Abstract
Objective
Pancreatic adenocarcinoma is a rapidly progressive malignancy characterized by its tendency for early metastatic spread. MDCT is the primary diagnostic modality for the preoperative staging of patients with pancreatic cancer, with an accuracy established in multiple studies. However, for a variety of reasons, there is often a prolonged interval between staging MDCT and the surgical intervention. This study examines the relationship between the interval between imaging and surgery and the accuracy of MDCT in determining the presence or absence of metastatic disease at surgery in patients with pancreatic cancer.
Materials and Methods
Patients were identified who had undergone surgery for pancreatic cancer at our institution with a dedicated preoperative pancreas-protocol MDCT performed in our department. Findings from the preoperative MDCT report were correlated with the operative findings, as well as the time between imaging and surgery.
Results
Two hundred ninety-two MDCT scans were performed on 256 patients who underwent exploration for pancreatic adenocarcinoma. The patients had a median age of 67 years (range, 30–95 years), and 51.6% (132/256) were male. The median time between MDCT and surgical exploration was 15.5 days (range, 1–198 days). MDCT correctly predicted the absence of metastatic disease at surgery in 233 of 274 (85.0%) studies. MDCT was more accurate in predicting the absence of metastatic disease if the study was performed within 25 days of surgery than it was if the study was performed within more than 25 days of surgery (89.3% vs 77.0%; p = 0.0097). Furthermore, regression models showed that the negative predictive value of a given MDCT significantly decreased after approximately 4 weeks.
Conclusion
MDCT is an accurate method to stage patients with pancreatic cancer, but its accuracy in excluding distant metastatic disease depreciates over time. Patients should undergo a repeat MDCT within 25 days of any planned definitive operative intervention for pancreatic cancer to avoid unexpectedly finding metastatic disease at surgery.
Keywords: MDCT, metastases, pancreatic adenocarcinoma, pancreatic cancer, staging
Pancreatic adenocarcinoma carries an extraordinarily poor prognosis, with a 5-year survival rate of less than 5%, and pancreatic cancer now is the fourth leading cause of cancer-related mortality in the United States [1]. Unfortunately, despite increasingly sophisticated chemotherapy and radiation regimens, the only truly curative option for these patients is complete surgical resection of localized disease, and the vast majority of patients are not candidates for resection at the time of diagnosis because of either distant metastatic disease or significant involvement of the major central mesenteric arterial and venous vasculature. Accurate staging before surgery is critical, because a nontherapeutic laparotomy (as a result of unexpectedly discovering metastatic disease or a locally advanced tumor at surgery) subjects patients to unnecessary morbidity, potentially delays the initiation of chemotherapy, and incurs an unnecessary monetary cost [2, 3]. As a result, accurate preoperative staging via imaging is critical in determining which patients truly have localized disease and will be appropriate candidates for surgical resection.
The primary role of MDCT in the diagnosis and staging of pancreatic adenocarcinoma is undeniable, and the role of CT is critical with regard to lesion identification, determination of locoregional resectability (i.e., tumoral involvement of the portal-superior mesenteric vein confluence, as well as the superior mesenteric artery, celiac trunk, and hepatic artery), and, most importantly, detection of distant metastatic disease (usually to the liver, peritoneum, or lung) [1, 4, 5]. The accuracy of MDCT in determining the resectability of pancreatic cancer has been reported to range from 70% to 85%. Arguably, these studies may understate the accuracy of MDCT given that much of this research was conducted on older scanner technology [1, 6–9].
Although there are significant data supporting the accuracy of MDCT in staging patients with pancreatic cancer, the accuracy of MDCT in appropriately staging patients with pancreatic cancer relative to the time of surgical exploration has received much less attention [10]. Unlike more indolent tumors, which are not likely to change appreciably in the weeks or months between an initial staging CT and surgical resection, pancreatic cancer can rapidly metastasize over sequential CT studies, and a tumor that was initially thought to be resectable can quickly become unresectable (because of metastatic disease or vascular involvement) over the course of just a few weeks. Accordingly, a study performed a few weeks or months before a patient's surgery may not be predictive of that patient's ability to ultimately undergo a definitive resection and of the tumor's being truly resectable on the planned date of surgery. Therefore, the goals of this study are to evaluate the accuracy of MDCT in identifying metastatic disease in a cohort of patients with pancreatic cancer relative to the “age” (i.e., interval between imaging and surgery) of the MDCT study and, more specifically, to evaluate the accuracy of MDCT for metastatic disease relative to the timing of surgery to establish time-sensitive cut-offs when the diagnostic accuracy of a given cross-sectional imaging study declines.
Materials and Methods
Study Population and Clinical Parameters
Before the study, institutional review board approval was obtained, and a waiver of informed consent was provided for medical record and CT review. The study complied with HIPAA regulations. A search was then conducted of a Radiology Department database, and radiology reports for all “pancreas-protocol” MDCT studies performed at our institution from January 1, 2006, to December 3, 2007, were reviewed. Subsequently, this group of patients acquired from the Radiology Department database was cross-referenced with patients from a Surgery Department database, and the study population ultimately consisted of patients who had undergone a dedicated preoperative pancreas-protocol CT at our institution, who had a pathologically proven pancreatic adenocarcinoma, and who had undergone surgery with the intent to perform curative resection, surgical palliation, or diagnostic exploration.
Imaging Review
As mentioned already, all the MDCT examinations in this study were performed at our institution with an identical technique (i.e., the pancreas protocol). MDCT examinations performed at other institutions, as well as scans performed at our institution without a dedicated pancreatic protocol, were not included in the study, thereby ensuring that CT technique and protocols and scanner quality had no influence on the results. All the MDCT examinations reviewed in this study were performed on a 64-MDCT scanner (Somatom Sensation 64, Siemens Healthcare), with detector collimation of 64 × 0.6 mm, reconstruction at 3-mm slice thickness and 3-mm slice interval for diagnostic interpretation, reconstruction at 0.75-mm slice thickness and 0.5-mm intervals for multiplanar reformation and interactive 3D rendering, 120 kVp, and 150–200 mA. All studies were conducted with a uniform technique (pancreas-protocol MDCT). Either iohexol (Omnipaque 350, GE Healthcare) or iodixanol (Visipaque 320, GE Healthcare) was used as the IV contrast agent and was injected rapidly through a peripheral IV line at 3–5 mL/s. Water was used as an oral contrast agent. All studies were performed using a dual-phase technique, with arterial and venous phase images acquired at roughly 30 and 60 seconds, respectively, after the injection of IV contrast agent. Multiplanar reformations were directly reconstructed at the scanner and sent to a PACS for imaging review [11]. Three-dimensional reconstructions were created at an independent workstation and were used for image interpretation in all cases [7].
MDCT and Surgical Comparison
Importantly, as a result of the unique involvement of one of our group's radiologists in the multidisciplinary pancreatic tumor board, all pancreas-protocol MDCT studies performed during the course of this study received a supplementary dictation from a single radiologist. Only diagnoses from this single radiologist were evaluated for the purposes of this study, and no other radiologist's interpretations were examined. The radiology reports from these MDCT studies were then compared with the operative reports and were scored for accuracy in evaluating the presence or absence of metastatic disease. Notably, the images from each MDCT were not rereviewed for this study, and the study relied solely on a review of the original radiology reports from 2006 to 2007 and whether metastatic disease was reported on the original radiology report. For the purposes of this study, the reference standard for metastatic disease was based on findings in the operative notes with confirmation by surgical pathology. The accuracy of MDCT for metastatic disease was graded in a binary fashion (i.e., presence or absence of metastatic disease), and the numbers of sites of metastatic disease on either MDCT or at surgery were not considered.
Statistics
To evaluate the diagnostic accuracy of MDCT for metastatic disease, the sensitivity, specificity, positive predictive value, negative predictive value (NPV), and AUC at different time points before surgery were assessed. The AUC is a test of the discriminatory power of a diagnostic test and establishes values between 50% (no discriminatory power) and 100% (perfect discrimination). Detection of metastatic disease at surgery was considered to be the reference standard. The locally weighted scatterplot smoothing technique was also used to evaluate the variation over time in the accuracy of MDCT and to possibly identify an inflection point in time after which accuracy decreased significantly. This was performed by fitting weighted regression models to localized subsets of the data to build up a function that describes the variation in the data, point by point [12]. All statistical analysis was performed using Stata software (version 11.0, StataCorp).
Results
Demographics and Descriptive Analysis
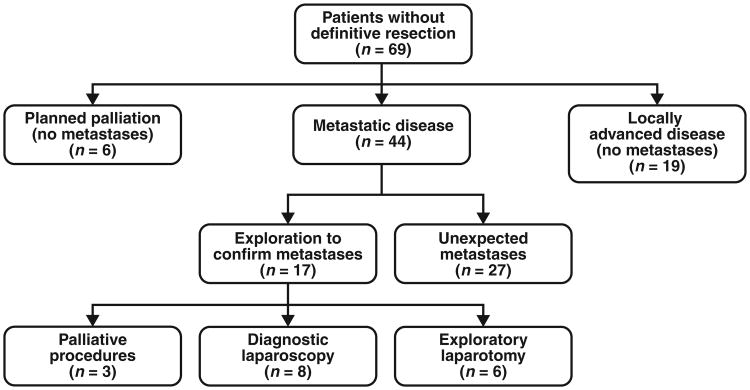
From January 1, 2006, to December 31, 2007, 2589 pancreas-protocol MDCT examinations were performed at our institution. Of these, 292 preoperative studies were performed on 256 patients who subsequently underwent surgery and had a final pathologic diagnosis of pancreatic adenocarcinoma. This cohort of patients had a median age of 67 years, and consisted of 51.6% male patients. Additional detailed demographic information is presented in Table 1. Of the 256 patients, 141 (55.1%) underwent pancreaticoduodenectomy, 32 (12.5%) underwent distal pancreatectomy, and 14 (5.5%) underwent total pancreatectomy, as opposed to 69 (27.0%) patients who did not undergo formal pancreatic resection at the time of operation. Among the 69 patients who did not undergo a definitive resection, six had a planned palliative procedure for failed nonoperative management, 44 had an exploration with biopsy of a metastatic lesion, and 19 had an exploration without resection as a result of a locally advanced tumor (Fig. 1).
Table 1. Demographics and Descriptive Characteristics of 256 Patients Who Underwent MDCT and Operative Intervention for Pancreatic Adenocarcinoma.
| Characteristic | Value |
|---|---|
|
| |
| Male sex | 132 (51.6) |
| Age (y), median (range) | 67 (30–95) |
| Operation | |
| Pancreaticoduodenectomy | 141 (55.1) |
| Distal pancreatectomy | 32 (12.5) |
| Total pancreatectomy | 14 (5.5) |
| Exploration without resection | 69 (27.0) |
Note—Except for patient age, data are number (%) of patients. Percentages may total more than 100% due to rounding.
Fig. 1.
Flowchart of patients who did not undergo definitive surgical resection.
MDCT Accuracy
One hundred eighty-seven patients underwent definitive curative surgery but 69 patients did not. Of these 69 patients, 17 patients had metastatic disease present on MDCT and underwent a confirmatory exploration or a palliative procedure (i.e., true-positive MDCT). Specifically, eight of these patients underwent only a diagnostic laparotomy, six patients underwent an exploratory laparotomy with biopsy to prove definitively the presence of metastatic disease thought to be equivocal on a preoperative CT scan, and three patients underwent a gastrojejunostomy secondary to gastric outlet obstruction. Twenty-seven patients (of the 69 patients who did not undergo definitive resection) had metastatic disease found unexpectedly at surgery (i.e., false-negative MDCT). In total, 44 (17.2%) patients were found to have metastatic disease at the time of operation (Fig. 1). Twenty-five of the 69 patients who did not undergo definitive resection either were found to have locally advanced unresectable disease at surgery (19 patients) or were planned to undergo palliative therapy (without suspicion for metastatic disease); these cases were also considered to be true-negative cases because no metastases were found at surgery.
Two hundred twelve patients (including 187 patients who underwent definitive resection) had true-negative MDCT findings (with regard to the presence or absence of metastatic disease). MDCT correctly identified the presence or absence of metastatic disease in 250 of 292 scans, yielding an overall accuracy rate for assessing metastatic disease of 85.6%. From the perspective of individual patients, CT correctly predicted the absence of metastatic disease in 229 of 256 patients, for an accuracy of 89.5%. As mentioned already, 27 of 256 patients (10.5%) had metastatic disease unexpectedly found at surgery (i.e., the most recently performed CT scan before surgery incorrectly did not detect metastatic disease).
The accuracy of MDCT (with regard to the presence or absence of metastatic disease) was then evaluated as a function of the interval between the scan and the operation (i.e., “scan age”). The median time between the most recent MDCT and surgery was 15.5 days (range, 1–198 days). No evidence of metastatic disease was identified in 274 of the 292 MDCT examinations (93.8%) in the 256 patients who went on to surgery. MDCT correctly predicted the absence of metastatic disease in 233 (85.0%) of these 274 scans. When the scans were stratified into scan ages of 0–10, 11–20, 21–30, and more than 30 days, the ability to predict the absence of metastatic disease (NPV) was stable, with a range of 85.1–90.6% within the first 30 days after the MDCT study (Table 2). However, the NPV decreased dramatically after 30 days to 72.6% (p = 0.004).
Table 2. Sensitivity, Specificity, Positive Predictive Value (PPV), and Negative Predictive Value (NPV) of MDCT to Detect Metastatic Disease at Different Time Points (10-Day Intervals).
| Parameter | 0–10 Days (n = 86) | 11–20 Days (n = 83) | 21–30 Days (n = 56) | > 30 Days (n = 67) |
|---|---|---|---|---|
|
| ||||
| Sensitivity | 46.7 | 35.3 | 16.7 | 15.0 |
| Specificity | 100 | 100 | 100 | 100 |
| PPV | 100 | 100 | 100 | 100 |
| NPV | 89.9 | 85.1 | 90.6 | 72.6 |
Note—Data are percentages.
When the scan ages were stratified into smaller segments of 5 days, the NPV for metastatic disease of MDCT scans performed up to 5, 10, 15, 20, and 25 days before surgery remained relatively stable, at 90.3%, 89.9%, 87.1%, 87.6%, and 88.6%, respectively. However, for patients with an interval between MDCT and surgery of more than 25 days, the NPV decreased to 76.2% and then decreased even further to 72.6% for patients with an interval of over 30 days (p = 0.004) (Table 3). MDCT was statistically significantly more accurate at predicting the absence of metastatic disease if the study was performed within 25 days of surgery (89.3% vs 77.0%; p = 0.0097).
Table 3. Comparison of AUC for Assessing Sensitivity, Specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV), and Discriminatory Power of MDCT to Detect Metastatic Disease at Different Time Points.
| Time Point (d) | Sensitivity (%) | Specifcity (%) | PPV (%) | NPV (%) | AUC |
|---|---|---|---|---|---|
|
| |||||
| ≤ 5 (n = 34) | 50.0 | 100 | 100 | 90.3 | 0.75 |
| > 5 (n = 258) | 26.9 | 100 | 100 | 84.0 | 0.63 |
| ≤ 10 (n = 86) | 46.7 | 100 | 100 | 89.9 | 0.73 |
| > 10 (n = 206) | 26.3 | 100 | 100 | 82.5 | 0.62 |
| ≤ 15 (n = 128) | 42.3 | 100 | 100 | 87.1 | 0.71 |
| > 15 (n = 206) | 18.7 | 100 | 100 | 82.9 | 0.59 |
| ≤ 20 (n = 169) | 40.6 | 100 | 100 | 87.6 | 0.70 |
| > 20 (n = 123) | 15.4 | 100 | 100 | 80.9 | 0.58 |
| ≤ 25 (n = 203) | 40.0 | 100 | 100 | 88.6 | 0.70 |
| > 25 (n = 89) | 13.0 | 100 | 100 | 76.2 | 0.56 |
| ≤ 30 (n = 225) | 36.8 | 100 | 100 | 88.3 | 0.68 |
| > 30 (n = 67) | 15.0 | 100 | 100 | 72.6 | 0.57 |
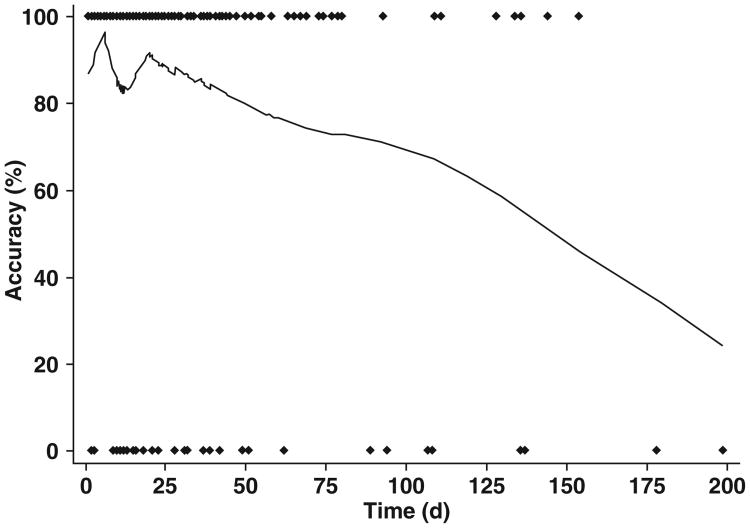
To better understand the declining accuracy in identifying metastatic disease as a function of time, a locally weighted robust regression analysis was performed (Fig. 2). The age of the MDCT studies included in this analysis ranged from 1 to 198 days and included a total of 292 scans. This analysis shows that the accuracy of MDCT remains stable up to 25 days, at which point a steady linear decline in accuracy occurs over the remaining interval (Fig. 2).
Fig. 2.
Locally weighted robust regression analysis of MDCT to determine presence of metastatic disease. Diamonds represent each individual scan, either accurate (top) or inaccurate (bottom), for identification of metastatic disease.
Discussion
Pancreatic adenocarcinoma is a rapidly progressive malignancy characterized by its tendency for early metastasis, direct invasion of adjacent structures and vasculature in the retroperitoneum, and dismal survival rates. The only hope for curative treatment is an R0 resection (margin negative surgical resection with no gross or microscopic tumor remaining in the tumor bed) in patients with completely localized disease, and, unfortunately, most patients are found to harbor metastases or locally invasive tumors at the time of diagnosis, precluding surgical resection. The correct determination of resectability on preoperative imaging and, in particular, the identification of distant metastatic disease are critical. Patients who undergo an aborted curative resection, after the discovery of unexpected metastatic disease at surgery, experience delays in the initiation of chemotherapy, unnecessary exposure to the complications of surgery, and potentially diminished quality of life due to surgical recovery.
Therefore, the primary goal of preoperative imaging evaluation is to select appropriately patients with localized disease who will truly benefit from surgical resection: MDCT serves as the primary diagnostic modality in the preoperative staging of patients with suspected pancreatic cancer. Multiple studies, although they were performed primarily using older-generation scanners, have evaluated the ability of MDCT to stage both local and distant disease in patients with pancreatic adenocarcinoma: studies by Shah et al. [8], Zamboni et al. [9], Karmazanovsky et al. [13], and Smith et al. [14] have reported NPVs for resectability on MDCT ranging between 88.2% and 100%. Reported accuracy in these same studies for resectability ranged between 72% and 90%, with reported NPVs with regard to metastatic disease ranging from 59% to 89%. With respect to metastatic disease, MDCT is relatively sensitive (91%) for liver metastases larger than 1 cm but is less effective in identifying smaller lesions, particularly along the capsular surface of the liver, resulting in an overall sensitivity for liver metastases of only 75% [15]. Similarly, although MDCT can easily identify larger sites of peritoneal carcinomatosis, the sensitivity of MDCT for small implants may be as low as 7–50% [5, 16–18]. Virtually all of these studies were conducted in 2007 or earlier, and accordingly, were performed on older-generation CT scanners (usually 16-MDCT scanners). As a result, it is conceivable that results with the most recent generation of CT scanners might be superior to the results in those studies.
Although these studies have examined the overall accuracy of MDCT with regard to resectability and detection of metastatic disease, to our knowledge, only one other study (in the surgical literature) has attempted to detail the relationship between the accuracy of MDCT and the interval between imaging and surgery. Glant et al. [10] suggested that there was a linear decrease in the accuracy of MDCT for metastatic disease as the time between the imaging study and the MDCT increases. However, notably, that study included a mixture of both CT and MRI examinations, studies were performed at different sites with variable techniques, imaging interpretations were apparently provided by both radiologists and surgeons (both internal and external), and many of the MDCT examinations were performed on older scanner technology dating back to 2004, all of which are factors that potentially limit the wider applicability of that study's results. Nevertheless, it is intuitive, given the aggressive nature of pancreatic adenocarcinoma, that tumors could metastasize during the interval between MDCT evaluation and surgery, particularly when there is a long interval between the two. This is a concept that is well understood by the pancreatic surgeons at our institution; as a result, our surgeons almost always prefer to have an MDCT performed within 2 weeks of a patient's surgery. Given that this change in practice pattern has occurred relatively recently in our institution, this study examined patients from 2006 to 2007, a period in which there was more variability at our hospital in the timing of MDCT relative to surgery.
As the data in this study suggest, the accuracy of a given MDCT examination in predicting the absence of distant metastatic disease at surgery decays over time, with the greatest decline occurring at 25 days after the CT is performed. Although the results of the study by Glant et al. [10] suggested a constant linear decrease in accuracy over time, the results of our study suggest a more complex picture, because accuracy rates appear to be relatively stable for the first 25 days after the imaging test is performed, and the need for reimaging a patient increases significantly after 25 days. The NPVs for metastatic disease of MDCT scans performed less than 5, 10, 15, 20, and 25 days before surgery are 90.3%, 89.9%, 87.1%, 87.6%, and 88.6%, respectively. Similarly the NPVs of patients with intervals of 0–10, 11–20, and 21–30 days were roughly comparable, with NPV values of 89.9%, 85.1%, and 90.6%, respectively. However, for those patients with an interval between MDCT and surgery of more than 25 days, the NPV decreased to 76.2% and then decreased further to 72.6% for patients with an interval of over 30 days (p = 0.004). This decline in MDCT accuracy over time for metastatic disease is confirmed in the locally weighted scatterplot smoothing analysis, which suggests relatively stable accuracy rates for the first 25 days after imaging, after which point there is a steady linear decrease in accuracy.
Nevertheless, some limitations of this study must be acknowledged. First, certainly, one could surmise that these same concepts could equally apply to the locoregional staging of patients, particularly with regard to vascular involvement by tumor. Unfortunately, because of the method of the study, which relied on retrospective review of MDCT and operative dictated reports, temporal changes in involvement of the central mesenteric vasculature could not be judged accurately, and this would certainly be an important component to consider in the overall accuracy of MDCT for tumor resectability.
Second, the reference standard for metastatic disease in this study was surgical exploration, and it is undoubtedly true that there are metastases that are missed at surgery, including deep liver metastases, lung metastases, and subtle carcinomatoses. As a result, the decline over time in MDCT accuracy is almost certainly greater than suggested in this study. Moreover, other imaging modalities (i.e., PET/CT and MRI) are not routinely used for preoperative evaluation of pancreatic cancer at our institution. It is, again, highly likely that these modalities would have identified lesions not visible at laparoscopy or laparotomy and potentially would have increased the rate of decline in MDCT accuracy over time [19–21].
Third, these studies were all acquired on 64-MDCT scanners from a single vendor and do not reflect the most recent scanner technology being used at our institution. It is possible, although unlikely, that performing scans on the latest generation of scanners could have an impact on the detection of metastatic lesions. Nevertheless, there is a wide range of different scanners being used in the community, and 64-MDCT scanners are likely reflective of the standard scanner technology being used at most major pancreatic surgery centers across the country.
Finally, our evaluation of the imaging was based on a review of the MDCT reports produced by a single radiologist, which could have introduced bias. Notably, we did not validate our study results using a second reader, and it is conceivable that the sensitivity and specificity of our single reader may not be reflective of a larger group of radiologists.
It is not rare for logistical reasons to result in a sizeable delay between a patient's initial staging MDCT and definitive surgical therapy, including patients seeking multiple opinions regarding treatment strategies, surgeons' schedules, and patients' own personal reasons. Unfortunately, partly as a result of the lack of supporting data in the literature, obtaining an additional MDCT closer to surgery has until now been quite difficult, because insurance carriers have been reluctant to provide reimbursement for repeat MDCT scans without a medical appeal from the treating physician, thereby placing a potentially large financial burden on the patient. This study provides evidence supporting the need to rescan patients with a long interval between their initial scan and the date of surgery to avoid an unnecessary nontherapeutic laparotomy, and in particular, to perform a CT scan as close in time as possible to the patient's surgery. Although it is still a topic of controversy in the surgical literature, it has been well described that diagnostic laparoscopy has a higher sensitivity for metastatic disease than does CT and can visualize metastases that are not visible on conventional imaging modalities. In cases for which a repeat preoperative CT is absolutely impossible, for either logistic or financial reasons, perhaps a diagnostic laparoscopy immediately before a formal laparotomy for resection might be considered [22]. Taken as a whole, these data strongly suggest that, to have the most accurate distant staging for pancreatic adenocarcinoma, MDCT should be performed within 25 days of any planned definitive therapy, especially surgical exploration with the intent to resect.
Conclusion
MDCT is a highly effective imaging modality for evaluating the resectability of pancreatic adenocarcinoma. However, given the aggressiveness of the tumor and its tendency for rapid metastatic spread, the accuracy of a given MDCT study declines over time. Even though a patient may not have had metastases on a scan performed 1 month before the surgery, there is no guarantee that the patient will be free of metastases at the time of surgery. Given the data in this study, all patients with potentially resectable pancreatic cancer should be imaged within 25 days of a planned resection.
References
- 1.Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–1733. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 2.Potter MW, Shah SA, McEnaney P, Chari RS, Callery MP. A critical appraisal of laparoscopic staging in hepatobiliary and pancreatic malignancy. Surg Oncol. 2000;9:103–110. doi: 10.1016/s0960-7404(01)00005-6. [DOI] [PubMed] [Google Scholar]
- 3.Raman SP, Horton KM, Cameron JL, Fishman EK. CT after pancreaticoduodenectomy: spectrum of normal findings and complications. AJR. 2013;201:2–13. doi: 10.2214/AJR.12.9647. [DOI] [PubMed] [Google Scholar]
- 4.Evans DB, Farnell MB, Lillemoe KD, Vollmer C, Jr, Strasberg SM, Schulick RD. Surgical treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1736–1744. doi: 10.1245/s10434-009-0416-6. [DOI] [PubMed] [Google Scholar]
- 5.Raman SP, Horton KM, Fishman EK. Multimodality imaging of pancreatic cancer: computed tomography, magnetic resonance imaging, and positron emission tomography. Cancer J. 2012;18:511–522. doi: 10.1097/PPO.0b013e318274a461. [DOI] [PubMed] [Google Scholar]
- 6.House MG, Yeo CJ, Cameron JL, et al. Predicting resectability of periampullary cancer with three-dimensional computed tomography. J Gastrointest Surg. 2004;8:280–288. doi: 10.1016/j.gassur.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Klauss M, Schobinger M, Wolf I, et al. Value of three-dimensional reconstructions in pancreatic carcinoma using multidetector CT: initial results. World J Gastroenterol. 2009;15:5827–5832. doi: 10.3748/wjg.15.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah D, Fisher WE, Hodges SE, Wu MF, Hilsenbeck SG, Charles Brunicardi F. Preoperative prediction of complete resection in pancreatic cancer. J Surg Res. 2008;147:216–220. doi: 10.1016/j.jss.2008.02.061. [DOI] [PubMed] [Google Scholar]
- 9.Zamboni GA, Kruskal JB, Vollmer CM, Baptista J, Callery MP, Raptopoulos VD. Pancreatic adenocarcinoma: value of multidetector CT angiography in preoperative evaluation. Radiology. 2007;245:770–778. doi: 10.1148/radiol.2453061795. [DOI] [PubMed] [Google Scholar]
- 10.Glant JA, Waters JA, House MG, et al. Does the interval from imaging to operation affect the rate of unanticipated metastasis encountered during operation for pancreatic adenocarcinoma? Surgery. 2011;150:607–616. doi: 10.1016/j.surg.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 11.Raman SP, Kawamoto S, Blackford A, et al. Histopathologic findings of multifocal pancreatic intraductal papillary mucinous neoplasms on CT. AJR. 2013;200:563–569. doi: 10.2214/AJR.12.8924. [DOI] [PubMed] [Google Scholar]
- 12.Cleveland WS, Devlin S. Locally-weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83:596–610. [Google Scholar]
- 13.Karmazanovsky G, Fedorov V, Kubyshkin V, Kotchatkov A. Pancreatic head cancer: accuracy of CT in determination of resectability. Abdom Imaging. 2005;30:488–500. doi: 10.1007/s00261-004-0279-z. [DOI] [PubMed] [Google Scholar]
- 14.Smith SL, Basu A, Rae DM, Sinclair M. Preoperative staging accuracy of multidetector computed tomography in pancreatic head adenocarcinoma. Pancreas. 2007;34:180–184. doi: 10.1097/01.mpa.0000250133.87520.d9. [DOI] [PubMed] [Google Scholar]
- 15.Horton KM, Fishman EK. Adenocarcinoma of the pancreas: CT imaging. Radiol Clin North Am. 2002;40:1263–1272. doi: 10.1016/s0033-8389(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 16.Tamm EP, Balachandran A, Bhosale PR, et al. Imaging of pancreatic adenocarcinoma: update on staging/resectability. Radiol Clin North Am. 2012;50:407–428. doi: 10.1016/j.rcl.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Tamm EP, Bhosale PR, Vikram R, de Almeida Marcal LP, Balachandran A. Imaging of pancreatic ductal adenocarcinoma: state of the art. World J Radiol. 2013;5:98–105. doi: 10.4329/wjr.v5.i3.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iafrate F, Ciolina M, Sammartino P, et al. Peritoneal carcinomatosis: imaging with 64-MDCT and 3T MRI with diffusion-weighted imaging. Abdom Imaging. 2012;37:616–627. doi: 10.1007/s00261-011-9804-z. [DOI] [PubMed] [Google Scholar]
- 19.van Kessel CS, van Leeuwen MS, van den Bosch MA, et al. Accuracy of multislice liver CT and MRI for preoperative assessment of colorectal liver metastases after neoadjuvant chemotherapy. Dig Surg. 2011;28:36–43. doi: 10.1159/000322390. [DOI] [PubMed] [Google Scholar]
- 20.Chandarana H, Taouli B. Diffusion-weighted MRI and liver metastases. Magn Reson Imaging Clin N Am. 2010;18:451–464. doi: 10.1016/j.mric.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Orlacchio A, Schillaci O, Fusco N, et al. Role of PET/CT in the detection of liver metastases from colorectal cancer. Radiol Med (Torino) 2009;114:571–585. doi: 10.1007/s11547-009-0393-7. [DOI] [PubMed] [Google Scholar]
- 22.Allen VB, Gurusamy KS, Takwoingi Y, Kalia A, Davidson BR. Diagnostic accuracy of laparoscopy following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer. Cochrane Database Syst Rev. 2013;11:CD009323. doi: 10.1002/14651858.CD009323.pub2. [DOI] [PubMed] [Google Scholar]