Abstract
The liver transplant allocation system is currently based upon the Model for End-Stage Liver Disease (MELD) score and allocates organs preferentially to patients with the highest scores (ie, the sickest patients) within a defined geographic unit. In addition, certain patient populations, such as patients with hepatocellular carcinoma and portopulmonary hypertension, receive MELD exception points to account for their increased waitlist mortality, which is not reflected by their MELD score. Significant geographic variation in the access to liver transplantation exists throughout the United States. Both the Organ Procurement and Transplant Network Board of Directors and the Health Resources and Services Administration have determined these geographic disparities to be unacceptable. The liver transplant community has worked to develop methods to reduce these geographic disparities and to reexamine how MELD exception points are granted to certain patient populations. As a result, numerous policy changes have been adopted throughout the years that have broadened the sharing of organs through wider geographic sharing. Despite all of these changes, variation in access to liver transplantation continues to exist, and, thus, the liver transplant community continues to examine new ways to address geographic disparities. This paper reviews several of the key changes to the liver allocation system that have occurred since the implementation of MELD allocation in 2002 and provides an overview of potential changes to the system.
Keywords: Liver transplantation, Model for End-Stage Liver Disease, allocation, geographic discrepancy, hepatocellular carcinoma
In 2000, the US Department of Health and Human Services published the Final Rule, which, in part, established principles for the allocation of deceased donor livers, including prioritizing candidates based upon medical urgency.1 The Final Rule also stated that access to a transplant should not be affected by place of residence or listing.1 Following publication of the Final Rule, the Organ Procurement and Transplant Network (OPTN) adopted the Model for End-Stage Liver Disease (MELD) score to prioritize patients on the liver transplant waiting list.2 The MELD score, which is calculated from serum bilirubin, international normalized ratio of prothrombin time, and serum creatinine, offers an objective score that accurately predicts the risk of shortterm mortality from chronic liver disease.2
The determination of which patient receives an organ is dependent upon 2 factors: allocation and distribution.3 Allocation is defined as the order of patients on a particular waiting list based upon medical urgency3 and has been based upon the MELD score since 2002.2 Distribution indicates the geographic units in the country where donated livers are matched to a priority-ranked list of candidates according to the allocation scheme within the distribution unit. The current liver distribution system follows a local-regional-national algorithm.4 The local distribution unit is defined as the donation service area (DSA), which is generally a single organ procurement organization. There are currently 58 organ procurement organizations in the United States that cover areas ranging from a single metropolitan area to multiple states. The United States is further divided into 11 regions.4
A shortage of livers and geographic disparities in access to liver transplantation continue in the United States.5 In 2013, 1767 patients died while waiting for a liver transplant, and another 1223 were removed from the list for being too sick to undergo the procedure.6 The difference between average MELD score at transplant varies as much as 10 points between DSAs.5 This variance suggests that candidates in certain parts of the country have to wait until they are much more ill before they receive a transplant as compared to other centers. The reasons for such disparities are complex and include geographic differences in the availability of deceased donor livers,5 the demand of liver transplantation in certain areas, and potential differences in listing criteria or organ acceptance rates. In addition, certain patient characteristics, such as serum sodium7 and hepatocellular carcinoma (HCC), may be associated with worse outcomes and may not be reflected by the MELD score. Since the initiation of MELD allocation in 2002, patients with HCC have been granted exception points to account for this difference.2,8
In an effort to achieve equity among liver transplant candidates and address geographic disparities in access to transplantation, the OPTN has implemented a series of changes to organ allocation policies, including the Regional Share 15 rule, which was implemented in 2005; the Regional Share 35/National Share 15 policy, implemented in June 2013; and revisions to awarding HCC exception points, implemented in October 2015. MELD-Na, a modified MELD score that incorporates serum sodium, was implemented on January 11, 2016 (Table 1). There are also ongoing discussions of new distribution units. This paper reviews recently approved and potential changes to the liver allocation system.
Table 1.
Liver Allocation Policy Changes Implemented Since 2002
| Year | Policy Implemented |
|---|---|
| 2002 | MELD score |
| 2005 | Regional Share 15 rule |
| 2013 | Regional Share 35/National Share 15 policy |
| 2015 | Modification of HCC MELD exception points |
| 2016 | MELD-Na score |
HCC, hepatocellular carcinoma; MELD, Model for End-Stage Liver Disease; MELD-Na, Model for End-Stage Liver Disease plus serum sodium.
Regional Share 15 Rule
According to Merion and colleagues, average survival benefit, truncated at 3 years, only occurred after liver transplantation in patients with MELD scores above 18, whereas patients with MELD scores less than 15 had reduced mean survival compared to matched candidates who remained on the waitlist.9 As a result of this analysis, the OPTN adopted the Regional Share 15 rule, which states that organs must first be offered to patients with MELD scores of 15 or greater locally and then regionally before making the organs available to local patients with MELD scores under 15.10 With this system, organs available within a certain DSA are offered to waitlist candidates within the DSA. If there are no suitable local candidates with a MELD score greater than or equal to 15, the organs are offered to candidates in other DSAs within the same region. With this system in place, there was a 36% decrease in the proportion of liver recipients with a MELD score less than 15 undergoing transplant. However, there was no change in the number of livers shared outside of the local DSA.8 Throughout the years, variations in median MELD score at the time of transplant increased between DSAs, and patients with a MELD score of 35 or greater had a waitlist mortality rate comparable to patients with acute liver failure who were listed as status 1a and eligible for regional sharing.11,12 Therefore, the transplant community proposed wider geographic sharing for patients with high MELD scores. Liver simulated allocation modeling (LSAM) projected that regional sharing of organs for high MELD patients would result in a decrease in waitlist mortality.3
Regional Share 35/National Share 15 Policy
The Regional Share 35/National Share 15 policy mandated that organs be offered to candidates with a MELD score greater than or equal to 35 in the region before being considered for local candidates with a MELD score below 35. In addition, if there was no suitable candidate with a MELD score greater than or equal to 15, the organs were offered nationally before coming back to centers in the local DSA for candidates with a MELD score less than 15.13 An analysis performed 1 year after the implementation of this policy revealed an increase in the total number of transplants, a 30% lower waitlist mortality in patients with a MELD score greater than 30, and fewer livers being discarded. This was not at the expense of increased cold ischemia times or in early posttransplant outcomes.14 A second analysis comparing outcomes in the pre— and post— Regional Share 35 eras noted an expected increase in transplant MELD scores following implementation of Regional Share 35 as well as an increase in the proportion of patients undergoing transplant while in the intensive care unit or on life-support devices.15 On a national level, no difference was noted in patient survival when comparing patients who underwent transplant prior to Regional Share 35 with patients who underwent transplant following Regional Share 35. However, patient survival was lower in 2 regions (regions 4 and 10) in the post—Regional Share 35 era with no significant differences in the remaining regions.15 The current allocation scheme, arranged from highest to lowest priority, is summarized in Table 2.
Table 2.
Current Liver Allocation Scheme
| Organ Allocation Offering (Highest to Lowest Priority) |
|---|
| 1. Combined local and regional status 1A |
| 2. Combined local and regional status 1B |
| 3. Local/regional MELD/PELD score 35-40 (offers made locally then regionally for each MELD score) |
| 4. Local MELD/PELD score 29-34 |
| 5. National Liver-Intestine MELD score >29 |
| 6. Local MELD/PELD score 15-28 |
| 7. Regional MELD/PELD score 15-34 |
| 8. National status 1A or 1B |
| 9. National MELD/PELD score >15 |
| 10. Local MELD/PELD score <15 |
| 11. Regional MELD/PELD score <15 |
| 12. National MELD/PELD score <15 |
MELD, Model for End-Stage Liver Disease; PELD, Pediatric End-Stage Liver Disease.
MELD-Na Score
Hyponatremia has been shown in several studies to be an independent predictor of mortality in patients with cirrhosis.16-18 This effect is most pronounced in patients with low MELD scores and has led to the development of the MELD-Na score, which has been shown to predict waitlist mortality more accurately than MELD score alone.7 The current formula of MELD-Na is calculated as: MELD-Na = MELD + 1.32 x (137 - Na) - [0.033 x MELD x (137 — Na)].19 The lower limit of sodium was set at 125, and the upper limit was set at 137. LSAM analysis suggested that implementation of this model will result in a decrease of 52 total deaths per year among patients awaiting transplant.20 The results of this analysis led to approval of the MELD-Na score by the United Network for Organ Sharing (UNOS) in June 2014 with subsequent implementation in January 2016 in the United States.20
Hepatocellular Carcinoma
In 1996, Mazzaferro and colleagues reported that liver transplantation for the treatment of small HCC, defined as 1 lesion smaller than 5 cm or 3 lesions smaller than 3 cm, produced a 4-year recurrence-free survival rate of 83%.21 The results of this study have led to the acceptance of liver transplantation as a main treatment modality for surgically unresectable HCC. It was understood prior to the implementation of MELD allocation that patients with HCC would not be well served by this policy; as a result, these patients are granted MELD exception points. Recently, patients with HCC were granted a MELD exception score of 22 at listing, with additional points granted every 3 months. In the latest Scientific Registry of Transplant Recipients report, HCC was the second most common indication for transplant.6 Patients with HCC had a lower dropout rate from the waitlist compared with patients without HCC.22 HCC patients also had a decreased risk of waitlist mortality and increased odds of transplant compared to non-HCC patients.23 These data suggested that HCC patients had an advantage over patients without HCC, which prompted a change in the MELD exception scheme for HCC recipients that was implemented in October 2015.24 In the new policy, HCC patients are listed with their native MELD score for the first 6 months. If patients are still within criteria after 6 months, they are granted a MELD score of 28, which will increase every 3 months to a maximum of 34 points.24 Data from the Scientific Registry of Transplant Recipients and LSAM showed that a 6-month delay in granting patients HCC MELD exception points would increase the number of transplants for non-HCC patients and decrease the number of HCC transplants.25 The revision should also decrease the geographic variability in the transplant rate for HCC.25 In addition, capping the MELD exception score at 34 will ensure that organs will not be allocated to HCC patients if there is a patient with a calculated MELD score higher than or equal to 35 in the region, who is in more imminent need for transplant.
Conclusion
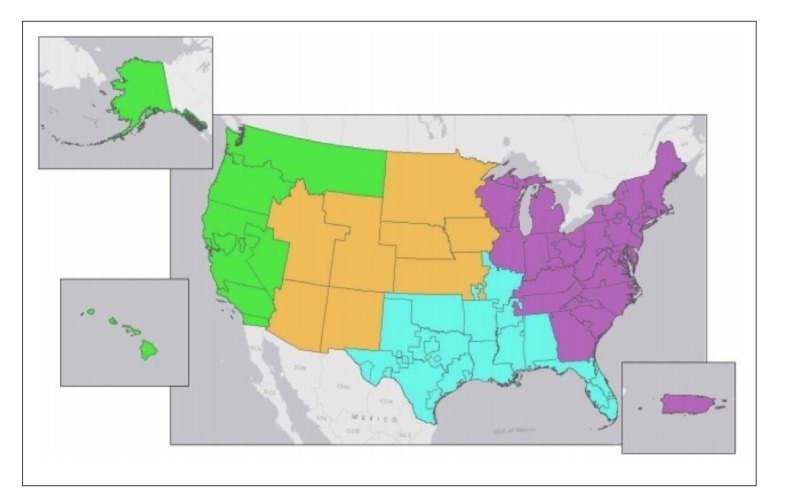
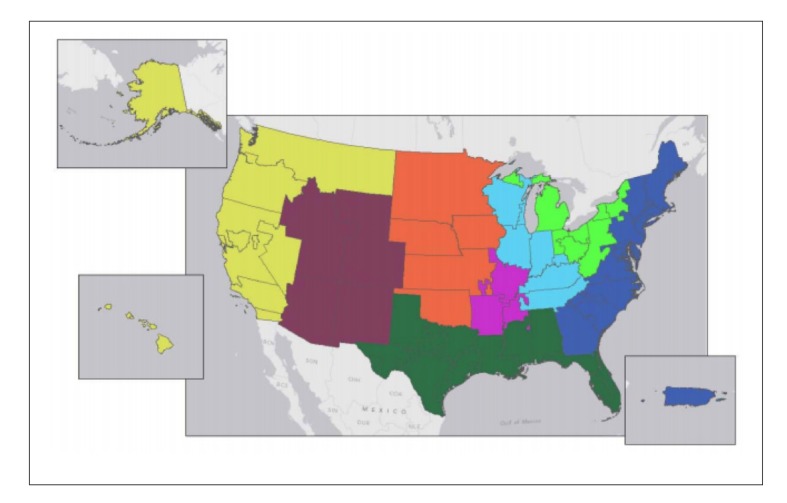
Changes in organ allocation policies throughout the years have resulted in decreased waitlist mortality rates and an increase in organs being offered to more ill patients.26 Despite these changes, significant geographic variations continue to exist between DSAs in terms of transplant rates, waitlist mortality, and median MELD score at transplant. Over the years, multiple methods for redistricting have been examined, including concentric circles and full regional sharing. While these methods represent broader sharing, they actually do little to address geographic disparities. In an attempt to address these geographic disparities, new districts were generated by attempting to match supply (the number of deceased donor livers) with demand (the number of incident listings with a MELD score >15).12 These new models divide the United States into either 4 districts (Figure 1) or 8 districts (Figure 2) and are subject to certain constraints imposed by the OPTN/UNOS Liver and Intestinal Organ Transplantation Committee. The primary outcome metric studied was median MELD score at transplant. Such redistricting would result in a median MELD score at time of transplant between 24 and 29 in most of the country.4 While not the primary outcome studied, redistricting is projected to save a net of 553 and 332 lives over the next 5 years for the 4-district vs 8-district model, respectively.4 Not surprisingly, redistricting will result in an increase in utilization of aircraft for organ transportation from 44% in the current system to 74% and 64% with 4 and 8 districts, respectively. Although aircraft utilization would increase the cost of organ transport, the expected overall decrease in costs of pretransplant care is projected to be larger, and, as a result, the total costs (pretransplant, transport, transplant plus 1 year follow-up, and transplant plus 3 years of follow-up) would decrease.4 Moreover, the increase in transportation costs can be addressed, at least in part, by awarding proximity points (ie, giving additional points to potential recipients who are listed at centers close to the donor hospital). These policies have been discussed at 2 recent public fora.
Figure 1.
Proposed 4-district distribution model for liver allocation.
Reproduced from the OPTN/UNOS Liver and Intestinal Organ Transplantation Committee.4
Figure 2.
Proposed 8-district distribution model for liver allocation.
Reproduced from the OPTN/UNOS Liver and Intestinal Organ Transplantation Committee.4
Footnotes
The authors have no relevant conflicts of interest to disclose.
References
- 1.Institute of Medicine (US) Committee on Organ Procurement and Transplantation Policy. Organ Procurement and Transplantation: Assessing Current Policies and the Potential Impact of the DHHS Final Rule. Washington, DC: National Academies Press; 1999. [PubMed] [Google Scholar]
- 2.Freeman RB, Jr, Wiesner RH, Harper A, et al. UNOS/OPTN Liver Disease Severity Score, UNOS/OPTN Liver and Intestine, and UNOS/OPTN Pediatric Transplantation Committees. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8(9):851–858. doi: 10.1053/jlts.2002.35927. [DOI] [PubMed] [Google Scholar]
- 3.Washburn K, Pomfret E, Roberts J. Liver allocation and distribution: possible next steps. Liver Transpl. 2011;17(9):1005–1012. doi: 10.1002/lt.22349. [DOI] [PubMed] [Google Scholar]
- 4.OPTN/UNOS Liver and Intestinal Organ Transplantation Committee. Redesigning liver distribution to reduce variation in access to liver transplantation. [February 8, 2016]. https://optn.transplant.hrsa.gov/contentdocuments/liver_concepts_20l4.pdf
- 5.Yeh H, Smoot E, Schoenfeld DA, Markmann JF. Geographic inequity in access to livers for transplantation. Transplantation. 2011;91(4):479–486. doi: 10.1097/TP.0b013e3182066275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2013 annual data report: liver. Am J Transplant. 2015;15(suppl 2):1–28. doi: 10.1111/ajt.13197. [DOI] [PubMed] [Google Scholar]
- 7.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl JMed. 2008;359(10):1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pomfret EA, Fryer JP, Sima CS, Lake JR, Merion RM. Liver and intestine transplantation in the United States, 1996-2005. Am J Transplant. 2007;7(5 pt 2):1376–1389. doi: 10.1111/j.1600-6143.2007.01782.x. [DOI] [PubMed] [Google Scholar]
- 9.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. Am J Transplant. 2005;5(2):307–313. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 10.Bittermann T, Makar G, Goldberg D. Exception point applications for 15 points: an unintended consequence of the share 15 policy. Liver Transpl. 2012;18(11):1302–1309. doi: 10.1002/lt.23537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma P, Schaubel DE, Gong Q, Guidinger M, Merion RM. End-stage liver disease candidates at the highest model for end-stage liver disease scores have higher wait-list mortality than status-1A candidates. Hepatology. 2012;55(1):192–198. doi: 10.1002/hep.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentry SE, Massie AB, Cheek SW, et al. Addressing geographic disparities in liver transplantation through redistricting. Am J Transplant. 2013;13(8):2052–2058. doi: 10.1111/ajt.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Organ Procurement and Transplantation Network. OPTN policies. [February 8, 2016]. http://optn.transplant.hrsa.gov/ContentDocuments/OPTN_Policies.pdf
- 14.Massie AB, Chow EK, Wickliffe CE, et al. Early changes in liver distribution following implementation of Share 35. Am J Transplant. 2015;15(3):659–667. doi: 10.1111/ajt.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halazun KJ, Mathur AK, Rana AA, et al. One size does not fit all-regional variation in the impact of the share 35 liver allocation policy. Am J Transplant. 2016;16(1):137–142. doi: 10.1111/ajt.13500. [DOI] [PubMed] [Google Scholar]
- 16.Biggins SW, Kim WR, Terrault NA, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130(6):1652–1660. doi: 10.1053/j.gastro.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Biggins SW, Rodriguez HJ, Bacchetti P, Bass NM, Roberts JP, Terrault NA. Serum sodium predicts mortality in patients listed for liver transplantation. Hepatology. 2005;41(1):32–39. doi: 10.1002/hep.20517. [DOI] [PubMed] [Google Scholar]
- 18.Ruf AE, Kremers WK, Chavez LL, Descalzi VI, Podesta LG, Villamil FG. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl. 2005;11(3):336–343. doi: 10.1002/lt.20329. [DOI] [PubMed] [Google Scholar]
- 19.Liver Transpl. 2015;21(3):279–281. doi: 10.1002/lt.24085. Biggins SW Use of serum sodium for liver transplant graft allocation: a decade in the making, now is it ready for primetime? [DOI] [PubMed] [Google Scholar]
- 20.Liver and Intestinal Organ Transplantation. Policy proposals: proposal to add serum sodium to the MELD score. [February 8, 2016]. http://optn.transplant.hrsa.gov/PublicCom- ment/pubcommentPropSub_317.pdf
- 21.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 22.Washburn K, Edwards E, Harper A, Freeman R. Hepatocellular carcinoma patients are advantaged in the current liver transplant allocation system. Am J Transplant. 2010;10(7):1643–1648. doi: 10.1111/j.1600-6143.2010.03127.x. [DOI] [PubMed] [Google Scholar]
- 23.Massie AB, Caffo B, Gentry SE, et al. MELD exceptions and rates of waiting list outcomes. Am J Transplant. 2011;11(11):2362–2371. doi: 10.1111/j.1600-6143.2011.03735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alcorn JB, United Network for Organ Sharing. Changes to OPTN bylaws and policies from actions at November board of directors meeting [February 8, 2016]. http://optn.trans- plant.hrsa.gov/media/1140/policy_notice_12-2014.pdf Published December 12, 2014.
- 25.Heimbach JK, Hirose R, Stock PG, et al. Delayed hepatocellular carcinoma model for end-stage liver disease exception score improves disparity in access to liver transplant in the United States. Hepatology. 2015;61(5):1643–1650. doi: 10.1002/hep.27704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Axelrod DA, Vagefi PA, Roberts JP. The evolution of organ allocation for liver transplantation: tackling geographic disparity through broader sharing. Ann Surg. 2015;262(2):224–227. doi: 10.1097/SLA.0000000000001340. [DOI] [PubMed] [Google Scholar]