Abstract
Background
Clinical trials of agents targeting the vascular endothelial growth factor A (VEGF-A) pathway in gastric adenocarcinoma (GA) suggest that these therapies may have varying efficacy in different races.
Methods
VEGF-A in serum and/or VEGF receptor 2 (VEGFR-2) in CD31-positive tumor vessels (VEGFR-2/CD31) were measured in 118 Caucasians and 263 Asians who underwent gastric resection at two institutions and correlated with overall survival (OS). Blood was drawn before any treatment. Patients receiving neoadjuvant treatment were excluded from VEGFR-2 analysis.
Results
Compared with Asians, Caucasians were older (mean age 66–73 vs 59–62 years), had more proximal tumors, and had more advanced TNM stage. In the VEGF-A cohort, Caucasians had a median VEGF-A level that was 95 % higher than that of Asians and a much higher standard deviation (88 ± 6.206 vs 45 ± 76 pg/ml, p < 0.001). The 5-year OS for patients with low versus high VEGF-A levels was 72 versus 43 % in Caucasians (p = 0.001) and 86 versus 77 % in Asians (p = 0.236). In the VEGFR-2 cohort, OS was worse in Caucasians with high VEGFR-2/CD31 levels (49 vs 73 %, p = 0.038), while there was no significant difference in OS in Asians (80 vs 90 %, p = 0.119). On multivariate analyses of significant prognostic factors (excluding treatment factors and margin status), serum VEGF-A and tumor VEGFR-2/CD31 levels were independent predictors of OS only in Caucasians.
Conclusions
In patients with resectable GA, VEGF-A and VEGFR-2/CD31 levels are independent predictors of OS in Caucasians but not in Asians, suggesting varying importance of this pathway in GA progression among different races.
Gastric cancer is the second leading cause of cancer death worldwide, accounting for nearly one million new cancer cases per year and almost 10 % of all cancer deaths.1 Several clinical studies support the idea of biological differences in gastric adenocarcinomas (GAs) arising in Western versus Asian patients.2,5 Given there may be differences in the biology of GA in Western and Asian patients, there may be varying responses to chemotherapy and targeted therapies depending on race or ethnicity.
Vascular endothelial growth factor A (VEGF-A) is one of the most important factors driving tumor angiogenesis; VEGF-A is produced by many different cell types and can act in an autocrine and paracrine manner on VEGF receptors (VEGF-R).6 VEGF-A exerts its effects primarily through VEGFR-1 (also called Flt-1) and VEGFR-2 (also called Flk-1 KDR).5 VEGFR-1 and VEGFR-2 are expressed primarily on endothelial cells and consist of 7 extracellular immunoglobulin-like domains, a transmembrane region, and an intracellular consensus tyrosine kinase domain.6 VEGFR-1 by itself is generally thought to transmit only weak mitogenic signals but can heterodimerize with VEGFR-2, forming a complex with strong signaling properties.7 VEGFR-2 seems to mediate the major growth and permeability actions of VEGF-A.8 VEGF receptor 2 (VEGFR-2) is the primary receptor for VEGF-A on endothelial cells.
VEGF-A levels in gastric tumors and circulating in the blood correlate with extent of disease, recurrence, and survival.7 The VEGF-A pathway has been a well-studied target in GA clinical trials. Clinical trials have examined VEGF-A and VEGFR-2 targeted agents in combination with chemotherapy in patients with metastatic GA, and results suggest the efficacy of these therapies may vary among patients of different races in Eastern versus Western countries.8,9
In this study, we sought to examine pretreatment levels of serum VEGF-A and levels of VEGFR-2 in tumor blood vessels in Caucasian and Asian patients with resectable GA and to correlate these levels with outcomes.
METHODS
Patients
Patients with adenocarcinomas arising in the stomach or gastroesophageal junction (GEJ) Siewert type II or III who underwent radical gastrectomy or esophagogastrectomy with potentially curative intent (R0 and R1) from May 2006 to March 2012 were included. The MSKCC, Seoul National University Hospital (SNUH), and Seoul National University Bundang Hospital (SNUBH) Institutional Review Boards approved this study, and informed consents for study of blood and tumor tissue were obtained preoperatively from all patients. For the VEGF-A cohort, 181 Caucasian patients were treated at MSKCC and designated as the Caucasian group. A total of 115 Asian patients from SNUH and SNUBH were designated as the Asian group. For the VEGFR-2/CD31 cohort, those patients who received neoadjuvant treatment and/or did not have tumor tissue available were excluded from the Caucasian group, leaving 42 patients. SNUH/SNUBH patients who did not have blood available but did have tumor tissue available were added to this cohort, giving us 263 Asian patients. Tumor staging was determined from the surgical specimen and was based on the seventh edition of American Joint Committee on Cancer TNM staging system.10
Blood Sample Collection and Enzyme-Linked Immunosorbent Assay (ELISA)
All patients had blood samples drawn before surgery and neoadjuvant therapy. Blood samples were collected in plain vacuum tubes and coagulated at room temperature. They were centrifuged at 1000×g for 10 min followed by serum collection. Serums were aliquoted and stored at −80 °C until analyses were performed. Serum samples were measured for VEGF-A using the commercially available Human VEGF Duoset ELISA kits (R&D Systems, Minneapolis, MN) as previously described.11 ELISA plates were read using the Emax Precision Microplate Reader (Molecular Devices, Sunnyvale, CA), and sample values were determined against a 4-parameter standard curve. The mean value of duplicate samples was used as the final concentration. Intra-assay and inter-assay validation were also performed and found to be <15 and 15 %, respectively. All samples were measured in one laboratory (S.S.Y.).
Analysis of VEGFR-2 Expression in Tumor Vessels
Immunohistochemical analysis of tissue arrays of paraffin-embedded GA was performed using antibodies for VEGFR-2 (55B11 Cell Signaling Technology, Beverly, MA) and CD31 (PECAM-,1 Dako, Carpentaria, CA), an endothelial cell marker. The mean number of VEGFR-2-and CD31-positive vessels per mm2 for each tumor was quantified. The percentage of VEGFR-2-positive vessels per tumor was calculated as: [(number of VEGFR-2 vessels per mm2/number of CD31 vessels per mm2) × 100]. All samples were analyzed in one laboratory (A.N.S.).
Statistical Analysis
Clinicopathological characteristics were compared between Caucasian and Asian groups using Chi square test and Mann–Whitney U test. Levels of serum VEGF-A and VEGFR-2/CD31 between patients were compared by the Kruskal–Wallis and Mann–Whitney U tests. Overall survival (OS) curves were plotted by the Kaplan–Meier method and compared using the log-rank test. Cutoff values for VEGF-A and VEGFR-2/CD31 were determined by analyzing receiver operating characteristics curves. A Cox proportional hazards regression was used for univariate and multivariate analyses of prognostic factors for OS. A p value <0.05 was considered statistically significant. Analyses were performed using SPSS software for Windows version 21 (SPSS, Chicago, IL).
RESULTS
Clinical and Pathological Characteristics
The demographics and treatment of Caucasian and Asian patients in the VEGF-A cohort (n = 296) and in the VEGFR-2/CD31 cohort (n = 305) are summarized in Supplemental Table 1 There was no difference in the ratio of male to female patients, but median age was 7–11 years older in Caucasian patients. For Asian patients, tumors were more commonly located in the middle or distal stomach, and thus about three-quarters of Asian patients underwent distal gastrectomy compared to only 27.6–50.0 % of Caucasian patients. Of patients in all subgroups, 90 % or more received a D2 lymphadenectomy. Nearly one-half of Caucasian patients received neoadjuvant chemotherapy or chemoradiation in the VEGF-A cohort, and about one-quarter received postoperative chemotherapy or chemoradiation.
The pathological characteristics of tumors in the VEGF-A and VEGFR-2/CD31 cohorts are also listed in Supplemental Table 1 Tumor size was similar in all groups. Caucasian patients had significantly more proximal and GE junction tumors while Asian patients had predominantly middle and distal stomach tumors. In terms of microscopic margin, 8.3–9.5 % of Caucasian patients had a positive microscopic margin compared with 0 % for Asian patients. There were more Lauren’s intestinal type tumors in Caucasian patients (50.0–56.9 vs 45.2 %–46.1 %) and more diffuse-type tumors in Asian patients (42.6–46.4 vs 23.8–24.9 %). Tumors from Caucasian patients also had a higher incidence of vascular invasion and neural invasion and had more advanced T status, N status, and TNM stage.
Serum VEGF-A Levels
In the VEGF-A cohort, serum VEGF-A levels were measured in all patients in the VEGF-A cohort prior to any neoadjuvant therapy or surgery (Fig. 1). The median level of serum VEGF-A in Caucasian patients was nearly twice that of Asian patients, and the range of serum VEGF-A values was much greater in Caucasian patient compared with Asian patients (88.1 pg/ml ± s.d. 6.206 vs 45.2 pg/ml ± s.d. 76.3, p <0.001).
FIG. 1.
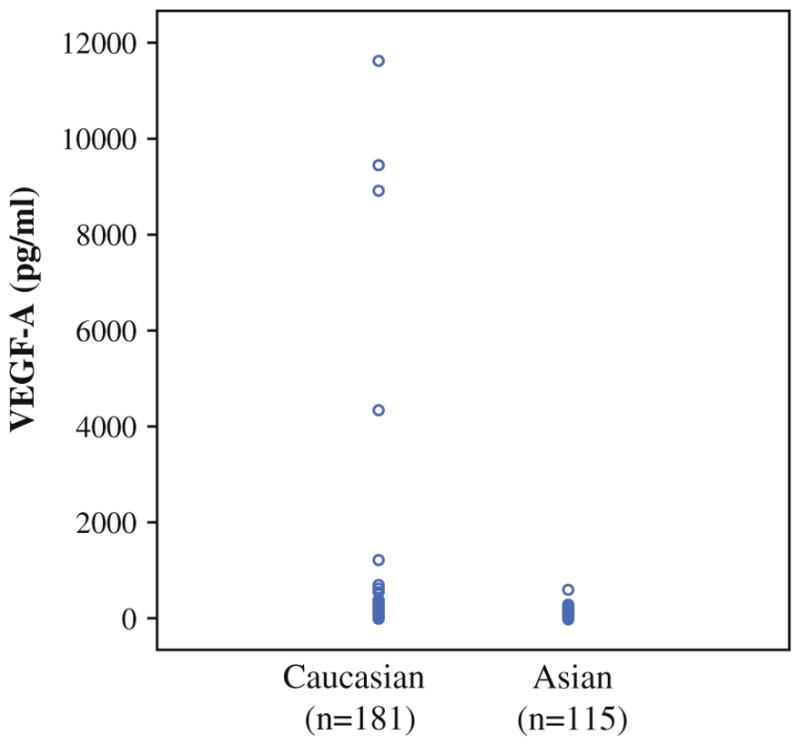
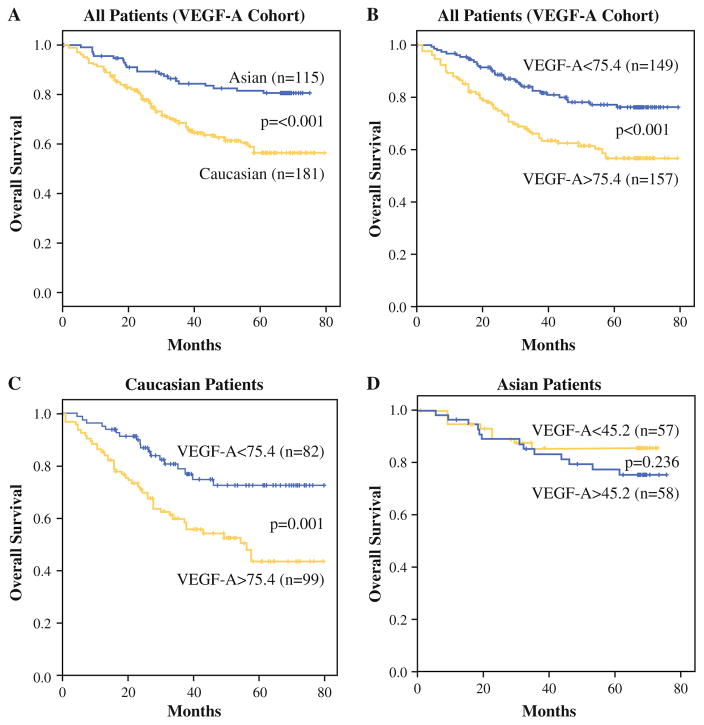
Scatter plot of serum VEGF-A levels in gastric and gastroesophageal junction cancers in Caucasian and Asian patients
Follow-up was conducted until January, 2013, and the median follow-up period was 46.8 months. At the time of last follow-up, 206 patients were alive and 90 patients were dead. Overall, Asian patients had significantly better OS compared with Caucasian patients, with the actuarial 5-year OS being 81.5 % for Asian patients compared with 56.5 % for Caucasian patients (Fig. 2a). The Kaplan–Meier OS curve according to serum VEGF-A level (cutoff value 75.4 pg/ml) for all patients is shown in Fig. 2b. For all patients, higher level of serum VEGF-A was associated with significantly poorer OS (p <0.001). When examining Caucasian and Asian patients separately, higher levels of VEGF-A correlated with worse OS in Caucasian patients (Fig. 2c) but not in Asian patients (Fig. 2d). The 5-year OS for patients with low versus high VEGF-A levels for Caucasians was 72.4 versus 43.4 % (p = 0.001) and for Asians was 85.6 versus 77.4 % (p = 0.236).
FIG. 2.
a OS of Caucasian versus Asian patients. OS of all patients (b), Caucasian patients (c), and Asian patients (d) based on low versus high VEGF-A levels
Prognostic factors of OS were investigated separately for Caucasian and Asian groups (Table 1). By univariate analysis, tumor location, margin status (R0 vs R1), neoadjuvant treatment, tumor size, grade, T status, N status, and serum VEGF-A level were statistically significant prognostic factors for OS for Caucasian patients. On multivariate analysis, serum VEGF-A remained an independent prognostic factor for OS in Caucasian patients along with tumor location, size, grade, and N status. For Asian patients, age, neoadjuvant treatment, size, vascular invasion, T status, and N status were statistically significant prognostic factors for OS for Asian patients. VEGF-A levels were not prognostic for OS in Asian patients. On multivariate analysis, age and N status were independent predictors of OS in Asian patients.
TABLE 1.
Univariate and multivariate analyses for overall survival for VEGF-A cohort
| Variable | Caucasian (n = 181)
|
Asian (n = 115)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis
|
Multivariate analysis | Univariate analysis
|
Multivariate analysi | |||||
| HR | 95 % CI | p value | p value | HR | 95 % CI | p value | p value | |
| Age | 1.015 | 0.992–1.039 | 0.200 | 1.077 | 1.031–1.125 | 0.001 | <0.001 | |
| Gender | 0.952 | 0.556–1.631 | 0.859 | 0.473 | 0.173–1.291 | 0.144 | ||
| Location | 1.295 | 1.048–1.600 | 0.017 | <0.001 | 1.145 | 0.694–1.890 | 0.596 | |
| Margin status | 4.066 | 2.152–7.680 | <0.001 | |||||
| Neoadjuvant treatment | 1.468 | 1.106–1.948 | 0.008 | 2.821 | 1.407–5.655 | 0.003 | ||
| Adjuvant treatment | 0.993 | 0.626–1.577 | 0.978 | 0.954 | 0.359–2.533 | 0.925 | ||
| Lauren classification | 0.987 | 0.728–1.338 | 0.933 | 0.930 | 0.488–1.775 | 0.827 | ||
| Tumor size | 1.134 | 1.069–1.202 | <0.001 | <0.001 | 1.225 | 1.037–1.447 | 0.017 | 0.264 |
| Vascular invasion | 1.334 | 0.808–2.201 | 0.260 | 2.662 | 1.129–6.274 | 0.025 | 0.866 | |
| Neural invasion | 1.350 | 0.819–2.228 | 0.239 | 1.989 | 0.822–4.810 | 0.127 | ||
| Grade | 2.437 | 1.344–4.417 | 0.003 | 0.010 | 0.647 | 0.273–1.537 | 0.324 | |
| T status | 1.618 | 1.277–2.050 | <0.001 | 0.151 | 1.973 | 1.314–2.962 | 0.001 | 0.051 |
| N status | 1.518 | 1.215–1.896 | <0.001 | <0.001 | 1.815 | 1.315–2.504 | <0.001 | <0.001 |
| VEGF-A level (high vs low) | 2.438 | 1.411–4.212 | 0.001 | 0.034 | 0.591 | 0.245–1.426 | 0.242 | |
Bold values are statistically significant (p < 0.05)
Location/Lauren Subtype and Serum VEGF-A Levels
In a prior study, we divided GAs into three distinct subtypes—proximal nondiffuse, distal nondiffuse, and diffuse—and found that these subtypes could be reliably distinguished by a classifier based on gene expression microarrays.12 When we divided our Caucasian patients into these three categories, there were 85 proximal non-diffuse tumors (47.5 %), 49 distal nondiffuse tumors (27.4 %), and 45 diffuse tumors (25.1 %) (Supplemental Table 2). There were significant differences between these three groups in terms of age, type of resection, neoadjuvant treatment, size, location, grade, and T stage. Furthermore, median VEGF-A levels were 83.1 and 142 % higher in proximal nondiffuse tumors compared with distal nondiffuse and diffuse tumors, respectively.
When examining OS based on these three subtypes in Caucasian patients, distal nondiffuse tumors had a better OS than proximal nondiffuse tumors or diffuse tumors (Supplemental Fig. 1a). VEGF-A levels correlated inversely with OS in all three subtypes, although this difference did not reach statistical significance for the diffuse group (Supplemental Fig. 1b–d). We performed the same analysis of the proximal nondiffuse, distal nondiffuse, and diffuse subtypes in Asian patients and found no difference in levels of VEGF-A (Supplemental Table 3) and found no correlation with OS (Supplemental Fig. 2a–c).
VEGFR-2 Expression in Tumor Vessels
We next analyzed the expression of the primary VEGF-A receptor VEGFR-2 on CD-31 positive tumor vessels (VEGFR-2/CD31) on a different cohort of Caucasian and Asian patients (VEGFR-2/CD31 cohort). All patients receiving neoadjuvant chemotherapy were excluded, and thus the size of the Caucasian cohort was reduced to 42. More Asian patients had tumor samples than blood samples available, and thus the size of the Asian cohort was increased to 263, giving a total of 305 patients. The median level of VEGFR-2/CD31 in Caucasian patients was 67.1 ± 22.2 and in Asian patients was 77.3 ± 25.4 (p = 0.050).
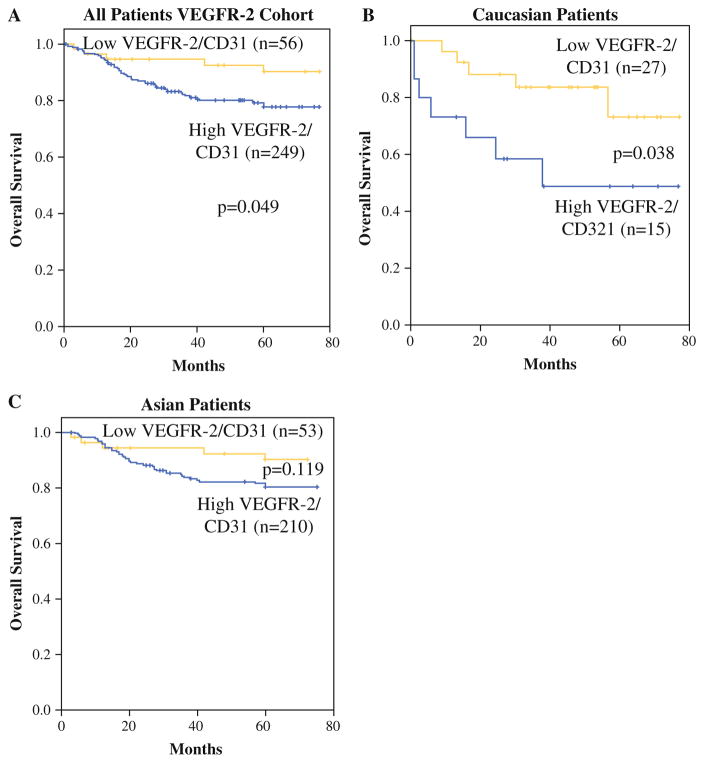
For this cohort, follow-up was conducted until January, 2013, and the median follow-up period was 60.0 months. At the time of last follow-up, 246 patients were alive and 59 patients were dead. Patients with higher VEGFR-2/CD31 levels had significantly worse OS compared with patients with lower VEGFR-2/CD31 levels (Fig. 3a). When examining Caucasian and Asian patients separately, higher levels of VEGFR-2/CD31 correlated with worse OS in Caucasian patients (Fig. 3b) but not Asian patients (Fig. 3c). The 5-year OS for patients with low versus high VEGFR-2/CD31 levels for Caucasians was 73.2 versus 48.9 % (p = 0.038) and for Asians was 90.0 versus 80.0 % (p = 0.119). On multivariate analysis for Caucasian patients, factors associated with OS included tumor size and VEGFR-2/CD31 level (Table 2). For Asian patients, T status and N status but not VEGFR-2/CD31 were independent predictors of OS.
FIG. 3.
OS of all patents (a), Caucasian patients (b), and Asian patients (c) based on low versus high tumor VEGFR-2/CD31 levels
TABLE 2.
Univariate and multivariate analyses for overall survival for VEGFR-2 cohort
| Variable | Caucasian (n = 42)
|
Asian (n = 263)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis
|
Multivariate analysis | Univariate analysis
|
Multivariate analysis | |||||
| HR | 95 % CI | p value | p value | HR | 95 % CI | p value | p value | |
| Age | 1.045 | 0.992–1.101 | 0.097 | 1.018 | 0.990–1.046 | 0.208 | ||
| Gender | 0.633 | 0.190–2.105 | 0.456 | 0.862 | 0.470–1.582 | 0.632 | ||
| Location | 0.924 | 0.524–1.631 | 0.786 | 1.321 | 1.002–1.742 | 0.048 | 0.700 | |
| Margin status | 4.401 | 1.152–16.810 | 0.030 | 0.622 | ||||
| Adjuvant treatment | 0.796 | 0.346–1.831 | 0.591 | 4.505 | 2.587–7.845 | <0.001 | ||
| Lauren classification | 1.307 | 0.729–2.344 | 0.369 | 1.345 | 0.870–2.079 | 0.183 | ||
| Tumor size | 1.309 | 1.124–1.525 | 0.001 | 0.001 | 1.26 | 1.178–1.349 | <0.001 | 0.202 |
| Vascular invasion | 2.179 | 0.476–9.986 | 0.316 | 9.935 | 3.923–25.161 | <0.001 | 0.288 | |
| Neural invasion | 2.588 | 0.690–9.708 | 0.159 | 7.181 | 3.711–13.896 | <0.001 | 0.200 | |
| Grade | 7.049 | 0.909–54.675 | 0.062 | 1.956 | 1.056–3.623 | 0.033 | 0.155 | |
| T status | 1.687 | 0.965–2.950 | 0.067 | 3.594 | 2.511–5.144 | <0.001 | <0.001 | |
| N status | 1.468 | 0.907–2.378 | 0.118 | 3.484 | 2.492–4.871 | <0.001 | <0.001 | |
| VEGFR2/CD31 (high vs low) | 3.163 | 0.999–10.008 | 0.050 | 0.047 | 2.057 | 0.813–5.206 | 0.128 | |
Bold values are statistically significant (p < 0.05)
DISCUSSION
In this study of Caucasian and Asian patients undergoing potentially curative resection of gastric and GEJ adenocarcinoma, there were significant differences between the Caucasian and Asian patients in terms of patient demographics, use of neoadjuvant and adjuvant therapy, and pathological characteristics. In addition to these differences, levels of serum VEGF-A were significantly higher in Caucasians versus Asians. Higher VEGF-A level was an independent predictor of worse OS in Caucasians and had no correlation to OS in Asians. When tumor VEGFR-2/CD31 levels were analyzed, higher VEGFR-2/CD31 level was also an independent predictor of worse OS in Caucasians but not in Asians. This study supports the notion suggested by some clinical studies that the VEGF-A/VEGFR-2 pathway may play a greater role in GAs progression in Caucasians compared with Asians.
We excluded 26 Asian-American patients treated at MSKCC from the VEGF-A cohort. The median level of VEGF-A in Asian-Americans treated at MSKCC was 55.5 pg/ml ± s.d. 123.4. This is compared with 88.1 pg/ml ± s.d. 6.206 for Caucasians at MSKCC (p = 0.027) and 45.2 ± s.d. 76.3 for Asians at SNUBH (p = 0.547). There were only 6 Asian-Americans at MSKCC excluded from the VEGFR-2 cohort, making comparisons difficult. Thus, Asian-Americans patients at MSKCC had significantly lower levels of VEGF-A compared with Caucasian patients at MSKCC and levels similar to Asian patients at SNUBH.
Analysis of circulating factors such as VEGF-A may be helpful in determining the prognosis of gastric cancer patients undergoing surgical resection. Gastric cancer patients are most commonly staged using the American Joint Committee on Cancer (AJCC) TNM system.10 In our previous analysis of the surveillance, epidemiology, and end results (SEER) database, we found the seventh AJCC staging system misclassifies or mispredicts the prognosis of more than half of SEER patients.13 There are several alternatives to TNM-based staging systems, and our group has developed a nomogram that incorporates additional prognostic clinical factors including site of age, sex, tumor size, site of primary tumor, and Lauren subtype.14 This nomogram has been validated at other Western institutions. However, as has been demonstrated most prominently in breast cancer patients, the addition of biological biomarkers to traditional clinical parameters can significantly improve the ability to determine prognosis and make treatment decisions.15
The clinical trials of bevacizumab combined with chemotherapy for metastatic gastric cancer suggest possible differences in efficacy based on patient race or ethnicity. In 2 phase II clinical trials performed at MSKCC, bevacizumab and chemotherapy as first-line therapy led to response rates of 41–67 % and median OS of 10.8–16.8 months.16,17 These studies led to the phase III randomized AVAGAST trial, which did not find a significant difference in OS when bevacizumab was added to chemotherapy (12.1 vs 10.1 months).8 Interestingly, subgroup analysis found that patients from Europe and the Americas derived more benefit from bevacizumab (11.5 vs 6.8 months; 95 % CI 0.43–0.94) than patients from the Asia–Pacific region (13.9 vs 12.1; 95 % CI 0.75–1.25). Another phase III study (AVATAR) in Chinese patients confirmed that lack of benefit in OS in Asian patients with the addition of bevacizumab to chemotherapy.18
More recent trials of ramucirumab, an anti-VEGFR-2 antibody, have enrolled a higher proportion of non-Asian patients and have yielded positive results. The REGARD study randomized patients with advanced gastric or GEJ adenocarcinoma with disease progression after first-line chemotherapy to ramucirumab or placebo.19 Only 7 % of patients in this study were from Asia. Median OS was 5.2 months in the ramucirumab group and 3.8 months in the placebo group (p = 0.047). The RAINBOW trial randomized 665 patients to paclitaxel plus ramucirumab or paclitaxel plus placebo, and median OS was significantly improved from 7.4 to 9.6 months with the addition of ramucirumab (p = 0.017).9 This study examined 140 patients from Japan and found that ramucirumab improved response rate and progression-free survival but not OS in Japanese patients.
There are several limitations to this study. First, we demonstrate some correlations between serum VEGF-A and tumor VEGFR-2/CD31 levels and OS in highly heterogeneous groups undergoing treatment at different institutions. Given there is multiple testing, any significant association should be considered hypothesis generating and not hypothesis proving. Perhaps in the future, differences in the VEGF-A pathway between Caucasians and Asians could be put into the context of broader molecular differences as seen in the Cancer Genome Atlas study of GAs.20 Second, there are more than 300 patients in this study, but a larger number of patients would have allowed for more definitive associations and conclusions. Third, 37.5 % of patients in the VEGF-A cohort received neoadjuvant treatment, which may have affected pathological factors such as T and N status. However, only 48 patients (16 %) had a pathological treatment effect of more than 50 %. Fourth, some patients in both the VEGF-A and VEGFR-2 cohorts were excluded, and thus selection bias may exist. For example, patients receiving neoadjuvant treatment were excluded from the VEGFR-2 cohort. However, there are clearly effects of chemotherapy on VEGFR-2 expression, and thus including such patients was necessary. Lastly, there are no correlations made in this study between levels of circulating factors and response to targeted therapies for patients who developed recurrence and receive these treatments in the metastatic setting, but current studies are underway to address this issue.
In summary, our understanding of GA and other cancers continues to evolve. From a tumor biology perspective, GA is a heterogeneous disease driven by numerous growth and signaling pathways, and specific pathways play a predominant role in only a minority of tumors. Quite interestingly, specific pathways may play greater roles in tumors arising in one race or ethnicity versus another. Here we demonstrate that serum VEGF-A levels are higher in Caucasian patients with resectable GA compared with Asian patients and that VEGF-A and VEGFR-2/CD31 levels are prognostic for OS in Caucasian patients but not in Asian patients.
Supplementary Material
Footnotes
DISCLOSURE None.
Presented at the 2015 Gastrointestinal Cancers Symposium (GI ASCO), San Francisco, CA, January 15, 2015.
Electronic supplementary material The online version of this article (doi:10.1245/s10434-015-4790-y) contains supplementary material, which is available to authorized users.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–30. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 4.Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–21. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 5.Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 6.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–80. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 7.Park DJ, Thomas NJ, Yoon C, Yoon SS. Vascular endothelial growth factor a inhibition in gastric cancer. Gastric Cancer. 2015;18:33–42. doi: 10.1007/s10120-014-0397-4. [DOI] [PubMed] [Google Scholar]
- 8.Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–76. doi: 10.1200/JCO.2011.36.2236. [DOI] [PubMed] [Google Scholar]
- 9.Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–35. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 10.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7. New York: Springer; 2010. [Google Scholar]
- 11.Park DJ, Yoon C, Thomas N, et al. Prognostic significance of targetable angiogenic and growth factors in patients undergoing resection for gastric and gastroesophageal junction cancers. Ann Surg Oncol. 2014;21:1130–7. doi: 10.1245/s10434-013-3429-0. [DOI] [PubMed] [Google Scholar]
- 12.Shah MA, Khanin R, Tang L, et al. Molecular classification of gastric cancer: a new paradigm. Clin Cancer Res. 2011;17:2693–701. doi: 10.1158/1078-0432.CCR-10-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Dang P, Raut CP, et al. Comparison of a lymph node ratio-based staging system with the 7th AJCC system for gastric cancer: analysis of 18,043 patients from the SEER database. Ann Surg. 2012;255:478–85. doi: 10.1097/SLA.0b013e31824857e2. [DOI] [PubMed] [Google Scholar]
- 14.Kattan MW, Karpeh MS, Mazumdar M, Brennan MF. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J Clin Oncol. 2003;21:3647–50. doi: 10.1200/JCO.2003.01.240. [DOI] [PubMed] [Google Scholar]
- 15.Oakman C, Santarpia L, Di LA. Breast cancer assessment tools and optimizing adjuvant therapy. Nat Rev Clin Oncol. 2010;7:725–32. doi: 10.1038/nrclinonc.2010.170. [DOI] [PubMed] [Google Scholar]
- 16.Shah MA, Ramanathan RK, Ilson DH, et al. Multicenter phase II study of irinotecan, cisplatin, and bevacizumab in patients with metastatic gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006;24:5201–6. doi: 10.1200/JCO.2006.08.0887. [DOI] [PubMed] [Google Scholar]
- 17.Shah MA, Jhawer M, Ilson DH, et al. Phase II study of modified docetaxel, cisplatin, and fluorouracil with bevacizumab in patients with metastatic gastroesophageal adenocarcinoma. J Clin Oncol. 2011;29:868–74. doi: 10.1200/JCO.2010.32.0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shen L, Li J, Xu J, et al. Bevacizumab plus capecitabine and cisplatin in Chinese patients with inoperable locally advanced or metastatic gastric or gastroesophageal junction cancer: randomized, double-blind, phase III study (AVATAR study) Gastric Cancer. 2015;18:168–76. doi: 10.1007/s10120-014-0351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–9. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 20.Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.