Abstract
Survivin overexpression is associated with poor prognosis of human gastric cancer, and is a target for gastric cancer therapy. YM155 is originally identified as a specific inhibitor of survivin. In this study, we investigated the antitumor effect of YM155 on human gastric cancer. Our results showed that YM155 treatment significantly inhibited cell proliferation, reduced colony formation and induced apoptosis of gastric cancer cells in a dose-dependent manner. Accordingly, YM155 treatment significantly decreased survivin expression without affecting XIAP expression and increased the cleavage of apoptosis-associated proteins caspase 3, 7, 8, 9. YM155 significantly inhibited sphere formation of gastric cancer cells, suppressed expansion and growth of the formed spheres (cancer stem cell-like cells, CSCs) and downregulated the protein levels of β-catenin, c-Myc, Cyclin D1 and CD44 in gastric cancer cells. YM155 infusion at 5 mg/kg/day for 7 days markedly inhibited growth of gastric cancer xenograft in a nude mouse model. Immunohistochemistry staining and Western Blot showed that YM155 treatment inhibited expression of survivin and CD44, induced apoptosis and reduced CD44+ CSCs in xenograft tumor tissues in vivo. No obvious pathological changes were observed in organs (e.g. heart, liver, lung and kidney) in YM155-treated mice. Our results demonstrated that YM155 inhibits cell proliferation, induces cell apoptosis, reduces cancer stem cell expansion, and inhibits xenograft tumor growth in gastric cancer cells. Our results elucidate a new mechanism by which YM155 inhibits gastric cancer growth by inhibition of CSCs. YM155 may be a promising agent for gastric cancer treatment.
Keywords: YM155, survivin, gastric cancer, xancer stem cells, cancer therapy
INTRODUCTION
Gastric cancer is one of the world's most common malignancies. Although diagnosis and treatment of gastric cancers have been greatly improved, the overall survival rate remains low. Less than 20% of patients with gastric cancer survive to 5 years [1]. Survivin, a member of inhibitor apoptosis (IAP) protein family, is implicated in both cell survival and regulation of mitosis in cancer cells [2, 3]. Survivin is expressed in various primary tumors, but is rare expressed in normal differentiated tissues [4]. Previous studies showed that an over expression of survivin is found in human gastric cancer tissues, and is a poor prognostic factor in gastric cancer patients [5-7]. Moreover, overexpression of survivin is detected in gastric cancer cells during drug treatment, indicating that survivin may contribute to chemo-resistance in gastric cancer [8-10]
Recent studies showed that survivin is also associated with cancer stem cells (CSCs). Survivin has been demonstrated to be a downstream gene of the Wnt signal pathway and is highly expressed in colon cancer stem cells [11]. Wnt/β-catenin signaling has also been demonstrated to be essential for the proliferation of gastric CSCs [12, 13]. Zhang et al. found that mutant APC up-regulates survivin, causing apoptosis inhibition and the expansion of colon tissue stem cells in the colon crypt, thereby initiating colon tumorigenesis [14, 15]. Carter et al. showed that survivin is overexpressed in AML stem/progenitor cells [16]. These results suggest that survivin is a potential target for inhibiting cancer stem cells proliferation. Whether YM155 downregulates other target genes of CSC signaling such as β-catenin, c-Myc, Cyclin D1 and CD44 remains unknown. CD44 has been demonstrated to be a marker of human gastric cancer CSC and CD44+ gastric cancer cells have stronger tumorigenic than CD44− gastric cancer cells [17, 18]. However, whether YM155 inhibits gastric CSCs remains to be investigated.
Several approaches have been developed to target inhibition of survivin for cancer therapy. Those approaches include, administering a molecular antagonist to target survivin mRNA and to inhibit survivin translation (including antisense oligonucleotides, ribozymes and small interfering RNAs), utilizing gene therapy approaches grounded on the use of survivin dominant-negative mutants (Cys84Ala, Thr34Ala) and the application of the survivin promoter to drive the expression of cytotoxic genes; survivin-based immunotherapy; small-molecule antagonists (suppressing survivin function) [19]. Considerable effects have also been made to target inhibition of survivin for gastric cancer therapy. For example, RNAi-mediated survivin knockdown can effectively inhibit the growth of gastric cancer cells [20, 21]. Our previous studies have shown that suppression of survivin expression or function by plasmid vectors encoding antisense survivin or survivin dominant-negative (DN) mutant (Cys84Ala) can inhibit gastric cancer carcinogenesis and angiogenesis in vivo [22]. While those approaches are effective, it is still difficult to use in clinic. Recent studies showed that YM155, a novel small, imidazolium-based compound can specifically inhibit survivin expression and induce apoptosis in human cancer cells [23]. Preclinical studies demonstrated that three to seven-day continuous infusion of YM155 (1-10mg/kg.d) significantly inhibited tumor growth in hormone-refractory prostate cancer, melanoma and non-small-cell lung cancer [24]. Moreover, recent results from completed phase I/II clinical studies show that YM155 was safe at a dose of 4.8 mg/m2/day for 168 hours every 3 weeks and exhibited encouraging anti-cancer effect in advanced cancer patients [25-29]. These results suggest that YM155 is a promising agent for cancer therapy.
However, there are no studies to show that YM155 inhibit gastric tumor growth in vivo. In this study, we have evaluated the antitumor effect of YM155 in gastric cancer cell lines. We found that YM155 induced apoptosis of gastric cancer cells, inhibited expansion of gastric CSCs and expression of CSC molecules CD44 and β-catanin and suppressed gastric cancer xenograft growth. Our results, for the first time, demonstrate that YM155 inhibits gastric cancer growth by inhibition expansion of CSCs and document a new mechanism that YM155 inhibits tumor growth.
RESULTS
YM15 inhibits cell proliferation in gastric cancer cells
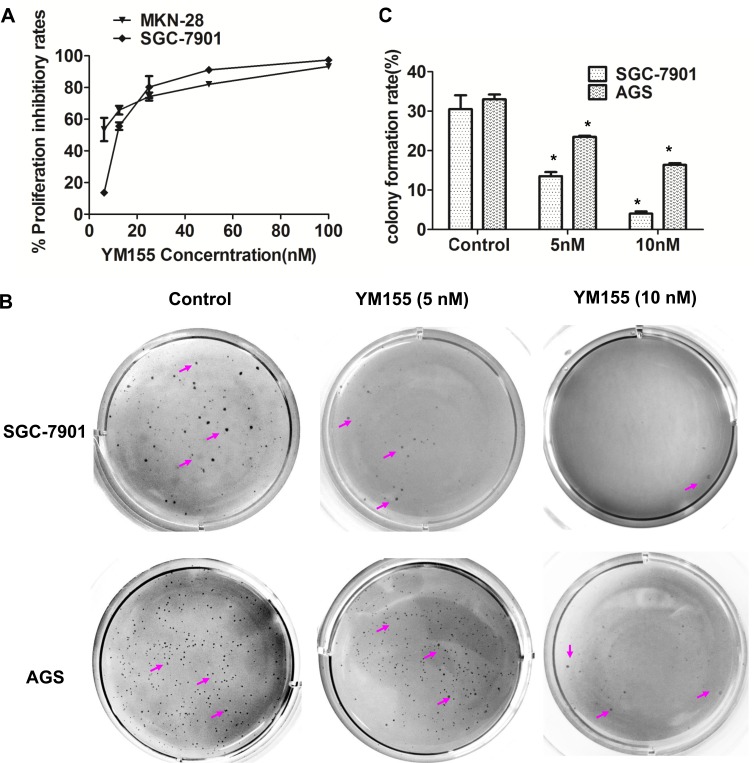
To investigate the effect of YM155 on cell proliferation of gastric cancer cells in vitro, gastric cancer SGC-7901 and MKN-28 cells were treated with YM155 for 48 hours; cell proliferation was measured by MTT. The results showed that YM155 significantly inhibited cell proliferation. The mean IC50 of SGC-7901 and MKN-28 cells were 13.2 nM and 11.6 nM (Figure 1A), respectively. YM155 has also shown a great activity against other gastric cancer cell lines, such as AGS and Hs 764T cell lines, with IC50 values 0.8 nM and 7.3 nM [24].
Figure 1. YM155 inhibits anchored-dependent and anchored-independent growth in gastric cancer cells.
A. YM155 inhibits cell proliferation of gastric cancer cells. MKN-28 and SGC-7901 cells were cultured in 96-well plates and treated with YM155 at indicated doses. Cell proliferation was detected by MTT method. The data presented is means ± SD of 3 independent experiments. B. YM155 inhibits the colony formation of gastric cancer cells. Colonies in soft agar assay were stained with 0.1% crystal violet at 16 days after culture. Representative colonies were photographed. Arrows indicate colonies formed in soft agar. C. The number of colonies in B. was counted under microscope. Data represented is the means ± SD of three independent experiments. *P < 0.05 compared with the control group.
To further investigated the effect of YM155 on cell transformation. Soft agar assay was conducted to determine cell transformation in vitro. The result showed that gastric cancer SGC-7901 cells treated with YM155 formed less number of colonies and smaller size colonies in soft agar compared to control cells treated with DMSO (Figure 1B, upper panel). Same results were obtained in AGS cells (Figure 1B, bottom panel). Quantification analysis showed that YM155 treatment significantly inhibited colony formation in gastric cancer SGC-7901 and AGS cells in a dose-dependent manner (Figure 1C). The rates of colony formation in SGC-7901 were reduced by 55.7% and 86.9% at dose of 5 nM and 10 nM of YM155, respectively. Similar results were obtained in AGS cells. YM155 at 5 nM and 10 nM reduced colony formation of AGS cells by 28.9% and 50.3%, respectively, compared with control DMSO treatment. These results suggest that YM155 inhibits anchored-dependent and anchored-independent growth of gastric cancer cells.
YM155 induces apoptosis of gastric cancer cells
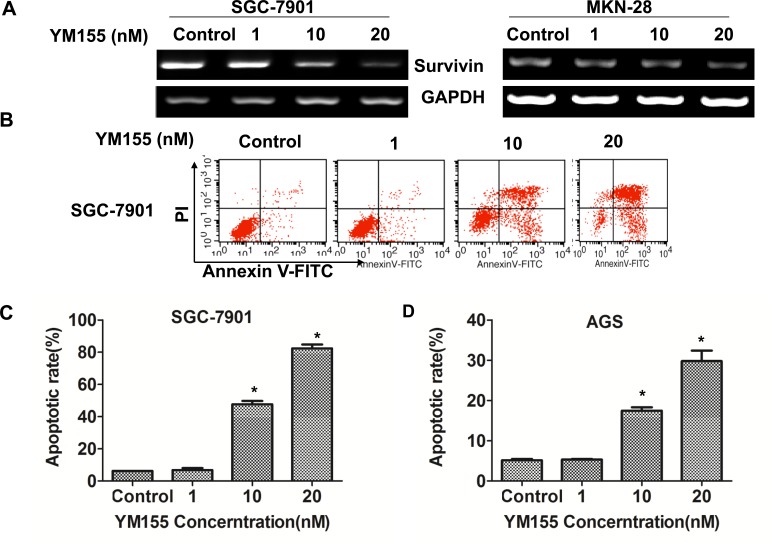
To investigate the effect of YM155 on apoptosis of gastric cancer cells, we first determined the effect of YM155 on survivin expression. The results showed that YM155 effectively inhibited mRNA expression of survivin in SGC-7901 and MKN-28 cells in a dose-dependent manner (Figure 2A). FACS analysis showed that YM155 induced apoptosis of SGC-7901 (Figure 2B) in a dose-dependent manner. Quantification analysis showed that apoptosis rates were significantly increased in YM155-treated gastric cancer cells compared to the control groups (Figure 2C), the apoptosis rates were 47.6 ± 2.9% and 82.4 ± 3.4% when SGC-7901 cells treated by YM155 at the doses of 10 nM and 20 nM YM155 for 24 hours, respectively. The apoptosis rate of control group was only 6.2 ± 0.2%. Same results were obtained in AGS cells (Figure 2D). The results demonstrate that YM155 induce apoptosis of gastric cancer cells efficiently.
Figure 2. YM155 down-regulates survivin expression and induces apoptosis of gastric cancer cells.
A. YM155 inhibits mRNA expression of survivin. SGC-7901 & MKN-28 cells were treated with YM155 at indicated dose for 24 hours. The mRNA expression of survivin was determined by RT-PCR. B.-D. YM155 induces apoptosis of gastric cancer cells. SGC-7901 cells and AGS cells were treated with YM155 at indicated doses for 24 hours. Apoptosis was determined by FACS analysis. Representative FACS Plots was shown in B. The apoptotic rates are the means ± SD of 3 independent experiments * P < 0.05 compared with the control group.
YM155 induces apoptosis by activating intrinsic and extrinsic apoptotic pathways
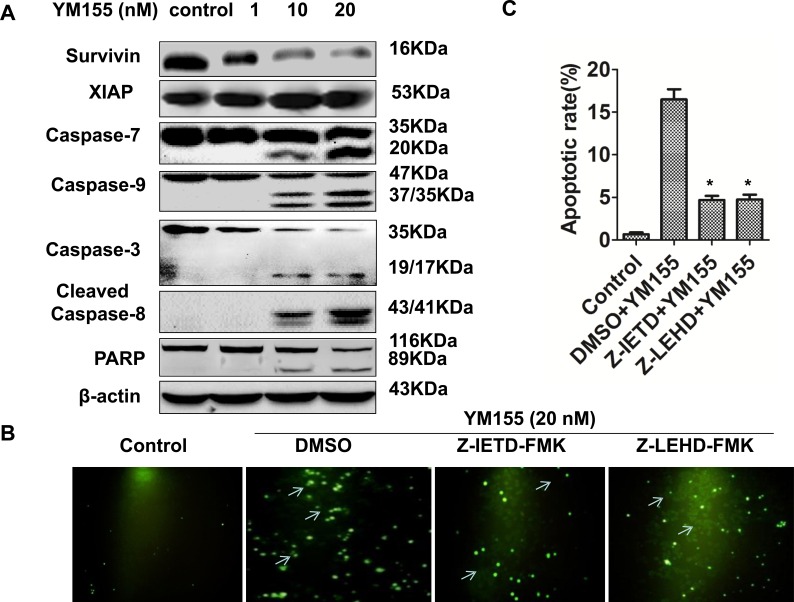
To investigate the underlying mechanisms by which YM155 induces apoptosis in gastric cancer cells, we determined the effect of YM155 on caspases signaling. Western Blot showed that YM155 could decrease protein level of survivin in a dose-dependent manner without affecting the level of XIAP (Figure 3A). Accordingly, YM155 treatment significantly increased the cleavage of effector caspases such as caspase-3, caspase-7, and PARP (the substrate of caspases 3). Furthermore, YM155 treatment could increase the cleavage of both caspase-9 and caspase-8, which are involved in intrinsic and extrinsic apoptosis signal pathways, respectively (Figure 3A).
Figure 3. YM155 triggers intrinsic and extrinsic apoptotic pathways.
A. YM155 treatment decreased survivin expression without affecting XIAP and induced cleavage of caspases. Protein expression (caspase-3, -7, -9, -8, PARP) was detected by Western Blot analysis. B. Pre-treatment of caspase inhibitors attenuates YM155-induced apoptosis. Gastric cancer cells were pre-treated with 20 μM caspase-8 inhibitor (Z-IETD-FMK) or 20 μM caspase-9 inhibitor (Z-LEHD-FMK) for 1 hour and treated with YM155 (20 nM) for another 24 hours. Apoptosis was detected by TUNEL assay. Representative photos were taken under fluorescence microscope 24 hours after the YM155 treatment. Arrows indicate apoptotic cells (green cells) (200 ×). C. Quantification of apoptotic cells. The apoptotic rates presented from B. are the means ± SD of 3 independent experiments.* P < 0.05 compared with the control group.
To further confirm whether YM155 induces apoptosis through activation of intrinsic and extrinsic apoptosis pathways, we tested the effects of caspase-8 and caspase-9 inhibitors on YM155-induced apoptosis. TUNEL results showed that pre-treatment with caspase-8 inhibitor Z-IETD-FMK (20 M) or caspase-9 inhibitor Z-LEHD-FMK (20 μM) for 1 hour significantly decreased YM155-induced apoptosis of SGC-7901 cells (Figure 3B). Quantification analysis showed that the apoptosis rate of SGC-7901 cells in YM155 treated group was 17.2% ± 7.2%, while pretreated with 20 M caspase-8 inhibitor or caspase-9 inhibitor for 1 hour, the apoptosis rates of SGC-7901 cells were 4.9% ± 1.8% and 5.2 ± 1.4%, respectively (Figure 3C), suggesting that caspase-8 and caspase-9 inhibitors inhibit YM155-induced apoptosis. These results demonstrate that YM155 induced apoptosis by triggering intrinsic and extrinsic apoptotic pathways.
YM155 inhibits stem-like sphere formation
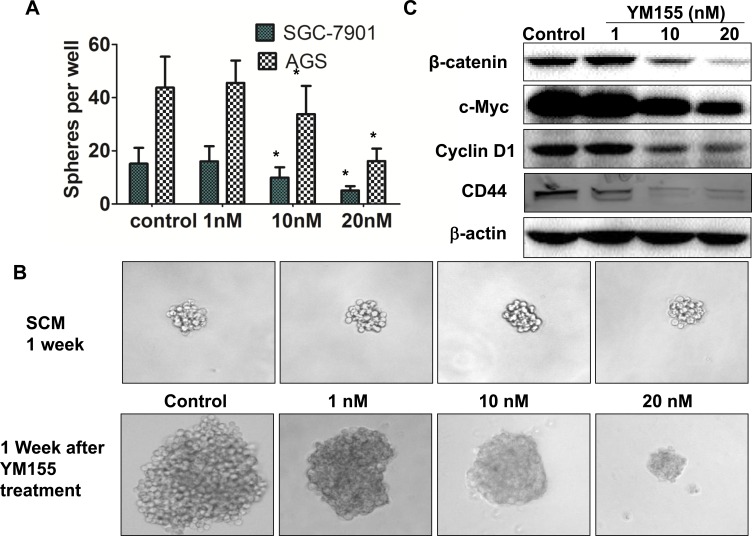
Overexpression of survivin has been shown to be associated with CSCs. Hypothesized that YM155 can suppress gastric CSCs by inhibition of survivin. Several approaches have been developed to evaluate CSCs [30-32]. One approach is to use a in vitro method, “spheroid colony formation”, that candidate CSCs were cultured in serum-free medium containing only EGF and bFGF (stem cell medium, SCM) using a ultra-low-attachment plates. We first investigated the effect of YM155 on sphere formation. SGC-7901 cells and AGS cells were cultured in SCM with and without YM155 for 2 weeks. Quantification analysis of spheres showed that YM155 reatment significantly reduced spheres formation in SGC-7901 and AGS cells in dose dependent patterns (Figure 4A). These results indicate that YM155 can inhibit formation of spheres in gastric cancer cells. To determine whether YM155 also reduces expansion of gastric CSCs, SGC-7901 cells were first cultured in SCM for one week to form sphere. One week after culturing, SGC-7901 cells formed small spheroid colonies (spheres) (Figure 4B, upper panel). These formed spheres of SGC-7901 cells were then treated with YM155 at the doses of 1 nM, 10 nM and 20 nM in SCM for one more week. We observed that vehicle-treated spheres greatly expanded and formed large size colonies, and that YM155-treated spheres grew slowly, and formed smaller size colonies compared to vehicle-treated spheres (Figure 4B, bottom panel). The similar inhibition of sphere growth was also observed in AGS cells treated with YM155 (data not shown). The results suggest that YM155 inhibits expansion of gastric CSCs.
Figure 4. YM155 inhibits formation and expansion of gastric cancer spheres.
A. YM155 inhibits sphere formation of gastric cancer cells. SGC-7901 and AGS cells (100 per well) were cultured for 2 weeks in SCM supplement with or without YM155 at indicated doses. The spheres were counted under microscope. The data presented are the means ± SD of 3 independent experiments.* P < 0.05 compared with the control group. B. YM155 inhibits expansion of spheres of gastric cancer cells. SGC-7901 cells were cultured for 1 week in SCM to form sphere (upper panel), and then treated with YM155 at indicated doses for another 1 week. YM155 treatment inhibits growth of spheres. Representative photos were taken at one week after the YM155 treatment (200 ×). C. YM155 inhibited expression of CSC molecules. SGC-7901 cells were treated with YM155 at indicated doses for 24 hours. The protein levels of genes were determined by Western Blot.
YM155 inhibits expression of CSC molecules
We then explored the underlying mechanisms by which YM155 inhibits the expansion of gastric CSCs. Since Wnt/β-catenin signaling has been demonstrated to be essential for the expansion of gastric CSCs [12, 13], we tested the effect of YM155 on Wnt signaling. Western Blot showed that YM155 decreased the level of β-catenin and the expression of c-Myc and Cyclin D1, which are downstream of Wnt/β-catenin signaling (Figure 4C). CD44 has been identified as a marker of gastric CSCs [17, 18, 33]. YM155 treatment downregulated expression of CD44 in gastric cancer cells (Figure 4C). These results suggest that YM155 inhibits expansion of gastric CSCs by inhibiting Wnt/β-catenin signaling and expression of CD44.
YM155 inhibits growth of gastric cancer xenograft
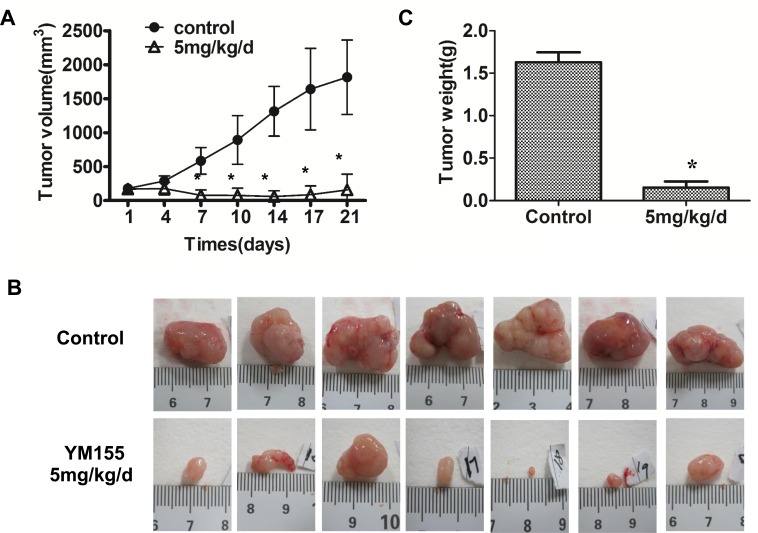
We finally investigated the effects of YM155 on tumor growth in a xenograft mouse model. SGC-7901 cells were injected into the frank of athymic nude mice to establish tumor xenograft. When tumor size reached around 80 mm3-100 mm3, mice received a 7-day continuous infusion of YM155 at 5 mg/kg/day or vehicle control. The results showed that tumors in the mice treated with YM155 grew slowly compared to tumor in the mice treated with vehicle control (Figure 5A-5B). Tumor growth curve showed that YM155 treatment resulted in persistent inhibition of tumor growth and tumor shrank (Figure 5B). Accordingly, YM155 treatment significantly reduced tumor weight compared to the control treatment (Figure 5C). The results demonstrate that YM155 inhibits gastric cancer tumor growth in vivo.
Figure 5. YM155 inhibits xenograft growth of gastric cancer.
Tumor xenografts were established by s.c. injection of SGC-7901 cells into the right flanks of the mice (5×106/0.1ml per mouse). When tumor size reached around 80 mm2−100 mm2, mice were administered with a 7-day YM155 continuous infusion using a micro-osmotic pump. Tumor size was measured every three days. The tumor growth curve was shown in A. Three weeks after treatment, xenograft tumors were excised and photographed. Representative tumor photos were shown in B. Data presented are the means ± SD of tumor weight per mouse (m = 7) C. *P < 0.05 compared with the control group.
YM155 induces apoptosis and inhibits gastric CSCs in vivo
To determine whether YM155 inhibits survivin expression in xenograft tissues, tumor tissues were subjected to Western Blot and IHC staining. Western Blot showed that the band densities of survivin protein were lower in the tumor tissues from YM155-treated mice than in those from vehicle-treated mice (Figure 6A, left and middle panel). IHC showed that the intensity of survivin+ staining in tumor tissues from YM155-treated mice were significantly weaker than in those from vehicle-treated mice (Figure 6B). Quantification analysis showed that the number of survivin+ staining cells in the tumors from YM155-treated mice were also significantly lower than in those from vehicle-treated mice (Figure 6C). The results suggest that YM155 inhibit survivin expression in vivo.
Figure 6. YM155 induces apoptosis and inhibits gastric CSCs in vivo.
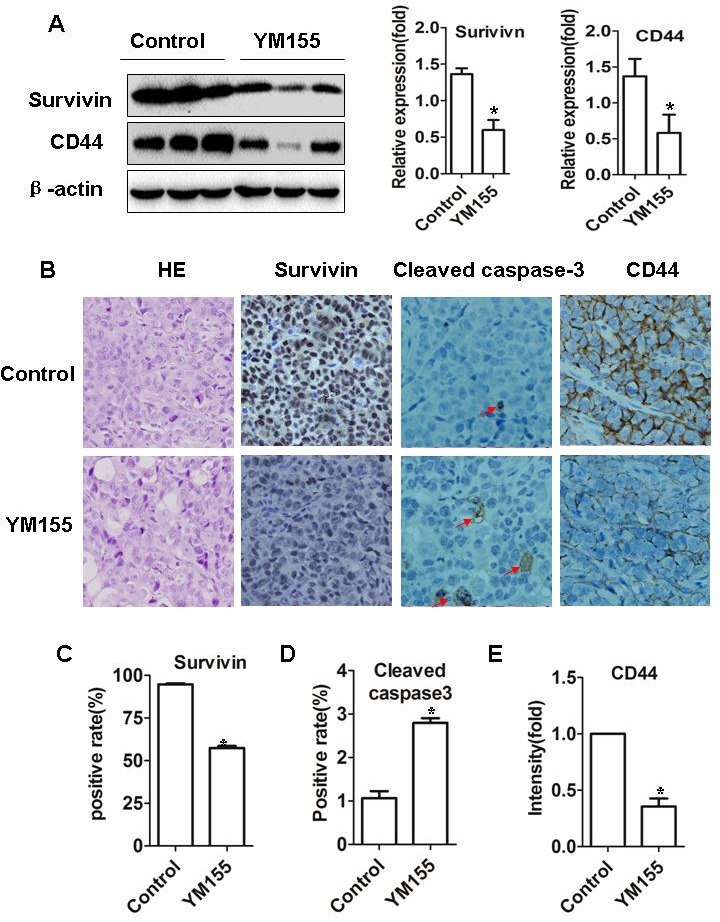
A. YM155 treatment decreased protein levels of survivin and CD44. Xenograft tumor tissues (from Figure 5) were extracted and protein concentrations were measured. The levels of protein were determined by Western blot (left panel). The data of the densities presented are means ± SD of 3 mice. B. YM155 treatment inhibits expression of surviving, cleaved caspase-3 and CD44. Tissue sections were subjected to surviving, cleaved caspase-3 and CD44 immunostaining. Representative photos were shown (Original magnification × 400). C.-D. Quantification analysis of survivin and cleaved caspase 3 positive staining cells. Data presented are means ± SD of 7 mice. *p < 0.01, compared to control treatment. E. Quantification analysis of intensities of CD44 positive staining cells. Data presented are means ± SD of five mice. *p < 0.01, compared to control treatment.
Accordingly, we found that YM155 treatment increased the number of cleaved caspases-3-positive cells (apoptotic cells) in xenograft tumor tissues compared to control treatment (Figure 6B). Quantification analysis showed that caspase 3 positive rates in the tumor was significantly higher in YM155-treated mice than vehicle-treated mice (2.80% ± 0.11% vs 1.07 ± 0.15 %, p < 0.01) (Figure 6D). These results indicate that YM155 induces apoptosis of gastric cancer in vivo.
To further explore whether YM155 also inhibits gastric CSCs in vivo we determined expression of CD44 in xenograft tissues by Western Blot and IHC staining. Western blot showed that the protein levels (densities of band) of CD44 in the tumors from YM155-treated mice were lower than those in the mice from vehicle-treated mice (Figure 6A left and right panel). Although IHC staining showed that the number of CD44+ staining cells was not significantly different between the groups, the intensity of CD44+ staining were markedly weaker in tumor tissues from YM155-treated mice compared to those in tumor tissues from vehicle-treated mice (Figure 6B). Quantification analysis showed that the densities of CD44+ positive staining were significantly lower in the tumor from the YM155-treated mice than those in tumor tissues from vehicle-treated mice (Figure 6E). The results indicate that YM155 reduces CD44 expression and inhibits gastric CSCs in vivo.
Assessment of YM155 treatment safety
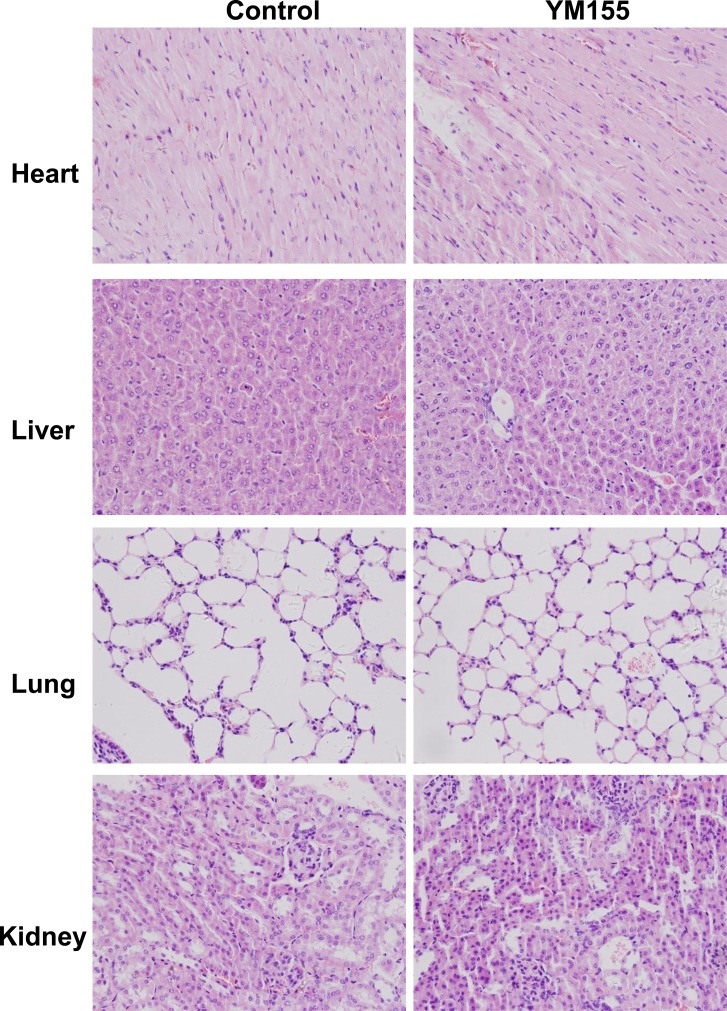
We also determined the effects of YM155 on normal tissues. Furthermore, we did not observe obvious pathological change in some organs (heart, liver, lung, and kidney) of YM155-treated mice (Figure 7), suggesting that YM155 treatment is safety in our mouse mice model.
Figure 7. YM155 treatment does not cause histopathological alteration of organs.
Main organs (heart, liver, lung and kidney) of mice treated with YM155 or control vehicle were subjected to H&E staining. Histology analysis (heart, liver, lung and kidney) were conducted by pathologist. Representative photos were shown (Original magnification × 400).
DISCUSSION
Survivin is highly expressed in the majority of human cancer tissues, including gastric cancer, and is associated with drug-resistance and poor prognosis [34, 35]. Suppression of survivin expression or function resulted in spontaneous apoptosis, increased sensitivity to cytotoxic drugs and suppression of tumor growth in a nude mouse model [22, 36]. These data indicate that targeted inhibition of survivin is a potential and promising approach for the treatment of gastric cancer [22]. In this study, we further found that YM155, a specific survivin suppressant [24], induced apoptosis of gastric cancer cells, inhibited expansion of gastric CSCs and reduced expression of CSC molecules (CD44 and β-catanin) and suppressed gastric cancer xenograft growth. Our results, for the first time, demonstrate that YM155 inhibit gastric cancer growth by inhibition CSCs and document a new mechanism that YM155 inhibits tumor growth.
Our previous studies have demonstrated that inhibition of survivin function by AAV-mediated survivin mutant or inhibition of survivin expression by antisense oligonucleotide suppress gastric and colon cancer growth [37, 38]. Although AAV vector can efficaciously mediated long-term expression of survivin mutant, its safety and inconvenience are still the obstacles for its clinical use. YM155 is a small imidazolium-based molecule that inhibits specifically both mRNA and protein expression of survivin [24]. YM155 has been demonstrated to suppress the growth of a large number of human cancers including non-small-cell lung cancer, breast cancer, hormone-refractory prostate cancer, ovarian cancer, leukemia, non-Hodgkin's lymphoma, sarcoma, melanoma, esophageal cancer, renal cancer, breast cancer, hepatoma [24, 39-42]. The combination of YM155 and chemotherapeutic drugs (including etoposide, platinum, doxetaxel) enhanced inhibition of survivin expression, resulting in stronger inhibition of tumor growth in some cancer types such as non-small cell lung cancer, melanoma and neuroblastoma. The mean log growth inhibition of 50% (GI50) value was around 15 nM for YM155 [43-45]. In this study, we have demonstrated the potent anti-proliferation activity of YM155 to gastric cancer cells with the mean IC50 of YM155 was 11.6∼13.2 nM. The data suggest that gastric cancer cells are also sensitive to YM155. Furthermore, we showed that YM155 significantly inhibited gastric xenograft tumor growth in a nude mouse model and reduced tumor weight, exhibiting strong anti-tumor effect. Our results demonstrated that survivin inhibitor, YM155, is able to inhibit gastric cancer growth.
Survivin has been demonstrated to inhibit caspase 9 and caspase 3, resulting in inhibition intrinsic apoptosis pathway [46]. Previous studies have suggested that YM155 specifically down regulates survivin expression in prostate cancer cells and non-small cell lung cancer (NSCLC) cells without affecting other proteins [23, 47]. Our results showed that YM155 downregulated survivin expression without affecting XIAP and induced cleavage of caspase 3, caspase 9 and caspase 8. Furthermore, the inhibitors of caspases 9 and caspase 8 significantly suppressed YM155-induced apoptosis, suggesting that YM155 induces apoptosis by activating intrinsic and extrinsic apoptotic pathway. A study showed that YM155 not only inhibits survivin, but also regulate expression of a large number of genes including in death receptor signaling and TNFR1 signaling that induce apoptosis through a extrinsic apoptosis pathway [48], consistent with our results.
While YM155 is originally identified as a survivin inhibitor, its mechanisms of antitumor actions are not fully understood. Recently, a number of studies have shown that YM155 could inhibit expression of EGFR, XIAP, Mcl-1 [49-51], suggesting that YM155 function is beyond to inhibition of survivin. We found that YM155 could inhibit expansion of gastric CSCs. CSCs are a subpopulation in tumors that have features to initiate tumor growth, sustain tumor self-renewal, promote relapse and metastasis by giving rise to new tumors [52]. However, the roles of CSCs in tumorigenesis remain in debate [53, 54]. Survivin is highly expressed in colon CSCs [11] and is upregulated by interleukin-4 [55]. Mutant APC up-regulates survivin, causing apoptosis inhibition and the expansion of colon tissue stem cells in the colon crypt, thereby initiating tumorigenesis [14, 15]. Survivin has been shown to be overexpressed in AML stem/progenitor cells [16]. The data suggested that survivin plays a role in regulation of CSCs. It is well known that Wnt/β-catenin signaling is essential for expansion of CSCs [12, 13]. Survivin has been shown to be a downstream gene of Wnt signaling pathway [11]. Therefore, survivin is a potential targeting for inhibition of CSCs. In this study, we observed that YM155 markedly suppressed formation and expansion of spheres in gastric cancer cells and downregulated expression of β-catenin, c-Myc and Cyclin D1, which are downstream genes of Wnt/β-catenin signaling. More importantly, YM155 inhibited CD44 expression in gastric cancer cells and gastric cancer tissues both in vitro and in vivo. CD44+ gastric cancer cells is a CSC marker of human gastric cancer and have stronger tumorigenic than CD44− gastric cancer cells [17, 18]. Our results, for the first time, show that YM155 inhibits gastric tumor growth by inhibition of expansion of CD44+ gastric CSCs.
YM155 has been studied in clinical trial in several cancer types. In phase I and II studies, YM155 has shown to be safe and well-tolerated, with a maximum tolerated dose of 8.0 mg/m2/d. In our xenograft mouse model, a 7-day continuous infusion of YM155 at 5 mg/kg induced massive tumor regression with decreased expression of survivin and CD44 in intratumoral. Furthermore, we did not observe serious side effects in some organisms. The data document that YM155 is a safety and promising agent for gastric cancer therapy.
MATERIALS AND METHODS
Cell lines and regents
The human gastric cancer cell lines SGC-7901, MKN28 and AGS were maintained in RPMI1640 (Thermo Scientific, China) supplemented with 10% fetal bovine serum (Life Technologies inc., Grand island, USA), 100 U/ml of penicillin sodium, and 100 mg/ml of streptomycin sulfate at 37°C in a humidified air atmosphere containing 5% CO2. YM155 monobromide (YM155) was purchased from ChemieTek Company (Indianapolis, USA).
MTT assay
In vitro cell proliferation was measured using MTT assay. Cells in the logarithmic phase of growth were seeded into 96-well culture plates at 1× 104 cells per well for 24 hours. After treatment with different concentrations of YM155 or vehicle control for 48 hours, 100 μl of MTT solution (1 mg/ml) were added to each well, and the cells were further incubated at 37°C for 4 hours. The supernatant was replaced with dimethyl sulfoxide (DMSO) to dissolve formazan production. The absorbance at wave length 595 nm was measured using micro-ELISA reader (Bio-Rad, Hercules, CA). The mean 50% growth inhibition (IC50) value was calculated by logistic analysis. The mean IC50 value was obtained from the results of 3 independent assays.
Soft agar colony formation assay
Anchorage-independent cell growth was determined by soft agar clone formation assay. Cells (5 × 102) in 0.5 ml complete culture medium containing 0.3% agar and YM155 or vehicle were cultured on the top of 0.7% agar in the same medium. Dishes were then transferred into culture incubator. Cultures were stained with 0.1% crystal violet at 16 days, colonies were counted under microscope (magnification 50 ×). Colonies containing ≥ 50 cells were considered viable.
Flow cytometry assay for apoptosis
Cells were collected and washed with cold PBS, then stained with FITC Annexin V/PI (BD Biosciences, San Diego, USA) according to production manuals. Apoptotic cells were analyzed by flow cytometry (Coulter, Luton, United Kingdom).
Western blot analysis
Cells were lysed in lysis buffer (1X PBS,1% NP40,0.1% SDS,5mM EDTA,0.5% sodium deoxyccholate, 1 mM sodium orthovanadate, and 1 mM phenylmethylsulfonyl fluoride). Protein samples were electrophoresed in 10% denaturing sodium dodecylsulfate polyacrylamide gel, and transferred to Immobilon-P transfer membrane (Millipore, Billerica, MA). The blots were incubated with specific primary antibodies, reacted with a peroxidase-conjugated second antibody (Cell Signaling Technology, Inc., Danvers, USA) and then visualized by chemiluminescence HRP Substrate (Millipore, Billerica, MA). Survivin (71G4B7), PARP, Caspase 3/9/7, cleaved caspase-8, β-catenin, c-Myc, Cyclin D1, CD44 antibodies were all purchased from Cell Signaling, (Cell Signaling Technology, Inc., Danvers, USA) and β-actin antibody was from Santa Cruz Biotechnology (Santa Cruz, California).
Polymerase chain reaction (PCR)
Total RNA from cultured cells was prepared with RNAprep pure cell kit (Tiangen). First-strand cDNA was synthesized with oligo (dT) primer by using PrimeScript® RT reagent Kit (Takara, Daliang, China). Primer sequences were designed using the Primer QuestSM and were as follows: human survivin (forward) 5-ATGGGTGCCCCGACGTTGCC-3 and (reverse) 5-TCAATCCATGGCAGCCAG CT-3; human CD44 (forward) 5-AAAGCAGGACCTTCATCCCAGTGA-3 and (reverse) 5-TTGCTCCACCTTCTTGACTCCCAT-3; human GAPDH (forward) GAAGACTGTGGATGGCCCCT and (reverse) GTCCACCACCCTGTTGCTGT. PCR was performed using the PCR Master Mix (Tiangen, China), according to the manufacturer's instructions.
In situ detection of apoptotic cells by TUNEL assay
Cells were harvested and fixed by 0.4% paraformaldehyde, TUNEL staining was carried out using In Situ Cell Death Detection Kit (Roche, Indianapolis, USA) according to the manufactory manuals. Apoptotic cells show strong nuclear green fluorescence. The apoptotic cells were counted in 5 randomly selected fields viewed at × 20 magnification. The apoptotic index was calculated as the number of apoptotic cells/total number of nucleated cells ×100%.
Spheroid colony formation assay
Gastric cancer cells (102 cells per well) were inoculated in a ultra-low-attachment 96-well plates (Corning, USA) supplemented with 200 μl of RPMI-1640 medium (Thermo Scientific, China) plus 0.3% albumin from bovine serum (BSA, Sigma-Alorich, USA), 20 ng/ml human recombinant epidermal growth factor (EGF, Invitrogen Corp., Frederick, MD, USA) and 10 ng/ml human recombinant basic fibroblast growth factor (bFGF, Invitrogen Corp., Frederick, MD, USA). Two weeks after culture, each well was examined under light microscope and the total spheroid colonies numbers per well were counted. The rates of sphere formation were calculated as the number of apoptotic cells/total number of nucleated cells 1×100%.
Gastric cancer xenograft experiment
Five weeks old female BALB/c nude mice were purchased from Shanghai Experimental Animals Centre of Chinese Academy of Sciences. All animal studies abode the rules of The Laboratory Animal Ethics Committee of Renji Hospital, Shanghai Jiaotong University School of Medicine. Xenografts were established by s.c. injection of SGC 7901 cells into the right flanks of the mice (5×106 /0.1ml per mouse. After one week, tumor size reached approximately 80 mm3-100 mm3, mice were randomly assigned to treatment and control groups. YM155 at 5mg/kg/day or vehicle control was administered subcutaneously as a 7-day continuous infusion using a micro-osmotic pump (Alzet model 1007D) implanted in the left dorsal flank under anesthesia. Body weight of mice was assessed twice weekly, and tumor diameter was measured using standard calipers. After two weeks observation, mice were sacrificed and tumors were weighed, subsequently processed for immunohistochemistry and Western Blot.
Immunohistochemistry
The expression of survivin, CD44, cleaved-caspase-3 in tumor tissues was detected with the Ultra-SensitiveTMS-P (Mouse/Rabbit) kit (Maxin-Bio, China) according to the manufacturer instructions. Briefly, sections were dewaxed in xylene. Antigen retrieval was performed with a microwave for 10 min at 100°C. The sections were then incubated with rabbit anti-survivin antibody (1:200), anti-CD44 antibody (1:50), anti-cleaved-caspase-3 antibody (1:100) (cell signaling) for 1 hour, followed by biotinylated anti-IgG Antibody and streptavidin-biotinylated-complex horseradish peroxidase. Antibody binding was visualized by incubated with DAB (Maxin-Bio, China). Sections were then stained with hematoxylin.
Statistical analysis
Data were expressed as the means of at least three different experiments ± SD. The results were analyzed by ONE-WAY ANOVA, and P ≤ 0.05 was considered statistically significant.
Acknowledgments
The project was supported by NSFC 8117259, NSFC 81272403, NSFC 81472727, NSFC 91029718, NSFC 91429307; Key Discipline and Specialty Foundation of Shanghai Municipal Commission of Health and Family Planning; Science and Technology Commission of Shanghai Municipality (15JC1403100).
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest.
REFERENCES
- 1.Smith MG, Hold GL, Tahara E, El-Omar EM. Cellular and molecular aspects of gastric cancer. World J Gastroenterol. 2006;12:2979–2990. doi: 10.3748/wjg.v12.i19.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 3.Giodini A, Kallio MJ, Wall NR, Gorbsky GJ, Tognin S, Marchisio PC, Symons M, Altieri DC. Regulation of microtubule stability and mitotic progression by survivin. Cancer Res. 2002;62:2462–2467. [PubMed] [Google Scholar]
- 4.Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, Reed JC. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315–5320. [PubMed] [Google Scholar]
- 5.Lu CD, Altieri DC, Tanigawa N. Expression of a novel antiapoptosis gene, survivin, correlated with tumor cell apoptosis and p53 accumulation in gastric carcinomas. Cancer Res. 1998;58:1808–1812. [PubMed] [Google Scholar]
- 6.Okada E, Murai Y, Matsui K, Isizawa S, Cheng C, Masuda M, Takano Y. Survivin expression in tumor cell nuclei is predictive of a favorable prognosis in gastric cancer patients. Cancer letters. 2001;163:109–116. doi: 10.1016/s0304-3835(00)00677-7. [DOI] [PubMed] [Google Scholar]
- 7.Liu JL, Gao W, Kang QM, Zhang XJ, Yang SG. Prognostic value of survivin in patients with gastric cancer: a systematic review with meta-analysis. PLoS One. 2013;8:e71930. doi: 10.1371/journal.pone.0071930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeguchi M, Liu J, Kaibara N. Expression of survivin mRNA and protein in gastric cancer cell line (MKN-45) during cisplatin treatment. Apoptosis. 2002;7:23–29. doi: 10.1023/a:1013556727182. [DOI] [PubMed] [Google Scholar]
- 9.Zheng WE, Chen H, Yuan SF, Wu LL, Zhang W, Sun HY, Chen WJ. Overexpression of betaIII-tubulin and survivin associated with drug resistance to docetaxel-based chemotherapy in advanced gastric cancer. J BUON. 2012;17:284–290. [PubMed] [Google Scholar]
- 10.Shen X, Zheng JY, Shi H, Zhang Z, Wang WZ. Survivin knockdown enhances gastric cancer cell sensitivity to radiation and chemotherapy in vitro and in nude mice. Am J Med Sci. 2012;344:52–58. doi: 10.1097/MAJ.0b013e318239c4ee. [DOI] [PubMed] [Google Scholar]
- 11.Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, Sprick MR, Kemper K, Richel DJ, Stassi G, Medema JP. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 12.Mao J, Fan S, Ma W, Fan P, Wang B, Zhang J, Wang H, Tang B, Zhang Q, Yu X, Wang L, Song B, Li L. Roles of Wnt/beta-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis. 2014;5:e1039. doi: 10.1038/cddis.2013.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai C, Zhu X. The Wnt/beta-catenin pathway regulates self-renewal of cancer stem-like cells in human gastric cancer. Mol Med Rep. 2012;5:1191–1196. doi: 10.3892/mmr.2012.802. [DOI] [PubMed] [Google Scholar]
- 14.Zhang T, Otevrel T, Gao Z, Ehrlich SM, Fields JZ, Boman BM. Evidence that APC regulates survivin expression: a possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res. 2001;61:8664–8667. [PubMed] [Google Scholar]
- 15.Zhang T, Fields JZ, Opdenaker L, Otevrel T, Masuda E, Palazzo JP, Isenberg GA, Goldstein SD, Brand M, Boman BM. Survivin-induced Aurora-B kinase activation: A mechanism by which APC mutations contribute to increased mitoses during colon cancer development. Am J Pathol. 2010;177:2816–2826. doi: 10.2353/ajpath.2010.100047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter BZ, Qiu Y, Huang X, Diao L, Zhang N, Coombes KR, Mak DH, Konopleva M, Cortes J, Kantarjian HM, Mills GB, Andreeff M, Kornblau SM. Survivin is highly expressed in CD34(+)38(−) leukemic stem/progenitor cells and predicts poor clinical outcomes in AML. Blood. 2012;120:173–180. doi: 10.1182/blood-2012-02-409888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen W, Zhang X, Chu C, Cheung WL, Ng L, Lam S, Chow A, Lau T, Chen M, Li Y, Nie Y, Wong BC, Pang R. Identification of CD44+ cancer stem cells in human gastric cancer. Hepatogastroenterology. 2013;60:949–954. doi: 10.5754/hge12881. [DOI] [PubMed] [Google Scholar]
- 19.Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy. Expert Opin Ther Targets. 2008;12:463–476. doi: 10.1517/14728222.12.4.463. [DOI] [PubMed] [Google Scholar]
- 20.Chen T, Deng C. Inhibitory effect of siRNA targeting survivin in gastric cancer MGC-803 cells. Int Immunopharmacol. 2008;8:1006–1011. doi: 10.1016/j.intimp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Li YH, Chen M, Zhang M, Zhang XQ, Zhang S, Yu CG, Xu ZM, Zou XP. Inhibitory effect of survivin-targeting small interfering RNA on gastric cancer cells. Genet Mol Res. 2014;13:6786–6803. doi: 10.4238/2014.August.28.22. [DOI] [PubMed] [Google Scholar]
- 22.Tu SP, Jiang XH, Lin MC, Cui JT, Yang Y, Lum CT, Zou B, Zhu YB, Jiang SH, Wong WM, Chan AO, Yuen MF, Lam SK, Kung HF, Wong BC. Suppression of survivin expression inhibits in vivo tumorigenicity and angiogenesis in gastric cancer. Cancer Res. 2003;63:7724–7732. [PubMed] [Google Scholar]
- 23.Nakahara T, Kita A, Yamanaka K, Mori M, Amino N, Takeuchi M, Tominaga F, Hatakeyama S, Kinoyama I, Matsuhisa A, Kudoh M, Sasamata M. YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Res. 2007;67:8014–8021. doi: 10.1158/0008-5472.CAN-07-1343. [DOI] [PubMed] [Google Scholar]
- 24.Nakahara T, Kita A, Yamanaka K, Mori M, Amino N, Takeuchi M, Tominaga F, Kinoyama I, Matsuhisa A, Kudou M, Sasamata M. Broad spectrum and potent antitumor activities of YM155, a novel small-molecule survivin suppressant, in a wide variety of human cancer cell lines and xenograft models. Cancer Sci. 2011;102:614–621. doi: 10.1111/j.1349-7006.2010.01834.x. [DOI] [PubMed] [Google Scholar]
- 25.Tolcher AW, Mita A, Lewis LD, Garrett CR, Till E, Daud AI, Patnaik A, Papadopoulos K, Takimoto C, Bartels P, Keating A, Antonia S. Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of survivin. J Clin Oncol. 2008;26:5198–5203. doi: 10.1200/JCO.2008.17.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheson BD, Bartlett NL, Vose JM, Lopez-Hernandez A, Seiz AL, Keating AT, Shamsili S, Papadopoulos KP. A phase II study of the survivin suppressant YM155 in patients with refractory diffuse large B-cell lymphoma. Cancer. 2012;118:3128–3134. doi: 10.1002/cncr.26510. [DOI] [PubMed] [Google Scholar]
- 27.Satoh T, Okamoto I, Miyazaki M, Morinaga R, Tsuya A, Hasegawa Y, Terashima M, Ueda S, Fukuoka M, Ariyoshi Y, Saito T, Masuda N, Watanabe H, Taguchi T, Kakihara T, Aoyama Y, et al. Phase I study of YM155, a novel survivin suppressant, in patients with advanced solid tumors. Clin Cancer Res. 2009;15:3872–3880. doi: 10.1158/1078-0432.CCR-08-1946. [DOI] [PubMed] [Google Scholar]
- 28.Lewis KD, Samlowski W, Ward J, Catlett J, Cranmer L, Kirkwood J, Lawson D, Whitman E, Gonzalez R. A multi-center phase II evaluation of the small molecule survivin suppressor YM155 in patients with unresectable stage III or IV melanoma. Invest New Drugs. 2011;29:161–166. doi: 10.1007/s10637-009-9333-6. [DOI] [PubMed] [Google Scholar]
- 29.Kelly RJ, Thomas A, Rajan A, Chun G, Lopez-Chavez A, Szabo E, Spencer S, Carter CA, Guha U, Khozin S, Poondru S, Van Sant C, Keating A, Steinberg SM, Figg W, Giaccone G. A phase I/II study of sepantronium bromide (YM155, survivin suppressor) with paclitaxel and carboplatin in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24:2601–2606. doi: 10.1093/annonc/mdt249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 31.Sharpe B, Beresford M, Bowen R, Mitchard J, Chalmers AD. Searching for prostate cancer stem cells: markers and methods. Stem Cell Rev. 2013;9:721–730. doi: 10.1007/s12015-013-9453-4. [DOI] [PubMed] [Google Scholar]
- 32.Vermeulen L, Sprick MR, Kemper K, Stassi G, Medema JP. Cancer stem cells—old concepts, new insights. Cell Death Differ. 2008;15:947–958. doi: 10.1038/cdd.2008.20. [DOI] [PubMed] [Google Scholar]
- 33.Zhang C, Li C, He F, Cai Y, Yang H. Identification of CD44+CD24+ gastric cancer stem cells. J Cancer Res Clin Oncol. 2011;137:1679–1686. doi: 10.1007/s00432-011-1038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krieg A, Baseras B, Tomczak M, Verde PE, Stoecklein NH, Knoefel WT. Role of survivin as prognostic and clinicopathological marker in gastric cancer: a meta-analysis. Mol Biol Rep. 2013;40:5501–5511. doi: 10.1007/s11033-013-2650-z. [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Li T, Liu Q, Jiao H, Yang W, Liu X, Huo Z. Clinical and prognostic significance of HIF-1alpha, PTEN, CD44v6, and survivin for gastric cancer: a meta-analysis. PLoS One. 2014;9:e91842. doi: 10.1371/journal.pone.0091842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wenying Z, Zhaoning J, Zhimin Y, Dongyun C, Lili S. Survivin siRNA inhibits gastric cancer in nude mice. Cell Biochem Biophys. 2012;62:337–341. doi: 10.1007/s12013-011-9315-0. [DOI] [PubMed] [Google Scholar]
- 37.Chiou SK, Hoa N, Hodges A, Ge L, Jadus MR. Indomethacin promotes apoptosis in gastric cancer cells through concomitant degradation of Survivin and Aurora B kinase proteins. Apoptosis. 2014;19:1378–1388. doi: 10.1007/s10495-014-1002-3. [DOI] [PubMed] [Google Scholar]
- 38.Tu SP, Cui JT, Liston P, Huajiang X, Xu R, Lin MC, Zhu YB, Zou B, Ng SS, Jiang SH, Xia HH, Wong WM, Chan AO, Yuen MF, Lam SK, Kung HF, et al. Gene therapy for colon cancer by adeno-associated viral vector-mediated transfer of survivin Cys84Ala mutant. Gastroenterology. 2005;128:361–375. doi: 10.1053/j.gastro.2004.11.058. [DOI] [PubMed] [Google Scholar]
- 39.Zhao N, Mao Y, Han G, Ju Q, Zhou L, Liu F, Xu Y, Zhao X. YM155, a survivin suppressant, triggers PARP-dependent cell death (parthanatos) and inhibits esophageal squamous-cell carcinoma xenografts in mice. Oncotarget. 2015;6:18445–18459. doi: 10.18632/oncotarget.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo K, Huang P, Xu N, Xu P, Kaku H, Zheng S, Xu A, Matsuura E, Liu C, Kumon H. A combination of YM-155, a small molecule survivin inhibitor, and IL-2 potently suppresses renal cell carcinoma in murine model. Oncotarget. 2015;6:21137–21147. doi: 10.18632/oncotarget.4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vequaud E, Seveno C, Loussouarn D, Engelhart L, Campone M, Juin P, Barille-Nion S. YM155 potently triggers cell death in breast cancer cells through an autophagy-NF-kB network. Oncotarget. 2015;6:13476–13486. doi: 10.18632/oncotarget.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia H, Chen J, Shi M, Deivasigamani A, Ooi LL, Hui KM. The over-expression of survivin enhances the chemotherapeutic efficacy of YM155 in human hepatocellular carcinoma. Oncotarget. 2015;6:5990–6000. doi: 10.18632/oncotarget.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwasa T, Okamoto I, Takezawa K, Yamanaka K, Nakahara T, Kita A, Koutoku H, Sasamata M, Hatashita E, Yamada Y, Kuwata K, Fukuoka M, Nakagawa K. Marked anti-tumour activity of the combination of YM155, a novel survivin suppressant, and platinum-based drugs. Br J Cancer. 2010;103:36–42. doi: 10.1038/sj.bjc.6605713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamanaka K, Nakahara T, Yamauchi T, Kita A, Takeuchi M, Kiyonaga F, Kaneko N, Sasamata M. Antitumor activity of YM155, a selective small-molecule survivin suppressant, alone and in combination with docetaxel in human malignant melanoma models. Clin Cancer Res. 2011;17:5423–5431. doi: 10.1158/1078-0432.CCR-10-3410. [DOI] [PubMed] [Google Scholar]
- 45.Clemens MR, Gladkov OA, Gartner E, Vladimirov V, Crown J, Steinberg J, Jie F, Keating A. Phase II, multicenter, open-label, randomized study of YM155 plus docetaxel as first-line treatment in patients with HER2-negative metastatic breast cancer. Breast Cancer Res Treat. 2015;149:171–179. doi: 10.1007/s10549-014-3238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamanaka K, Nakata M, Kaneko N, Fushiki H, Kita A, Nakahara T, Koutoku H, Sasamata M. YM155, a selective survivin suppressant, inhibits tumor spread and prolongs survival in a spontaneous metastatic model of human triple negative breast cancer. Int J Oncol. 2011;39:569–575. doi: 10.3892/ijo.2011.1077. [DOI] [PubMed] [Google Scholar]
- 47.Iwasa T, Okamoto I, Suzuki M, Nakahara T, Yamanaka K, Hatashita E, Yamada Y, Fukuoka M, Ono K, Nakagawa K. Radiosensitizing effect of YM155, a novel small-molecule survivin suppressant, in non-small cell lung cancer cell lines. Clin Cancer Res. 2008;14:6496–6504. doi: 10.1158/1078-0432.CCR-08-0468. [DOI] [PubMed] [Google Scholar]
- 48.Tao YF, Lu J, Du XJ, Sun LC, Zhao X, Peng L, Cao L, Xiao PF, Pang L, Wu D, Wang N, Feng X, Li YH, Ni J, Wang J, Pan J. Survivin selective inhibitor YM155 induce apoptosis in SK-NEP-1 Wilms tumor cells. BMC Cancer. 2012;12:619. doi: 10.1186/1471-2407-12-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Na YS, Yang SJ, Kim SM, Jung KA, Moon JH, Shin JS, Yoon DH, Hong YS, Ryu MH, Lee JL, Lee JS, Kim TW. YM155 induces EGFR suppression in pancreatic cancer cells. PLoS One. 2012;7:e38625. doi: 10.1371/journal.pone.0038625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng SM, Chang YC, Liu CY, Lee JY, Chan HH, Kuo CW, Lin KY, Tsai SL, Chen SH, Li CF, Leung E, Kanwar JR, Huang CC, Chang JY, Cheung CH. YM155 down-regulates survivin and XIAP, modulates autophagy and induces autophagy-dependent DNA damage in breast cancer cells. Br J Pharmacol. 2015;172:214–234. doi: 10.1111/bph.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagner V, Hose D, Seckinger A, Weiz L, Meissner T, Reme T, Breitkreutz I, Podar K, Ho AD, Goldschmidt H, Kramer A, Klein B, Raab MS. Preclinical efficacy of sepantronium bromide (YM155) in multiple myeloma is conferred by down regulation of Mcl-1. Oncotarget. 2014;5:10237–10250. doi: 10.18632/oncotarget.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei B, Chen L, Li R, Tian J. Stem cells in gastrointestinal cancers: a matter of choice in cell fate determination. Expert Rev Anticancer Ther. 2010;10:1621–1633. doi: 10.1586/era.10.52. [DOI] [PubMed] [Google Scholar]
- 53.Blagosklonny MV. Cancer stem cell and cancer stemloids: from biology to therapy. Cancer Biol Ther. 2007;6:1684–1690. doi: 10.4161/cbt.6.11.5167. [DOI] [PubMed] [Google Scholar]
- 54.Blagosklonny MV. Target for cancer therapy: proliferating cells or stem cells. Leukemia. 2006;20:385–391. doi: 10.1038/sj.leu.2404075. [DOI] [PubMed] [Google Scholar]
- 55.Waclawczyk S, Buchheiser A, Flogel U, Radke TF, Kogler G. In vitro differentiation of unrestricted somatic stem cells into functional hepatic-like cells displaying a hepatocyte-like glucose metabolism. J Cell Physiol. 2010;225:545–554. doi: 10.1002/jcp.22237. [DOI] [PubMed] [Google Scholar]