Abstract
The effect of red and processed meats on cancer survival is unclear. We sought to examine the role of total and processed red meat consumption on all-cause mortality among patients with cancers of the upper aerodigestive tract (UADT) and lung, in order to test our hypothesis that red or processed meat was associated with overall mortality in these patients. Using data from a population-based case-control study conducted in Los Angeles County, we conducted a case-only analysis to examine the association of red or processed meat consumption on mortality after 12 years of follow-up, using a diet history questionnaire. Cox regression was used to estimate adjusted hazards ratios (HRs) with 95% confidence intervals (CIs), adjusting for potential confounders. Of 601 UADT cancer cases and 611 lung cancer cases, there were 248 and 406 deaths, respectively, yielding crude mortality rates of 0.07 and 0.12 deaths per year. Comparing the highest with lowest quartile of red meat consumption, the adjusted HR was 1.64 (95% CI: 1.04, 2.57) among UADT cancer cases; for red or processed meat the adjusted HR was 1.76 (95% CI: 1.10, 2.82). A dose-response trend was observed. A weaker association was observed with red meat consumption and overall mortality among lung cancer cases.
In conclusion, this case-only analysis demonstrated that increased consumption of red or processed meats was associated with mortality among UADT cancer cases, and weakly associated with mortality among lung cancer cases.
Keywords: red meat, Cox regression, mortality, lung cancer, UADT cancer, cohort, epidemiology
1. Introduction
There is considerable evidence for the role of nutrition and other lifestyle behaviors in cancers of the upper aero digestive tract (UADT) [1]. Malnutrition is frequently observed in patients with head and neck cancers. Red or processed meats may be associated with cancer susceptibility or progression, as a consequence of production of carcinogens generated by heterocyclic amines, polyaromatic hydrocarbons, and N-nitroso compounds [2], among other potentially harmful compounds or intermediates. A few studies have reported an association between red meat consumption and head and neck cancers. Most of the evidence has been obtained from studies of esophageal cancer, where meta-analyses have demonstrated associations between red meat and cancer susceptibility when comparing highest versus lowest intake categories [3-8]. As far as other UADT cancers, studies from Uruguay have demonstrated an association between cancers of the oral cavity and pharynx, larynx, and esophagus with consumption of red meat [9]. Results from European studies have demonstrated strong associations between red meat and UADT cancers, including a 3-fold risk for laryngeal cancer [10-12], while other studies have not reported this association [13]. Many studies have been done to examine the role of red meat consumption on lung cancer with inconsistent results. However, a recent meta-analysis summarizing these studies showed an association between high consumption of red meat and lung cancer (OR: 1.34, 95% CI: 1.18 – 1.52) [14]. According to a population-based study in Iowa, red meat consumption was associated with lung cancer susceptibility even after controlling for total and saturated fat intake [15]. Such an association has been reported particularly among smokers [16].
There is a great need for understanding the significance of diet to head and neck, and lung cancer outcomes. Very little is known about the relation between red meat and cancer survival. Examination of red and processed meat in cancer has potential utility in informing public health nutrition guidelines for cancer patients. Due to the potentially harmful and inflammation-triggering compounds present in red and processed meats, we hypothesized that consumption of these meats was associated with increased mortality among cancer patients. We sought to analyze the association between red and/or processed meat consumption and survival of 1)UADT and 2) lung cancers. To test this, we performed a case-only survival analysis, using recently diagnosed cancer cases obtained from a population-based case-control study of residents of Los Angeles County [17].
2. Methods and Materials
2.1.Study Population
The study population of the Los Angeles case-control study of lung and UADT cancers has been described previously [17]. Participants were residents of Los Angeles County at the time of recruitment or diagnosis, aged 18 to 65 years during the study period, 1999-2004, and able to speak either English or Spanish or having a translator available. Cancer cases, including oral, pharyngeal, laryngeal and esophageal cancers, were identified through the rapid ascertainment system of the Cancer Surveillance Program at the University of Southern California (USC). Vital status was obtained through the social security death index. In-person interviews were conducted, and standardized questionnaires were used to collect information on demographics, lifestyle behaviors such as smoking and drinking, diet history, occupational and environmental exposure, employment history, family cancer history, and clinical information. The food frequency questionnaire (FFQ), described previously [18], was based on the National Cancer Institute's “Brief Block FFQ” [19], which inquired about diet history over the last 12 months, one year prior to diagnosis. This was expanded to include more fruit and vegetable items. Frequencies of consumption were sought for 78 food items, with categories including “Vegetables”, “Fruits”, “Meat and Mixed Dishes”, “Starches, Breads, Salty Snacks, Spreads”, “Breakfast Foods”, “Sweets”, and “Dairy Products, Beverages”. Frequency of intake was assessed as per day, week, month, year, or rarely/never, based on a specified serving size. For 89% of cases, interviews were conducted within six months post-diagnosis. A total of 601 UADT cancer cases, which were interviewed in the case-control study, are included in this cohort study: 497 squamous cell cancers, 74 adenocarcinomas (esophageal), and 30 other cancer cases. Of 611 lung cancer cases, 95 were squamous cases, 297 adenocarcinomas, 115 large cell carcinomas (LCC), and 75 small cell carcinomas (SCC). Cases with missing information on dietary and behavioral factors were excluded (eleven UADT and nine lung cancer cases).
2.2 Statistical Analyses
Consumption of red or processed meat was measured in grams per day. Four categories of meat consumption were considered in separate categorical models: 1) total red meat (RM), 2) processed red meat only (PRM), all processed or fried meats, which included poultry and fish in addition to processed red meat (PFM), and 3) total red, processed, or fried meat (RPFM).
Meat intake was analyzed by comparing the highest with lowest quartiles of consumption. For lung cancer cases, the cut points were as follows: RM, Q1 and Q2 – 43.31, Q2 and Q3 – 83.16, Q3 and Q4 – 131.97; PRM, Q1 and Q2 – 6.31, Q2 and Q3 – 22.34, Q3 and Q4 – 48.57; PFM, Q1 and Q2 – 13.78, Q2 and Q3 – 34.67, Q3 and Q4 – 65.28; RPFM, Q1 and Q2 – 51.04, Q2 and Q3 – 95.21, Q3 and Q4 – 145.15. The cut points among UADT cancer cases were as follows: RM, Q1 and Q2 – 46.83, Q2 and Q3 – 87.98, Q3 and Q4 – 148.84; PRM, Q1 and Q2 – 8.10, Q2 and Q3 – 25.53, Q3 and Q4 – 53.66; PFM, Q1 and Q2 – 15.22, Q2 and Q3 – 39.96, Q3 and Q4 – 70.60; RPFM, Q1 and Q2 – 54.95, Q2 and Q3 – 99.09, Q3 and Q4 – 168.05.
We conducted a case-only survival analysis of lung and UADT cancer cases. All cases of lung cancer and UADT cancers, including oral, pharyngeal, laryngeal and esophageal, were pathologically confirmed new cancer incidences identified by the rapid ascertainment system of the Cancer Surveillance Program, a population-based SEER registry for Los Angeles County at the University of Southern California. A summary of selection of cases for the present study is shown in Figure 1. Follow-up time for each cancer case was the duration of the period between the date of diagnosis and the date of death or last follow-up, which was October 10, 2013. The median follow-up time was 12.1 years for UADT cancer cases and 12.8 for lung cancer cases. Cox regression was used to obtain adjusted hazard ratios (aHR) and corresponding 95% confidence intervals (CIs). Tests for trend (P-values) were calculated using the exposure of interest as a continuous variable, based on the Wald chi-square statistic. Meat intake was analyzed by comparing quartiles of intake, using the lowest quartile as the reference. We adjusted for the following potential confounders: age, gender, race/ethnicity (non-Hispanic White, non-Hispanic African American, Non-Hispanic Asian/Pacific Islander, other non-Hispanic race, and Hispanic), tobacco smoking (ever/never and pack-years), education, body mass index (BMI), caloric intake, pathology type (squamous, adenocarcinoma, large/small cell carcinoma, other) and differentiation grade (well-differentiated, poorly differentiated, undetermined) in Model 1. Alcohol drinking (ever/never and drinks per day) was included in models considering UADT cancer cases. In addition to these variables, Model 2 (expanded model) included saturated fat (grams/day), fruit consumption (servings/day), and vegetable consumption (servings/day). The mean and standard deviation (SD) were calculated for continuous variables, and relative proportions for categorical variables are presented.
Figure 1.
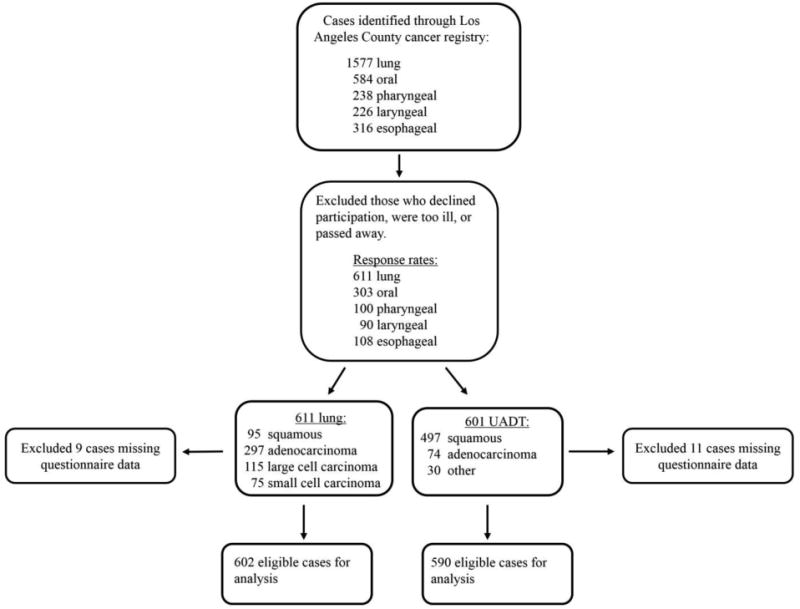
Flow chart of case selection in the Los Angeles Study. After exclusions, a total of 611 and 601 lung and UADT cancer cases, respectively, were included. A total of 602 lung and 590 UADT cancer cases with complete data on diet and behavioral factors were included in the regression analysis of red/processed meat and all-cause mortality.
Missing caloric intake or dietary fat data were imputed using multiple imputation with the Proc MI procedure in SAS to generate five imputed datasets, and Proc MIANALYZE to combine results. The imputation included a total of eight covariates: saturated fat, total dietary fat, daily caloric intake, BMI, education, gender, cancer diagnosis, and age. Informed consent was obtained from all participants. The study was approved by the institutional review boards of UCLA and USC.
3. Results
3.1. Demographic and Clinical Characteristics
A summary of demographic and clinical characteristics of UADT and lung cancer cases is presented in Table 1. There were 406 (66%) deaths from lung cancer and 248 (41%) deaths among UADT cancer cases during follow-up, with crude mortality rates of 0.12 and 0.07 per year, respectively.
Table 1.
Demographic and clinical characteristics of lung and UADT cancer cases.
| LUNG | UADT | |||
|---|---|---|---|---|
| All, n | Death | All, n | Death | |
| Survival, n (%) | 611 | 406 (66) | 601 | 248 (41) |
| Age, means, SD | 52.6 ±5.3 | 51.2 ±7.3 | ||
| Age, n (%) | ||||
| < 45 | 61 | 38 (62) | 109 | 38 (35) |
| 45-54 | 301 | 188 (62) | 267 | 205 (39) |
| ≥ 55 | 249 | 179 (72) | 225 | 105 (47) |
| Gender, n (%) | ||||
| Male | 303 | 215 (71) | 454 | 191 (42) |
| Female | 308 | 191 (62) | 147 | 57 (39) |
| Ethnicity, n (%) | ||||
| White | 359 | 245 (68) | 341 | 135 (40) |
| Hispanic | 70 | 44 (63) | 109 | 46 (42) |
| Black | 96 | 60 (62) | 69 | 39 (56) |
| Asian/Pacific | 70 | 46 (66) | 64 | 21 (33) |
| Other | 15 | 10 (67) | 16 | 6 (38) |
| Pack-years, means, SD | 33.1 ± 24.8 | 28.3 ± 25.8 | ||
| Pack-years, n (%) | ||||
| <20 | 98 | 63 (64) | 145 | 51 (35) |
| 20-40 | 201 | 139 (69) | 146 | 71 (49) |
| ≥ 40 | 202 | 143 (71) | 128 | 73 (57) |
| Drink-days, means, SD | 1.6 ± 3.1 | 3.1 ± 4.6 | ||
| Drinking, n (%) | ||||
| No | 170 | 111 (65) | 117 | 45 (38) |
| Yes | 440 | 294 (67) | 482 | 202 (42) |
| BMI means, SD | 26.0 ± 6.2 | 26.5 ± 7.4 | ||
| BMI (Kg/m2), n (%) | ||||
| <25 | 291 | 195 (67) | 242 | 113 (47) |
| ≥25 | 318 | 210 (66) | 357 | 135 (38) |
| Education, means, SD | 13.2 ± 3.3 | 12.9 ± 3.6 | ||
| Education, n (%) | ||||
| 0-12 | 265 | 181 (68) | 273 | 117 (43) |
| 13-16 | 275 | 181 (66) | 259 | 110 (42) |
| >16 | 71 | 44 (62) | 69 | 21 (30) |
| Tumor histology, n (%) | ||||
| Well differentiated | 169 | 90 (53) | 399 | 172 (43) |
| Poorly differentiated | 222 | 154 (69) | 121 | 42 (35) |
| Undetermineda | 219 | 161 (74) | 81 | 34 (42) |
| Tumor pathology, n (%) | ||||
| Squamous | 95 | 53 (56) | 497 | 195 (39) |
| Adenocarcinoma | 297 | 186 (63) | 74 | 42 (57) |
| Large | 115 | 85 (74) | ||
| Small | 75 | 60 (80) | ||
| Other | 29 | 22 (76) | 30 | 11 (37) |
Not graded because of prior hormone therapy
3.2. Meat Distribution Among Cases
Consumption of red and processed meat among all cancer cases is shown in Table 2. UADT cancer cases consumed an average of 119 grams per day of any category of red or processed meat and lung cancer cases consumed 108.7 grams per day, mostly attributable to red meat.
Table 2. Red and processed meat consumption among cancer cases.
| UADT Cancer | Lung Cancer | |
|---|---|---|
| Meat means, SDa | ||
| Total red or processed meat | 119.0 ± 89.0 | 108.7 ± 81.8 |
| Red meat (all) | 105.3 ± 81.1 | 97.3 ± 77.1 |
| Processed meat (all) | 52.2 ± 52.1 | 46.9 ± 49.9 |
| Processed red | 38.5 ± 43.0 | 35.3 ± 45.1 |
| Processed (fried) poultry/fish | 13.7 ± 23.1 | 11.4 ± 14.9 |
| Total calories, means, SD | 1784.9 ± 1011.1 | 1526.2 ± 700.0 |
Meat calculated as grams/day
3.3. Red and Processed Meat and UADT Cancer Mortality
Associations of consumption of red or processed meat with mortality among UADT cancer cases are presented in Table 3. A positive association was observed between red meat consumption and mortality. Comparing the highest quartile with the lowest quartile, the aHR for the estimated effect of red meat was 1.71 (95% CI: 1.13, 2.57) in Model 1 (p for trend = 0.009) and 1.64 (95% CI: 1.04, 2.57) in Model 2 (p for trend = 0.04). Similarly, the aHR for the estimated effect of total red and processed meat was 1.85 (95% CI: 1.21, 2.83) in Model 1 (p for trend = 0.009) and 1.76 (95% CI: 1.10, 2.82) in Model 2 (p for trend = 0.04). This estimated effect was due primarily to the association with oropharyngeal cancer (results not shown). Associations were weaker for the estimated effects of fried or processed meats on mortality.
Table 3. Hazard ratios for UADT cancer mortality according to consumption of red or processed meat.
| Meat grams/day | Dead/All | aHR (95% CI)a | P* | aHR (95% CI)b | P* | ||
|---|---|---|---|---|---|---|---|
| RM | Q1 | (0-46.82) | 43/147 | 1.0 | 1.0 | ||
| Q2 | (46.83-87.97) | 56/148 | 1.25 (0.83, 1.88) | 1.24 (0.83, 1.87) | |||
| Q3 | (87.98-148.83 | 63/148 | 1.27 (0.84, 1.91) | 1.23 (0.81, 1.89) | |||
| Q4 | (> 148.84) | 79/147 | 1.71 (1.13, 2.57) | 0.009 | 1.64 (1.04, 2.57) | 0.04 | |
| PRM | Q1 | (0-8.0) | 48/145 | 1.0 | 1.0 | ||
| Q2 | (8.1-25.52) | 55/150 | 1.05 (0.70, 1.57) | 1.02 (0.68, 1.53) | |||
| Q3 | (25.53-53.65) | 61/149 | 1.08 (0.72, 1.60) | 1.02 (0.68, 1.54) | |||
| Q4 | (> 53.66) | 77/146 | 1.39 (0.94, 2.05) | 0.09 | 1.30 (0.85, 1.98) | 0.23 | |
| PFM | Q1 | (0-15.21) | 47/147 | 1.0 | 1.0 | ||
| Q2 | (15.22-39.95) | 56/146 | 1.11 (0.74, 1.67) | 1.07 (0.71, 1.61) | |||
| Q3 | (39.96-70.59) | 58/149 | 1.07 (0.71, 1.61) | 1.02 (0.67, 1.56) | |||
| Q4 | (> 70.60) | 80/148 | 1.47 (0.98, 2.21) | 0.07 | 1.39 (0.90, 2.14) | 0.15 | |
| RPFM | Q1 | (0-54.94) | 39/148 | 1.0 | 1.0 | ||
| Q2 | (54.95-99.08) | 59/147 | 1.59 (1.05, 2.41) | 1.56 (1.03, 2.37) | |||
| Q3 | (99.09-168.04) | 67/148 | 1.50 (0.98, 2.28) | 1.47 (0.96, 2.27) | |||
| Q4 | (> 168.05) | 76/147 | 1.85 (1.21, 2.83) | 0.009 | 1.76 (1.10, 2.82) | 0.04 | |
Model 1 adjusted for gender, race, age, smoking, alcohol drinking, education, caloric intake, BMI, tumor grade, and pathology
Model 2 adjusted for all variables in Model 1 and additionally adjusted for saturated fat, and fruit and vegetable consumption
P-value for trend
3.4 Red and Processed Meat and Lung Cancer Mortality
Table 4 presents hazard ratios depicting the association between red or processed meat and lung cancer mortality. Associations were weaker overall than those observed with UADT cancers. Comparing the highest with lowest quartiles, the aHR for the effect of red meat on mortality was 1.38 (95% CI: 0.98, 1.93) in Model 2. A similar association was observed when considering the effect of total red or processed meat on mortality (aHR: 1.39, 95% CI: 0.99, 1.96).
Table 4. Hazard ratios for lung cancer mortality according to consumption of red or processed meat.
| Meat grams/day | Dead/All | aHR (95% CI)a | P* | aHR (95% CI)b | P* | ||
|---|---|---|---|---|---|---|---|
| RM | Q1 | (0-43.30) | 94/150 | 1.0 | 1.0 | ||
| Q2 | (43.31-83.15) | 94/150 | 1.00 (0.74, 1.35) | 1.01 (0.75, 1.37) | |||
| Q3 | (83.16-131.96) | 92/150 | 0.90 (0.66, 1.23) | 0.92 (0.67, 1.25) | |||
| Q4 | (> 131.97) | 118/151 | 1.34 (0.96, 1.86) | 0.17 | 1.38 (0.98, 1.93) | 0.13 | |
| PRM | Q1 | (0-6.30) | 90/150 | 1.0 | |||
| Q2 | (6.31-22.33) | 93/146 | 1.02 (0.76, 1.37) | 1.03 (0.77, 1.39) | |||
| Q3 | (22.34-48.56) | 108/154 | 1.17 (0.86, 1.57) | 1.19 (0.88, 1.61) | |||
| Q4 | (> 48.57) | 107/151 | 1.07 (0.78, 1.49) | 0.5 | 1.11 (0.80, 1.55) | 0.38 | |
| PFM | Q1 | (0-13.77) | 92/149 | 1.0 | 1.0 | ||
| Q2 | (13.78-34.66) | 95/151 | 0.95 (0.71, 1.29) | 0.98 (0.72, 1.32) | |||
| Q3 | (34.67-65.27) | 104/150 | 1.08 (0.80, 1.48) | 1.11 (0.81, 1.52) | |||
| Q4 | (> 65.28) | 107/151 | 0.99 (0.71, 1.39) | 0.84 | 1.03 (0.73, 1.46) | 0.67 | |
| RPFM | Q1 | (0-51.03) | 92/149 | 1.0 | 1.0 | ||
| Q2 | (51.04-95.20) | 99/151 | 1.10 (0.82, 1.48) | 1.12 (0.83, 1.50) | |||
| Q3 | (95.21-145.14) | 90/150 | 0.83 (0.60, 1.13) | 0.84 (0.61, 1.15) | |||
| Q4 | (> 145.15) | 117/151 | 1.35 (0.97, 1.89) | 0.29 | 1.39 (0.99, 1.96) | 0.23 | |
Model 1 adjusted for gender, race, age, smoking, education, caloric intake, BMI, tumor grade, and pathology
Model 2 adjusted for variables in Model 1 and additionally adjusted for saturated fat, and fruit and vegetable consumption
P-value for trend
4. Discussion
This study sought to examine associations between consumption of red and processed meat and overall survival among UADT and lung cancer cases. Herein, in favor of our hypothesis, we report an association between red meat consumption and all-cause mortality for UADT cancers, and a weaker association between the consumption of red meats and all-cause mortality among lung cancer patients. This association persisted after controlling for saturated fat in the diet, an important fact considering the potential relevance of saturated fat to cancer [20, 21]. It was somewhat surprising that stronger associations were not observed when considering processed red meat alone. Perhaps this is explained by lower overall consumption of red processed meat. While red or processed meat consumption has been reported to be associated with overall mortality from cardiovascular disease and cancer in general, as well as mortality among colorectal cancer patients [22-24], such an association with head and neck cancers has not been reported previously, in spite of the reported associations between red meat consumption and UADT cancer risk. It should be noted that we did not detect such an association with UADT cancer susceptibility in the original population-based Los Angeles County case-control study (unpublished results). Similarly, there is evidence supporting an association between red meat consumption and risk of lung cancer [14, 15], but the evidence of such an association with mortality is lacking.
Other dietary factors, such as fruit and vegetable consumption, may contribute to mortality of UADT and lung cancers. Daily consumption of fruits and vegetables may reduce risk of oral and lung cancers, in some cases with a dose-response effect [25-28]. In our analyses, we did not observe such a protective effect with stratification according to consumption of fruits and vegetables (data not shown). However, overall consumption of fruits and vegetables was very low among the study population with median values of only 1 and 1.7 servings per day, respectively.
There are several proposed mechanisms whereby red or processed meat potentially promotes cancer mortality. Carcinogenic heterocyclic amines, polyaromatic hydrocarbons, and N-nitroso compounds may be generated through processing, preservation, and cooking at high temperatures, and may be carcinogenic [29-31]. Particularly, heme-iron toxicity associated with meat is a potential mechanism for cancer promotion. Heme-iron may promote formation of some of these carcinogenic compounds and aldehydes, damaging cells and DNA [32]. Interestingly, genes associated with heme-iron related processes have been correlated with lung cancer [33]. Meat and animal products may also contain endotoxins, arachidonic acid-polyunsaturated omega-6 fatty acid which stimulate inflammatory factors (interleukin-6, tumor necrosis factor-α, toll-like receptor) and a heightened inflammatory response [34]. Because of the potentially lower levels of saturated fat, hormones and endotoxins, it might be interesting to analyze the role of organic meats from grass-fed cattle on UADT cancers in future studies.
Our study is limited by numbers of deaths, and consequently there was limited power to detect interactions with potential confounding factors, as well as associations with mortality among cancer subtypes. Additionally, there is the possibility of recall bias, and misclassification of meat consumption, potentially adding bias in estimates, as well as residual bias due to unmeasured or mismeasured confounders such as human papilloma virus status for UADT cancers, and other factors. Similarly, it was not possible to obtain complete information on patient treatment regimens; however, all models were adjusted for pathology and grade, which are highly correlated with treatment. Furthermore, the diet history reflected by the questionnaire does not necessarily consistently reflect diet during follow-up. Clearly, such memory-based approaches have been labeled as inaccurate or unrelated to actual consumption, with recall being subjective and unable to be independently quantified [35]. However, the Block FFQ has shown correlation with estimated truth for energy and nutrient intakes [19, 36]. Nonetheless, our results provide preliminary evidence that consumption of red and processed meats may negatively affect survival of cancer patients, although prospective studies are needed to validate this finding and the details concerning the intervals and duration of consumption should be elucidated. Lastly, the possibility of selection bias due to loss of eligible UADT and lung cancer cases as a result of early death or hospitalization precluding interviewing, patient refusal to participate, or physician refusal for patient contact can not be disregarded.
In conclusion, we have found a positive association between red meat consumption and mortality among UADT cancer cases. Larger, prospective cohort studies examining the association between red meat consumption and overall or cancer-specific survival among patients with UADT and lung cancers are warranted. Validation of such findings in prospective or intervention studies might ultimately be used to inform public health guidelines for cancer control.
Acknowledgments
The authors appreciate all participants of the Los Angeles Study for their valuable time, support and contributions to this study. This research was partially supported by the National Institutes of Health (Grant Numbers ES011667, CA90833, CA09142, DA11386) and the Alper Research Funds for Environmental Genomics of the UCLA Jonsson Comprehensive Cancer Center. The authors have no conflicts of interest to disclose.
Abbreviations
- UADT
upper aerodigestive tract
- LCC
large cell carcinoma
- SCC
small cell carcinoma
- aHR
adjusted hazard ratio
- HHHQ
health habits and history questionnaire
- USC
University of Southern California
- UCLA
University of California, Los Angeles
- RM
red meat
- PRM
processed red meat
- PFM
processed fried meat
- RPFM
red, processed, or fried meat
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chuang SC, Jenab M, Heck JE, Bosetti C, Talamini R, Matsuo K, et al. Diet and the risk of head and neck cancer: a pooled analysis in the INHANCE consortium. Cancer Causes Control. 2012;23:69–88. doi: 10.1007/s10552-011-9857-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santarelli RL, Pierre F, Corpet DE. Processed meat and colorectal cancer: a review of epidemiologic and experimental evidence. Nutr Cancer. 2008;60:131–44. doi: 10.1080/01635580701684872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi Y, Song S, Song Y, Lee JE. Consumption of red and processed meat and esophageal cancer risk: meta-analysis. World J Gastroenterol. 2013;19:1020–9. doi: 10.3748/wjg.v19.i7.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cross AJ, Leitzmann MF, Gail MH, Hollenbeck AR, Schatzkin A, Sinha R. A prospective study of red and processed meat intake in relation to cancer risk. PLoS Med. 2007;4:e325. doi: 10.1371/journal.pmed.0040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Navarro Silvera SA, Mayne ST, Risch H, Gammon MD, Vaughan TL, Chow WH, et al. Food group intake and risk of subtypes of esophageal and gastric cancer. Int J Cancer. 2008;123:852–60. doi: 10.1002/ijc.23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Doherty MG, Cantwell MM, Murray LJ, Anderson LA, Abnet CC. Dietary fat and meat intakes and risk of reflux esophagitis, Barrett's esophagus and esophageal adenocarcinoma. Int J Cancer. 2011;129:1493–502. doi: 10.1002/ijc.26108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu HC, Yang X, Xu LP, Zhao LJ, Tao GZ, Zhang C, et al. Meat consumption is associated with esophageal cancer risk in a meat- and cancer-histological-type dependent manner. Dig Dis Sci. 2014;59:664–73. doi: 10.1007/s10620-013-2928-y. [DOI] [PubMed] [Google Scholar]
- 8.De Stefani E, Deneo-Pellegrini H, Ronco AL, Boffetta P, Brennan P, Munoz N, et al. Food groups and risk of squamous cell carcinoma of the oesophagus: a case-control study in Uruguay. Br J Cancer. 2003;89:1209–14. doi: 10.1038/sj.bjc.6601239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aune D, De Stefani E, Ronco A, Boffetta P, Deneo-Pellegrini H, Acosta G, et al. Meat consumption and cancer risk: a case-control study in Uruguay. Asian Pac J Cancer Prev. 2009;10:429–36. [PubMed] [Google Scholar]
- 10.Bosetti C, La Vecchia C, Talamini R, Negri E, Levi F, Dal Maso L, et al. Food groups and laryngeal cancer risk: a case-control study from Italy and Switzerland. Int J Cancer. 2002;100:355–60. doi: 10.1002/ijc.10485. [DOI] [PubMed] [Google Scholar]
- 11.Levi F, Pasche C, Lucchini F, Bosetti C, La Vecchia C. Processed meat and the risk of selected digestive tract and laryngeal neoplasms in Switzerland. Ann Oncol. 2004;15:346–9. doi: 10.1093/annonc/mdh060. [DOI] [PubMed] [Google Scholar]
- 12.Lagiou P, Talamini R, Samoli E, Lagiou A, Ahrens W, Pohlabeln H, et al. Diet and upper-aerodigestive tract cancer in Europe: the ARCAGE study. Int J Cancer. 2009;124:2671–6. doi: 10.1002/ijc.24246. [DOI] [PubMed] [Google Scholar]
- 13.Steffen A, Bergmann MM, Sanchez MJ, Chirlaque MD, Jakszyn P, Amiano P, et al. Meat and heme iron intake and risk of squamous cell carcinoma of the upper aero-digestive tract in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer Epidemiol Biomarkers Prev. 2012;21:2138–48. doi: 10.1158/1055-9965.EPI-12-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang WS, Wong MY, Vogtmann E, Tang RQ, Xie L, Yang YS, et al. Meat consumption and risk of lung cancer: evidence from observational studies. Ann Oncol. 2012;23:3163–70. doi: 10.1093/annonc/mds207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alavanja MC, Field RW, Sinha R, Brus CP, Shavers VL, Fisher EL, et al. Lung cancer risk and red meat consumption among Iowa women. Lung Cancer. 2001;34:37–46. doi: 10.1016/s0169-5002(01)00227-6. [DOI] [PubMed] [Google Scholar]
- 16.Gnagnarella P, Maisonneuve P, Bellomi M, Rampinelli C, Bertolotti R, Spaggiari L, et al. Red meat, Mediterranean diet and lung cancer risk among heavy smokers in the COSMOS screening study. Ann Oncol. 2013;24:2606–11. doi: 10.1093/annonc/mdt302. [DOI] [PubMed] [Google Scholar]
- 17.Hashibe M, Morgenstern H, Cui Y, Tashkin DP, Zhang ZF, Cozen W, et al. Marijuana use and the risk of lung and upper aerodigestive tract cancers: results of a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2006;15:1829–34. doi: 10.1158/1055-9965.EPI-06-0330. [DOI] [PubMed] [Google Scholar]
- 18.Cui Y, Morgenstern H, Greenland S, Tashkin DP, Mao JT, Cai L, et al. Dietary flavonoid intake and lung cancer--a population-based case-control study. Cancer. 2008;112:2241–8. doi: 10.1002/cncr.23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–35. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 20.Alavanja MC, Brown CC, Swanson C, Brownson RC. Saturated fat intake and lung cancer risk among nonsmoking women in Missouri. J Natl Cancer Inst. 1993;85:1906–16. doi: 10.1093/jnci/85.23.1906. [DOI] [PubMed] [Google Scholar]
- 21.Kesteloot H, Lesaffre E, Joossens JV. Dairy fat, saturated animal fat, and cancer risk. Prev Med. 1991;20:226–36. doi: 10.1016/0091-7435(91)90022-v. [DOI] [PubMed] [Google Scholar]
- 22.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, et al. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. 2012;172:555–63. doi: 10.1001/archinternmed.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohrmann S, Overvad K, Bueno-de-Mesquita HB, Jakobsen MU, Egeberg R, Tjonneland A, et al. Meat consumption and mortality--results from the European Prospective Investigation into Cancer and Nutrition. BMC Med. 2013;11:63. doi: 10.1186/1741-7015-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCullough ML, Gapstur SM, Shah R, Jacobs EJ, Campbell PT. Association between red and processed meat intake and mortality among colorectal cancer survivors. J Clin Oncol. 2013;31:2773–82. doi: 10.1200/JCO.2013.49.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boeing H, Dietrich T, Hoffmann K, Pischon T, Ferrari P, Lahmann PH, et al. Intake of fruits and vegetables and risk of cancer of the upper aero-digestive tract: the prospective EPIC-study. Cancer Causes Control. 2006;17:957–69. doi: 10.1007/s10552-006-0036-4. [DOI] [PubMed] [Google Scholar]
- 26.Pavia M, Pileggi C, Nobile CG, Angelillo IF. Association between fruit and vegetable consumption and oral cancer: a meta-analysis of observational studies. Am J Clin Nutr. 2006;83:1126–34. doi: 10.1093/ajcn/83.5.1126. [DOI] [PubMed] [Google Scholar]
- 27.Miller AB, Altenburg HP, Bueno-de-Mesquita B, Boshuizen HC, Agudo A, Berrino F, et al. Fruits and vegetables and lung cancer: Findings from the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2004;108:269–76. doi: 10.1002/ijc.11559. [DOI] [PubMed] [Google Scholar]
- 28.Wu QJ, Xie L, Zheng W, Vogtmann E, Li HL, Yang G, et al. Cruciferous vegetables consumption and the risk of female lung cancer: a prospective study and a meta-analysis. Ann Oncol. 2013;24:1918–24. doi: 10.1093/annonc/mdt119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baghurst K. Red meat consumption in Australia: intakes, contributions to nutrient intake and associated dietary patterns. Eur J Cancer Prev. 1999;8:185–91. doi: 10.1097/00008469-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Cross AJ, Pollock JR, Bingham SA. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 2003;63:2358–60. [PubMed] [Google Scholar]
- 31.Puangsombat K, Gadgil P, Houser TA, Hunt MC, Smith JS. Heterocyclic amine content in commercial ready to eat meat products. Meat Sci. 2011;88:227–33. doi: 10.1016/j.meatsci.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 32.Bastide NM, Pierre FH, Corpet DE. Heme iron from meat and risk of colorectal cancer: a meta-analysis and a review of the mechanisms involved. Cancer Prev Res (Phila) 2011;4:177–84. doi: 10.1158/1940-6207.CAPR-10-0113. [DOI] [PubMed] [Google Scholar]
- 33.Lam TK, Rotunno M, Ryan BM, Pesatori AC, Bertazzi PA, Spitz M, et al. Heme-related gene expression signatures of meat intakes in lung cancer tissues. Mol Carcinog. 2014;53:548–56. doi: 10.1002/mc.22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erridge C. The capacity of foodstuffs to induce innate immune activation of human monocytes in vitro is dependent on food content of stimulants of Toll-like receptors 2 and 4. Br J Nutr. 2011;105:15–23. doi: 10.1017/S0007114510003004. [DOI] [PubMed] [Google Scholar]
- 35.Archer E, Pavela G, Lavie CJ. The Inadmissibility of What We Eat in America and NHANES Dietary Data in Nutrition and Obesity Research and the Scientific Formulation of National Dietary Guidelines. Mayo Clin Proc. 2015;90:911–26. doi: 10.1016/j.mayocp.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol. 2001;154:1089–99. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]


