Figure 5. ART-838 inhibited growth of acute leukemia xenografts and primagrafts in NSG mice more potently than did AS.
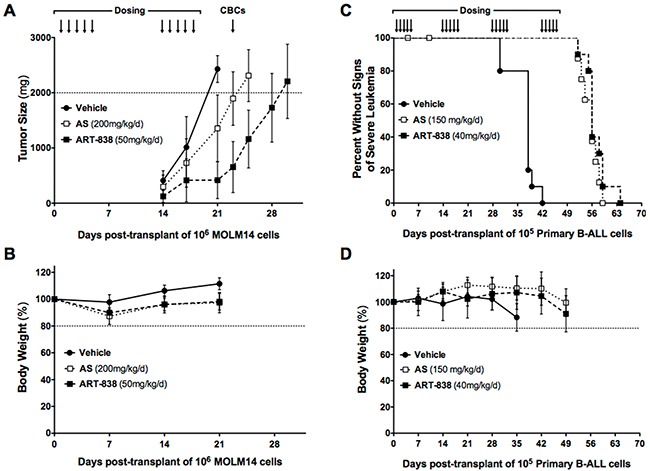
A, B. NSG mice transplanted subcutaneously with MOLM14 AML cells (106/mouse), were treated by oral gavage with two 5-day cycles of ART-838 (50 mg/kg/d, n = 8), AS (200 mg/kg/d, n = 9), or vehicle (n=10) beginning on days 1 and 14 post-transplantation (arrows indicate days of drug administration). Mice were weighed weekly and tumors measured at least twice weekly. CBCs were performed on day 23 (Supplementary Figure S8). Mice were euthanized when tumors reached an estimated 2000mg. Ascites was not observed in any mouse. A. Average tumor mass (estimated by: [length x width2]/2); (p<.001 for ART-838 or AS vs vehicle tumor mass at day 21). B. Percent of Day 0 body weight. (C,D) NSG mice (n=10 per group) transplanted with primary B-ALL case #109 (105 cells/mouse), were treated by oral gavage with four 5-day cycles of ART-838 (40 mg/kg/d), AS (150 mg/kg/d), or vehicle beginning on days 1, 15, 29, and 43 post-transplantation (arrows indicate days of drug administration). Mice were weighed weekly and observed daily for clinical signs of severe leukemia. C. Kaplan-Meier survival analysis. Mean time to severe clinical signs of leukemia (surrogate for survival): vehicle (37), AS (56), and ART-838 (57); (p<.001 for ART-838 vs vehicle or AS vs vehicle). 2 mice from the AS group that died on days 4 and 10 were censored from analysis, as they showed no evidence of leukemia or drug toxicity at necropsy. D. Percent of Day 0 body weight.
