Abstract
We report data from the final analysis of the Chinese subset of the GIDEON (the Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib) study, which evaluated the safety and efficacy of sorafenib in Child-Pugh A, B and C patients with unresectable hepatocellular carcinoma (uHCC) in real-life clinical practice. Patient demographics, disease characteristics and treatment history were recorded at enrollment; dose, adverse events (AEs) and efficacy were recorded at follow-up. Of the 338 evaluable patients, 98.5% started on 800 mg/day sorafenib, regardless of their Child-Pugh status. The median treatment duration (21.1 vs. 18.8 weeks) and median overall survival (322 vs 240 days) were longer in patients with Child-Pugh A compared with the Child-Pugh B, progression-free survival were 183 vs. 208 days, respectively). AEs (all grades) were comparable in the Child-Pugh B vs A group (56.3% vs. 50.4%, respectively), moreover, the Child-Pugh B group also had comparable rates of drug-related AEs (35.4% vs. 27.2%, respectively) and serious AEs (25.0% vs. 23.0%, respectively) compared with the Child-Pugh A group. The overall dosing strategy was consistent in Chinese patients across Child-Pugh subgroups. Tolerability and safety data suggest that Child-Pugh B patients might be safely treated with sorafenib. The findings from our study showed that safety profile of sorafenib in terms of rate and type of AEs is similar to the global international GIDEON study as well as other pivotal studies.
Keywords: sorafenib, unresectable hepatocellular carcinoma, GIDEON, Child-Pugh, Chinese subset
INTRODUCTION
Primary liver cancer (PLC) represents the sixth most common malignancy worldwide and is the third leading cause of cancer-related deaths [1]. Hepatocellular carcinoma (HCC) is the most common type of PLC. Globally, ∼75% of the deaths occurring due to HCC worldwide is expected to rise, due partly to the increasing incidence of hepatitis C [2]. Owing to its large population China has a very high prevalence of PLCs, contributing to more than 50% of all PLCs worldwide, with estimated cases of 293,000 in males and 101,000 in females, as reported in GLOBOCAN 2012 [3, 4]. Data from various studies have reflected that China has the highest prevalence of hepatitis B virus (HBV) carriers in the world and an increasing trend in the rate of hepatitis C virus (HCV) infection. All these factors contribute to a heavy disease burden in China. Although healthcare advancements have been noted in the management of HCC, there has not been any considerable reduction in the mortality over the past few decades. The Ministry of Health of China has also taken several measures to control the disease, but it still remains a health issue of major concern [4–8].
Most HCC patients present unresectable disease (uHCC), wherein surgery is contraindicated. Moreover, survival benefits obtained from medical interventions are controversial. Nonsurgical loco-regional treatment options, such as image-guided ablation and transcatheter arterial chemoembolization (TACE) or use of 90Y microspheres (TheraSphere), have limited applicability and may lead to high rates of recurrence [9,10]. Despite the magnitude of the burden of uHCC patients with uHCC still have poor prognosis and limited treatment options. Therefore, further studies are warranted in the field of management of uHCC in terms of treatment morbidity, mortality, and survival.
Sorafenib is an orally bioavailable multitargeted tyrosine kinase inhibitor with potential antiangiogenic and antiproliferative properties that acts by blocking a number of protein kinases[11, 12]. Based on the promising results observed in several clinical trials, sorafenib has been suggested as a first-line therapy in patients with uHCC patients for several years [13–17], and, was approved by the China Food and Drug Administration in 2008 [18]. Two placebo-controlled, Phase III studies (SHARP and Asia-Pacific) acted as milestones in the treatment of patients with uHCC (Child-Pugh A), revealing that sorafenib caused significant improvements in overall survival (OS) [5, 19]. Yao et al, in an analysis concluded that discontinuation of sorafenib treatment for a long period can lead to uHCC disease progression, and, appropriate management of sorafenib-related AEs could result in better clinical outcomes in patients with HCC [20].
GIDEON (the Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib) is a global, international, prospective, non-interventional study undertaken to fulfill post-approval commitments to licensing agencies for compiling a large, more comprehensive, and robust database of HCC treatment patterns and outcomes among patients with advanced disease [21]. The GIDEON trial evaluated the safety and efficacy of sorafenib in uHCC patients under real-life clinical practice conditions, including patients with Child-Pugh B liver function as well, who were usually excluded from randomized clinical trials [21]. Being one of the largest studies undertaken in patients with uHCC, GIDEON allows the conduct of multiple predefined sub-analyses, focusing on potentially predictive or prognostic factors, including the Child-Pugh score, staging systems, etiology, and prior or concomitant TACE. Results from these sub-analyses would be useful to analyze differences that would influence prognosis and management of HCC, and, thus, will aid physicians in effectively treating HCC to improve patient outcomes [22–25]. Here we report the final analysis of safety and efficacy of sorafenib treatment in the Chinese subset of GIDEON study.
RESULTS
Patient disposition and baseline characteristics by Child-Pugh status
During January 2009 and April 2012, a total of 3371 patients from 39 countries of 5 regions were included in the GIDEON study (NCT00812175). The Chinese subset included a total of 345 patients, of which, 338 patients (92.3% were male) were included in the ITT population. The safety (SAF) population included 331 patients. At the start of sorafenib therapy, 74.0% of patients (median age: 50 years) had Child-Pugh A status, 15.1% of patients (median age: 52 years) had Child-Pugh B status, and 0.6% of patients (median age: 58 years) had Child-Pugh C status. Majority of the patients belonged to Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 (Child-Pugh A: 90%; Child-Pugh B: 66.7%; Child-Pugh C: 50%). Tumor node metastasis (TNM) stage IV was more prominent in Child-Pugh A (41.2%) patients, whereas most of the Child-Pugh B and C patients reported TNM stage III (51% vs. 50%, respectively). Barcelona Clinic Liver Cancer (BCLC) stage C was prominently noted in the majority of the patients (63.2% of patients with Child-Pugh A and 68.6% with Child-Pugh B). Baseline characteristics by Child-Pugh status are summarized in Table 1.
Table 1. Baseline characteristics by Child-Pugh status.
| Child-Pugh A | Child-Pugh B | Child-Pugh C | Total* | |
|---|---|---|---|---|
| Number of patients (%) | 250 (74.0) | 51 (15.1) | 2 (0.6) | 338(100) |
| Median age (years) | 50.0 | 52.0 | 58.0 | 50.0 |
|
Gender Male (%) Female (%) |
231 (92.4) 19 (7.6) |
47 (92.2) 4 (7.8) |
2 (100) 0 (0) |
312 (92.3) 26 (7.7) |
|
ECOG PS (%) 0 or 1 ≥2 |
225 (90) 25 (10) |
34 (66.7) 17 (33.3) |
1 (50) 1 (50) |
286 (84.6) 52 (15.4) |
|
TNM stage (%) I II III IV |
13 (5.2) 28 (11.2) 88 (35.2) 103 (41.2) |
0 (0) 2 (3.9) 26 (51.0) 19 (37.3) |
0 (0) 0 (0) 1 (50) 1 (50) |
13 (3.8) 32 (9.5) 124 (36.7) 137 (40.5) |
|
BCLC stage (%) A B C D |
15 (6.0) 49 (19.6) 158 (63.2) 10 (4.0) |
1 (2.0) 8 (15.7) 35 (68.6) 4 (7.8) |
0 (0) 0 (0) 0 (0) 2 (100) |
18 (5.3) 59 (17.5) 210 (62.1) 18 (5.3) |
BCLC, Barcelona Clinic Liver Cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; TNM, tumor node metastasis.
Includes 35 non-evaluable patients.
Sorafenib dosing and duration
Overall, the majority of patients received the recommended initial dose of 800 mg (98.5%); the median daily dose was 800 mg. These were consistent across all Child-Pugh subgroups. The duration of treatment was slightly longer in Child-Pugh A (21.1 weeks) patients than in Child-Pugh B patients (18.8 weeks) (Table 2).
Table 2. Sorafenib administration by Child-Pugh status.
| Child-Pugh A (N=250) |
Child-Pugh B (N=51) |
Child-Pugh C (N=2) |
Total* (N=338) |
|
|---|---|---|---|---|
| Initial dose of 800 mg (%) | 245 (98.0) | 51 (100.0) | 2 (100.0) | 333 (98.5) |
| Initial dose of 400 mg (%) | 5 (2.0) | 0 | 0 | 5 (1.5) |
| Median daily dose (mg) | 800 | 800 | 800 | 800 |
| Median treatment duration (weeks) | 21.1 | 18.8 | 63.3 | 21.1 |
Includes 35 non-evaluable patients.
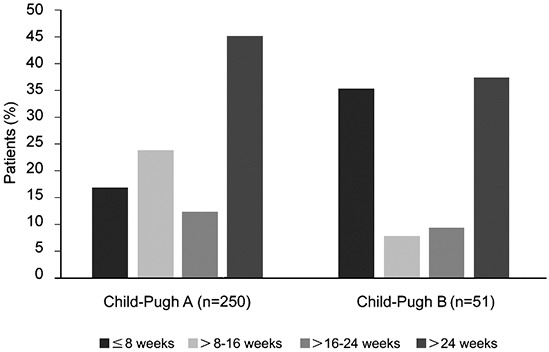
The majority of patients in the Child-Pugh B group received sorafenib for <8 weeks. However, a considerable percentage of patients also received sorafenib for >24 weeks, though the number was fewer than that in the Child-Pugh A group. The percentages of patients treated for >28 weeks in the Child-Pugh A and B groups were 40.8% and 31.4%, respectively (Figure 1).
Figure 1. Duration of treatment by Child-Pugh status.
Safety assessment
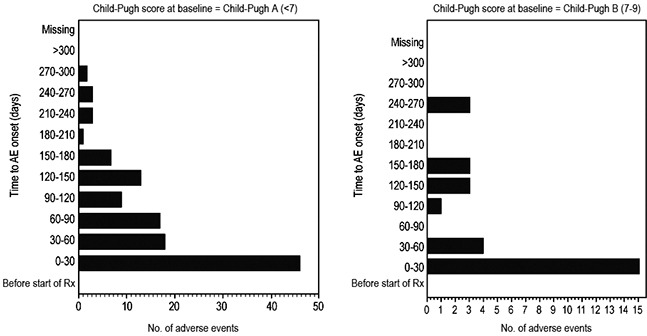
Based on the SAF dataset, a comparison of the incidence of AEs and serious AEs in the Child-Pugh A and B subgroups is presented in Table 3A. No AEs were observed in the Child-Pugh C group. AEs (all grades) were comparable in the Child-Pugh B group compared with the Child-Pugh A group (56.3% vs. 50.4%, respectively). Similarly, Child-Pugh B group also had comparable rates of drug-related AEs (35.4% vs. 27.2%, respectively) and serious AEs (25.0% vs. 23.0%, respectively) as compared with the Child-Pugh A group. The most common drug-related AEs overall were diarrhea, hand-foot skin reaction (HFSR), and rash/desquamation. No unexpected AEs were observed in patients with liver dysfunction (Table 3B). The incidence of drug-related HFSR was higher in the Child-Pugh A group than in the Child-Pugh B group (20.7% vs 14.6%, respectively), although incidence of diarrhea was higher in the Child-Pugh B group than in the Child-Pugh A group (14.6% vs 10.2%). The rates of drug-related AEs, calculated as the number of events per patient-year, in the Child-Pugh A and B groups are presented in Table 3C. The time to onset of AEs greater than grade 1 in the Child-Pugh A group was comparable to that in the Child-Pugh B group, with the majority of AEs occurring within the first 30 days of treatment in both groups (Figure 2). Overall, comparable rates of treatment-emergent AEs were reported in Child-Pugh B and Child-Pugh A patients. Discontinuation of treatment due to AEs was slightly more common in Child-Pugh B than in Child-Pugh A patients (10.4% vs 8.9%). Also, the number of treatment-emergent deaths occurring up to 30 days after the last sorafenib dose was comparable in Child-Pugh B patients and Child-Pugh A patients.
Table 3A. Treatment-emergent adverse events (AEs) by Child-Pugh status.
| % | Child-Pugh A (N=246) | Child-Pugh B (N=48) | Child-Pugh C (N=2) | Total* (N=331) |
|---|---|---|---|---|
| AEs (all grades) | 124 (50.4) | 27 (56.3) | 0 | 167 (50.5) |
| Drug-related AEs (all grades) | 67 (27.2) | 17 (35.4) | 0 | 95 (28.7) |
| Serious AEs (all grades)** | 58 (23.6) | 12 (25.0) | 0 | 77 (23.3) |
| Drug-related serious AEs (all grades) | 0 | 1 (2.1) | 0 | 1 (0.3%) |
| All grade 3 or 4 | 14 (5.7) | 4 (8.3) | 0 | 20 (6.0) |
| Drug-related grade 3 or 4 | 9 (3.7) | 2 (4.2) | 0 | 12 (3.6) |
| AEs resulting in permanent discontinuation of sorafenib | 22 (8.9) | 5 (10.4) | 0 | 29 (8.8) |
| Deaths*** | 127 (51.6) | 27 (56.3) | 0 | 166 (50.2) |
Includes 35 non-evaluable patients.
Any AE occurring at any dose that results in any of the following consequences: death; life-threatening conditions; hospitalization or prolongation of existing hospitalization; persistent or significant disability/incapacity; congenital anomaly/birth defect; and medically important event.
Treatment-emergent deaths occurring up to 30 days after the last sorafenib dose.
Table 3B. Drug-related adverse events by Child-Pugh status.
| % | Child-Pugh A (N=246) | Child-Pugh B (N=48) | Child-Pugh C (N=2) |
|---|---|---|---|
| Diarrhea | 25 (10.2) | 7 (14.6) | 0 |
| HFSR | 51 (20.7) | 7 (14.6) | 0 |
| Fatigue | 1 (0.4) | 0 | 0 |
| Rash/desquamation | 8 (3.3) | 3 (6.3) | 0 |
| Hypertension | 7 (2.8) | 1 (2.1) | 0 |
| Alopecia | 6 (2.4) | 2 (4.2) | 0 |
| Nausea | 0 | 1 (2.1) | 0 |
| Pain, abdomen NOS | 2 (0.8) | 0 | 0 |
HFSR, hand-foot skin reaction; NOS, not otherwise specified
Table 3C. Rate of drug-related adverse events (AEs) by Child-Pugh status.
| Rate (events per patient-year*) | Child-Pugh A (N=246) |
Child-Pugh B (N=48) | Child-Pugh C (N=2) | Total** (N=331) |
|---|---|---|---|---|
| Any AE | 0.43 | 0.64 | 0 | 0.46 |
| Diarrhea | 0.16 | 0.26 | 0 | 0.17 |
| HFSR | 0.33 | 0.26 | 0 | 0.30 |
| Liver dysfunction | 0 | 0 | 0 | 0 |
Rate calculation based on treatment-emergent AEs with .10% incidence and 365.25 days per year.
Includes 35 non-evaluable patients.
HFSR, hand-foot skin reaction.
Figure 2. Time to onset of adverse events greater than grade 1 by Child-Pugh status.
Efficacy outcomes
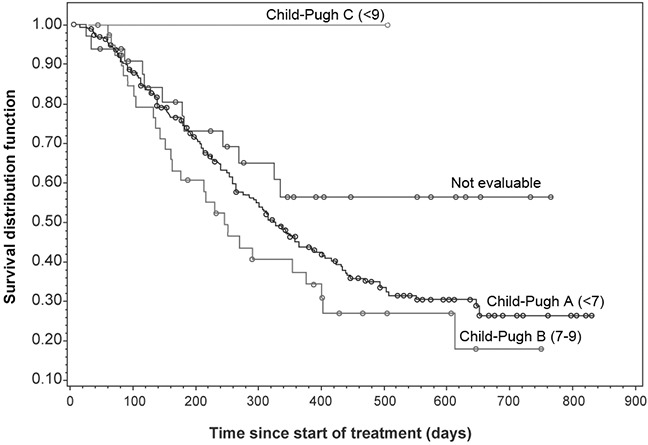
Median OS was 322 days in the Chinese subset (Figure 3). The OS was longer in Child-Pugh A patients than in Child-Pugh B patients (Table 4, Figure 4). Child-Pugh B patients showed a similar progression-free survival (PFS) to that of Child-Pugh A patients. Median time to progression (TTP) could not be compared between subgroups due to the high censoring rate in the Child-Pugh B and C groups.
Figure 3. Overall survival in the Chinese subset.
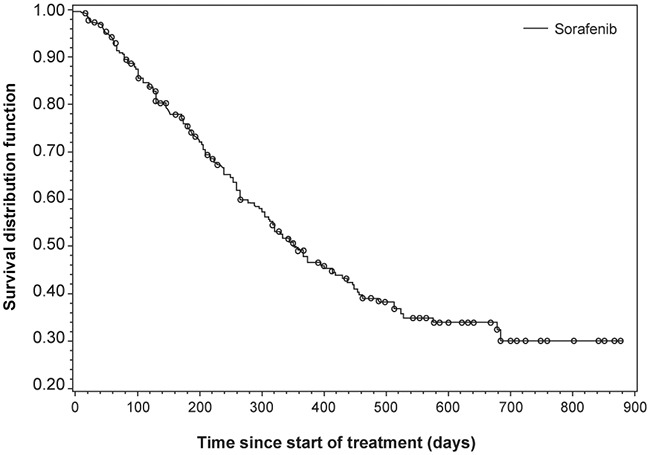
Table 4. Survival outcomes by Child-Pugh status.
| Child-Pugh A | Child-Pugh B | Child-Pugh C | |
|---|---|---|---|
|
Overall survival Number failed Number censored Median (days) |
127 (50.8%) 123 (49.2%) 322 |
27 (52.9%) 24 (47.1%) 240 |
– 2 (100%) – |
|
Progression-free survival Number failed Number censored Median (days) |
173 (69.2%) 77 (30.8%) 183 |
31 (60.8%) 20 (39.2%) 208 |
– 2 (100%) – |
|
Time to progression Number failed Number censored Median (days) |
85 (34.0%) 165 (66.0%) 214 |
4 (7.8%) 47 (92.2%) – |
– 2 (100%) – |
Figure 4. Overall survival by Child-Pugh score.
DISCUSSION
Previously conducted large clinical trials enrolled Child-Pugh class A patients, and thus, the viability of sorafenib treatment in patients with uHCC and poor liver function was largely unknown[5,19]. GIDEON is of considerable clinical interest and relevance, as the study provides an opportunity to assess a wider patient population as compared with randomized clinical trials. Observations from this study will aid physicians in effectively treating HCC and improving patient outcomes. To our knowledge, GIDEON is amongst the largest study undertaken in patients with HCC involving 3371 patients from 39 countries of 5 regions [21].
The results of the Chinese subset of GIDEON reveal that patient demographics in China are largely in line with the previous global GIDEON report in terms of gender, staging, Child-Pugh status, and performance status [25]. However, in our study, patients appeared to be younger, and most of them belonged to BCLC Stage C (62.1%), in whom sorafenib could be considered an appropriate treatment option [26].
Several studies have indicated that sorafenib is beneficial in Child-Pugh B patients, with an acceptable safety profile [25, 27–32]. In our study, Child-Pugh status did not appear to influence the approach to sorafenib dosing. A considerable percentage of Chinese patients in the Child-Pugh B group received sorafenib for >28 weeks. In contrast to the global report, almost all Chinese patients received the recommended initial daily dose of sorafenib (800 mg/day), suggesting that sorafenib was well tolerated in Chinese patients. Notably, the median treatment duration was 63.3 weeks in 2 Chinese Child-Pugh C patients, which might imply that patients with liver dysfunction could still receive sorafenib therapy. However, the extremely small sample size in this subgroup does not allow us to draw a definite conclusion and hence more studies are warranted.
With the exception that fatigue was not a common AE in Chinese patients, the safety profile was largely consistent with that in the global report, with diarrhea, HFSR, and rash/desquamation being the most commonly reported drug-related AEs. Although the Child-Pugh B group had most of the drug-related AEs, the Child-Pugh A group had more HFSR cases. Comparable rates of treatment-emergent AEs were reported among Child-Pugh B and Child-Pugh A patients. Discontinuation of treatment due to AEs was slightly more common in Child-Pugh B than in Child-Pugh A patients (10.4% vs 8.9%). The majority of AEs greater than grade 1 occurred within the first 30 days of treatment in both Child-Pugh A and B groups. Overall, most commonly reported drug-related AEs across Child-Pugh subgroups were comparable in both type and incidence.
Consistent with previous reports, OS was longer in Child-Pugh A patients compared with Child-Pugh B or C patients; suggesting that Child-Pugh status is a strong prognostic indicator of OS in uHCC patients, regardless of treatment with sorafenib. PFS was similar between Child-Pugh A and B patients. As shown in Table 4, many patients in the Child-Pugh B (TTP analysis) and C (OS, PFS, and TTP analyses) groups may have been lost to follow-up. These findings are in line with the previously conducted SHARP and Asia-Pacific study. The SHARP trial enrolled 602 patients and showed prolonged median OS (10.7 vs. 7.9 months; P<.001) and prolonged time to radiological disease progression (5.5 vs. 2.8 months; P<.001) for sorafenib compared with placebo [19]. Similarly, the Asia-Pacific study demonstrated that sorafenib had a substantially longer median OS (6.4 vs 4.2 months) and median TTP (2.8 vs 1.4 months) over placebo [5].
Our study has certain limitations. First, being an observational study, it is subjected to known and unknown confounders and biases. Second, lack of randomization and a placebo or a comparative group may change the study outcomes. Third, statistical limitation of our data must be considered before drawing any definitive conclusion. Owing to all the above factors, it becomes mandatory that the implications of this observational study must be interpreted with caution. Further prospective trials are much needed to evaluate the efficacy and safety of sorafenib in Child-Pugh class subgroups, particularly in those patients with less compromised liver function. Nevertheless, the primary strength of our analysis to observe both practice patterns and the safety of an intervention in real-life practice should not be ignored.
The GIDEON study has now been completed, fulfilling a licensing commitment to assess the safety of sorafenib in clinical practice. Our study results suggest that sorafenib at 800 mg/day appears to be safe and better tolerated in Chinese clinical setting. Child-Pugh status was found to be a strong prognostic indicator of OS in uHCC patients. Tolerability and safety data suggest that Child-Pugh B patients might be safely treated with sorafenib. The findings from our study showed that safety profile of sorafenib in terms of rate and type of AEs is similar to GIDEON study, in Chinese uHCC patients with Child-Pugh B, as well other pivotal GIDEON subset studies [21, 33, 34].
MATERIALS AND METHODS
Study design
GIDEON was the largest, international, prospective, open-label, non-interventional study that presented data from a global perspective. Design and rationale of the GIDEON study have already been published by Lencioni et al [21]. As a non-interventional study, assignment to a particular therapeutic strategy was not mandated by the study protocol but imitated the current practice of participating physicians. The decision to include a patient in the study was separate from the treatment decision. The final analysis was conducted upon completion of the 12-month follow-up assessment [21].
Study objectives
The primary objective was to evaluate the safety of sorafenib under real-life clinical setting; secondary objectives included evaluation of efficacy (OS, PFS, TTP, response rate, and rate of stable disease) and duration of therapy; regional and global methods of patient evaluation, diagnosis, and follow-up; evaluation of comorbidities and their influence on treatment and outcome; and evaluation of practice patterns of the physicians involved in the care of these patients. Computed tomography (CT) and other radiographic methods were used by the physicians for assessing the tumor at baseline, at follow-up visits, or at a frequency determined by the treating physician.
Patient population
The inclusion criteria were similar to that of the principal GIDEON trial [21]. Patients were eligible for participating in GIDEON if they were ≥18 years of age; were diagnosed histologically, cytologically, or radiographically with uHCC; had a life expectancy of >8 weeks; had not undertaken previous sorafenib treatment; were candidates for systemic therapy; and in whom the decision to be treated with sorafenib had been made by their physician (radiographic diagnosis required the typical observations of HCC by a radiographic method, i.e., multidimensional dynamic CT, CT hepatic arteriography/CT arterial portography, or MRI). Moreover, the patients' physicians must be willing to duly complete and submit all case report forms and must be ready to submit to a site audit with verification of the source documents along with all the other related records/testimonials and validation of the reported data. Exclusion criteria were based on the local product information for sorafenib.
Informed consent
Signed patient consent form and eligibility criteria form were completed for all patients who were included in the study and were approved by local Ethics Review Board. All patients' data were kept anonymous. All the participants received a written explanation of the study and provided written informed consent before participating.
Ethical considerations
This study followed the ethical principles approved by the local institutional review board, ethics committees, and health authorities according to Good Clinical Practice and local laws and regulations in China, as well as established recommendations and regulations pertaining to non-interventional and post-authorization safety studies [26]. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Data collection
Baseline characteristics were recorded, and information regarding AEs, dosing, and outcomes was collected during follow-up visits. AEs and other safety parameters, including blood pressure, Child-Pugh grade, and ECOG PS, were summarized using the safety population. All data were collected using case report forms at study entry and at the initiation of sorafenib treatment, and then at intervals normally decided by the prescribing physician (usually ≥6 weeks or ≥12 weeks), or until patient's death, withdrawal, or loss to follow-up, or if significant changes in a patient's disease were observed [17]. Analyses of the final data were conducted to evaluate dosing patterns, AEs, and outcomes [21].
Statistical analyses
Patients who received at least one dose of sorafenib and underwent at least one follow-up assessment were evaluable for safety and efficacy. All AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. Descriptive statistics, such as frequency, proportion, mean, median, standard deviation, 95% confidence intervals, and quartiles, were calculated for all baseline, safety, and efficacy data. Kaplan–Meier estimates were calculated for the OS, PFS, and TTP efficacy end points.
The recruitment number was based on an overall sample of 3000 patients, a number considered sufficient for comprehensive evaluation of safety for the overall population, as well as planned subgroup analyses [13]. No P-values are presented as all data are summarized using descriptive statistics.
Acknowledgments
The authors would like to acknowledge Dr. Amit Bhat and Mrs. Nishi Gupta (Indegene Lifesystems Pvt. Ltd., Bangalore), for providing medical writing assistance in the preparation of the manuscript, with funding from Bayer China. All the authors planned this study and contributed to interpretation of the data, revisions, and inputs at all stages of the study. All the authors have approved the final version of the manuscript.
Footnotes
CONFLICTS OF INTEREST
The authors have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship, and contributorship, as well as compliance with ethical requirements with respect to treatment of human and animal test subjects. All the authors–Sheng-Long Ye, Xiaoping Chen, Jiamei Yang, Ping Bie, Shuijun Zhang, Fengyong Liu, Luming Liu, Jie Zhou, Kefeng Dou, Chunyi Hao, Guoliang Shao, Qiang Xia, Yajin Chen, Jijin Yang, Xiaxing Deng, Yunpeng Liu, Yunfei Yuan, Zhiren Fu, Keiko Nakajima, Christina Sm Yip, and Zhengguang LU–declared that they have no conflicts of interest to disclose.
GRANT SUPPORT
The GIDEON study is funded by Bayer HealthCare Pharmaceuticals and Onyx Pharmaceuticals.
REFERENCES
- 1.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Facts and Figures 2015 American Cancer Society. 2015 Available from: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf Accessed Jul 31, 2015.
- 3.Liver Cancer Estimated Incidence, Mortality and Prevalence Worldwide in 2012 GLOBOCAN 2012, World Health Organization Website. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx Accessed August 19, 2015.
- 4.Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42:S206–S214. doi: 10.1016/S1590-8658(10)60507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka M, Katayama F, Kato H, Tanaka H, Wang J, Qiao YL, Inoue M. Hepatitis B and C virus infection and hepatocellular carcinoma in China: a review of epidemiology and control measures. J Epidemiol. 2011;21:401–416. doi: 10.2188/jea.JE20100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15:5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 8.Wu Q, Qin SK. Features and treatment options of Chinese hepatocellular carcinoma. Chin Clin Oncol. 2013;2:38. doi: 10.3978/j.issn.2304-3865.2013.09.07. [DOI] [PubMed] [Google Scholar]
- 9.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 11.Smalley KS, Xiao M, Villanueva J, Nguyen TK, Flaherty KT, Letrero R, Van BP, Elder DE, Wang Y, Nathanson KL, Herlyn M. CRAF inhibition induces apoptosis in melanoma cells with non-V600E BRAF mutations. Oncogene. 2009;28:85–94. doi: 10.1038/onc.2008.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PubMed] [Google Scholar]
- 13.Berretta M, Di BF, Dal ML, Cacopardo B, Nasti G, Facchini G, Bearz A, Spina M, Garlassi E, De R V, Fiorica F, Lleshi A, Tirelli U. Sorafenib for the treatment of unresectable hepatocellular carcinoma in HIV-positive patients. Anticancer Drugs. 2013;24:212–218. doi: 10.1097/CAD.0b013e32835c032f. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furuse J. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Biologics. 2008;2:779–788. doi: 10.2147/btt.s3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Wang L, Cong N, Shi C, Bu W, Song J, Chen H. Efficacy of Sorafenib for Advanced Hepatocellular Carcinoma and Prognostic Factors. Hepatogastroenterology. 2014;61:954–957. [PubMed] [Google Scholar]
- 17.Ozenne V, Paradis V, Pernot S, Castelnau C, Vullierme MP, Bouattour M, Valla D, Farges O, Degos F. Tolerance and outcome of patients with unresectable hepatocellular carcinoma treated with sorafenib. Eur J Gastroenterol Hepatol. 2010;22:1106–1110. doi: 10.1097/MEG.0b013e3283386053. [DOI] [PubMed] [Google Scholar]
- 18.Guidelines for the diagnosis and treatment of primary liver cancer (2011 edition). Ministry of Health of China 2011. Chin Clin Oncol. 2012;1:10. doi: 10.3978/j.issn.2304-3865.2012.07.01. [DOI] [PubMed] [Google Scholar]
- 19.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 20.Yao X, Yan D, Liu D, Zeng H, Li H. Efficacy and adverse events of transcatheter arterial chemoembolization in combination with sorafenib in the treatment of unresectable hepatocellular carcinoma. Mol Clin Oncol. 2015;3:929–935. doi: 10.3892/mco.2015.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lencioni R, Marrero J, Venook A, Ye SL, Kudo M. Design and rationale for the non-interventional Global Investigation of Therapeutic DEcisions in Hepatocellular Carcinoma and Of its Treatment with Sorafenib (GIDEON) study. Int J Clin Pract. 2010;64:1034–1041. doi: 10.1111/j.1742-1241.2010.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daniele B, Ratziu V, Stal PI, Serejo F, Bodoky G, Croitoru AE, et al. Final analysis of European patients from the Global Investigation of Therapeutic Decisions in Hepatocellular Carcinoma and of its Treatment with Sorafenib (GIDEON) study: Baseline characteristics and staging systems. 2014 ASCO Annual Meeting 2015; Available from: http://meetinglibrary.asco.org/content/127583-144. Accessed Aug 1, 2015. [Google Scholar]
- 23.Lencioni R, Kudo M, Ye SL, Bronowicki JP, Chen XP, Dagher L, Furuse J, Geschwind JF, Ladron de GL, Papandreou C, Sanyal AJ, Takayama T, Yoon SK, et al. First interim analysis of the GIDEON (Global Investigation of therapeutic decisions in hepatocellular carcinoma and of its treatment with sorafeNib) non-interventional study. Int J Clin Pract. 2012;66:675–683. doi: 10.1111/j.1742-1241.2012.02940.x. [DOI] [PubMed] [Google Scholar]
- 24.Lencioni R, Kudo M, Ye SL, Bronowicki JP, Chen XP, Dagher L, Furuse J, Geschwind JF, de Guevara LL, Papandreou C, Takayama T, Yoon SK, Nakajima K, et al. GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib): second interim analysis. Int J Clin Pract. 2014;68:609–617. doi: 10.1111/ijcp.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marrero JA, Lencioni R, Ye SL, Kudo M, Bronowicki JP, Chen XP, et al. Final analysis of GIDEON (Global Investigation of Therapeutic Decisions in Hepatocellular Carcinoma [HCC] and of Its Treatment with Sorafenib [Sor]) in >3000 Sor-treated patients (pts): Clinical findings in pts with liver dysfunction. 2013 ASCO Annual Meeting 2015; Available from: http://meetinglibrary.asco.org/content/116738-132. Accessed Aug 1, 2015. [Google Scholar]
- 26.European Medical Agency; 2007. EUDRALEX : The rules governing medicinal products in the European Union. Available from: http://ec.europa.eu/enterprise/newsroom/cf/itemdetail.cfm?&item_id=897. Accessed Jun 1, 2014. [Google Scholar]
- 27.Hollebecque A, Cattan S, Romano O, Sergent G, Mourad A, Louvet A, Dharancy S, Boleslawski E, Truant S, Pruvot FR, Hebbar M, Ernst O, Mathurin P. Safety and efficacy of sorafenib in hepatocellular carcinoma: the impact of the Child-Pugh score. Aliment Pharmacol Ther. 2011;34:1193–1201. doi: 10.1111/j.1365-2036.2011.04860.x. [DOI] [PubMed] [Google Scholar]
- 28.Iavarone M, Cabibbo G, Piscaglia F, Zavaglia C, Grieco A, Villa E, Camma C, Colombo M. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology. 2011;54:2055–2063. doi: 10.1002/hep.24644. [DOI] [PubMed] [Google Scholar]
- 29.Kim JE, Ryoo BY, Ryu MH, Chang HM, Suh DJ, Lee HC, Lim YS, Kim KM, Kang YK. Sorafenib for hepatocellular carcinoma according to Child-Pugh class of liver function. Cancer Chemother Pharmacol. 2011;68:1285–1290. doi: 10.1007/s00280-011-1616-x. [DOI] [PubMed] [Google Scholar]
- 30.Pinter M, Sieghart W, Graziadei I, Vogel W, Maieron A, Konigsberg R, Weissmann A, Kornek G, Plank C, Peck-Radosavljevic M. Sorafenib in unresectable hepatocellular carcinoma from mild to advanced stage liver cirrhosis. Oncologist. 2009;14:70–76. doi: 10.1634/theoncologist.2008-0191. [DOI] [PubMed] [Google Scholar]
- 31.Pressiani T, Boni C, Rimassa L, Labianca R, Fagiuoli S, Salvagni S, Ferrari D, Cortesi E, Porta C, Mucciarini C, Latini L, Carnaghi C, Banzi M, et al. Sorafenib in patients with Child-Pugh class A and B advanced hepatocellular carcinoma: a prospective feasibility analysis. Ann Oncol. 2013;24:406–411. doi: 10.1093/annonc/mds343. [DOI] [PubMed] [Google Scholar]
- 32.Worns MA, Weinmann A, Pfingst K, Schulte-Sasse C, Messow CM, Schulze-Bergkamen H, Teufel A, Schuchmann M, Kanzler S, Duber C, Otto G, Galle PR. Safety and efficacy of sorafenib in patients with advanced hepatocellular carcinoma in consideration of concomitant stage of liver cirrhosis. J Clin Gastroenterol. 2009;43:489–495. doi: 10.1097/MCG.0b013e31818ddfc6. [DOI] [PubMed] [Google Scholar]
- 33.Daniele B, Croitoru A, Papandreou C, Bronowicki JP, Mathurin P, Serejo F, Stål P, Turnes J, Ratziu V, Bodoky G. Impact of sorafenib dosing on outcome from the European patient subset of the GIDEON study. Future Oncol. 2015;11:2553–2562. doi: 10.2217/fon.15.163. [DOI] [PubMed] [Google Scholar]
- 34.Turnes J, Díaz R, Hernandez-Guerra M, Gómez M, Castells L, Bustamante J, Espinosa MD, Fernández-Castroagudín J, Serrano T, Rendón P, Andrade R, Salgado M, Arenas J, et al. Therapeutic decisions in the treatment of hepatocellular carcinoma and patterns of sorafenib use. Results of the international observational GIDEON trial in Spain. Gastroenterol Hepatol. 2015;38:263–273. doi: 10.1016/j.gastrohep.2014.11.001. [DOI] [PubMed] [Google Scholar]


