values are dominated by the uncertainties of the potential; for the mixtures, the uncertainties of the transport properties also include contributions from approximations in the transport theory. In all cases, the uncertainties are smaller than the corresponding experimental uncertainties; therefore, we recommend the ab initio results be used as standards for calibrating instruments relying on these thermophysical properties. We present the calculated thermophysical properties in easy-to-use tabular form.
Keywords: diffusion coefficient, helium, intermolecular potential, second virial, thermal conductivity, thermal diffusion factor, thermophysical standards, transport properties, viscosity
1. Introduction
Today, the most accurate values of the thermophysical properties of helium at low densities can be obtained from two, very lengthy, calculations. The first calculation uses quantum mechanics and the fundamental constants to obtain, ab initio, a potential energy φ(r) for the helium-helium (He2) interaction at discrete values of the interatomic separation r and also limiting forms of φ(r) at large r (see Fig. 1). The second calculation uses standard formulae from quantum-statistical mechanics and the kinetic theory of gases to obtain the thermophysical properties of low-density helium from φ(r). Here, we report the results of the second calculation spanning the temperature range 1 K to 104 K for the second virial coefficient B(T), the viscosity η(T), the thermal conductivity λ(T), the mass diffusion coefficient D(T), and the thermal diffusion factor αT(T) for 3He, 4He, and their equimolar mixture. Our results, together with estimates of their uncertainties, are presented in easy-to-use tabular form in Appendix A. For the pure fluids, the statistical-mechanics calculations make negligible contributions to the uncertainties of the tabulated properties; therefore, we estimated the uncertainties of the results by varying φ(r) within its uncertainty and examining the consequences. For the equimolar mixture, the results from different orders of approximation in the transport theory are compared to estimate their contribution to the uncertainties.
Fig. 1.

Top: the helium-helium pair potential φ00(r). Note: a logarithmic scale is used for positive values of φ00(r) and a linear scale is used for negative values of φ00(r). Bottom: the uncertainties of the ab initio results and their fractional deviations from φ00(r). Also shown are the fractional deviations of the considered potentials fit to the various ab initio values. The fractional deviations diverge near r = 5.0 bohr where φ00(r) passes through zero. Key: (— —) φA; (• • • • • •) φSAPT; (— • •) φB; □ Ceperley and Partridge [15]; ■ Komasa [24]; ● Korona et al. [18]; ○ van Mourik and Dunning [21]; ◇ van de Bovenkamp and van Duijneveldt [20]; △ Gdanitz [19].
The present results can be applied to many problems in metrology; here we mention a few. Low-density helium is used in primary, constant-volume, gas thermometry [1]; primary, dielectric-constant gas thermometry [2]; and in interpolating gas thermometry (required by ITS-90 in the temperature range 3 K to 24.6 K) [3]. These applications require the extrapolation of measurements to zero pressure. If the present values of B(T) are used for such extrapolations, the results may be more accurate and the probability of detecting systematic errors in the measurements will be increased. Low-density helium can be used to calibrate acoustic resonators for acoustic thermometry and for measuring the speed of sound in diverse gases. Spherical acoustic resonators [4] may be calibrated using the present values of λ(T), B(T), and temperature derivatives dB/dT and d2B/dT2. The same properties together with η(T) may be used to calibrate cylindrical acoustic resonators [5]. Other instruments that might be calibrated with the help of the present results include the vibrating wire viscometer [6], the Greenspan acoustic viscometer [7], and the Burnett apparatus [8] for making very accurate measurements of the equation of state of moderately dense fluids.
The present work contrasts with a long tradition of using semi-empirical models for φ(r) to correlate the thermophysical property data for helium and the other monatomic gases [9, 10, 11]. These semi-empirical models combined limited ab initio results with critically evaluated and judiciously selected experimental data to determine the function φ(r) that correlates as much data as possible. In this work, we did not consider experimental results until all of the calculations were completed as in [12, 13]. The ab initio results were then compared to the sets of data that others had selected as inputs to semi-empirical models. In every case that we examined, the ab initio values of the thermophysical properties agreed with the data within plausible estimates of their combined uncertainties.
This manuscript is organized as follows: Sec. 2 reviews the ab initio results for φ(r) and our analytic representation of them. Section 3 outlines the steps in calculating the thermophysical properties of helium from φ(r). Each step includes a description of the precautions that were taken to insure that imperfections of the numerical methods did not adversely affect the results. Section 4 estimates the uncertainty of the ab initio helium pair potential and how it propagates into the uncertainties of the calculated properties. Section 5 describes the tabulated results and methods for their use. Section 6 compares the calculated properties with selected measurements. Section 7 summarizes the present results and the prospects for future refinements.
2. Ab Initio Values for the He2 Potential Energy Functions φ(r)
Table 1 lists recent ab initio values of φ(r) at selected values of r (3.0 bohr, 4.0 bohr, and 5.6 bohr, where 1 bohr = 0.052917721 nm) and, where available, the uncertainties estimated by the original authors. As is conventional in this field, the potential energy is divided by kB K and thus has the unit K (kB is the Boltzmann constant [14] and K is the unit symbol for the kelvin). The various calculations almost, but not quite, agree within their uncertainties. The discrepancies near 4.0 bohr are particularly significant in determining the uncertainties of thermophysical properties of helium near ambient temperatures. A detailed evaluation of each calculation in Table 1 is beyond the scope of this paper. Here, we mention the observations that guided our selection among the sources cited in Table 1 to obtain φ00(r), the function that we used to calculate the thermophysical properties of helium.
Table 1.
Selected ab initio values of φ(r). (1 bohr = 0.052 917 721 nm)
| φ(3.0 bohr)/K | φ(4.0 bohr)/K | φ(5.6 bohr)/K | Remarks | |
|---|---|---|---|---|
| Ceperley and Partridge [15] | 3800 ± 100 | “exact” QMC | ||
| Anderson et al. [16] | 3812 ± 96.0 | −11.01 ± 0.10 | “exact” QMC | |
| Klopper and Noga [17] | 294.5 292.6 |
−10.68 −11.00 |
corrected to FCI | |
| Korona et al. [18] | 3759.959 ± 11.3 | 291.64 ± 0.9 | −11.06 ± 0.03 | SAPT |
| Komasa [24] | 3768.469 | 292.784 | −10.947 −10.978 |
(1200 term) (2048 term) upper bound |
| Gdanitz [19] | 3768.813 3768.0 ± 0.8 |
293.025 292.7 ± 0.4 |
−10.947 −11.05 ± 0.10 |
extrapolated to ∞ basis set |
| van de Bovenkamp and Duijneveldt [20] | 293.48 292.72 ± 0.02 |
−10.95 −10.99 ± 0.02 |
corrected to FCI | |
| van Mourik and Dunning [21] | 293.498 292.578 |
−11.00 ± 0.03 −10.99 |
corrected to FCI |
2.1 Long-Ranges: r ≳ 8 bohr
The asymptotic long-range attractive behavior of our preferred potential φ00(r) is represented by the two-body dispersion coefficients Cn (n = 6, 8, …) in the multipole expansion. These coefficients have been calculated, ab initio, by two independent groups [22, 23] using a sum-over-states formalism with explicitly electron-correlated wave functions to describe the states. The independent calculations [22, 23] differed by less than 1 in the fourth digit. This small difference makes a negligible contribution to the uncertainties of the thermophysical properties calculated from φ00(r).
2.2 Short-Ranges: r ≲ 3 bohr
Ceperley and Partridge [15] obtained values of φ(r) at small r using a quantum Monte Carlo (QMC) method. The QMC method is exact insofar as it requires no mathematical or physical approximations beyond those in the Schrödinger equation and the method yields estimates of the uncertainties of φ(r). Komasa [24] used a variational method to obtain rigorous upper bounds to φ(r) in the range 0.01 bohr ≤ r ≤ 15 bohr. At some values of r, the variational values of φ(r) are less than the QMC values; however the differences between the values are usually within twice the QMC uncertainties. Thus, we used the variational values to determine φ00(r) and we have evidence that the QMC uncertainties are reasonable. At smaller values of r the variational and QMC results are inconsistent. For example, at r = 1 bohr (not plotted), Komasa reports φ(1 bohr) = (286.44 ± 0.03) × 103 K, and Ceperley and Partridge report φ(1 bohr) = (291.9 ± 0.6) × 103 K. We are unable to resolve this inconsistency; however, the inconsistency does not affect the thermophysical properties in the temperature range 1 K to 104 K.
Komasa provides two values for the well depth at 5.6 bohr, ε/kB = −10.947 K using a 1200-term basis set and ε/kB = −10.978 K using a 2048 term basis set. The second value is 0.3 % lower. Komasa’s calculations at other values of r used the 1200-term basis set. We speculate that comparable reductions in φ(r) would occur if Komasa’s variational calculation were repeated with the larger basis set at all values of r.
2.3 Intermediate Ranges: 3 ≳ r ≳ 8 bohr
At intermediate ranges, we considered the seven relevant publications cited in Table 1. Anderson et al. [16] report exact QMC results that have relatively large uncertainties. Klopper and Noga [17] used an explicitly correlated coupled cluster [CCSD(T)] method that resulted in the limiting value for the well depth of ε/kB = −10.68 K at 5.6 bohr. Then, they estimated the effects of quadruple substitutions to be −0.32 K at 5.6 bohr (and −1.9 K at 4.0 bohr) by comparing their results to the full configuration interaction (FCI) calculation of van Mourik and van Lenthe [25]. This extrapolation to a complete basis set resulted in ε/kB = −(11.0 ± 0.03) K, which agrees with the QMC results of Anderson [16].
Korona et al. [18] used symmetry-adapted perturbation theory (SAPT) to calculate values for φ(r) with uncertainties that they estimated to be the larger of 0.3 % or 0.03 K in the range 3 bohr ≤ r ≤ 7 bohr. The SAPT well-depth is ε/kB = −(11.06 ± 0.03) K, the lowest of all ab initio results; however, it also agrees with the QMC result [16] within the latter’s uncertainty.
While this project was in progress, two groups extended the CCSD(T) calculations of Klopper and Noga [17]. These groups (de Bovenkamp and Duijneveldt [20]; and van Mourik and Dunning [21]) used different techniques to extrapolate the results of Klopper and Noga [17] to an infinite basis set. Gdanitz [19] also published calculations labeled r12-MRACPF in which he extrapolated his results to an infinite basis set by yet another method. These three recent publications and the variational results of Komasa [24] indicate that the SAPT [18] results in the region around r = 4.0 bohr are too attractive by approximately 0.05 K (Fig. 1, lower panel). Nevertheless, we used the SAPT intermediate-range results in determining the potential φ00(r) and we used the differences between the SAPT and the other results to determine alternative potentials that were used to estimate the uncertainties of the thermophysical properties. Our decisions are based on three observations. First, we recalled that Komasa’s [24] variational result at 5.6 bohr decreased 0.3 % upon increasing the basis set from 1200 terms to 2048 terms. If Komasa’s result at 4.0 bohr (292.784 K) were decreased by 0.3 %, it would be 291.906 K, in agreement with the SAPT value of 291.64 K. Gdanitz suggested that the decrease at 4.0 bohr might be less than 0.3 % because the variation method is more accurate at smaller separations [26]. Second, we noted that the two extensions of Klopper and Noga’ work [17] are not independent. The two decompose φ(r) in several components, the largest of which were calculated best by Klopper and Noga. Thus, the uncertainties of these results may be dominated by those of Klopper and Noga. (van Mourik and Dunning [21] state, “It is likely that the corrected curve is the most accurate available to date for He2 interactions”. In effect, they asserted that Klopper and Noga’s interaction energies are more accurate than their own complete basis set extrapolated energies.) Third, Bukowski et al. [27] argue that their own Gaussian-type geminals (GTG) computation bounds the larger components of Klopper and Noga’s CCSD(T) computations and they suggest that Klopper and Noga’s results may be too high by approximately 0.3 K at 4 bohr and by approximately 0.04 K at 5.6 bohr. If Bukowski et al.’s suggestion is correct and if one decreases the CCSD(T) values of φ(r) accordingly, then they all would agree with the SAPT results. Ultimately, additional calculations will resolve these issues.
2.4 Algebraic Representations of ab initio Values of φ(r)
We calculated the thermophysical properties of helium six times, each using a different function to represent ab initio values of φ(r). We fitted two of these six functions, φ00 and φB, to our own selections among the published ab initio values. The third function, φSAPT, had already been fitted by others to ab initio results and used to calculate thermophysical properties. [18] We fitted the fourth, φA, to the same ab initio results used to obtain φSAPT; however, we added one additional fitting parameter. Thus, differences between the thermophysical properties computed from φSAPT and φA provide one indication of the sensitivity of the properties to the algebraic representation of the ab initio “data”. The last two functions are denoted, , and . To obtain , we decreased the ab initio short-range results [15] by their claimed uncertainties and decreased the intermediate-range SAPT results by 0.1 % and re-fitted them. Then, we increased the ab initio results by their claimed uncertainties and the SAPT results by 0.1 % and fitted them to obtain . The differences between the thermophysical properties calculated using φA, , and φSAPT are analogous to the uncertainties of measured values of thermophysical properties conducted in a single laboratory and analyzed using different methods. In the present case, the differences between the thermophysical properties calculated from φA, , , and φSAPT are much smaller than the differences between those calculated from φ00, φA, and φB.
2.4.1 φ00
We used φ00 to calculate the thermophysical properties tabulated in Appendix A. In our judgement, φ00 is the best representation of the ab initio results available at the time of this writing. The subscript “00” identifies φ00 by the year in which we began using it. The ab initio results fitted by φ00(r) come from three sources: (1) at small r (1 < r < 2.5 bohr), the results of the variational calculation from Komasa [24], (2) at intermediate r (3 bohr < r < 7 bohr), the SAPT results from Korona et al. [18], (3) at large r, the asymptotic constants from the “exact” dispersion coefficients of Bishop and Pipin [22] and the higher order dispersion coefficients determined from the approximate relations presented by Thakkar [29]. The algebraic representation of φ00(r) is a modification of the form given by Tang and Toennies [9]. The representation is the sum of repulsive (φrep) and attractive (φatt) terms:
| (1) |
Equation (1) includes the factor f2n(r) that accounts for the relativistic retardation of the dipole-dipole (n = 3) term applied over all r. This factor changes the behavior of the dipole-dipole term from r−6 to r−7 at very large r, and it was taken from Jamieson et al. [30]. When the expressions for the retardation of the higher dispersion terms C8 and C10 given by Chen and Chung [23] were applied to φ00, the well depth changed by only 0.0014 K out of 11 K. The resulting changes in the calculated thermophysical properties were much smaller than their uncertainties; thus, we used the approximation f2n(r) ≡ 1 for n > 3. (Note: retardation is included when calculating the thermophysical properties; however, by convention, it is not included when comparing Eq. (1) to the ab initio results.) We also considered the adiabatic correction of the helium dimer given by Komasa et al. [28]. The effects of this correction were also much smaller than those from the uncertainties in φ(r); thus, we omitted this correction.
The definition of φ00(r) in Eq. (1) is broken into two ranges. If this were not done, φ00(r) would have a spurious maximum at very small values of r. As indicated in Eq. (1), the break-point was set at 0.3 bohr.
The dispersion coefficients (C6, C8, … C16) in Eq. (1) and Table 1 were held fixed [22, 29]. The values of the remaining parameters in Table 1 (a−2, a−1, a1, a2, and δ) were determined by fitting φ00(r) to the ab initio results. When fitting φ00 the ab initio results were weighted in proportion to the reciprocal of the uncertainty squared, where the uncertainties were taken (when available) from the publications that presented the results. [15, 18, 20, 21, 24].
2.4.2 φSAPT
Korona et al. fitted their SAPT results and the QMC values of Ceperley and Partridge [15] to the algebraic expression of Tang and Toennies [9] while holding constant the asymptotic dispersion coefficients of Bishop and Pipin [22]. They included higher order dispersion coefficient determined with combining rules of Thakkar [29] and retardation effects of the C6 dispersion coefficient as given by Jamieson et al. [30]. Janzen and Aziz [11] calculated the thermophysical properties of helium using φSAPT and they “judged it to be the most accurate characterization of the helium interaction yet proposed.” We believe that φ00 is more accurate than φSAPT because it uses the recent, accurate variational results of Komasa [24] instead of the earlier short range QMC values of Ceperley and Partridge [15].
2.4.3 φA , and
In an attempt to ascertain how uncertainties in the interaction energies propagate into the thermophysical properties we constructed alternative potentials which differed in the choice of ab initio results, and in the form of the algebraic expression. The first alternative, denoted φA, was obtained by fitting the exact same ab initio results from [18, 22, 24, 29] as φSAPT. The algebraic expression of Tang and Toennies [9] was modified by adding a a3r3 to the exponent of the repulsive term, such that φrep = A exp(a1r + a2r2 + a3r3). The additional a3r3 term enables φA to fit the φSAPT ab initio results within 0.1 % in two regions r = 3 bohr and at r > 6 bohr where φSAPT [18] deviates from the ab initio results slightly greater than 0.1 %.
To obtain , we decreased the ab initio short-range [15] and long-range [22] results by their claimed uncertainties and decreased the intermediate-range SAPT results by 0.1 % and the long-range dispersion coefficients by 0.08 %. Equation (1) was then re-fitted to obtain . We then increased the ab initio results by their claimed uncertainties and the intermediate-range SAPT results by 0.1 % and again fitted them to obtain .
2.4.4 φB
The potential φB, uses the CCSD(T) results of van Mourik and Dunning [21] and of van de Bovenkamp and van Duijneveldt [20] instead of the SAPT results of Korona et al. [18] in the intermediate range of 3 bohr < r < 7 bohr. To fit these values the algebraic expression of Tang and Toennies [9] was modified again by adding a a−1r−1 and a−2r−2 to the exponent of the repulsive term, such that φrep. = A exp(a1r + a2r2 + a−1r−1 + a−2r−2).
2.5 Comparison of φ00, φSAPT, φA, and φB
Table 2 and the lower panel of Fig. 1 display the changes in φ(r) resulting from alternate choices among the ab initio results. The differences between the thermophysical properties calculated using φ00, φSAPT, φA, and φB are analogous to the differences between measurements of thermophysical properties conducted in different laboratories using different methods and they are used to estimate the uncertainties of the results for pure 3He and pure 4He.
Table 2.
Parameters for Eq. (1) in atomic units (1 bohr = 1 Bo = 0.052 917 721 nm, 1 hartree = 1 Ha = 3.157 746 5 × 105 K)
| Property (unit) | φ00 | φA | φB | φSAPT | ||
|---|---|---|---|---|---|---|
| 10−6 A (K) | 2.83379199 | 2.02311 | 2.03130 | 2.01529 | 3.12631 | 2.07436426 |
| a1 (Bo−1) | −1.986231822 | −1.84827 | −1.85059 | −1.84616 | −2.01639 | −1.88648251 |
| 102 a2 (Bo−2) | −5.034284240 | −7.55879 | −7.50314 | −7.60470 | −4.67475 | 6.20013490 |
| 103 a3 (Bo−3) | 0.0 | 1.82924 | 1.71078 | 1.93491 | 0.0 | 0.0 |
| a−1 (Bo) | −0.3514929118 | 0.0 | 0.0 | 0.0 | −0.47972 | 0.0 |
| a−2 (Bo2) | 0.1101468439 | 0.0 | 0.0 | 0.0 | 0.16755 | 0.0 |
| δ (Bo−1) | 2.00788607 | 2.03451 | 2.02137 | 2.04780 | 2.01997 | 1.94861295 |
| C6 (Ha·Bo−6) | 1.46097780 | 1.46098 | 1.45981 | 1.46215 | 1.46098 | 1.46097780 |
| 10−1 C8 (Ha·Bo−8) | 1.4117855 | 1.41179 | 1.41066 | 1.41291 | 1.41179 | 1.4117855 |
| 10−2 C10 (Ha·Bo−10) | 1.83691250 | 1.83691 | 1.83544 | 1.83838 | 1.83691 | 1.83691250 |
| a10−3 C12 (Ha·Bo−12) | 3.265 | 3.265 | 3.262 | 3.268 | 3.265 | 3.265 |
| a10−4 C14 (Ha·Bo−14) | 7.644 | 7.644 | 7.638 | 7.650 | 7.644 | 7.644 |
| a10−6 C16 (Ha·Bo−16) | 2.275 | 2.275 | 2.273 | 2.277 | 2.275 | 2.275 |
Calculated using combining rules of Thakkar [29]
Table 3 lists some characteristic properties of the potentials that we have used. They include the well depth ε/kB, the locations of the zero (σ) and of the minimum (rm) of the potential, and the energy of the bound state (Eb) of a pair of 4He atoms. Following Janzen and Aziz [31], we estimated the number of Efimov states NE from the scattering length and the effective range with the result NE = 0.77 ± 0.01 for φ00. Because NE < 1 for all potentials in Table 2, Efimov states are unlikely to exist. A discussion of these properties of the interatomic potential for helium can be found in Ref. [31].
Table 3.
Properties of the fitted helium potentials. (1 Å = 10−10 m)
| Property (unit) | φ00 | φA | φB | ||
|---|---|---|---|---|---|
| ε/kB (K) | 11.054 | 11.063 | 10.974 | 11.074 | 11.052 |
| rm (bohr) | 5.6039 | 5.6034 | 5.6097 | 5.6034 | 5.6034 |
| rm (Å) | 2.9654 | 2.9652 | 2.9685 | 2.9625 | 2.9652 |
| σ (bohr) | 4.9873 | 4.9870 | 4.9922 | 4.9868 | 4.9873 |
| scattering length (Å) | 83.68 | 82.00 | 96.91 | 85.30 | 78.90 |
| effective range (Å) | 7.24 | 7.24 | 7.30 | 7.26 | 7.22 |
| bound state/kB (mK) | 1.90 | 1.98 | 1.39 | 1.83 | 2.51 |
3. Numerical Calculations and Their Uncertainties
Here, we outline the steps required to calculate the thermophysical properties of helium from the interatomic potential. We also describe the precautions that were taken to insure that the uncertainties in the results from approximations in statistical mechanics and in the numerical methods were both smaller than the uncertainties results from different choices for φ(r).
The initial steps of calculating the thermophysical properties that depend upon pairs of helium atoms are all the same. (1) The Schrödinger equation for the scattering of a helium atom at the energy E in the potential φ(r) is separated in spherical coordinates, (2) the radial part of the wave function is expanded in partial waves ψℓ(r) of angular momentum ℓ, (3) several nodes of the scattered wave are located far from the scattering atom, and (4) the phase shifts δℓ of the scattered wave are determined and (5) summed with appropriate statistics to obtain cross sections. The summations account for large symmetry effects at low temperatures [32]. Thus, separate summations are required for 3He and 4He and their mixtures when calculating the second virial coefficient and the transport properties. The final step (6) is an integration over energy that is appropriate to the thermophysical property under consideration.
3.1 Integration of the Radial Schrödinger Equation
The Schrödinger equation is separated in spherical coordinates and decomposed into angular momentum states to obtain
| (2) |
where ħ is Planck’s constant [14] divided by 2π, µ is the reduced mass µ= (m1 + m2)/m1m2, k = (2µE)1/2/ħ is the wave number, and E is the energy of the incoming wave. Equation (2) is integrated to obtain the perturbed wave functions ψℓ(k, r). The location rn of the nth zero (or node) of the wave function ψℓ(k, r) was found using a five point Aiken interpolation formula with values of ψℓ(k, r) near the nth node. The integration was performed using Numerov’s method [33] as implemented in [34] and [35]. At each energy, rn was recalculated using successively smaller step sizes. The calculation was terminated when halving the step size changed rn less than 10−9 × rn. We verified that the tolerance 10−9 × rn was sufficiently small that further reductions of the step-size did not change the thermophysical properties beyond the tolerances given in Table 6. The final sizes of the integration steps are listed in Table 4.
Table 6.
Relative uncertainty of thermophysical properties of pure 4He and 3He propagated from the differences between potentials
| 2000 | ΔB/B × 104 | Δη/η × 104 | Δλ/λ × 104 | ΔD12/D12 ×104 | ΔαT/αT × 104 |
|---|---|---|---|---|---|
| 2 | 80 | 40 | 40 | 56 | 301 |
| 5 | 89 | 17 | 17 | 15 | 84 |
| 10 | 125 | 6.3 | 6.5 | 5.8 | 32 |
| 20 | 559 | 5.4 | 5.2 | 4.5 | 10 |
| 50 | 91 | 8 | 8 | 6.6 | 7.5 |
| 100 | 43 | 7.7 | 7.7 | 6.4 | 4.4 |
| 200 | 29 | 6.7 | 6.8 | 5.8 | 4.5 |
| 300 | 22 | 6.1 | 6.2 | 5.4 | 4.1 |
| 400 | 19 | 5.7 | 5.8 | 5.1 | 7.1 |
| 500 | 17 | 5.5 | 5.5 | 4.9 | 9.8 |
| 1000 | 14 | 4.8 | 4.8 | 4.6 | 19.9 |
| 2000 | 11 | 4.6 | 4.6 | 5.6 | 33 |
Table 4.
Integration step sizes used in a given energy range to locate the nth zero of ψℓ(k, r)
| Integration step size (cm−1) |
Applicable range (cm−1) |
|---|---|
| 0.0001 | 0.0 ≤ k ≤ 0.01 |
| 0.001 | 0.01 ≤ k ≤ 0.4 |
| 0.01 | 0.4 ≤ k ≤ 10.0 |
| 0.05 | 10.0 ≤ k ≤ 100.0 |
| 0.1 | 100.0 ≤ k |
3.2 Calculation of Phase Shifts, δℓ(k,n)
The relative phase shifts, δℓ(k,n) of the outgoing partial wave were evaluated from the relation
| (3) |
where jℓ(k,rn) and nℓ(k,rn) are the Bessel and Neuman functions for angular momentum quantum number ℓ and wave number k. In practice, the phase shifts were evaluated at groups of three consecutive nodes. If the phase shift did not change by more than 10−8 × δℓ(k,n) between the first and last of the three nodes, it was assumed that n (and rn) were sufficiently large that additional effects of the potential were negligible, and the calculation was terminated. Otherwise, the calculation was continued to larger values of r, and the test was repeated. We verified that the tolerance 10−8 × δℓ(k,n) is consistent with the uncertainties of the thermophysical properties listed in Table 6.
3.3 Calculation of the Second Virial Coefficient, B(T)
The second virial coefficient was obtained by adding two or three terms; the first term is a thermal average Bth(T), the second term is that of an ideal gas Bideal(T), and the third term is the bound state term Bbound(T), which applies to 4He, but not to 3He because a bound state exists only for 4He.
3.3.1 The Thermal Average Term Bth(T)
The thermal average term Bth(T) is
| (4) |
where kB is the Boltzmann constant, and δℓ(k,n ≃ ∞) is the phase shift at large enough separation that the potential no longer perturbs the outgoing wave function [32, 36].
Equation (4) contains both a sum and an integral with the limits 0 and ∞. Truncating the sum and the integral at a finite upper bound is a potential source of error. At each value of k, the sum was computed until the addition of six phase shifts did not change the sum by more than 10−8 of its value. At the lowest energies, this condition was met after adding seven phase shifts; at the highest energies, hundreds of phase shifts were added.
At this step of the calculation, symmetry effects are incorporated. The unweighted sum [Eq. (4)] is carried out over all values of ℓ only when calculating the interaction virial coefficient for mixtures of 3He and 4He, because these atoms are distinguishable and follow Boltzmann statistics. For pure 3He and 4He, weighted sums are performed over the even and odd values of ℓ using the formulas
| (5) |
where s is the spin quantum number (0 for 4He; ½ for 3He), BE stands for Bose-Einstein statistics for bosons (4He), and FD stands for Fermi-Dirac statistics for fermions (3He). Details on this calculation can be found in Ref. [32].
The integral in Eq. (4) was evaluated using a standard integration routine, DQAGI [37]. This routine is designed for semi-infinite or infinite intervals and automatically uses nonlinear transformation and extrapolation to achieve user-specified absolute and relative tolerances for a user-specified function. The relative error was set to 10−8. If the integrator could not achieve this accuracy, an error message would have been reported the problem.
3.3.2 The Ideal-Gas and Bound State Terms Bideal(T) and Bbound(T)
The ideal-gas contribution Bideal(T) is negative for BE and positive for FD, and zero for Boltzmann statistics as given by
| (6) |
where NA is the Avagodro constant and λ ≡ [ħ/(µkBT)]1/2 is the “thermal wavelength.” The ideal-gas term is important only at low temperatures; it is 1/10 of B(T) at 5 K and 1/100 of B(T) at 75 K. The ideal-gas term is a function of fundamental physical constants and the resulting standard uncertainty is on the order of 10−6.
For 4He, the bound state term Bbound(T) is
| (7) |
where Eb is the energy of the bound state. The bound state term is 1/1000 of B(T) at 3 K and 1/100 of B(T) at 0.4 K. Eb was determined from integrating the Schrödinger equation; thus, it depended upon the integration step size. Decreasing step sizes were used until consecutive values of Eb differed by less than 10−6 × Eb. This numerical uncertainty is much smaller than the 18 % difference between Eb determined from and that determined from (Table 3).
The sum of the numerical uncertainties in the calculation of B(T) is at most 10−5 × B(T). This is insignificant compared with the uncertainty of B(T) which arises from the uncertainty of the potential φ(r). For example, the uncertainty of B(T) resulting from the uncertainty of φ(r) is 0.0022 × B(T) at 300 K; the relative uncertainties at other temperatures are listed in Table 6.
3.4 Calculation of the Transport Properties
In order to calculate the transport properties, we used the numerical methods outlined above to obtain the phase shifts as functions of the wave number and angular momentum quantum number. Then we computed the sums over the phase shift that determine the quantum cross sections, Q(1), Q(2), Q(3)….Q(n), etc. [38]. The cross sections were integrated with respect to energy to obtain the temperature-dependent collision integrals. Finally, the transport properties were calculated using the appropriate combinations of the collision integrals.
3.4.1 Calculation of the Quantum Cross Sections Q(n)
The quantum cross sections are functions involving the sums of the phase shifts that depend upon the symmetry of the interacting atoms. The sums over the even and the odd values of ℓ are needed separately:
| (8) |
and then weighted sums are computed. To evaluate Q(1) for Bose-Einstein (BE) or Fermi-Dirac (FD) statistics the sums are weighted with the spin-dependent quotients, as shown in Eq. (5). As for the case of the second virial coefficient, the sums in Eq. (8) extend to ℓ = ∞. The sum was continued until the addition of six more phase shifts changed the cross section by less than 10−8 of its value. Cross sections with moments up to n = 6 are required to calculate the collision or omega integrals used in the higher order approximations for the transport properties. The equations for these calculations are given by Ref. [38].
3.4.2 Calculation of the Collision Integrals Ω(n,s)
The reduced collision integrals were evaluated from the equation
| (9) |
where the superscript ★ indicates that both the energy and the temperature were scaled by the well-depth of φ00 and Q(n)★ was scaled by the value Q(n)★ for a rigid sphere of radius rm, the location of the minimum of φ00 (Table 3; See Ref. [32]).
In order to evaluate of Eq. (9), the quantum cross sections Q(n)★ must be calculated at each energy E used for the quadrature. We calculated a table of Q(n)★ as a function of E★ and used a 5 point Aiken interpolation to determine values of Q(n)★ between tabulated values. The intervals in the table were determined such that the interpolated values had a uncertainty of less that 10−6 × Q(n)★. Equation (9) was integrated using the automated quadrature routine DQAGI [37], discussed in Sec. 3.1.3, with the tolerance set to 10−8. The numerical methods used to calculate the collision integrals yielded results with a relative uncertainty of less than 10−5.
3.4.3 Calculation of the Transport Properties From the Collision Integrals
The transport properties of dilute gases are calculated using combinations of the collision integrals in approximations of increasing complexity and accuracy. The viscosity and thermal conductivity of pure 3He and 4He were calculated to the 5th order approximation [39]. The equimolar mixture thermal conductivity [40] and thermal diffusion factors [41] were calculate to the 3rd order, and the diffusion coefficient and mixture viscosity were calculated to 2nd order. Figure 2 shows the effects of truncating the order of the calculation. The changes in η and λ for 4He and equimolar mixtures of 4He and 3He are compared at four temperatures upon increasing order of the approximation. The calculations converge very well; 2nd to 3rd order results in less than a 0.1 % change, 3rd to 4th order results in less than a 0.01 % change, and 4th to the 5th order less than 0.001 %. The behavior of the other transport properties (η and λ for pure 3He, D12, and αT) is similar to that shown in Fig. 2. Figure 2 shows that the change in η and λ of the equimolar mixture from 1st to 2nd order, is very close to that of pure 4He. These results show that only calculations of the 2nd order contribute any significant uncertainty to the calculated properties. From these observations, we conclude that the relative uncertainty of η and D12 for the equimolar mixture ranges from 0.01 % to 0.04 % in the temperature range 10 K ≤ T ≤ 104 K. Figure 2, together with the equivalent figure for pure 3He, suggest that, at T > 100 K, the accuracy of the calculated η and D12 for the equimolar mixture might be improved if one extrapolated from 2nd order to 5th order by following the curves for pure 4He and 3He.
Fig. 2.

Asymptotic approach of viscosity and thermal conductivity of 4He as a function of increasing order of approximation along the four indicated isotherms. The open symbols are for the equimolar mixture of 3He and 4He.
The rapid reduction of the uncertainty of the calculated viscosity with increasing order of approximation is not sensitive to φ(r); Viehland et al. [39] obtained similar results for the viscosity of rigid atoms that interact via (12-6) Lennard-Jones potentials.
3.5 Interpolation as a Function of Temperature
The tables in Appendix A list values of the second virial coefficient, the transport properties, and their first derivatives as functions of temperature. The temperature intervals were chosen so that the errors from linear interpolation would be smaller than the uncertainties propagated from the uncertainties of the interatomic potential. Table 5 lists bounds of the interpolation errors, and the unweighted average over the entire temperature range. Below 10 K, the interpolation errors increase because the temperature derivatives of the properties increase.
Table 5.
Relative uncertainties from interpolating between tabulated temperatures
| Max (1 K to 10 K) |
Max (10 K to 104 K) |
Average (1 K to 104 K) |
|
|---|---|---|---|
| ΔB/B × 106 | 187 | 95.3 | 18.0 |
| Δη/η × 106 | 107 | 3.23 | 3.01 |
| Δλ/λ × 106 | 85.5 | 3.24 | 3.05 |
| ΔD12/D12 × 106 | 1.95 | 1.93 | 0.38 |
| ΔαT/αT × 106 | 288 | 6.55 | 6.43 |
3.6 Classical Calculation
We made an important check of the entire calculation of each thermophysical property. To do so, we performed the relatively simple classical calculation [32] which is valid at high temperatures where the ratio of the de Broglie wavelength h(2πmkT)−1/2 to atomic diameter σ is much less than 1. Figures 3 and 4 show that the classical calculations of the viscosity and of the second virial coefficient asymptotically approach the quantum results.
Fig. 3.

Fractional deviation of the calculated viscosity using the considered potentials. The base line is the viscosity calculated to the 5th approximation for 4He from φ00. The other curves are identified in the figure. The shaded region around the curve for φA shows how and vary the predicted viscosity. Also shown is the classically calculated viscosity asymptotically approaching the quantum values with increasing temperature.
Fig. 4.

Fractional deviation of the calculated second virial coefficient using the considered potentials. The base line is B00 calculated for 4He using φ00. The other curves are as identified in the figure. The shaded region around the curve for BA shows how and influence the predicted B. Also shown is the classically calculated value for B(T) asymptotically approaching the quantum values with increasing temperature.
4. Uncertainties of the Thermophysical Properties From the Uncertainty of the Potential
We now evaluate how the uncertainty of the ab initio values of φ(r) propagates into the uncertainty of calculated thermophysical properties. To do so, we calculated the properties with each of the potentials discussed in Sec. 2 and we plotted the results as deviations from the results obtained for φ00(r). Figure 3 shows these deviations for the viscosity of 4He. In Fig. 3, the width of the shaded band surrounding the curve φA spans the range of results obtained with to those obtained with . Similar bands could have been placed about the results from those obtained with φ00, φB, and φSAPT; they were omitted for clarity.
We took the differences in the alternative potentials as an accurate estimate of the uncertainty in φ00. By comparing the properties calculated from each alternative potential, we estimated the actual uncertainty propagated into each reported thermophysical property. Figure 3 shows that as the temperature is increased from 1 K to 10 K, the relative uncertainty of the viscosity ur(η) of 4He decreases from 0.4 % to 0.1 %. In this temperature range, the discrepancies among the potentials are comparable to the uncertainty of each potential, as indicated by the width of the shaded band. In the range 10 K < T < 1000 K, the difference between the results obtained using φ00 and the results obtained with φB and φSAPT lead us to conclude that ur(η) is approximately 0.08 %. If the discrepancies between the ab initio results around 4.0 bohr could be resolved, then ur(η) would be reduced by nearly a factor of three in this temperature range. In the range 1000 K < T < 104 K, we also conclude ur(η) ≈ 0.08 %. In this temperature range, the results from φ00 and φB are more reliable than the results from φA and φSAPT having been fit to the short-range variational calculations of Komasa [5] as discussed in Sec. 2, above.
The relative uncertainty of the second virial coefficient ur(B) of 4He can be judged from Fig. 4. In the range 1 K < T < 10 K, ur(B) ≈ 1 %. At T ≈ 23.4 K, B(T) passes through zero. There, ur(B) diverges; however, the uncertainty of B, u(B) ≈ 0.3 cm3·mol−1. In the range 100 K < T < 104 K, ur(B) of 4He gradually declines from 0.4 % to 0.1 %.
The uncertainties for each property are summarized in Table 6 from comparisons similar to those provided in Figs. 3 and 4 and described in the preceding paragraphs. These uncertainties are much lower than those from measurements; thus, the corresponding values of the properties listed in the Appendices can be used as standards.
5. Results
The results of the present calculations for 4He, 3He, and their equimolar mixture are listed in Tables A1, A2, and A3 in Appendix A. These tables contain the second virial coefficient B for the pure species and the interaction second virial B12 where . The zero-density viscosity, thermal conductivity and their equimolar mixture. The diffusion coefficient at 101.325 kPa (one atmosphere), and the thermal diffusion factor. Derivatives with respect to temperature are provided to facilitate interpolation and for use in calculating acoustic virial coefficients. The tables for pure 4He and 3He contain the self-diffusion coefficient calculated without symmetry effects (Boltzmann statistics), and the thermal diffusion factor of a mixture of 99.999 % 4He or 3He respectively. The tables span the temperature interval 1 K ≤ T ≤ 104 K. The highest temperature is well below the first excited state of helium (2 × 105 K) and well below 2.91 × 105 K, the highest value of the ab initio results used to determine φA. In order to calculate the thermophysical properties between the tabulated temperatures, we recommend interpolation using the cubic polynomial f(T) such that
| (10) |
where f′ = df/dT and ΔT = T2 − T1. The calculated values listed in the Tables are accurate to the uncertainties discussed in Sec. 4. Equation (10) contributes an additional uncertainty from the interpolation discussed as in Sec. 3.
6. Comparison With Measurements
In this section we compare the values of the thermophysical properties calculated using φ00 with the best experimental values. In nearly every case, the experimental values agree with the calculated values within their combined uncertainties, and the calculated properties have the smaller uncertainties.
6.1 Second Virial Coefficient
Figure 5 displays the deviations of various experimental values of B(T) for 4He from B00(T) calculated using φ00. The dashed curves in Fig. 5 represent the values of BA(T), calculated using φA, and the dash-dot-dot curves the values of BB(T), calculated from φB. Also shown in Fig. 5 and summarized in Table 7, are measured values of B(T) along with their reported uncertainties. In nearly every case, B00(T) agrees with the experimental values within the uncertainties of the experimental values. The maximum uncertainties of B00(T) are estimated by comparing the variances with BA(T) and BB(T). These uncertainties are much smaller than the experimental uncertainties (see the dash-dot-dot curve in Fig. 5.).
Fig. 5.

Deviations of Bexp of 4He from B00 calculated using φ00. Key: ● Ref. [42]; ○ Ref. [42] adjusted by Ref. [43]; ■ Ref. [43]; ▲ Ref. [43]; ▼ Ref. [44]; ♦ Ref [45]; □ Ref. [46]; △ Ref. [8] Eqs. (37) and (38); — — Values of BA(T) calculated using φA; — • • — BB(T) calculated using φB.
Table 7.
Deviations of Bexp from B00 calculated using φ00
| Authors [reference] | Temp. range | <|Bexp − B00|> | Range (Bexp − B00) | Reported uncertainties |
|---|---|---|---|---|
| (K) | (cm3·mol−1) | (cm3·mol−1) | (cm3·mol−1) | |
| Berry [42] | 2.60 to 27.10 | 0.41 | 0.14 to 0.83 | 0.20 to 1.00 |
| Gugan and Michel [43] [corrected for C(T)] | 4.22 to 27.10 | 0.13 | 0.04 to 0.20 | 0.20 to 0.70 |
| Gugan and Michel [43] | 4.23 to 27.17 | 0.15 | 0.03 to 0.33 | 0.01 to 0.07 |
| Kemp et al. [44] | 27.10 to 172.01 | 0.06 | −0.05 to 0.11 | 0.13 to 0.16 |
| Gammon [45] | 98.15 to 1474.85 | 0.05 | −0.07 to 0.07 | 0.05 to 0.06 |
| Kell et al. [46] | 273.15 to 623.15 | 0.03 | −0.05 to 0.05 | 0.01 to 0.15 |
| Waxman and Davis [8] | 298.15 | 0.08 | 0.08 | 0.01 |
| Matacotta et al. [47] | 1.47 to 20.30 | 0.42 | −0.14 to 0.96 | 0.20 to 1.00 |
At very low temperatures B(T) is sensitive to the shape of the potential well. Figure 5 shows that the lower well depth of φ00 predicted by Korona et al. [18] reproduces the low temperature measurements better than the shallower well depth predicted by Van de Bovenkamp and van Duijneveldt [20] and by Van Mourik and Dunning [21]. To further strengthen this argument, it is known that the low temperature second virial measurements have not been corrected for contributions from the third virial coefficient C(T). For 4He [42], the size of this “third virial correction” can be seen in the top panel of Figure 5. In that panel, the solid circles show the values B(T) before they were corrected in Ref. [43], and the open circles show the values after the correction. The correction for C(T) lowers the second virial values bringing them further in line with B00(T) and away from BB(T) indicating a preference for the lower well depth.
Table 7 provides two numerical measures of the differences between experimental values of B(T) and those calculated using φ00. One measure is the mean of the absolute values of the differences Bexp − B00 and the second is the range of these differences. The final column of Table 7 lists the range of the uncertainties reported by the experimenters. In nearly all cases the experimental uncertainties exceed the differences Bexp − B00.
Figure 6 compares Bexp(T) of 3He, deduced from the measurements of Matacotta et al. [47], with B00(T). There is an obvious trend in the deviations which is larger than the experimental uncertainties below 5 K. Probably, the trend would be removed if Bexp(T) was corrected for the for effects of the third virial coefficient of 3He [48] as discussed above for 4He [42].
Fig. 6.

Deviation of Bexp of 3He from B00 calculated using φ00(r). ■ Ref. [47]; — — Values of BA calculated using φA; — • • — BB calculated using φB.
6.2 Viscosity
Figures 7 and 8 and Table 8 compare the zero-density viscosity η00, calculated using φ00, with measured values from many sources. The experimental results are typically reported at 101.325 kPa where the density dependence is negligible in comparison with experimental uncertainties. Figure 7 shows the viscosity of 3He and 4He at low temperatures where the large quantum effects lead to important differences between the isotopes. The η00(T) values are in good agreement with the measurements of Becker et al. [49].
Fig. 7.
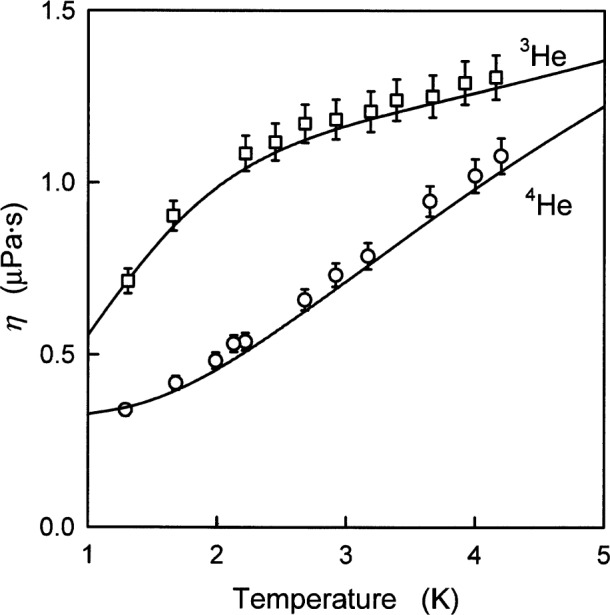
The viscosity of 3He and 4He at low temperature. Experimental values: □ for 3He and ○ for 4He from Ref. [49], ±5 % uncertainty bars are shown. The curves are η00 calculated using φ00.
Fig. 8.

Relative deviations of ηexp of 4He from η00 calculated using φ00(r). Experimental values: ● Ref. [51]; ■ Ref. [58]; Δ Ref. [54]; ▲ Ref [56] (smoothed); ○ Ref. [52]; □ Ref. [50]; ∇ Ref. [61]; — — Values of ηA calculated using φA; — • • — ηB calculated using φB.
Table 8.
Relative deviations of ηexp from η00 calculated using φ00
| Temperature range (K) | 100× (Δη/η00)rms | Range of 100 × Δη/η00 | Reported uncertainties (%) | |
|---|---|---|---|---|
| Wakeham et al. [50] | 298 to 793 | 0.22 | 0.12 to 0.32 | 0.2 to 0.5 |
| Maitland and Smith [51] | 80 to 2000 | −0.51 | −2.27 to −0.51 | 1.5 |
| Vogel [52] | 294.5 to 647.9 | 0.06 | 0.02 to 0.12 | 0.3 |
| Kestin et al. [53] | 298 to 973 | 0.20 | 0.08 to 0.30 | 0.1 to 0.3 |
| Clark and Smith [54] | 77.5 to 373 | 0.58 | 0.18 to 1.21 | 0.5 |
| Dawe and Smith [55] | 293 to 1600 | −1.16 | −2.43 to 0.45 | 1.0 |
| Coremans et al. [56] | 20.4 to 77.8 | 4.03 | 1.77 to 6.33 | 3.0 |
| Kestin and Wakeham [57] | 298 to 473 | 0.16 | 0.10 to 0.22 | 0.3 |
| Johnston and Grilly [58] | 79 to 296 | −0.96 | −1.60 to −0.18 | 3.0 |
| Kalelkar and Kestin [59] | 298 to 1121 | −0.31 | −2.16 to 0.46 | 0.5 |
| Becker et al. [49] 4He | 1.3 to 4.2 | 5.15 | −1.65 to 9.57 | 5.0 |
| Becker et al. [49] 3He | 1.3 to 4.2 | 2.73 | −0.13 to 4.37 | 5.0 |
| Kestin et al. [60] | 298 to 778 | 0.35 | 0.08 to 0.57 | 0.1 to 0.3 |
| Guevara et al. [61] | 1100 to 2150 | −0.90 | −3.93 to 0.31 | 0.65 |
Figure 8 displays the fractional deviations of various values of ηexp of 4He from η00. In nearly every case they are smaller than the uncertainties provided by the experimenters. In Fig. 8, the barely visible dashed curve represents ηA calculated using φA, and the dash-dot-dot curve ηB, calculated from φB. The differences between these curves are a measure of the ab initio uncertainties which are much smaller than the reported experimental uncertainties.
6.3 Thermal Conductivity
Figure 9 and Table 9 compare the values of the zero-density thermal conductivity of 4He calculated using φ00 with measured values from several sources. The experimental thermal conductivities are typically reported at 101.325 kPa, however the density dependence is negligible compared to the experimental uncertainties. As it was the case for B and η, most of the values of λexp differ from λ00 by an amount comparable to the uncertainty of the measurements. The differences between the values λ00, λA, and λB calculated using φ00, φA, and φB are much smaller than the uncertainties of the measurements. Table 9 lists the root mean square of the relative differences Δλ/λ00 ≡ (λexp − λ00)/λ00 and the range of these relative differences. The final column of Table 9 lists the range of the uncertainties reported by the experimenters.
Fig. 9.

Deviations of λexp from λ00 calculated using φ00 for 4He. Key: ○ Ref. [50]; □ Ref. [62]; Δ Ref. [65]; ∇ Ref. [64]; ◇ Ref [66]; — — λA calculated using φA; — • • — λB calculated using φB.
Table 9.
Relative deviations of λexp from λ00
| Temperature range (K) | 100 × (Δλ/λ00)rms | Range of 100 × Δλ/λ00 | Reported uncertainties (%) | |
|---|---|---|---|---|
| Wakeham et al. [50] | 298.15 to 973.15 | 0.23 | 0.07 to 0.36 | 0.2 to 0.5 |
| Haarman [62] | 328.15 to 468.15 | 0.43 | −0.54 to −0.32 | 0.3 |
| Jody et al. [63] | 400 to 2500 | 2.34 | −4.67 to −0.41 | 2.0 to 4.7 |
| Assael et al. [64] | 308.15 | 0.20 | −0.20 | 0.2 |
| Acton and Kellner [65] | 3.3 to 20.0 | 0.66 | −0.34 to −1.30 | 1.0 |
| Kestin et al. [66] | 300.65 | 0.13 | −0.13 | 0.3 |
6.4 Diffusion Coefficient D12(T)
Figure 10 and Table 10 compare the values of the mutual diffusion coefficient for an equimolar mixture of 3He and 4He at one atmosphere (101325 Pa). Figure 10 shows the deviations of D12,exp taken from three sources, from D12,00, where D12,00 was calculated using φ00. Because the diffusion coefficient is difficult to measure, the uncertainties of the experimental values are comparatively large; therefore, the relative deviations of the values calculated using φA and φB are not visible in Fig. 10. The difference in D12 on going from the first to the second order approximation is practically the same as seen for the viscosity in Fig. 2. Table 10 lists the root-mean-square of the relative differences ΔD/D00 ≡ (Dexp − D00)/D00 and the range of these relative differences as well as the range of the uncertainties reported by the experimenters.
Fig. 10.

Fractional deviations of D12,exp from D12,00 calculated using φ00, for an equimolar mixture of 3He and 4He. Key: ○ Ref. [67]; □ Ref. [68]; Δ Ref. [69]; — — Values of D12,A calculated using φA; — • • — D12,B calculated using φB.
Table 10.
Deviations of the D12,exp from D12,00 calculated using φ00
6.4 Thermal Diffusion Factor αT
The thermal diffusion factor αT is a complicated function of temperature and concentration and only a few, relatively inaccurate measurements are available. Figure 11 compares αT,exp for an equimolar mixture of 3He and 4He to the αT,00 values calculated from φ00. The values of αT,A and αT,B calculated from φA and φB are also shown, only differing at low temperatures. In the first-order approximate calculation of the transport properties, αT is identically zero; thus, we compared the second-order transport-theory results to the third-order results to estimate the uncertainties of the ab initio results from truncating the transport theory. Going from the second to third order increased αT by 0.56 % at 10 K and by 0.36 % at 10,000 K. The thermal diffusion factor is very difficult to measure the typical relative uncertainties are 4 % to 8 %. Owing to the experimental difficulties, the calculated values would be more accurate than any experimentally determined value.
Fig. 11.

Thermal diffusion factor αT as a function of temperature for an equimolar mixture of 3He and 4He. The solid curve represents αT,00 calculated using φ00. Key: ○ Ref. [71]; □ Ref. [72]; Δ Ref. [73]; Δ Ref. [74]; ◇ Ref [75]; — — Values of αT,A calculated using φA; — • • — αT,B calculated using φB.
7. Conclusion
We have reviewed the recent ab initio calculations of φ(r) for helium. We represented one of the most accurate ab initio values of φ(r) by the algebraic expression φ00(r) and we estimated its uncertainty by comparing the various ab initio calculations. For the thermophysical properties, the most significant uncertainties occur near 4.0 bohr. Using φ00(r), we calculated B, η, λ, D12, and αT. The numerical methods used in these calculations contributed negligible uncertainty to the results. In all cases, the uncertainties of the calculated thermophysical properties propagated from the uncertainties in φ00(r) were much less than the uncertainties of published measurements. Therefore, the calculated values should be used as standard reference values.
The large number of recent ab initio calculations of φ(r) demonstrate that this is an active field of research. In the near future, ab initio calculations will surely reduce the uncertainty of φ(r) near 4.0 bohr, further reducing the uncertainties in the calculated properties. Improved ab initio calculations of the molar polarizability of helium and of the dielectric virial coefficients are also under way. These may well lead to an ab initio standard of pressure based on measurements of the dielectric constant of helium near 273.16 K [70].
Acknowledgments
We gratefully acknowledge valuable discussions with Krzysztof Szalewicz and Robert Gdanitz.
Biography
About the authors: John J. Hurly is a Research Chemist in the Fluid Science Group of the NIST Chemical and Science Technology Laboratory. Michael R. Moldover leads the Fluid Science Group and is a NIST Fellow. The National Institute of Standards and Technology is an agency of the Technology Administration, U.S. Department of Commerce.
8. Appendix A. Calculations
The results of the present calculations for 4He, 3He, and their equimolar mixture are listed in Tables A1, A2, and A3, respectively. These tables contain the second virial coefficient for the pure species, the interaction second virial coefficient B12, the zero-density viscosity and thermal conductivity, the diffusion coefficient, and the thermal diffusion coefficient. Derivatives with respect to temperature are provided to facilitate interpolation and for use in calculating acoustic virial coefficients. The tables for pure 4He and 3He contain the self-diffusion coefficient, and the thermal diffusion factor of binary mixtures of 4He and 3He with mole fractions of 0.99999 and 0.00001, respectively.
Table A1.
Thermophysical properties of 4He as a function of temperature, where (−2) is × 10−2
| T | B | dB/dT | d2B/dT2 | η | dη/dT | λ | dλ/dT | D(101.3 kPa) | αT |
|---|---|---|---|---|---|---|---|---|---|
| (K) | (cm3·mol−1) | (cm3·mol−1·K−1) | (cm3·mol−1·K−2) | (μ Pa·s) | (μ Pa·K−1) | (mW·m−1·K−1) | (mW·m−1·K−2) | (10−4·m2·s−1) | |
| 1.0 | −474.449 | 664.861 | −1771.941 | 3.279(−1) | 5.296(−2) | 2.624 | 4.512(−1) | 7.154(−5) | 4.147(−2) |
| 1.2 | −369.743 | 411.102 | −891.823 | 3.395(−1) | 6.980(−2) | 2.713 | 5.030(−1) | 9.622(−5) | 5.098(−2) |
| 1.4 | −302.255 | 276.706 | −500.007 | 3.573(−1) | 1.096(−1) | 2.839 | 7.759(−1) | 1.240(−4) | 5.716(−2) |
| 1.6 | −255.395 | 198.357 | −304.265 | 3.834(−1) | 1.504(−1) | 3.026 | 1.097 | 1.560(−4) | 6.162(−2) |
| 1.8 | −220.996 | 149.180 | −197.490 | 4.171(−1) | 1.860(−1) | 3.276 | 1.395 | 1.927(−4) | 6.501(−2) |
| 2.0 | −194.640 | 116.448 | −135.022 | 4.573(−1) | 2.149(−1) | 3.581 | 1.642 | 2.345(−4) | 6.764(−2) |
| 2.25 | −169.200 | 88.972 | −89.028 | 5.146(−1) | 2.419(−1) | 4.022 | 1.872 | 2.943(−4) | 7.011(−2) |
| 2.5 | −149.421 | 70.388 | −61.860 | 5.775(−1) | 2.600(−1) | 4.510 | 2.023 | 3.624(−4) | 7.190(−2) |
| 2.75 | −133.560 | 57.209 | −44.818 | 6.440(−1) | 2.705(−1) | 5.028 | 2.108 | 4.386(−4) | 7.318(−2) |
| 3.0 | −120.530 | 47.499 | −33.586 | 7.123(−1) | 2.749(−1) | 5.560 | 2.143 | 5.228(−4) | 7.409(−2) |
| 3.5 | −100.334 | 34.377 | −20.391 | 8.492(−1) | 2.708(−1) | 6.628 | 2.110 | 7.138(−4) | 7.525(−2) |
| 4.0 | −85.360 | 26.103 | −13.372 | 9.815(−1) | 2.573(−1) | 7.658 | 2.005 | 9.328(−4) | 7.594(−2) |
| 4.5 | −73.788 | 20.526 | −9.272 | 1.106 | 2.407(−1) | 8.630 | 1.878 | 1.178(−3) | 7.646(−2) |
| 5 | −64.566 | 16.578 | −6.706 | 1.222 | 2.244(−1) | 9.537 | 1.754 | 1.446(−3) | 7.693(−2) |
| 6 | −50.774 | 11.477 | −3.849 | 1.433 | 1.974(−1) | 11.180 | 1.547 | 2.050(−3) | 7.784(−2) |
| 7 | −40.944 | 8.419 | −2.415 | 1.620 | 1.783(−1) | 12.650 | 1.400 | 2.735(−3) | 7.871(−2) |
| 8 | −33.581 | 6.440 | −1.615 | 1.791 | 1.649(−1) | 14.000 | 1.295 | 3.495(−3) | 7.949(−2) |
| 9 | −27.859 | 5.084 | −1.134 | 1.951 | 1.551(−1) | 15.250 | 1.218 | 4.326(−3) | 8.014(−2) |
| 10 | −23.285 | 4.114 | −8.269(−1) | 2.102 | 1.474(−1) | 16.440 | 1.157 | 5.226(−3) | 8.068(−2) |
| 11 | −19.547 | 3.397 | −6.215(−1) | 2.246 | 1.412(−1) | 17.570 | 1.108 | 6.190(−3) | 8.110(−2) |
| 12 | −16.435 | 2.850 | −4.790(−1) | 2.385 | 1.359(−1) | 18.660 | 1.066 | 7.217(−3) | 8.143(−2) |
| 14 | −11.556 | 2.087 | −3.021(−1) | 2.648 | 1.274(−1) | 20.720 | 9.990(−1) | 9.451(−3) | 8.186(−2) |
| 16 | −7.910 | 1.591 | −2.026(−1) | 2.895 | 1.206(−1) | 22.660 | 9.458(−1) | 1.191(−2) | 8.208(−2) |
| 18 | −5.088 | 1.251 | −1.425(−1) | 3.131 | 1.151(−1) | 24.510 | 9.021(−1) | 1.459(−2) | 8.216(−2) |
| 20 | −2.842 | 1.007 | −1.039(−1) | 3.356 | 1.104(−1) | 26.270 | 8.654(−1) | 1.749(−2) | 8.214(−2) |
| 22 | −1.017 | 8.27(−1) | −7.81(−2) | 3.573 | 1.064(−1) | 27.970 | 8.338(−1) | 2.058(−2) | 8.206(−2) |
| 23 | −0.227 | 7.54(−1) | −6.84(−2) | 3.678 | 1.046(−1) | 28.800 | 8.196(−1) | 2.220(−2) | 8.200(−2) |
| 24 | 0.494 | 6.90(−1) | −6.02(−2) | 3.782 | 1.029(−1) | 29.610 | 8.063(−1) | 2.387(−2) | 8.193(−2) |
| 25 | 1.155 | 6.33(−1) | −5.32(−2) | 3.884 | 1.013(−1) | 30.410 | 7.938(−1) | 2.558(−2) | 8.185(−2) |
| 26 | 1.762 | 5.83(−1) | −4.73(−2) | 3.985 | 9.980(−2) | 31.200 | 7.821(−1) | 2.734(−2) | 8.177(−2) |
| 28 | 2.840 | 4.98(−1) | −3.78(−2) | 4.181 | 9.706(−2) | 32.740 | 7.606(−1) | 3.101(−2) | 8.159(−2) |
| 30 | 3.766 | 4.30(−1) | −3.07(−2) | 4.373 | 9.460(−2) | 34.240 | 7.412(−1) | 3.485(−2) | 8.140(−2) |
| 35 | 5.587 | 3.08(−1) | −1.93(−2) | 4.833 | 8.941(−2) | 37.840 | 7.004(−1) | 4.522(−2) | 8.087(−2) |
| 40 | 6.917 | 2.29(−1) | −1.28(−2) | 5.269 | 8.523(−2) | 41.260 | 6.675(−1) | 5.664(−2) | 8.034(−2) |
| 45 | 7.921 | 1.76(−1) | −8.94(−3) | 5.686 | 8.176(−2) | 44.530 | 6.402(−1) | 6.907(−2) | 7.980(−2) |
| 50 | 8.698 | 1.38(−1) | −6.46(−3) | 6.087 | 7.882(−2) | 47.670 | 6.171(−1) | 8.246(−2) | 7.928(−2) |
| 60 | 9.806 | 8.86(−2) | −3.66(−3) | 6.851 | 7.406(−2) | 53.650 | 5.798(−1) | 1.120(−1) | 7.831(−2) |
| 70 | 10.537 | 5.98(−2) | −2.25(−3) | 7.572 | 7.035(−2) | 59.290 | 5.507(−1) | 1.452(−1) | 7.741(−2) |
| 80 | 11.038 | 4.16(−2) | −1.47(−3) | 8.260 | 6.734(−2) | 64.680 | 5.270(−1) | 1.818(−1) | 7.659(−2) |
| 90 | 11.389 | 2.95(−2) | −9.98(−4) | 8.920 | 6.483(−2) | 69.846 | 5.073(−1) | 2.216(−1) | 7.584(−2) |
| 100 | 11.640 | 2.11(−2) | −7.03(−4) | 9.558 | 6.270(−2) | 74.833 | 4.906(−1) | 2.646(−1) | 7.514(−2) |
| 120 | 11.947 | 1.07(−2) | −3.78(−4) | 10.775 | 5.923(−2) | 84.360 | 4.633(−1) | 3.597(−1) | 7.390(−2) |
| 140 | 12.098 | 4.92(−3) | −2.18(−4) | 11.932 | 5.650(−2) | 93.405 | 4.419(−1) | 4.666(−1) | 7.281(−2) |
| 160 | 12.160 | 1.50(−3) | −1.32(−4) | 13.039 | 5.428(−2) | 102.063 | 4.245(−1) | 5.847(−1) | 7.184(−2) |
| 180 | 12.167 | −6.18(−4) | −8.32(−5) | 14.105 | 5.242(−2) | 110.403 | 4.099(−1) | 7.137(−1) | 7.097(−2) |
| 200 | 12.140 | −1.96(−3) | −5.34(−5) | 15.137 | 5.083(−2) | 118.474 | 3.975(−1) | 8.532(−1) | 7.017(−2) |
| 225 | 12.077 | −2.99(−3) | −3.10(−5) | 16.386 | 4.914(−2) | 128.241 | 3.842(−1) | 1.042 | 6.927(−2) |
| 250 | 11.994 | −3.59(−3) | −1.78(−5) | 17.596 | 4.769(−2) | 137.701 | 3.729(−1) | 1.246 | 6.845(−2) |
| 275 | 11.900 | −3.93(−3) | −9.74(−6) | 18.772 | 4.644(−2) | 146.897 | 3.630(−1) | 1.466 | 6.769(−2) |
| 300 | 11.799 | −4.10(−3) | −4.69(−6) | 19.919 | 4.534(−2) | 155.863 | 3.544(−1) | 1.700 | 6.699(−2) |
| 325 | 11.695 | −4.18(−3) | −1.46(−6) | 21.040 | 4.436(−2) | 164.625 | 3.467(−1) | 1.949 | 6.634(−2) |
| 350 | 11.591 | −4.19(−3) | 6.15(−7) | 22.138 | 4.348(−2) | 173.205 | 3.398(−1) | 2.212 | 6.573(−2) |
| 375 | 11.486 | −4.15(−3) | 1.95(−6) | 23.215 | 4.268(−2) | 181.621 | 3.336(−1) | 2.489 | 6.516(−2) |
| 400 | 11.383 | −4.09(−3) | 2.81(−6) | 24.272 | 4.196(−2) | 189.889 | 3.279(−1) | 2.780 | 6.462(−2) |
| 450 | 11.183 | −3.93(−3) | 3.66(−6) | 26.338 | 4.069(−2) | 206.028 | 3.179(−1) | 3.403 | 6.361(−2) |
| 500 | 10.991 | −3.74(−3) | 3.88(−6) | 28.344 | 3.960(−2) | 221.707 | 3.094(−1) | 4.078 | 6.270(−2) |
| 600 | 10.637 | −3.36(−3) | 3.66(−6) | 32.211 | 3.783(−2) | 251.926 | 2.955(−1) | 5.584 | 6.108(−2) |
| 700 | 10.319 | −3.01(−3) | 3.19(−6) | 35.922 | 3.643(−2) | 280.913 | 2.846(−1) | 7.290 | 5.968(−2) |
| 800 | 10.033 | −2.72(−3) | 2.72(−6) | 39.506 | 3.529(−2) | 308.912 | 2.757(−1) | 9.189 | 5.843(−2) |
| 900 | 9.774 | −2.47(−3) | 2.32(−6) | 42.986 | 3.434(−2) | 336.095 | 2.682(−1) | 11.278 | 5.731(−2) |
| 1000 | 9.538 | −2.25(−3) | 1.99(−6) | 46.378 | 3.353(−2) | 362.590 | 2.618(−1) | 13.551 | 5.628(−2) |
| 1200 | 9.124 | −1.91(−3) | 1.48(−6) | 52.946 | 3.221(−2) | 413.878 | 2.515(−1) | 18.639 | 5.445(−2) |
| 1400 | 8.770 | −1.65(−3) | 1.14(−6) | 59.280 | 3.117(−2) | 463.334 | 2.434(−1) | 24.430 | 5.286(−2) |
| 1600 | 8.462 | −1.44(−3) | 9.01(−7) | 65.427 | 3.033(−2) | 511.329 | 2.368(−1) | 30.909 | 5.144(−2) |
| 1800 | 8.190 | −1.28(−3) | 7.27(−7) | 71.421 | 2.963(−2) | 558.122 | 2.313(−1) | 38.060 | 5.015(−2) |
| 2000 | 7.947 | −1.15(−3) | 5.97(−7) | 77.286 | 2.904(−2) | 603.905 | 2.267(−1) | 45.872 | 4.897(−2) |
| 2500 | 7.437 | −9.08(−4) | 3.90(−7) | 91.499 | 2.904(−2) | 714.835 | 2.267(−1) | 68.240 | 4.637(−2) |
| 3000 | 7.026 | −7.45(−4) | 2.73(−7) | 105.216 | 2.904(−2) | 821.876 | 2.267(−1) | 94.577 | 4.415(−2) |
| 3500 | 6.684 | −6.28(−4) | 2.00(−7) | 118.560 | 2.904(−2) | 925.992 | 2.267(−1) | 124.803 | 4.219(−2) |
| 4000 | 6.393 | −5.41(−4) | 1.53(−7) | 131.612 | 2.904(−2) | 1027.819 | 2.267(−1) | 158.862 | 4.043(−2) |
| 4500 | 6.141 | −4.73(−4) | 1.20(−7) | 144.429 | 2.904(−2) | 1127.805 | 2.267(−1) | 196.713 | 3.882(−2) |
| 5000 | 5.918 | −4.19(−4) | 9.68(−8) | 157.054 | 2.904(−2) | 1226.279 | 2.267(−1) | 238.324 | 3.734(−2) |
| 6000 | 5.542 | −3.39(−4) | 6.62(−8) | 181.847 | 2.904(−2) | 1419.639 | 2.267(−1) | 332.735 | 3.466(−2) |
| 7000 | 5.232 | −2.83(−4) | 4.79(−8) | 206.173 | 2.904(−2) | 1609.339 | 2.267(−1) | 441.969 | 3.229(−2) |
| 8000 | 4.972 | −2.41(−4) | 3.61(−8) | 230.154 | 2.904(−2) | 1796.322 | 2.267(−1) | 565.960 | 3.014(−2) |
| 9000 | 4.747 | −2.09(−4) | 2.81(−8) | 253.873 | 2.904(−2) | 1981.249 | 2.267(−1) | 704.683 | 2.816(−2) |
| 10000 | 4.551 | −1.84(−4) | 2.24(−8) | 277.393 | 2.904(−2) | 2164.607 | 2.267(−1) | 858.143 | 2.633(−2) |
Table A2.
Thermophysical properties of 3He as a function of temperature, where (−2) is × 10−2
| T | B | dB/dT | d2B/dT2 | η | dη/dT | λ | dλ/dT | D(101.3 kPa) | αT |
|---|---|---|---|---|---|---|---|---|---|
| (K) | (cm3·mol−1) | (cm3·mol−1·K−1) | (cm3·mol−1·K−1) | (μ Pa·s) | (μ Pa·K−1) | (mW·m−1·K−1) | (mW·m−1·K−2) | (10−4·m2·s−1) | |
| 1.0 | −237.503 | 174.583 | −218.592 | 5.561(−1) | 5.280(−1) | 5.756 | 5.459 | 1.910(−4) | 6.797(−2) |
| 1.2 | −206.501 | 137.501 | −156.563 | 6.608(−1) | 5.126(−1) | 6.839 | 5.317 | 2.706(−4) | 8.532(−2) |
| 1.4 | −181.829 | 110.586 | −115.265 | 7.593(−1) | 4.688(−1) | 7.864 | 4.893 | 3.629(−4) | 9.794(−2) |
| 1.6 | −161.811 | 90.544 | −86.881 | 8.472(−1) | 4.086(−1) | 8.786 | 4.309 | 4.654(−4) | 1.077(−1) |
| 1.8 | −145.295 | 75.286 | −66.837 | 9.224(−1) | 3.439(−1) | 9.585 | 3.677 | 5.756(−4) | 1.150(−1) |
| 2.0 | −131.470 | 63.444 | −52.348 | 9.850(−1) | 2.830(−1) | 10.259 | 3.073 | 6.913(−4) | 1.200(−1) |
| 2.25 | −117.097 | 52.073 | −39.409 | 1.047 | 2.187(−1) | 10.942 | 2.421 | 8.412(−4) | 1.235(−1) |
| 2.5 | −105.208 | 43.422 | −30.305 | 1.096 | 1.701(−1) | 11.481 | 1.909 | 9.952(−4) | 1.247(−1) |
| 2.75 | −95.226 | 36.710 | −23.736 | 1.134 | 1.360(−1) | 11.909 | 1.535 | 1.152(−3) | 1.242(−1) |
| 3.0 | −86.736 | 31.410 | −18.897 | 1.165 | 1.136(−1) | 12.258 | 1.275 | 1.312(−3) | 1.224(−1) |
| 3.5 | −73.089 | 23.709 | −12.431 | 1.215 | 9.298(−2) | 12.816 | 1.004 | 1.639(−3) | 1.171(−1) |
| 4.0 | −62.616 | 18.504 | −8.637 | 1.261 | 9.003(−2) | 13.294 | 9.302(−1) | 1.980(−3) | 1.112(−1) |
| 4.5 | −54.332 | 14.836 | −6.209 | 1.306 | 9.430(−2) | 13.762 | 9.487(−1) | 2.338(−3) | 1.057(−1) |
| 5 | −47.616 | 12.159 | −4.605 | 1.355 | 1.006(−1) | 14.249 | 1.002 | 2.715(−3) | 1.012(−1) |
| 6 | −37.392 | 8.599 | −2.732 | 1.461 | 1.114(−1) | 15.310 | 1.116 | 3.531(−3) | 9.498(−2) |
| 7 | −29.972 | 6.405 | −1.752 | 1.576 | 1.172(−1) | 16.466 | 1.187 | 4.431(−3) | 9.149(−2) |
| 8 | −24.336 | 4.958 | −1.191 | 1.695 | 1.191(−1) | 17.671 | 1.217 | 5.413(−3) | 8.964(−2) |
| 9 | −19.909 | 3.953 | −8.465(−1) | 1.814 | 1.185(−1) | 18.890 | 1.218 | 6.475(−3) | 8.868(−2) |
| 10 | −16.338 | 3.226 | −6.238(−1) | 1.931 | 1.166(−1) | 20.102 | 1.204 | 7.612(−3) | 8.819(−2) |
| 11 | −13.397 | 2.682 | −4.732(−1) | 2.047 | 1.141(−1) | 21.295 | 1.181 | 8.822(−3) | 8.795(−2) |
| 12 | −10.933 | 2.264 | −3.676(−1) | 2.159 | 1.115(−1) | 22.463 | 1.155 | 1.010(−2) | 8.782(−2) |
| 14 | −7.038 | 1.675 | −2.350(−1) | 2.377 | 1.062(−1) | 24.720 | 1.102 | 1.287(−2) | 8.769(−2) |
| 16 | −4.101 | 1.287 | −1.593(−1) | 2.584 | 1.014(−1) | 26.876 | 1.054 | 1.589(−2) | 8.757(−2) |
| 18 | −1.811 | 1.018 | −1.130(−1) | 2.783 | 9.726(−2) | 28.939 | 1.011 | 1.916(−2) | 8.743(−2) |
| 20 | 0.022 | 8.242(−1) | −8.309(−2) | 2.974 | 9.365(−2) | 30.923 | 9.735(−1) | 2.266(−2) | 8.724(−2) |
| 22 | 1.519 | 6.795(−1) | −6.286(−2) | 3.158 | 9.049(−2) | 32.837 | 9.407(−1) | 2.639(−2) | 8.702(−2) |
| 23 | 2.168 | 6.206(−1) | −5.517(−2) | 3.248 | 8.906(−2) | 33.770 | 9.258(−1) | 2.834(−2) | 8.690(−2) |
| 24 | 2.762 | 5.688(−1) | −4.870(−2) | 3.336 | 8.771(−2) | 34.688 | 9.117(−1) | 3.035(−2) | 8.678(−2) |
| 25 | 3.308 | 5.229(−1) | −4.318(−2) | 3.423 | 8.643(−2) | 35.593 | 8.985(−1) | 3.240(−2) | 8.665(−2) |
| 26 | 3.810 | 4.822(−1) | −3.847(−2) | 3.509 | 8.523(−2) | 36.486 | 8.859(−1) | 3.451(−2) | 8.652(−2) |
| 28 | 4.703 | 4.132(−1) | −3.091(−2) | 3.677 | 8.301(−2) | 38.234 | 8.628(−1) | 3.889(−2) | 8.626(−2) |
| 30 | 5.471 | 3.573(−1) | −2.520(−2) | 3.841 | 8.101(−2) | 39.939 | 8.420(−1) | 4.348(−2) | 8.599(−2) |
| 35 | 6.987 | 2.568(−1) | −1.593(−2) | 4.235 | 7.674(−2) | 44.033 | 7.976(−1) | 5.580(−2) | 8.531(−2) |
| 40 | 8.097 | 1.915(−1) | −1.067(−2) | 4.610 | 7.328(−2) | 47.928 | 7.615(−1) | 6.933(−2) | 8.464(−2) |
| 45 | 8.936 | 1.467(−1) | −7.476(−3) | 4.969 | 7.038(−2) | 51.658 | 7.313(−1) | 8.400(−2) | 8.400(−2) |
| 50 | 9.586 | 1.148(−1) | −5.423(−3) | 5.314 | 6.792(−2) | 55.249 | 7.057(−1) | 9.978(−2) | 8.339(−2) |
| 60 | 10.509 | 7.365(−2) | −3.091(−3) | 5.973 | 6.392(−2) | 62.088 | 6.640(−1) | 1.345(−1) | 8.226(−2) |
| 70 | 11.114 | 4.929(−2) | −1.905(−3) | 6.596 | 6.078(−2) | 68.559 | 6.313(−1) | 1.734(−1) | 8.125(−2) |
| 80 | 11.524 | 3.384(−2) | −1.243(−3) | 7.190 | 5.823(−2) | 74.735 | 6.047(−1) | 2.161(−1) | 8.033(−2) |
| 90 | 11.808 | 2.355(−2) | −8.470(−4) | 7.762 | 5.609(−2) | 80.667 | 5.825(−1) | 2.627(−1) | 7.949(−2) |
| 100 | 12.005 | 1.642(−2) | −5.961(−4) | 8.313 | 5.427(−2) | 86.394 | 5.635(−1) | 3.128(−1) | 7.872(−2) |
| 120 | 12.237 | 7.620(−3) | −3.186(−4) | 9.367 | 5.130(−2) | 97.341 | 5.326(−1) | 4.236(−1) | 7.735(−2) |
| 140 | 12.336 | 2.752(−3) | −1.826(−4) | 10.369 | 4.896(−2) | 107.740 | 5.082(−1) | 5.479(−1) | 7.616(−2) |
| 160 | 12.360 | −1.030(−4) | −1.096(−4) | 11.328 | 4.705(−2) | 117.698 | 4.883(−1) | 6.852(−1) | 7.510(−2) |
| 180 | 12.339 | −1.842(−3) | −6.771(−5) | 12.253 | 4.545(−2) | 127.293 | 4.717(−1) | 8.349(−1) | 7.415(−2) |
| 200 | 12.291 | −2.924(−3) | −4.241(−5) | 13.148 | 4.408(−2) | 136.581 | 4.574(−1) | 9.968(−1) | 7.329(−2) |
| 225 | 12.206 | −3.728(−3) | −2.354(−5) | 14.231 | 4.262(−2) | 147.821 | 4.422(−1) | 1.216 | 7.231(−2) |
| 250 | 12.107 | −4.168(−3) | −1.253(−5) | 15.280 | 4.137(−2) | 158.711 | 4.293(−1) | 1.452 | 7.142(−2) |
| 275 | 12.000 | −4.392(−3) | −5.884(−6) | 16.301 | 4.029(−2) | 169.298 | 4.180(−1) | 1.707 | 7.060(−2) |
| 300 | 11.889 | −4.485(−3) | −1.787(−6) | 17.296 | 3.933(−2) | 179.621 | 4.081(−1) | 1.978 | 6.985(−2) |
| 325 | 11.776 | −4.495(−3) | 7.679(−7) | 18.268 | 3.848(−2) | 189.710 | 3.992(−1) | 2.266 | 6.915(−2) |
| 350 | 11.664 | −4.455(−3) | 2.364(−6) | 19.220 | 3.772(−2) | 199.590 | 3.913(−1) | 2.571 | 6.849(−2) |
| 375 | 11.554 | −4.383(−3) | 3.350(−6) | 20.155 | 3.703(−2) | 209.283 | 3.842(−1) | 2.891 | 6.787(−2) |
| 400 | 11.445 | −4.291(−3) | 3.939(−6) | 21.073 | 3.641(−2) | 218.804 | 3.777(−1) | 3.228 | 6.729(−2) |
| 450 | 11.236 | −4.080(−3) | 4.413(−6) | 22.865 | 3.531(−2) | 237.392 | 3.662(−1) | 3.948 | 6.621(−2) |
| 500 | 11.038 | −3.857(−3) | 4.428(−6) | 24.606 | 3.437(−2) | 255.451 | 3.564(−1) | 4.729 | 6.524(−2) |
| 600 | 10.673 | −3.434(−3) | 3.961(−6) | 27.962 | 3.283(−2) | 290.258 | 3.404(−1) | 6.469 | 6.351(−2) |
| 700 | 10.349 | −3.068(−3) | 3.370(−6) | 31.183 | 3.162(−2) | 323.649 | 3.278(−1) | 8.439 | 6.201(−2) |
| 800 | 10.058 | −2.758(−3) | 2.842(−6) | 34.294 | 3.063(−2) | 355.903 | 3.176(−1) | 10.633 | 6.068(−2) |
| 900 | 9.796 | −2.496(−3) | 2.400(−6) | 37.314 | 2.981(−2) | 387.217 | 3.090(−1) | 13.044 | 5.948(−2) |
| 1000 | 9.557 | −2.275(−3) | 2.042(−6) | 40.259 | 2.910(−2) | 417.739 | 3.016(−1) | 15.669 | 5.839(−2) |
| 1200 | 9.139 | −1.923(−3) | 1.515(−6) | 45.959 | 2.796(−2) | 476.824 | 2.897(−1) | 21.541 | 5.645(−2) |
| 1400 | 8.782 | −1.658(−3) | 1.160(−6) | 51.457 | 2.706(−2) | 533.800 | 2.804(−1) | 28.225 | 5.476(−2) |
| 1600 | 8.472 | −1.452(−3) | 9.133(−7) | 56.793 | 2.633(−2) | 589.092 | 2.728(−1) | 35.699 | 5.325(−2) |
| 1800 | 8.199 | −1.288(−3) | 7.355(−7) | 61.996 | 2.572(−2) | 643.000 | 2.665(−1) | 43.949 | 5.189(−2) |
| 2000 | 7.955 | −1.155(−3) | 6.031(−7) | 67.087 | 2.521(−2) | 695.745 | 2.611(−1) | 52.961 | 5.064(−2) |
| 2500 | 7.443 | −9.112(−4) | 3.926(−7) | 79.424 | 2.521(−2) | 823.544 | 2.611(−1) | 78.760 | 4.790(−2) |
| 3000 | 7.031 | −7.471(−4) | 2.741(−7) | 91.331 | 2.521(−2) | 946.864 | 2.611(−1) | 109.131 | 4.556(−2) |
| 3500 | 6.688 | −6.296(−4) | 2.013(−7) | 102.913 | 2.521(−2) | 1066.813 | 2.611(−1) | 143.985 | 4.349(−2) |
| 4000 | 6.396 | −5.417(−4) | 1.535(−7) | 114.243 | 2.521(−2) | 1184.125 | 2.611(−1) | 183.256 | 4.164(−2) |
| 4500 | 6.143 | −4.737(−4) | 1.206(−7) | 125.369 | 2.521(−2) | 1299.316 | 2.611(−1) | 226.896 | 3.996(−2) |
| 5000 | 5.920 | −4.196(−4) | 9.704(−8) | 136.328 | 2.521(−2) | 1412.766 | 2.611(−1) | 274.868 | 3.841(−2) |
| 6000 | 5.543 | −3.393(−4) | 6.638(−8) | 157.849 | 2.521(−2) | 1635.532 | 2.611(−1) | 383.707 | 3.561(−2) |
| 7000 | 5.234 | −2.829(−4) | 4.799(−8) | 178.965 | 2.521(−2) | 1854.081 | 2.611(−1) | 509.625 | 3.314(−2) |
| 8000 | 4.973 | −2.412(−4) | 3.616(−8) | 199.781 | 2.521(−2) | 2069.499 | 2.611(−1) | 652.548 | 3.090(−2) |
| 9000 | 4.748 | −2.093(−4) | 2.813(−8) | 220.370 | 2.521(−2) | 2282.550 | 2.611(−1) | 812.447 | 2.885(−2) |
| 10000 | 4.552 | −1.842(−4) | 2.243(−8) | 240.786 | 2.521(−2) | 2493.792 | 2.611(−1) | 989.327 | 2.695(−2) |
Table A3.
Thermophysical properties of an equimolar binary mixture of 3He 4He as a function of temperature, where (−2) is × 10−2
| T | B | dB/dT | d2B/dT2 | η | dη/dT | λ | dλ/dT | D(101.3 kPa) | αT |
|---|---|---|---|---|---|---|---|---|---|
| (K) | (cm3·mol−1) | (cm3·mol−1·K−1) | (cm3·mol−1·K−2) | (μPa·s) | (μPa·K−1) | (mW·m−1·K−1) | (mW·m−1·K−2) | (10−4·m2·s−1) | |
| 1.0 | −338.460 | 362.783 | −761.653 | 4.147(−1) | 2.503(−1) | 3.802 | 2.449 | 1.152(−4) | 5.168(−2) |
| 1.2 | −278.477 | 248.027 | −428.348 | 4.629(−1) | 2.363(−1) | 4.260 | 2.185 | 1.616(−4) | 6.401(−2) |
| 1.4 | −236.166 | 180.498 | −264.495 | 5.102(−1) | 2.378(−1) | 4.690 | 2.129 | 2.143(−4) | 7.237(−2) |
| 1.6 | −204.675 | 137.383 | −174.857 | 5.582(−1) | 2.416(−1) | 5.117 | 2.149 | 2.732(−4) | 7.859(−2) |
| 1.8 | −180.297 | 108.166 | −121.639 | 6.067(−1) | 2.437(−1) | 5.550 | 2.175 | 3.379(−4) | 8.327(−2) |
| 2.0 | −160.849 | 87.440 | −88.058 | 6.555(−1) | 2.433(−1) | 5.986 | 2.183 | 4.081(−4) | 8.672(−2) |
| 2.25 | −141.430 | 69.017 | −61.471 | 7.159(−1) | 2.397(−1) | 6.529 | 2.160 | 5.032(−4) | 8.962(−2) |
| 2.5 | −125.902 | 55.906 | −44.632 | 7.751(−1) | 2.338(−1) | 7.063 | 2.108 | 6.063(−4) | 9.129(−2) |
| 2.75 | −113.192 | 46.238 | −33.437 | 8.327(−1) | 2.267(−1) | 7.582 | 2.039 | 7.168(−4) | 9.206(−2) |
| 3.0 | −102.590 | 38.900 | −25.720 | 8.884(−1) | 2.191(−1) | 8.082 | 1.961 | 8.346(−4) | 9.219(−2) |
| 3.5 | −85.895 | 28.672 | −16.178 | 9.943(−1) | 2.046(−1) | 9.024 | 1.810 | 1.091(−3) | 9.134(−2) |
| 4.0 | −73.329 | 22.034 | −10.848 | 1.093 | 1.921(−1) | 9.896 | 1.685 | 1.372(−3) | 8.988(−2) |
| 4.5 | −63.518 | 17.477 | −7.632 | 1.187 | 1.820(−1) | 10.714 | 1.589 | 1.679(−3) | 8.838(−2) |
| 5 | −55.638 | 14.211 | −5.579 | 1.276 | 1.737(−1) | 11.490 | 1.517 | 2.008(−3) | 8.708(−2) |
| 6 | −43.756 | 9.939 | −3.248 | 1.443 | 1.612(−1) | 12.952 | 1.415 | 2.734(−3) | 8.531(−2) |
| 7 | −35.212 | 7.347 | −2.058 | 1.599 | 1.519(−1) | 14.328 | 1.341 | 3.542(−3) | 8.444(−2) |
| 8 | −28.767 | 5.654 | −1.387 | 1.747 | 1.446(−1) | 15.639 | 1.282 | 4.428(−3) | 8.412(−2) |
| 9 | −23.731 | 4.486 | −9.800(−1) | 1.889 | 1.384(−1) | 16.894 | 1.230 | 5.389(−3) | 8.407(−2) |
| 10 | −19.687 | 3.646 | −7.184(−1) | 2.024 | 1.331(−1) | 18.101 | 1.185 | 6.420(−3) | 8.415(−2) |
| 11 | −16.369 | 3.021 | −5.425(−1) | 2.155 | 1.284(−1) | 19.265 | 1.144 | 7.520(−3) | 8.427(−2) |
| 12 | −13.597 | 2.543 | −4.199(−1) | 2.281 | 1.242(−1) | 20.391 | 1.108 | 8.686(−3) | 8.439(−2) |
| 14 | −9.233 | 1.872 | −2.667(−1) | 2.522 | 1.171(−1) | 22.541 | 1.045 | 1.121(−2) | 8.456(−2) |
| 16 | −5.957 | 1.433 | −1.799(−1) | 2.751 | 1.113(−1) | 24.578 | 9.932(−1) | 1.397(−2) | 8.462(−2) |
| 18 | −3.411 | 1.130 | −1.270(−1) | 2.968 | 1.064(−1) | 26.519 | 9.498(−1) | 1.697(−2) | 8.458(−2) |
| 20 | −1.380 | 9.125(− 1) | −9.303(−2) | 3.177 | 1.022(−1) | 28.381 | 9.127(−1) | 2.019(−2) | 8.448(−2) |
| 22 | 0.276 | 7.508(−1) | −7.015(−2) | 3.377 | 9.863(−2) | 30.174 | 8.807(−1) | 2.362(−2) | 8.433(−2) |
| 23 | 0.993 | 6.851(−1) | −6.148(−2) | 3.475 | 9.701(−2) | 31.047 | 8.663(−1) | 2.542(−2) | 8.425(−2) |
| 24 | 1.649 | 6.273(−1) | −5.418(−2) | 3.571 | 9.549(−2) | 31.906 | 8.527(−1) | 2.726(−2) | 8.415(−2) |
| 25 | 2.250 | 5.763(−1) | −4.799(−2) | 3.666 | 9.406(−2) | 32.753 | 8.399(−1) | 2.916(−2) | 8.405(−2) |
| 26 | 2.803 | 5.311(−1) | −4.271(−2) | 3.760 | 9.271(−2) | 33.587 | 8.279(−1) | 3.111(−2) | 8.394(−2) |
| 28 | 3.786 | 4.545(−1) | −3.424(−2) | 3.942 | 9.024(−2) | 35.220 | 8.058(−1) | 3.515(−2) | 8.372(−2) |
| 30 | 4.631 | 3.927(−1) | −2.786(−2) | 4.121 | 8.800(−2) | 36.811 | 7.858(−1) | 3.939(−2) | 8.349(−2) |
| 35 | 6.296 | 2.818(−1) | −1.754(−2) | 4.548 | 8.328(−2) | 40.631 | 7.436(−1) | 5.080(−2) | 8.290(−2) |
| 40 | 7.514 | 2.100(−1) | −1.172(−2) | 4.955 | 7.945(−2) | 44.260 | 7.094(−1) | 6.333(−2) | 8.230(−2) |
| 45 | 8.433 | 1.609(−1) | −8.188(−3) | 5.344 | 7.627(−2) | 47.734 | 6.810(−1) | 7.694(−2) | 8.171(−2) |
| 50 | 9.146 | 1.260(−1) | −5.929(−3) | 5.718 | 7.357(−2) | 51.077 | 6.569(−1) | 9.160(−2) | 8.115(−2) |
| 60 | 10.160 | 8.104(−2) | −3.370(−3) | 6.431 | 6.919(−2) | 57.442 | 6.177(−1) | 1.239(−1) | 8.010(−2) |
| 70 | 10.827 | 5.450(−2) | −2.074(−3) | 7.105 | 6.576(−2) | 63.460 | 5.871(−1) | 1.601(−1) | 7.915(−2) |
| 80 | 11.282 | 3.769(−2) | −1.353(−3) | 7.748 | 6.297(−2) | 69.202 | 5.622(−1) | 1.999(−1) | 7.829(−2) |
| 90 | 11.599 | 2.650(−2) | −9.213(−4) | 8.366 | 6.065(−2) | 74.717 | 5.414(−1) | 2.432(−1) | 7.749(−2) |
| 100 | 11.823 | 1.874(−2) | −6.493(−4) | 8.963 | 5.866(−2) | 80.039 | 5.237(−1) | 2.900(−1) | 7.677(−2) |
| 120 | 12.092 | 9.153(−3) | −3.476(−4) | 10.102 | 5.544(−2) | 90.212 | 4.948(−1) | 3.934(−1) | 7.547(−2) |
| 140 | 12.217 | 3.832(−3) | −2.001(−4) | 11.185 | 5.290(−2) | 99.873 | 4.722(−1) | 5.094(−1) | 7.433(−2) |
| 160 | 12.260 | 6.941(−4) | −1.209(−4) | 12.221 | 5.083(−2) | 109.125 | 4.537(−1) | 6.375(−1) | 7.332(−2) |
| 180 | 12.253 | −1.232(−3) | −7.540(−5) | 13.220 | 4.909(−2) | 118.039 | 4.382(−1) | 7.774(−1) | 7.241(−2) |
| 200 | 12.215 | −2.444(−3) | −4.786(−5) | 14.187 | 4.761(−2) | 126.667 | 4.249(−1) | 9.286(−1) | 7.159(−2) |
| 225 | 12.142 | −3.361(−3) | −2.725(−5) | 15.357 | 4.603(−2) | 137.109 | 4.108(−1) | 1.133 | 7.065(−2) |
| 250 | 12.051 | −3.879(−3) | −1.515(−5) | 16.490 | 4.468(−2) | 147.224 | 3.988(−1) | 1.354 | 6.980(−2) |
| 275 | 11.950 | −4.159(−3) | −7.805(−6) | 17.592 | 4.351(−2) | 157.059 | 3.883(−1) | 1.592 | 6.901(−2) |
| 300 | 11.844 | −4.294(−3) | −3.233(−6) | 18.667 | 4.248(−2) | 166.649 | 3.791(−1) | 1.846 | 6.829(−2) |
| 325 | 11.736 | −4.336(−3) | −3.446(−7) | 19.717 | 4.156(−2) | 176.022 | 3.709(−1) | 2.115 | 6.762(−2) |
| 350 | 11.628 | −4.320(−3) | 1.491(−6) | 20.746 | 4.074(−2) | 185.200 | 3.635(−1) | 2.400 | 6.699(−2) |
| 375 | 11.520 | −4.268(−3) | 2.653(−6) | 21.755 | 4.000(−2) | 194.204 | 3.569(−1) | 2.700 | 6.639(−2) |
| 400 | 11.414 | −4.192(−3) | 3.375(−6) | 22.746 | 3.932(−2) | 203.050 | 3.509(−1) | 3.015 | 6.583(−2) |
| 450 | 11.209 | −4.004(−3) | 4.043(−6) | 24.681 | 3.813(−2) | 220.320 | 3.402(−1) | 3.688 | 6.480(−2) |
| 500 | 11.014 | −3.797(−3) | 4.155(−6) | 26.562 | 3.711(−2) | 237.099 | 3.312(−1) | 4.419 | 6.385(−2) |
| 600 | 10.655 | −3.395(−3) | 3.811(−6) | 30.186 | 3.545(−2) | 269.441 | 3.163(−1) | 6.047 | 6.219(−2) |
| 700 | 10.334 | −3.040(−3) | 3.286(−6) | 33.664 | 3.415(−2) | 300.470 | 3.047(−1) | 7.891 | 6.074(−2) |
| 800 | 10.045 | −2.737(−3) | 2.784(−6) | 37.023 | 3.308(−2) | 330.444 | 2.951(−1) | 9.943 | 5.945(−2) |
| 900 | 9.785 | −2.481(−3) | 2.361(−6) | 40.285 | 3.219(−2) | 359.549 | 2.872(−1) | 12.200 | 5.830(−2) |
| 1000 | 9.548 | −2.263(−3) | 2.014(−6) | 43.465 | 3.143(−2) | 387.918 | 2.804(−1) | 14.656 | 5.724(−2) |
| 1200 | 9.132 | −1.915(−3) | 1.499(−6) | 49.621 | 3.019(−2) | 442.843 | 2.693(−1) | 20.153 | 5.536(−2) |
| 1400 | 8.776 | −1.652(−3) | 1.150(−6) | 55.558 | 2.922(−2) | 495.813 | 2.607(−1) | 26.409 | 5.373(−2) |
| 1600 | 8.467 | −1.448(−3) | 9.072(−7) | 61.320 | 2.843(−2) | 547.223 | 2.537(−1) | 33.405 | 5.227(−2) |
| 1800 | 8.194 | −1.285(−3) | 7.313(−7) | 66.938 | 2.778(−2) | 597.352 | 2.478(−1) | 41.128 | 5.094(−2) |
| 2000 | 7.951 | −1.152(−3) | 6.002(−7) | 72.436 | 2.722(−2) | 646.403 | 2.428(−1) | 49.565 | 4.973(−2) |
| 2500 | 7.440 | −9.097(−4) | 3.913(−7) | 85.759 | 2.613(−2) | 765.270 | 2.332(−1) | 73.716 | 4.707(−2) |
| 3000 | 7.029 | −7.461(−4) | 2.734(−7) | 98.617 | 2.534(−2) | 879.990 | 2.261(−1) | 102.149 | 4.479(−2) |
| 3500 | 6.686 | −6.289(−4) | 2.008(−7) | 111.126 | 2.472(−2) | 991.593 | 2.206(−1) | 134.778 | 4.279(−2) |
| 4000 | 6.395 | −5.412(−4) | 1.532(−7) | 123.361 | 2.424(−2) | 1100.756 | 2.162(−1) | 171.543 | 4.098(−2) |
| 4500 | 6.142 | −4.733(−4) | 1.204(−7) | 135.376 | 2.384(−2) | 1207.960 | 2.127(−1) | 212.399 | 3.934(−2) |
| 5000 | 5.919 | −4.193(−4) | 9.690(−8) | 147.211 | 2.351(−2) | 1313.554 | 2.098(−1) | 257.310 | 3.783(−2) |
| 6000 | 5.542 | −3.391(−4) | 6.630(−8) | 170.451 | 2.300(−2) | 1520.927 | 2.052(−1) | 359.205 | 3.510(−2) |
| 7000 | 5.233 | −2.827(−4) | 4.795(−8) | 193.255 | 2.263(−2) | 1724.410 | 2.019(−1) | 477.089 | 3.268(−2) |
| 8000 | 4.972 | −2.411(−4) | 3.613(−8) | 215.734 | 2.235(−2) | 1925.011 | 1.994(−1) | 610.893 | 3.049(−2) |
| 9000 | 4.748 | −2.092(−4) | 2.811(−8) | 237.969 | 2.213(−2) | 2123.436 | 1.975(−1) | 760.589 | 2.848(−2) |
| 10000 | 4.551 | −1.841(−4) | 2.242(−8) | 260.016 | 2.197(−2) | 2320.201 | 1.961(−1) | 926.183 | 2.661(−2) |
Contributor Information
John J. Hurly, Email: john.hurly@nist.gov.
Michael R. Moldover, Email: michael.moldover@nist.gov.
9. References
- 1.Guildner LA, Edsinger RE. Deviation of International Practical Temperatures from Thermodynamic Temperatures in the Temperature Range from 273.16 to 730 K. J Res Natl Bur Stand (US) 1976;80A(5–6):703–737. doi: 10.6028/jres.080A.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White MP, Gugan D. Direct Measurements of the Dielectric Virial Coefficients of 4He between 3 K and 18 K. Metrologia. 1992;29:37–57. [Google Scholar]
- 3.Meyer CW, Reilly ML. In: Marcarino P, editor. Proceedings of Tempmeko ’96, 6th International Symposium on Temperature and Thermal Measurements in Industry and Science; Torino, Levrotto & Bella. 1997. pp. 39–44. [Google Scholar]
- 4.Moldover MR, Mehl JB, Greenspan J. Gas-filled spherical resonators: Theory and experiment. J Acoust Soc Am. 1986;79(2):253–272. [Google Scholar]
- 5.Gillis KA. Thermodynamic Properties of two Gaseous halogenated Ethers from speed-of-Sound measurements: Difluoromethoxy-Difluoromethane and 2-Difluoromethoxy-1,1,1-Trifluoroethane. Int J Thermophys. 1994;15(5):821–847. [Google Scholar]
- 6.Wilhelm J, Vogel E, Lehmann JK, Wakeham WA. A vibrating-wire viscometer for dilute and dense gases. Int J Thermophys. 1998;19(2):391–401. [Google Scholar]
- 7.Gillis KA, Mehl JB, Moldover MR. Greenspan acoustic viscometer for gases. Rev Sci Instrum. 1996;67(5):1850–1857. [Google Scholar]
- 8.Waxman M, Davis H. Density of Ultra-Pure Air at 298.15 K for Mass Transfer Buoyancy Corrections. J Res Natl Bur Stand (US) 1978;83(5):415–418. doi: 10.6028/jres.083.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang KT, Toennies JP. An improved simple model for the van der Waals potential based on universal damping functions for the dispersion coefficients. J Chem Phys. 1984;80(8):3726–3741. [Google Scholar]
- 10.Aziz RA, Slaman MJ. An Analysis of the ITS-90 Relations for the Nonideality of He-3 and He-4− Recommended Relations Based on a New Interatomic Potential for Helium. Metrologia. 1990;27:211–219. [Google Scholar]
- 11.Aziz RA, Slaman MJ. An examination of ab initio results for the helium potential energy curve. J Chem Phys. 1991;94(12):8047–8053. [Google Scholar]
- 12.Aziz RA, Janzen AR, Moldover MR. Ab initio Calculations for Helium: A standard for Transport Property Measurements. Phys Rev Lett. 1995;74(9):1586–1589. doi: 10.1103/PhysRevLett.74.1586. [DOI] [PubMed] [Google Scholar]
- 13.Janzen AR, Aziz RA. An accurate potential energy curve for helium based on ab initio calculations. J Chem Phys. 1997;107(3):914–919. [Google Scholar]
- 14.Mohr PJ, Taylor BN. The 1998 CODATA Recommended Values of the Fundamental Physical Constants, Web Version 3.1. National Institute of Standards and Technology; Gaithersburg, MD 20899: Dec 3, 1999. available at physics.nist.gov/constants. [Google Scholar]
- 15.Ceperley DM, Partridge H. The He2 potential at small distances. J Chem Phys. 1986;84(2):820–821. [Google Scholar]
- 16.Anderson JB, Traynor CA, Boghosian BM. An exact quantum Monte Carlo calculation of the helium-helium intermolecular potential. J Chem Phys. 1993;99(1):345–351. doi: 10.1063/1.1704638. [DOI] [PubMed] [Google Scholar]
- 17.Klopper W, Noga J. An explicitly correlated coupled cluster calculation of the helium-helium interatomic potential. J Chem Phys. 1995;103(14):6127–6132. [Google Scholar]
- 18.Korona T, Williams HL, Bukowski R, Jeziorski B, Szalewicz K. Helium dimer potential from symmetry-adapted perturbation theory calculations using large Gaussian geminal and orbital basis sets. J Chem Phys. 1997;106(12):5109–5122. [Google Scholar]
- 19.Gdanitz RJ. Accurately solving the electronic Schrödinger equation of atoms and molecules using explicitly correlated (r12)MR-CI IV. The helium dimer (He2) Mol Phys. 1999;96(9):1423–1434. [Google Scholar]
- 20.Van de Bovenkamp J, van Duijneveldt FB. MRCI calculations on the helium dimer employing and interaction optimized basis set. J Chem Phys. 1999;110(23):11141–11151. [Google Scholar]
- 21.Van Mourik T, Dunning TH., Jr A new ab initio potential energy curve for the helium dimer. J Chem Phys. 1999;111(20):9248–9258. [Google Scholar]
- 22.Bishop DM, Pipin J. Dipole, Quadrupole, Octupole, and Dipole-Octupole Polarizabilities at Real and imaginary Frequencies for H, He, and H2 and the Dispersion-Energy Coefficients for Interactions Between Them. Inter J Quant Chem. 1993;45:349–361. [Google Scholar]
- 23.Chen M, Chung KT. Retardation long-range potentials between two helium atoms. Phys Rev A. 1996;55(3):1439–1446. doi: 10.1103/physreva.53.1439. [DOI] [PubMed] [Google Scholar]
- 24.Komasa J. Exponentially correlated Gaussian functions in variational calculations: Energy expectation values in the ground state helium dimer. J Chem Phys. 1999;110(16):7909–7916. [Google Scholar]
- 25.Van Mourik T, van Lenthe JH. Benchmark full configuration interaction calculations of the helium dimer. J Chem Phys. 1995;102(19):7479–7483. [Google Scholar]
- 26.R. Gdanitz, private communication.
- 27.Bukowski R, Jeziorski B, Szalewicz K. Basis set superposition problem in interaction energy calculations with explicitly correlated bases: Saturated second- and third-order energies for He2. J Chem Phys. 1996;104(9):3306–3319. Szalewicz, private communication. [Google Scholar]
- 28.Komasa J, Cencek JW, Rychlewski J. Adiabatic corrections of the helium dimer from exponentially correlated Gaussian functions. Chem Phys Lett. 1999;304:293–298. [Google Scholar]
- 29.Thakkar AJ. Higher dispersion coefficients: Accurate values for hydrogen atoms and simple estimates for other systems. J Chem Phys. 1988;89(4):2092–2098. [Google Scholar]
- 30.Jamieson JM, Drake GWF, Dalgarno A. Retarded dipole-dipole dispersion interaction potential for helium. Phys Rev A. 1995;51(4):3358–3361. doi: 10.1103/physreva.51.3358. [DOI] [PubMed] [Google Scholar]
- 31.Jansen AR, Aziz RA. Modern He-He Potentials—Another Look at Binding-energy, Effective Range Theory, Retardation, and Efimov States. J Chem Phys. 1995;103(22):9626–9630. [Google Scholar]
- 32.Hirschfelder JO, Curtiss CF, Bird RB. Molecular Theory of Gases and Liquids. New York: Wiley; 1964. [Google Scholar]
- 33.Hartree DR. Numerical Analysis. 2nd ed. Oxford University Press; London: 1958. [Google Scholar]
- 34.LeRoy RJ. University of Waterloo Chemical Physics Report. Waterloo; Ontario, Canada: (Computer Code CP-107R). [Google Scholar]
- 35.Hurly JJ, McConville GT, Taylor WL. Algorithms and Fortran Programs to Calculate Quantum Collision integrals for Realistic Intermolecular Potentials, MLM-3635. Miamisburg: EG&GMAT; 1990. [Google Scholar]
- 36.Maitland GC, Rigby M, Smith EB, Wakeham WA. Intermolecular Forces. Oxford: Clarendon Press; 1981. [Google Scholar]
- 37.Kahaner DK, Moler C, Nash S. Numerical Methods and Software. Prentice Hall; Englewood Cliffs, NJ: 1989. [Google Scholar]
- 38.Meeks FR, Cleland TJ, Hutchinson KE, Taylor WL. On the quantum cross sections in dilute gases. J Chem Phys. 1984;100(5):3813–3820. [Google Scholar]
- 39.Viehland LA, Janzen AR, Aziz RA. High approximations to the transport properties of pure atomic gases. J Chem Phys. 1995;102(13):5444–5450. [Google Scholar]
- 40.Assael MJ, Wakeham WA, Kestin J. Higher-Order Approximation to the Thermal Conductivity of Monatomic Gas Mixtures. Int J Thermophys. 1980;1(1):7–32. [Google Scholar]
- 41.Mason EA. Higher Approximations for the Transport Properties of Binary Gas Mixtures I. General Formulas. J Chem Phys. 1957;27(1):75–84. [Google Scholar]
- 42.Berry KH. NPL-75: A low Temperature Gas Thermometry Scale from 2.6 K to 27.1 K. Metrologia. 1979;15:89–115. [Google Scholar]
- 43.Gugan D, Michel GW. Dielectric Constant Gas Thermometry from 4.2 to 27.1 K. Metrologia. 1980;16:149–167. [Google Scholar]
- 44.Kemp RC, Kemp WRG, Besley LM. A Determination of Thermodynamic Temperatures and Measurements of the Second Virial Coefficient of 4He Between 13.81 K and 287 K Using a Constant-Volume Gas Thermometer. Metrologia. 1986;23:61–86. [Google Scholar]
- 45.Gammon BE. The velocity of sound with derived state properties in helium at −175 to 150 °C with pressure to 150 atm. J Chem Phys. 1979;64(6):2556–2568. [Google Scholar]
- 46.Kell GS, McLaurin GE, Whalley E. Second virial coefficient of helium from 0 to 500 °C by the two-temperature gas-expansion method. J Chem Phys. 1978;68(5):2199–2205. [Google Scholar]
- 47.Matacotta FC, McConville GT, Steur PPM, Durieux M. Measurements and Calculations of the 3He Second Virial Coefficient Between 1.5 K and 20.3 K. Metrologia. 1978;24:61–67. [Google Scholar]
- 48.McConville GT, Hurly JJ. An Analysis of the Accuracy of the Calculation of the Second Virial Coefficient of Helium from Interatomic Potential Functions. Metrologia. 1991;28:375–383. [Google Scholar]
- 49.Becker EW, Misenta R, Schmeissner F. Die Zähigkeit von gasförmigem He3 and He4 zwischen 1,3 °K. Z Phys. 1954;137:126–136. [Google Scholar]
- 50.Wakeham WA, Nagashima A, Sengers JV. Measurement of the transport properties of fluids. London: Blackwell; 1991. pp. 442–450. [Google Scholar]
- 51.Maitland GC, Smith EB. Critical Reassessment of Viscosities of 11 Common Gases. J Chem Eng Data. 1971;17(2):151–156. [Google Scholar]
- 52.Vogel E. Präzisionsmessungen des Viskositätskoeffizienten von Stickstoff und den Edelgases zwischen Raumtemperatur und 650 K. Ber Bunsenges Phys Chem. 1984;88:997–1002. [Google Scholar]
- 53.Kestin J, Ro ST, Wakeham WA. Viscosity of the Noble Gases in the Temperature Range 25-700 °C. J Chem Phys. 1972;56(8):4119–4122. [Google Scholar]
- 54.Clarke AG, Smith EB. Low-Temperature Viscosities and Intermolecular Forces of Simple Gases. J Chem Phys. 1969;51(9):4156–4161. [Google Scholar]
- 55.Dawe RA, Smith EB. Viscosities of the Inert Gases at High Temperatures. J Chem Phys. 1970;52(2):693–703. [Google Scholar]
- 56.Coremans JMJ, Van Itterbeek A, Beenakker JJM, Knaap HFP, Zandbergen P. The viscosity of gaseous He, Ne, H2, and D2 below 80 °K. Physica. 1958;24:557–576. [Google Scholar]
- 57.Kestin J, Wakeham WA. The Viscosity and Diffusion Coefficient of Binary Mixtures of Nitrous Oxide with He, Ne and CO. Ber Bunsenges Phys Chem. 1983;87:309–311. [Google Scholar]
- 58.Johnston HL, Grilly ER. Viscosities of Carbon Monoxide, Helium, Neon, and Argon between 80° and 300 °K. Coefficients of Viscosity. J Phys Chem. 1942;46:948–963. [Google Scholar]
- 59.Kalelkar AS, Kestin J. Viscosity of He-Ar and He-Kr Binary Gaseous Mixtures in the Temperature Range 25-700 °C. J Chem Phys. 1970;52(8):4248–4267. [Google Scholar]
- 60.Kestin J, Khalifa HE, Wakeham WA. The Viscosity and Diffusion Coefficients of the Binary Mixtures of Xenon with the other Noble Gases. Physica. 1978;90A:215–228. [Google Scholar]
- 61.Guevara FA, McInteer BB, Wageman WE. High-Temperature Viscosity Ratios for Hydrogen, Helium, Argon, and Nitrogen. Phys Fluids. 1969;12(12):2493–2502. [Google Scholar]
- 62.Haarman JW. Thermal Conductivity Measurements of He, Ne, Ar, Kr, N2, and CO2 with a Transient Hot Wire Method. AIP Conf Proc. 1973;11:193–198. [Google Scholar]
- 63.Jody BJ, Saxena SC, Nain VPS, Aziz RA. Thermal Conductivity of Helium: A Probe for the Repulsive Wall of the Interatomic Potential. Chem Phys. 1977;22:53–58. [Google Scholar]
- 64.Assael MJ, Dix M, Lucas A, Wakeham WA. Absolute Determination of the Thermal Conductivity of the Noble Gases and Two of their Binary Mixtures as a Function of Density. J Chem Soc, Faraday Trans. 1981;177:439–464. [Google Scholar]
- 65.Acton A, Kellner K. The Low Temperature Thermal Conductivity of 4He. Physica. 1977;90B:192–204. [Google Scholar]
- 66.Kestin J, Paul R, Clifford AA, Wakeham WA. Absolute Determination of the Thermal Conductivity of the Noble Gases at Room Temperature up to 35 MPa. Physica. 1980;100A:349–369. [Google Scholar]
- 67.Liner JC, Weissman S. Determination of the Temperature Dependence of Gaseous Diffusion Coefficients Using gas Chromatographic Apparatus. J Chem Phys. 1971;56(5):2288–2290. [Google Scholar]
- 68.Bendt PJ. Measurements of He3-He4 and H2-D2 gas diffusion Coefficients. Phys Rev. 1958;110(1):85–89. [Google Scholar]
- 69.DuBro GA, Weissman S. Measurements of Gaseous Diffusion Coefficients. Phys Fluids. 1970;13(11):2682–2688. [Google Scholar]
- 70.Moldover MR. Can a pressure standard be based on capacitance measurements? J Res Natl Inst Stand Technol. 1998;103(2):167–175. doi: 10.6028/jres.103.011. K. Szalewicz, private communication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hurly JJ, Taylor WL, Meeks FR. Thermal-Diffusion Factors at Low-Temperatures for Gas-Phase Mixtures of Isotopic Helium. J Chem Phys. 1992;96(5):3775–3781. [Google Scholar]
- 72.Taylor WL. Thermal Diffusion factor for the 3He-4He system in the quantum region. J Chem Phys. 1972;58(3):834–840. [Google Scholar]
- 73.Taylor WL, Weissman S. Thermal Diffusion Factors for the 3He-4He system. J Chem Phys. 1971;55(8):4000–4004. [Google Scholar]
- 74.McInteer BB, Aldrich LT, Nier AO. The Thermal Diffusion Constant of helium and the Separation of He3 by Thermal Diffusion. Phys Rev. 1947;72(6):510–511. [Google Scholar]
- 75.Watson WW, Howard AJ, Miller NE, Shiffrin RM. Isotopic Thermal Diffusion Factors for Helium and neon at Low Temperatures. Z Naturforsch Teal A. 1963;18A:242–245. [Google Scholar]


