Abstract
Purpose
Pulmonary support (PS) on day-of-life-30 (DOL-30) has been shown to be the strongest predictor of subsequent morbidity and in-patient mortality in Congenital Diaphragmatic Hernia (CDH). We hypothesized that PS on DOL-30 can also predict long-term outcomes in CDH survivors.
Methods
We analyzed records of 201 CDH survivors followed by a single multidisciplinary clinic (1995–2010). Follow-up was 83% and 70% at 1 and 5 years respectively. PS was defined as: (1) invasive support (n=44), (2) noninvasive support (n=54), or (3) room air (n=103). Logistic regression was used to estimate the adjusted association of PS on DOL-30 with outcomes at 1 and 5-years.
Results
Use of PS on DOL-30 was significantly associated with pulmonary and developmental morbidities at 1 and 5-years. Even after adjusting for defect-size and presence of ventilation/perfusion mismatch, greater PS on DOL-30 was associated with a significantly increased odds of requiring supplemental oxygen and developmental referral at 1-year, and asthma and developmental referral at 5-years.
Conclusion
CDH survivors continue to have significant long-term pulmonary and developmental morbidities. PS on DOL-30 is a strong independent predictor of morbidity at 1 and 5-years and may be used as a simple prognostic tool to identify high-risk infants.
Index Words: extracorporeal membrane oxygenation, mechanical ventilation, risk assessment, congenital anomaly
Introduction
Congenital diaphragmatic hernia (CDH) is associated with long hospital stays and significant morbidity and mortality. (1–3) With substantial variation in treatment and long-term outcomes in CDH (4, 5), it has become increasingly important to develop ways to identify high-risk infants who may benefit from long-term multidisciplinary follow-up. (6–8) The degree of pulmonary support, including use of a nasal cannula, continuous positive pressure support, mechanical ventilation or extracorporeal membrane oxygenation (ECMO), is routinely recorded for all patients in member hospitals of the CDH Study Group (CDHSG) at both day of life 30 (DOL-30) and discharge. The need for pulmonary support at one month of age has been shown to be highly associated with several important risk factors for poor outcome in CDH, including the need for patch repair, large defects, the presence of a cardiac anomaly, and low birthweight– suggesting that this measure may be an excellent summary indicator for the severity of pulmonary disease.(9) A recent analysis of the CDHSG registry found that the degree of pulmonary support on DOL-30 (PS on DOL-30) is a measure that is both simple to use and the strongest independent predictor of both subsequent mortality and pulmonary morbidity prior to discharge.(10)
In patients with CDH, the degree of long-term pulmonary, neurodevelopmental and gastrointestinal morbidity has previously been associated with various measures of disease severity – including the use of patch repair, size of defect, the need for oxygen at discharge or the need for ECMO. (11–17) Ventilation and perfusion scans, which are used by some multidisciplinary clinics to measure pulmonary function in CDH patients, have also been shown to be predictive of long-term outcome. Although some studies have shown that early ipsilateral V/Q mismatch can be a sensitive predictor of subsequent pulmonary function in survivors of CDH, (12, 18–20) others suggest that it may lack specificity and conclude that it does not warrant the costs of repeated nuclear medicine evaluation. (21) Given the significant long-term morbidity associated with the disease, many authors have promoted the use of extended multidisciplinary clinics for survivors of CDH. (3, 22) Despite this, current follow-up is extremely variable across institutions. (23)
We hypothesized that PS on DOL-30 would be a strong surrogate marker for the severity of pulmonary hypoplasia in congenital diaphragmatic hernia, and the best predictor of both short and long-term outcome in survivors of CDH. As more centers begin to enhance their long-term care strategies for these high-risk and high-cost patients, we aimed to determine if the degree of pulmonary support on DOL-30 could be a simple cross-institutional tool for identifying those patients with a higher risk of long-term morbidity who may benefit from more intensive extended multidisciplinary care.
Methods
Data
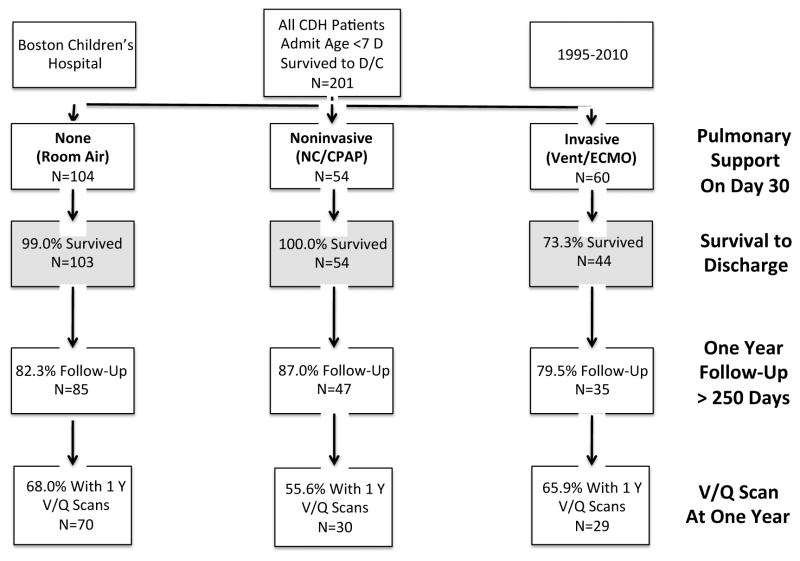
We reviewed the records of all infants treated at Boston Children’s Hospital for a diagnosis of congenital diaphragmatic hernia within the first 7 days of life between 1995 and 2010. Patients with diaphragmatic eventration, morgagni hernias, or those admitted after 7 days of life were excluded. Two hundred and sixty nine patients were confirmed to have a diagnosis of congenital diaphragmatic hernia on retrospective review. For the present study we included only the 201 patients who survived until discharge (Figure 1). The presence and type of pulmonary support was recorded on DOL-30 (PS on DOL-30) and on the day of discharge. Patients were grouped by the degree of pulmonary support on day of life 30: (i) invasive support (ventilator or extracorporeal membrane oxygenation), (ii) noninvasive support (nasal cannula/continuous positive airway pressure support), or (iii) room air (RA). Patients were followed in a multidisciplinary clinic, comprised of pediatric surgeons, pulmonologists, gastroenterologists, developmental medicine specialists, nutritionists and dedicated nursing. In the first year, patients were seen every 3–6 months depending on the acuity of the disease. After 1 year, patients were typically seen on an annual basis. We defined the “one-year” follow-up date and V/Q scan to be the appointment or scan that was closest to 1 year of age and at least 250 days from birth. “Long-term” follow-up or V/Q scan was defined as the appointment or scan closest to 5 years and at least 3 years from birth. Ventilation/Perfusion (V/Q) scans were obtained at roughly 1 year for all patients who consented for testing. A ventilation/perfusion ratio > 1.2 ipsilateral to the side of the diaphragmatic defect was considered a mismatch.(12, 18) A V/Q ratio in the upper tercile (>1.44) was considered severe. If a patient was determined to have a V/Q mismatch, an attempt was made to obtain follow up scans in roughly 2 to 3 year increments or until the mismatch resolved. V/Q scans were obtained for 129 patients. Variables compared between groups included gender, birth-weight (kilograms), estimated gestational age at birth (EGA, weeks), 1-minute and 5-minute APGAR, the presence of major cardiac abnormalities, the presence of a chromosomal abnormality, size of defect, type of repair (primary versus patch), and use of extracorporeal membrane oxygenation. Major cardiac defects were defined according to the CDHSG as any defect more physiologically severe than an isolated septal defect or patent ductus arteriosis. (24, 25). Given the limited power of the study, we elected to categorize graded size of diaphragmatic defect as either A/B or C/D, with A/B representing small defects often amenable to primary repair or only requiring a small patch and C/D representing larger defects or agenesis of the diaphragm.(25) Pulmonary and developmental outcomes were recorded including: use of any inhaler, inhaled steroids, any diuretics, or oxygen, referral for developmental or early intervention therapy (motor or verbal) by a developmental medicine specialist, or an empiric diagnosis of asthma as assigned by the pulmonary specialist. Pulmonary function tests were rarely obtained due to the young age of the patients at asthma diagnosis. Secondary outcome measures included hernia recurrence or bowel obstruction resulting in surgical intervention, the creation of a gastric fundoplication, placement of a gastric or jejunal feeding tube, and hospital readmission in the first year of life. Operations performed at other institutions were counted if they were mentioned in the inpatient record.
Figure 1.
Flow chart demonstrating survival and follow-up of patients with Congenital Diaphragmatic Hernia treated at Boston Children’s Hospital within 7 days of birth between 1995 and 2010.
Analysis
Bivariate analysis was used to compare known risk factors and outcomes by PS on DOL-30. All categorical analyses were performed using the Fisher’s exact test. Normally distributed data were compared with non-paired t-test and ANOVA, and non-parametric data were compared with the Wilcoxon rank sum test. A logistic regression model was used to examine the adjusted association of PS on DOL-30 with selected developmental and pulmonary outcomes at 1 and 5 years, adjusted empirically for the size of defect and the presence of a V/Q mismatch (V/Q ratio ≥1.2) before 1-year of life. Patients on invasive and noninvasive oxygen support on DOL-30 were combined to increase power for the five-year analysis. A sensitivity analysis was performed to examine the possible effects of “loss to follow up” on our findings. By assuming that all patients lost to follow up had either universally good or universally poor outcomes we provided a range for each estimate of long-term morbidity (Appendix 1 and 2). A p-value ≤ 0.05 was considered significant. All analyses were performed using JMP Pro 64-bit 10.0.0 (SAS Institute Inc. Cary, NC).
Results
Bivariate Analysis
Two hundred and one survivors of congenital diaphragmatic hernia were followed in a multidisciplinary clinic at Boston Children’s Hospital. There were 103 patients on room air on DOL-30, and 98 (48.8%) required some form of pulmonary support. Of those who required oxygen, 54 (55.1%) required only nasal cannula or CPAP, and 44 (44.9%) needed some form of invasive pulmonary support, including mechanical ventilation or ECMO (Figure 1).
PS on DOL-30 was associated with significant differences among known predictors of disease severity (Table 1). More invasive PS on DOL-30 was associated with significantly lower median apgar scores at 1 and 5 minutes (p<.001 for both), more cardiac anomalies (p=.01), more patch repairs (p<.001), larger defects (p<.001) and greater likelihood of being discharged home on oxygen (p<.001).
Table 1.
Comparison of patient factors by degree of pulmonary support on day of life 30 in survivors of Congenital Diaphragmatic Hernia (Birth Year 1995–2010, N=201).
| Patient Factors | Invasive Support (n=44) N (%) |
Noninvasive Support (n=54) N (%) |
Room Air (n=103) N (%) |
p-value |
|---|---|---|---|---|
|
| ||||
| Gender, Female (%) | 21 (47.7) | 20 (37.0) | 32 (31.1) | .16 |
|
| ||||
|
Birthweight, kilograms Median (IQR) |
3.0 (2.5–3.5) | 3.0 (2.7–3.3) | 3.2 (2.8–3.6) | .11 |
|
| ||||
|
Gestational Age, weeks Median (IQR) |
38 (37–39) | 38 (37–39) | 39 (38–39) | .14 |
|
| ||||
|
APGAR 1 minute Median (IQR) |
4.0 (2.0–6.0) | 5.0 (3.5–7.0) | 7.0 (4.0–8.0) | <.001* |
|
| ||||
|
APGAR 5 minutes Median (IQR) |
7.0 (5.0–8.0) | 8.0 (6.0–8.0) | 8.0 (7.0–9.0) | <.001* |
|
| ||||
| Major Cardiac Anomalies | 7 (16.3) | 7 (13.0) | 3 (3.1) | .01* |
|
| ||||
| Side (Left) | 33 (76.7) | 39 (72.2) | 86 (86.9) | .07 |
|
| ||||
| ECMO Used | 31 (70.5) | 23 (42.6) | 3 (2.9) | <.001* |
|
| ||||
| Patch Repair | 39 (90.7) | 35 (64.8) | 17 (17.2) | <.001* |
|
| ||||
| Defect Size | ||||
| A/B (Small/Medium) | 6 (15.8) | 22 (43.1) | 82 (82.8) | <.001* |
| C/D (Large/Agenesis) | 32 (84.2) | 29 (56.9) | 17 (17.2) | |
|
| ||||
| Discharge on Oxygen | 29 (70.7) | 27 (51.9) | 2 (1.9) | <.001* |
One-Year Outcomes
One hundred and sixty seven (83%) patients were followed through at least their first year of life, with comparable follow-up across all groups (Table 2, p=.60). Median age at the one-year follow-up appointment was 379 days (25th–75th percentile: 344–427 days). There were only three confirmed deaths after first hospital discharge in the entire study cohort (late death rate of 1.5%), all of which were in the group that required invasive PS on DOL-30. Across all measured outcomes, greater PS on DOL-30 was associated with significantly greater pulmonary and developmental morbidity. While more than half of the study patients on invasive PS on DOL-30 required supplemental oxygen at one year of age, only 12.8% and 2.4% of patients that were on noninvasive support or room air on DOL-30, respectively, were on oxygen at the same time point (p<.001). The use of invasive PS on DOL-30 was associated with a sensitivity of 69.2% (95%CI 48.2–85.6) and specificity of 87.9% (95%CI 81.4–92.8) in predicting the use of supplementary oxygen at one year (Positive Predictive Value: 51.43%, Negative Predictive Value: 93.9%).
Table 2.
One Year Outcomes of CDH Survivors by level of pulmonary support on Day of Life 30 (Birth Year 1995–2010, N=201). One hundred and sixty seven (83%) were followed for at least 250 days. Median age at one-year clinic appointment was 379 days (IQR: 344–427).
| Birth Year 1995–2010 | Invasive Support (n=44) N (%) |
Noninvasive Support (n=54) N (%) |
Room Air (n=103) N (%) |
p-value |
|---|---|---|---|---|
|
| ||||
| Death After First Admission (Reported) | 3 (6.8) | 0 (0.0) | 0 (0.0) | .01* |
|
| ||||
| No one year follow up | 9 (20.4) | 7 (13.0) | 18 (17.5) | .60 |
|
| ||||
| One-year clinical outcomes of followed cohort (≥250 days of follow up): | ||||
|
| ||||
| Pulmonary Outcomes | ||||
| Inhaler Use | 29/35 (82.9) | 17/47 (40.0) | 25/85 (29.4) | <.001* |
| Inhaled Steroids Use | 19/35 (54.3) | 12/47 (25.5) | 16/85 (18.8) | <.001* |
| On Diuretics | 25/35 (71.4) | 13/47 (27.7) | 5/85 (5.9) | <.001* |
| On Oxygen | 18/35 (51.4) | 6/47 (12.8) | 2/85 (2.4) | <.001* |
|
| ||||
| Developmental Outcomes | ||||
| Referred for Developmental Therapy | 25/35 (71.4) | 25/47 (53.2) | 16/85 (19.1) | <.001* |
| -------------------------------------------- | ------------ | ------------ | ------------ | -------- |
| • Motor | 14/35 (40.0) | 17/47 (36.2) | 12/85 (14.3) | |
| • Verbal | 2/35 (5.7) | 4/47 (8.5) | 2/85 (2.4) | <.001* |
| • Both | 9/35 (25.0) | 4/47 (8.5) | 2/85 (2.4) | |
|
| ||||
| First Ventilation/Perfusion (V/Q) scans of followed cohort | ||||
|
| ||||
| V/Q Scan Performed | 29/35 (82.9) | 30/47 (63.8) | 69/85 (81.2) | .06 |
|
| ||||
| Any Ipsilateral V/Q Mismatch: | ||||
| V/Q Ratio ≥ 1.2 | ||||
| Yes | 21 (72.4) | 8 (26.7) | 38 (54.3) | .10 |
| No | 8 (27.6) | 22 (73.3) | 31 (44.9) | |
|
| ||||
| Severe Ipsilateral V/Q Mismatch: | ||||
| V/Q Ratio ≥ 1.44 | ||||
| Yes | 13 (44.8) | 16 (53.3) | 14 (20.3) | .002* |
| No | 16 (55.2) | 14 (46.7) | 55 (79.7) | |
|
| ||||
| Surgeries and complications within 1st year of life of followed cohort | ||||
|
| ||||
| GI Surgeries | ||||
| G or J Tube Placed | 23/35 (65.7) | 16/47 (34.0) | 9/85 (10.6) | <.001* |
| Nissen Performed | 14/35 (40.0) | 11/47 (23.4) | 7/85 (8.2) | <.001* |
|
| ||||
| Surgical Complications | ||||
| Surgery for Hernia Recurrence | 7/35 (20.6) | 8/47 (17.0) | 4/85 (4.7) | .02* |
| Surgery for Bowel Obstruction | 3/35 (8.6) | 0/47 (0.0) | 3/85 (3.5) | .14 |
|
| ||||
| Readmissions | ||||
| Readmitted within First Year | 18/35 (51.4) | 16/47 (34.0) | 19/85 (22.4) | .008* |
| ------------------------------------------- | ------------ | ------------ | ------------ | --------- |
| At First Readmission | ||||
| • Failure to Thrive +/− Fever | 5/18 (29.4) | 6/16 (37.5) | 7/19 (38.9) | |
| • Respiratory Distress +/− Fever | 6/18 (35.3) | 7/16 (43.8) | 8/19 (44.4) | .87 |
| • Surgical Complication | 5/18 (29.4) | 2/16 (12.5) | 3/19 (16.7) | |
| • Other | 1/18 (5.9) | 1/16 (6.3) | 0/19 (0.0) | |
Furthermore, increased pulmonary support on DOL-30 was also associated with increased use of inhalers, steroids, and diuretics at one year (all p<.001). Seventy-one percent and 53% of patients on invasive or noninvasive pulmonary support on DOL-30, respectively, were referred by a developmental specialist for either early intervention or developmental therapy, compared to only 19% of patients on room air on DOL-30 (p<.001). In all groups, motor-associated delays were the most commonly cited reason for developmental referral.
Ventilation/Perfusion (V/Q) scans were performed in 128 patients, representing 64% of all survivors, and 77% of patients followed until at least one year of age. The proportion of followed patients who received V/Q scans at one year was 82.9, 63.8 and 81.2% in the invasive, noninvasive and room air groups respectively (p=.06). Seventy-two percent of patients on invasive PS on DOL-30 and 73% on noninvasive PS on DOL-30 had an ipsilateral V/Q mismatch at 1 year, compared to 54% on room air at DOL-30 (p=.10). When comparing patients on any form of PS on DOL-30 (either noninvasive or invasive PS) to patients on room air at the same time point, the need for PS on DOL-30 was associated with an increased odds of V/Q mismatch at 1 year of age (OR 2.30 95% CI 1.09–4.99, p=.03). Furthermore, there was a significantly lower proportion of patients on RA at DOL-30 who had a severe V/Q mismatch at 1 year (p=.002).
More gastric and jejunal feeding tubes were placed in the patients on invasive PS on DOL-30 (p<0.001). Fifteen percent of patients on invasive or noninvasive PS on DOL-30 experienced a hernia recurrence requiring operative intervention in the first year of life, compared to 4% in the patients on room air (p=.02). Greater PS on DOL-30 was also associated with a significantly higher rate of readmission in the first year (p=.02).
Long-term Outcomes
The proportion of patients lost to long-term follow up was not significantly different across study groups (p=.29). Long-term follow-up (3–5 years) was obtained for 105 of 151 patients (70%, in Table 3) born between 1995 and 2006. Median age at the long-term follow up appointment was 5.1 years (25th–75th percentile: 4.73–5.80 years). Patients on any form of PS on DOL-30 (including either noninvasive or invasive PS) were associated with significantly greater use of inhalers and steroids and a greater likelihood of asthma at 5-years (p=.003, p=.01, and p=.01 respectively). Patients on PS on DOL-30 was also associated with a significantly greater likelihood on referral for developmental therapy or early intervention at five-years (p=.005). Forty five percent of survivors first treated between 1995 and 2006 had V/Q scans after 3 years of age and the proportion of patients receiving V/Q scans at this time point was not different across PS groups (p=1.00). In the cohort of patients still receiving scans after 3 years of age, the presence of an ipsilateral V/Q mismatch was similarly common in patients on oxygen or room air on DOL-30 (87.9% and 75.7% respectively, p=.23). However a significantly lower proportion of patients who were on RA on DOL-30 were found to have a severe long-term V/Q mismatch (p=.008).
Table 3.
Long-Term Outcomes of CDH Survivors by level of pulmonary support on Day of Life 30 (Birth Year 1995–2006, N=151). One hundred and five (70%) patients had long-term clinical follow-up. Median age at five-year clinic appointment was 5.10 years (IQR: 4.73–5.8 years).
| Birth Year 1995–2006 | Invasive or Noninvasive Support (n=66) N (%) |
Room Air (n=85) N (%) |
p-value |
|---|---|---|---|
|
| |||
| No Long-term Follow-up | 17 (25.8) | 29 (34.1) | .29 |
|
| |||
| 5-Year Clinical Outcomes of Followed Cohort (At least 3 years of follow-up): | |||
|
| |||
| Pulmonary Outcomes | |||
| Inhaler Use | 34/49 (69.4) | 22/56 (39.3) | .003* |
| Inhaled Steroids Use | 23/49 (46.9) | 13/56 (23.2) | .01* |
| Diagnosis of Asthma | 28/49 (57.1) | 17/56 (30.4) | .01* |
|
| |||
| Developmental Outcomes | |||
| Referred for Developmental Therapy | 27/49 (55.1) | 15/56 (26.8) | .005* |
| -------------------------------------------- | ------------ | ------------ | ------------ |
| • Motor | 14/27 (51.9) | 7/15 (46.7) | |
| • Verbal | 3/27 (11.1) | 3/15 (20.0) | .82 |
| • Both | 10/27 (37.0) | 5/15 (33.3) | |
|
| |||
| 5-Year Ventilation/Perfusion (V/Q) Scan (At least 3 years of age): | |||
|
| |||
| V/Q Scan Performed | 33/49 (67.3) | 37/56 (66.1) | 1.0 |
|
| |||
| Any Ipsilateral V/Q Mismatch: | |||
| V/Q Ratio ≥ 1.2 | |||
| Yes | 29 (87.9) | 28 (75.7) | .23 |
| No | 4 (12.1) | 9 (24.3) | |
|
| |||
| Severe Ipsilateral V/Q Mismatch: | |||
| V/Q Ratio ≥ 1.44 | |||
| Yes | 20 (60.6) | 10 (27.0) | .008* |
| No | 13 (39.4) | 27 (73.0) | |
Sensitivity Analysis
Loss to follow up was not different across PS groups at either the one-year (p=.60) or five-year (p=.29) time points. Estimates of the rates of pulmonary and developmental morbidities, including all patients lost to follow up, are represented in Appendix 1 and 2. Patients on greater PS on DOL-30 continued to be associated with greater rates of pulmonary and developmental morbidities at 1 and 5-years whether all patients lost to follow up were assumed to have either universally positive or negative outcomes.
Multivariate Analysis
PS on DOL-30 was a better independent predictor of the odds of oxygen use at one year of age than either defect size or the presence of a V/Q mismatch (Table 4). PS on DOL-30 was also the best predictor of the odds of a developmental referral at one year. Furthermore, PS on DOL-30 was a significant independent predictor of an asthma diagnosis and developmental referral at long-term (3–5 year) follow up (Table 5).
Table 4.
Logistic regression analysis of one-year pulmonary and developmental outcomes adjusted for defect size, Ipsilateral V/Q Mismatch, and degree of pulmonary support on day of life 30.
| At One Year: | On Oxygen | Referral for Developmental Delays | ||
|---|---|---|---|---|
|
| ||||
| Variable | Odds Ratio (95%CI) | p-value | Odds Ratio (95%CI) | p-value |
|
| ||||
|
Defect Size: C/D (Large/Agenesis) |
2.02 (.51–8.92) | .32 | 2.30 (.89–5.92) | .09 |
|
| ||||
| Ipsilateral V/Q Mismatch At 1 Year | 0.67 (.19–2.33) | .53 | 3.11 (1.28–8.05) | .01* |
|
| ||||
| Pulmonary Support on DOL-30 | ||||
| Invasive Support | 28.75 (5.62–232.94) | <.001* | 5.09 (1.61–17.19) | .006* |
| Noninvasive Support | 5.35 (.93–43.14) | .06 | 3.29 (1.17–9.41) | .02* |
| Room Air | 1.0 | Ref. | 1.0 | Ref. |
Table 5.
Logistic regression analysis of Long-term pulmonary and developmental outcomes adjusted for defect size, Ipsilateral V/Q Mismatch, and degree of pulmonary support on day of life 30.
| At Long-term 5 Year Follow-Up: | Asthma + Inhaler | Referral for Developmental Delays | ||
|---|---|---|---|---|
|
| ||||
| Variable | Odds Ratio (95%CI) | p-value | Odds Ratio (95%CI) | p-value |
|
| ||||
| Defect Size: | ||||
|
| ||||
| C/D (Large/Agenesis) | 0.74 (.21–2.30) | .60 | 0.78 (.20–2.63) | .70 |
|
| ||||
| Ipsilateral V/Q Mismatch At 1 Year | 1.30 (.52–3.28) | .57 | 0.63 (.23–1.67) | .35 |
|
| ||||
| Pulmonary Support on DOL-30 | ||||
| On Oxygen | 3.26(1.04–11.49) | .04* | 5.51 (1.66–22.01) | .005* |
| Room Air | 1.0 | Ref. | 1.0 | Ref. |
Discussion
Survivors of congenital diaphragmatic hernia have significant long-term morbidity and mortality. A recent large-scale study of CDH patients using ECMO found that even in those patients who survive at least 90 days, only 74% are alive at five years. (26) Interestingly, in the present study of patients followed in a multidisciplinary clinic, we found a much lower rate of late death for patients surviving the initial hospitalization (1.5%). While it is difficult to determine if this low rate of late deaths was secondary to the center’s multidisciplinary clinic, other smaller studies of CDH patients with multi-specialty follow up have suggested similar outcomes. (27) Our previous analysis of the CDHSG registry found that pulmonary support (PS) on DOL-30 is the greatest predictor of inpatient mortality, length-of-stay and short-term pulmonary morbidity.(10) In the present study we found that PS on DOL-30 is also a strong independent predictor of longer-term outcomes, including pulmonary and developmental morbidity at both 1 and 5 years of age. Similar to our previous analysis, we again noted that PS on DOL-30 is a good summary indicator for the severity of pulmonary disease and highly correlated with most other predictors of outcome. (9, 10) We found that PS on DOL-30 was a stronger independent predictor of long-term morbidities than either defect size or presence of a V/Q mismatch. Taken together with the previous analyses of the CDHSG registry, we believe that the degree of pulmonary support on DOL-30 may be used as a simple cross-institutional tool to identify patients with a higher risk of long-term morbidity. While all CDH survivors could benefit from long-term multidisciplinary follow up, (3, 22) patients on room air at DOL-30 may be at lower risk of long-term complications, and therefore may require less intensive or less frequent follow up visits. We speculate that for the higher risk groups requiring pulmonary support on DOL-30, more intensive long-term multidisciplinary follow-up, including surgical, pulmonary, nutritional and developmental specialists could be of even greater benefit.
Though the aim of this paper was to compare outcomes between high and low risk patients according to their pulmonary support status on DOL-30, we found that all groups in the study had considerable long-term morbidity. Even in patients on room air on DOL-30, the lowest risk group, we noted significant rates of inhaler use, V/Q mismatch, and asthma, years after their initial hospitalization. In the first year, over 18% of patients on room air on DOL-30 were readmitted, most often for poor weight gain/failure to thrive, respiratory distress or fever.
Previous analyses have also found high rates of long-term pulmonary morbidities in survivors of CDH. In a study of adolescent CDH survivors, Trachsel et al. found significantly higher rates of asthma in survivors of CDH than in age-matched controls. (28) Other studies have found that in 8–12 year old CDH survivors may have reduced exercise tolerance compared to controls. (29) The need for ECMO and other predictors of disease severity such as the need for patch repair, have also been associated with ventilation/perfusion mismatches years later. (12, 18–20) The presence of a V/Q mismatch itself has also been demonstrated to be a sensitive predictor of future pulmonary morbidities such as obstructive pulmonary disease. (12, 19) We found that PS on DOL-30 may be at least as good of a predictor of outcome as the presence of V/Q mismatch. In our experience with the multidisciplinary clinic we have speculated that the degree of pulmonary mismatch may be closely linked with long-term pulmonary outcomes. As the group of long-term patients at the center grows, future research at will need to examine this observation. We found that greater pulmonary support on DOL-30 is associated with more severe V/Q mismatch 1 and 5-years later. Given this finding, the measurement of V/Q mismatch will continue to be an important part of our multidisciplinary clinic. We speculate that the measurement of V/Q mismatch is a useful means of predicting long-term outcomes and quantitatively following patients over time.
While we found that there was some persistent pulmonary disease even in our lowest risk group, it is clear that higher risk groups fared far worse. Greater PS on DOL-30 was associated with poorer outcomes across all measured variables. Patients with greater PS on DOL-30 were more likely to be referred for developmental therapy or early intervention at both 1 and 5 years than those only on room air on DOL-30. Previous studies from our group have also noted considerable neurodevelopmental morbidity in a subset of the present study’s cohort. Friedman et al. found that time on the ventilator significantly predicted the development of motor problems at age 1. Similar to the present study, they found that over half of their cohort had notable neurologic or neurodevelopmental concerns.(30) Several other studies have also correlated severity of disease with objective measures of neurodevelopmental deficits and functional status.(17, 31) Similarly, the severity of disease in CDH has also been frequently linked to gastrointestinal and nutritional outcomes, including the use of gastric or jejunal feeding tubes for nutritional support.(13) We also found that patients on greater PS on DOL-30 were associated with greater rates of surgical enteral access and fundoplication.
Limitations
The objective of this study was to examine the association between graded levels of pulmonary support and outcome. With inherent variability in both assessment and treatment across institutions it is likely that the absolute rates of morbidities may vary by center. The long-term outcomes of patients at our institution may not be similar to those of CDH patients at other institutions. A multicenter study will be needed to validate the present findings across the CDH study group. Given the retrospective nature of the analysis we were also limited to adjusting for risk factors available in the chart. The clinical decision to provide a given level of pulmonary support is likely affected by multiple objective and subjective variables. Prospective research will be needed to examine this decision making process. Regardless of the reasons for the use of pulmonary support, this variable appears to be highly correlated with long-term outcome and we believe it is an accurate surrogate marker for pulmonary dysfunction.
Conclusions
PS on DOL-30 is a strong independent predictor of long-term morbidity in survivors of CDH. We found that patients on room air on DOL-30 experienced near universal survival to discharge, <2% were on oxygen at 1 year, less than 20% were referred for developmental therapy by one year and only 20–30% had a diagnosis of asthma at 5 years. Conversely, patients who required more invasive PS on DOL-30 appeared to have far worse outcomes in terms of pulmonary morbidity, the reported need for developmental referrals, and the rate of future readmissions and surgical procedures. PS on DOL-30 is both a simple and strong independent predictor of long-term morbidity in CDH survivors and could be a useful tool both for family counseling and for the optimization of long-term care.
Supplementary Material
One Year Outcomes of CDH Survivors by level of pulmonary support on Day of Life 30 (Birth Year 1995–2010, N=201) assuming that all patients lost to follow up had either uniformly positive or negative outcomes.
Five Year Outcomes of CDH Survivors by level of pulmonary support on Day of Life 30 (Birth Year 1995–2006, N=151) assuming that all patients lost to follow up had either uniformly positive or negative outcomes.
Acknowledgments
This work was supported in part by Agency for Healthcare Research and Quality (AHRQ) Grant number T32HS019485 (RC), and National Institute of Child Health and Human Development (NICHD) Grant number K24HD060786 (JAF). The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S Government.
Footnotes
Author Contributions: RPC and JMW authors contributed to the study design, data collection, analysis and the drafting of this manuscript. KP, NF, SB and CS contributed to the data collection, analysis, editing and proofreading of the manuscript. JAF and DAG contributed to the study design, analysis, and drafting of this manuscript.
Funding/Disclosure:
None of the authors have commercial associations to disclose.
References
- 1.Raval MV, Wang X, Reynolds M, Fischer AC. Costs of congenital diaphragmatic hernia repair in the United States-extracorporeal membrane oxygenation foots the bill. Journal of pediatric surgery. 2011;46(4):617–24. doi: 10.1016/j.jpedsurg.2010.09.047. Epub 2011/04/19. [DOI] [PubMed] [Google Scholar]
- 2.Hospital stays, hospital charges, and in-hospital deaths among infants with selected birth defects--United States, 2003. MMWR Morbidity and mortality weekly report. 2007;56(2):25–9. Epub 2007/01/19. [PubMed] [Google Scholar]
- 3.Delacourt C, Hadchouel A, Toelen J, Rayyan M, de Blic J, Deprest J. Long term respiratory outcomes of congenital diaphragmatic hernia, esophageal atresia, and cardiovascular anomalies. Seminars in fetal & neonatal medicine. 2012;17(2):105–11. doi: 10.1016/j.siny.2012.01.008. Epub 2012/02/03. [DOI] [PubMed] [Google Scholar]
- 4.Baird R, Eeson G, Safavi A, Puligandla P, Laberge JM, Skarsgard ED. Institutional practice and outcome variation in the management of congenital diaphragmatic hernia and gastroschisis in Canada: a report from the Canadian Pediatric Surgery Network. Journal of pediatric surgery. 2011;46(5):801–7. doi: 10.1016/j.jpedsurg.2011.02.008. Epub 2011/05/28. [DOI] [PubMed] [Google Scholar]
- 5.van den Hout L, Sluiter I, Gischler S, De Klein A, Rottier R, Ijsselstijn H, et al. Can we improve outcome of congenital diaphragmatic hernia? Pediatric surgery international. 2009;25(9):733–43. doi: 10.1007/s00383-009-2425-8. Epub 2009/08/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seetharamaiah R, Younger JG, Bartlett RH, Hirschl RB. Factors associated with survival in infants with congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation: a report from the Congenital Diaphragmatic Hernia Study Group. Journal of pediatric surgery. 2009;44(7):1315–21. doi: 10.1016/j.jpedsurg.2008.12.021. Epub 2009/07/04. [DOI] [PubMed] [Google Scholar]
- 7.Lally KP, Lally PA, Lasky RE, Tibboel D, Jaksic T, Wilson JM, et al. Defect size determines survival in infants with congenital diaphragmatic hernia. Pediatrics. 2007;120(3):e651–7. doi: 10.1542/peds.2006-3040. Epub 2007/09/04. [DOI] [PubMed] [Google Scholar]
- 8.Estimating disease severity of congenital diaphragmatic hernia in the first 5 minutes of life. The Congenital Diaphragmatic Hernia Study Group. Journal of pediatric surgery. 2001;36(1):141–5. doi: 10.1053/jpsu.2001.20032. Epub 2001/01/11. [DOI] [PubMed] [Google Scholar]
- 9.van den Hout L, Reiss I, Felix JF, Hop WC, Lally PA, Lally KP, et al. Risk factors for chronic lung disease and mortality in newborns with congenital diaphragmatic hernia. Neonatology. 2010;98(4):370–80. doi: 10.1159/000316974. Epub 2010/11/03. [DOI] [PubMed] [Google Scholar]
- 10.Cauley RP, Stoffan A, Potanos K, Fullington N, Graham DA, Finkelstein JA, et al. Pulmonary Support On Day 30 As A Predictor Of Morbidity And Mortality In Congenital Diaphragmatic Hernia. Journal of pediatric surgery. 2013;48(6):1183–9. doi: 10.1016/j.jpedsurg.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortes RA, Keller RL, Townsend T, Harrison MR, Farmer DL, Lee H, et al. Survival of severe congenital diaphragmatic hernia has morbid consequences. Journal of pediatric surgery. 2005;40(1):36–45. doi: 10.1016/j.jpedsurg.2004.09.037. discussion -6. Epub 2005/05/04. [DOI] [PubMed] [Google Scholar]
- 12.Muratore CS, Kharasch V, Lund DP, Sheils C, Friedman S, Brown C, et al. Pulmonary morbidity in 100 survivors of congenital diaphragmatic hernia monitored in a multidisciplinary clinic. Journal of pediatric surgery. 2001;36(1):133–40. doi: 10.1053/jpsu.2001.20031. Epub 2001/01/11. [DOI] [PubMed] [Google Scholar]
- 13.Muratore CS, Utter S, Jaksic T, Lund DP, Wilson JM. Nutritional morbidity in survivors of congenital diaphragmatic hernia. Journal of pediatric surgery. 2001;36(8):1171–6. doi: 10.1053/jpsu.2001.25746. Epub 2001/08/02. [DOI] [PubMed] [Google Scholar]
- 14.Peetsold MG, Heij HA, Kneepkens CM, Nagelkerke AF, Huisman J, Gemke RJ. The long-term follow-up of patients with a congenital diaphragmatic hernia: a broad spectrum of morbidity. Pediatric surgery international. 2009;25(1):1–17. doi: 10.1007/s00383-008-2257-y. Epub 2008/10/09. [DOI] [PubMed] [Google Scholar]
- 15.Frisk V, Jakobson LS, Unger S, Trachsel D, O’Brien K. Long-term neurodevelopmental outcomes of congenital diaphragmatic hernia survivors not treated with extracorporeal membrane oxygenation. Journal of pediatric surgery. 2011;46(7):1309–18. doi: 10.1016/j.jpedsurg.2010.12.023. Epub 2011/07/19. [DOI] [PubMed] [Google Scholar]
- 16.Gischler SJ, van der Cammen-van Zijp MH, Mazer P, Madern GC, Bax NM, de Jongste JC, et al. A prospective comparative evaluation of persistent respiratory morbidity in esophageal atresia and congenital diaphragmatic hernia survivors. Journal of pediatric surgery. 2009;44(9):1683–90. doi: 10.1016/j.jpedsurg.2008.12.019. Epub 2009/09/09. [DOI] [PubMed] [Google Scholar]
- 17.Chen C, Jeruss S, Chapman JS, Terrin N, Tighiouart H, Glassman E, et al. Long-term functional impact of congenital diaphragmatic hernia repair on children. Journal of pediatric surgery. 2007;42(4):657–65. doi: 10.1016/j.jpedsurg.2006.12.013. Epub 2007/04/24. [DOI] [PubMed] [Google Scholar]
- 18.Hayward MJ, Kharasch V, Sheils C, Friedman S, Dunleavy MJ, Utter S, et al. Predicting inadequate long-term lung development in children with congenital diaphragmatic hernia: an analysis of longitudinal changes in ventilation and perfusion. Journal of pediatric surgery. 2007;42(1):112–6. doi: 10.1016/j.jpedsurg.2006.09.011. Epub 2007/01/09. [DOI] [PubMed] [Google Scholar]
- 19.Okuyama H, Kubota A, Kawahara H, Oue T, Kitayama Y, Yagi M. Correlation between lung scintigraphy and long-term outcome in survivors of congenital diaphragmatic hernia. Pediatric pulmonology. 2006;41(9):882–6. doi: 10.1002/ppul.20466. Epub 2006/07/20. [DOI] [PubMed] [Google Scholar]
- 20.Pal K, Gupta DK. Serial perfusion study depicts pulmonary vascular growth in the survivors of non-extracorporeal membrane oxygenation-treated congenital diaphragmatic hernia. Neonatology. 2010;98(3):254–9. doi: 10.1159/000278820. Epub 2010/04/24. [DOI] [PubMed] [Google Scholar]
- 21.Kamata S, Usui N, Kamiyama M, Tazuke Y, Nose K, Sawai T, et al. Long-term follow-up of patients with high-risk congenital diaphragmatic hernia. Journal of pediatric surgery. 2005;40(12):1833–8. doi: 10.1016/j.jpedsurg.2005.08.022. Epub 2005/12/13. [DOI] [PubMed] [Google Scholar]
- 22.Chiu PP, Ijsselstijn H. Morbidity and long-term follow-up in CDH patients. European journal of pediatric surgery : official journal of Austrian Association of Pediatric Surgery [et al] = Zeitschrift fur Kinderchirurgie. 2012;22(5):384–92. doi: 10.1055/s-0032-1329412. Epub 2012/11/02. [DOI] [PubMed] [Google Scholar]
- 23.Safavi A, Synnes AR, O’Brien K, Chiang M, Skarsgard ED, Chiu PP. Multi-institutional follow-up of patients with congenital diaphragmatic hernia reveals severe disability and variations in practice. Journal of pediatric surgery. 2012;47(5):836–41. doi: 10.1016/j.jpedsurg.2012.01.032. Epub 2012/05/19. [DOI] [PubMed] [Google Scholar]
- 24.Tsao K, Lally PA, Lally KP. Minimally invasive repair of congenital diaphragmatic hernia. Journal of pediatric surgery. 2011;46(6):1158–64. doi: 10.1016/j.jpedsurg.2011.03.050. Epub 2011/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsao K, Lally KP. Innovations in the surgical management of congenital diaphragmatic hernia. Clinics in perinatology. 2012;39(2):363–74. doi: 10.1016/j.clp.2012.04.002. Epub 2012/06/12. [DOI] [PubMed] [Google Scholar]
- 26.Iguchi A, Ridout DA, Galan S, Bodlani C, Squire K, O’Callaghan M, et al. Long-term survival outcomes and causes of late death in neonates, infants, and children treated with extracorporeal life support. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2013;14(6):580–6. doi: 10.1097/PCC.0b013e3182917a81. Epub 2013/07/05. [DOI] [PubMed] [Google Scholar]
- 27.Rocha G, Azevedo I, Pinto JC, Guimaraes H. Follow-up of the survivors of congenital diaphragmatic hernia. Early human development. 2011 doi: 10.1016/j.earlhumdev.2011.08.025. Epub 2011/09/29. [DOI] [PubMed] [Google Scholar]
- 28.Trachsel D, Selvadurai H, Bohn D, Langer JC, Coates AL. Long-term pulmonary morbidity in survivors of congenital diaphragmatic hernia. Pediatric pulmonology. 2005;39(5):433–9. doi: 10.1002/ppul.20193. Epub 2005/02/11. [DOI] [PubMed] [Google Scholar]
- 29.van der Cammen-van Zijp MH, Gischler SJ, Hop WC, de Jongste JC, Tibboel D, Ijsselstijn H. Deterioration of exercise capacity after neonatal extracorporeal membrane oxygenation. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 2011;38(5):1098–104. doi: 10.1183/09031936.00076410. Epub 2011/05/05. [DOI] [PubMed] [Google Scholar]
- 30.Friedman S, Chen C, Chapman JS, Jeruss S, Terrin N, Tighiouart H, et al. Neurodevelopmental outcomes of congenital diaphragmatic hernia survivors followed in a multidisciplinary clinic at ages 1 and 3. Journal of pediatric surgery. 2008;43(6):1035–43. doi: 10.1016/j.jpedsurg.2008.02.029. Epub 2008/06/19. [DOI] [PubMed] [Google Scholar]
- 31.Madderom MJ, Toussaint L, van der Cammen-van Zijp MH, Gischler SJ, Wijnen RM, Tibboel D, et al. Congenital diaphragmatic hernia with(out) ECMO: impaired development at 8 years. Archives of disease in childhood Fetal and neonatal edition. 2012 doi: 10.1136/archdischild-2012-303020. Epub 2012/12/14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
One Year Outcomes of CDH Survivors by level of pulmonary support on Day of Life 30 (Birth Year 1995–2010, N=201) assuming that all patients lost to follow up had either uniformly positive or negative outcomes.
Five Year Outcomes of CDH Survivors by level of pulmonary support on Day of Life 30 (Birth Year 1995–2006, N=151) assuming that all patients lost to follow up had either uniformly positive or negative outcomes.