Atrial fibrillation (AF) is the most common sustained clinical arrhythmia, and is an increasing problem in terms of prevalence, complications, and health costs.1 The options for treating AF are limited. Drug therapy has limited efficacy and non-trivial risks, such as ventricular arrhythmia. Although non-pharmacological treatment options like ablation are constantly improving, complications and recurrences continue to be problematic.1,2 Progression from paroxysmal to persistent to long-term persistent forms often appears to be inexorable and makes rhythm-control increasingly difficult.2 Novel approaches are therefore needed for AF management to improve.3 Significant therapeutic advancement is closely linked to improved understanding of underlying basic mechanisms.4,5 In this issues of Cardiovascular Research, Zahid et al.6 report the results of studies that use innovative methods to understanding the mechanisms maintaining persistent AF, providing information of pathophysiological and potential practical significance. We will consider the problem that they address, the approach that they take, the importance of their findings and the limitations of their work.
1. The problem
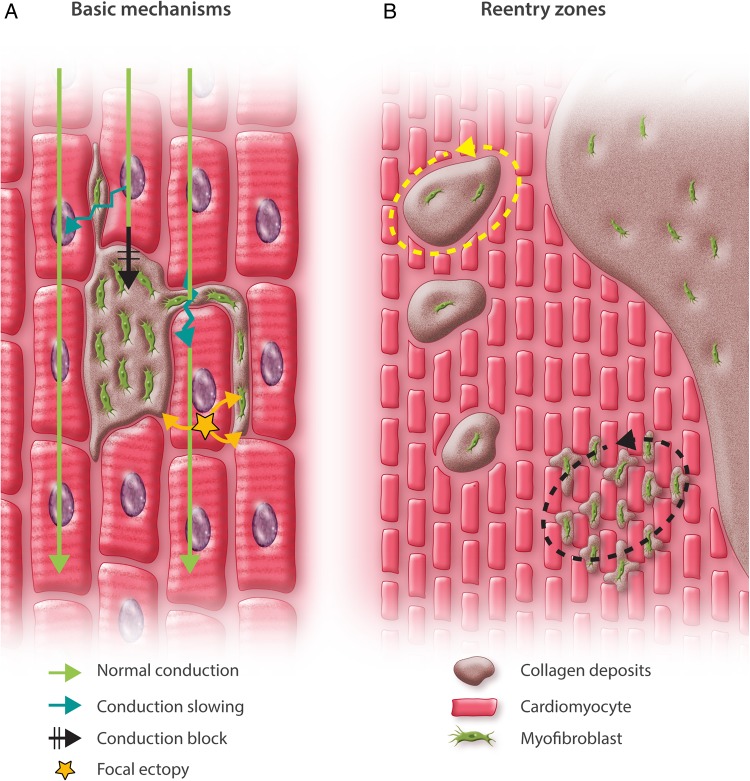
The first evidence directly associating atrial fibrosis with AF was published in 1999.7 Since then, there has been increasing data supporting the clinical importance of this association.1 Early suggestions that fibrosis promotes AF through local conduction abnormalities7 were reinforced by studies showing that fibrosis and associated conduction abnormalities support AF maintenance in the absence of cardiomyocyte ion-current abnormalities.8,9 In addition to direct interference with conduction via excess extracellular matrix (ECM) deposition, fibrosis is associated with fibroblast proliferation and differentiation into collagen-secreting myofibroblasts. Fibroblasts and myofibroblasts are capable of connecting electrically to cardiomyocytes and modulating their electrical activity10 to promote reentry11 and enhance automaticity.12 Thus, fibrosis can contribute to AF in a number of ways (Figure 1A).
Figure 1.
(A) Basic mechanisms through which fibrosis affects atrial electrophysiology. Cardiomyocytes (red) are arranged in tissue bundles. Fibrosis involves fibroblast proliferation and differentiation into myofibroblasts (green), which produce increased extracellular matrix protein (ECM, brown). The resulting ECM deposits (consisting principally of collagen) can interrupt muscle-bundle continuity and interfere with connexin-containing tight-junction formation, interfering with conduction and causing conduction-slowing and conduction-block. In addition, coupling between cardiomyocytes and fibroblasts/myofibroblasts can alter cardiomyocyte electrical activity, promoting ectopic firing (yellow star) and contributing to conduction-slowing. (B) Possible explanations for the localization of re-entrant drivers (dashed arrows) in zones with intermingling between cardiomyocytes and fibrotic tissue. For discussion, see text.
There is extensive evidence for the clinical importance of fibrosis in AF. Magnetic resonance imaging (MRI) methods have been developed using late gadolinium enhancement (LGE) to detect and non-invasively quantify atrial fibrosis.13 The extent of fibrosis has a close relationship to the risk of clinical complications.13 AF progression is a major clinical problem,2 with clinical and experimental evidence pointing to atrial fibrosis as a central contributor.14–16 Although it is clear that fibrosis is associated with AF, and a variety of candidate mechanisms have been suggested to explain the association, the precise role of fibrosis in AF initiation and maintenance is unclear.
2. The approach
Zahid et al. create in silico representations of the atria of 20 patients with persistent AF who had undergone electrophysiological study at the University of Bordeaux from June 2013 to October 2014.6 Atrial anatomy and fibrosis distribution are simulated based on MRI data. Key metrics included local fibrosis density (proportion of local elements composed of fibrous tissue) and fibrosis entropy (the fraction of elements adjacent to any given element that are fibrotic)—fibrosis density and fibrosis entropy are clearly closely related. They also use a human atrial action potential model,17 modified to reproduce AF-related remodelling,18 to simulate cardiomyocyte electrical activity. The action potential model was altered in fibrotic zones to represent presumed effects of fibrosis on cardiac ion-currents. Fibrosis was further modelled by reducing tissue conductivity to account for the effects of collagen deposition and gap-junction remodelling. Simulated decremental cycle-length pacing was applied at 30 atrial sites to induce AF. Reentrant drivers (reentries of consistent location with at least two rotations) were identified during 2.5-s simulations and their phase singularities (PSs) were tracked. PSs are identified by a mathematical transformation of activation electrophysiological data that allows for clearer identification of the PS central pivot-zone around which a reentrant circuit rotates. A particular strength of the study was the correlation that it provided between experimental results and clinical observations with electrocardiographic imaging (ECGi) in the same patients.
3. The results
AF was inducible in 13 of 20 patient models, with fibrosis density and entropy being significantly greater in inducible cases. Reentrant drivers occurred in spatially distinct regions, with an average of 2.7 ± 1.5 (mean ± standard deviation) reentrant drivers in each model. PSs were located in zones of substantial but incomplete fibrosis and intermingling between fibrotic and non-fibrotic tissues, with fibrotic densities averaging 0.63 ± 0.17 in regions with reentry PSs vs. 0.13 ± 1.9 in non-reentry zones. The use of non-remodelled membrane properties in fibrotic zones eliminated AF inducibility. Sensitivity to the MRI definition of fibrous tissue was examined by raising the LGE threshold for fibrous tissue definition in non-inducible cases so that the biatrial fibrosis burden increased from 16 ± 4 to 21 ± 4%. With this change, AF became inducible in five of the seven models in which it could not be induced before. Comparison with clinical data showed that just over half (57 ± 9%) of reentry-zone PSs in clinical studies corresponded to PS areas in simulations.
4. The importance
These studies provide support for the mechanistic role of atrial fibrosis in persistent AF patients and provide insights into the underlying basis. The results are consistent with clinical observations that reentrant drivers localize to border zones between fibrotic and non-fibrotic tissues and suggest potential explanations (illustrated in Figure 1B). One possibility (yellow dashed arrow) is that reentrant drivers are stabilized by discrete fibrotic areas surrounded by viable cardiomyocytes, with the fibrosis-density requirement reflecting areas of contiguous electrically active cardiomyocytes around fibrotic reentry-stabilizing axes that act as a kind of anchor for reentry. Another possibility (black dashed arrow) is that reentrant drivers require critical intermingling of collagen deposits and fibroblasts/myofibroblasts coupled to cardiomyocytes to create the conditions for reentry. Reentry does not occur in densely fibrotic areas because there are not enough cardiomyocytes to allow for impulse propagation. Nor does it occur in mostly normal tissue because any reentry circuits that are established are unstable and self-terminate promptly. These possibilities are not mutually exclusive and some combination may be operative. Recently, the stabilization of atrial reentry in regions of heterogeneous fibrosis distribution has been attributed to complex conduction patterns through such zones via a process called ‘percolation’.19
The approach developed in this study may lead to practical improvements in AF management. Simulated ablation lesions can be applied in the model and related to the ablation response in the same patient, providing insights into the basis for success or failure of ablation in suppressing reentrant drivers and preventing AF. Further development might allow for online simulations to be performed either before or during ablation procedures to plan patient-specific lesion sets.
5. The limitations
Despite the great strength of the Zahid studies, they do have some limitations. Cardiomyocytes in fibrotic zones were assumed to have remodelled ion-current properties and these were found to be necessary for AF-induction. However, studies in fibrotically remodelled but haemodynamically recovered dog atria show full normalization of cardiomyocyte ion-channel remodelling.8 Further work is therefore needed to clarify ion-current properties in fibrotic zones of human atria and how they differ from non-fibrotic regions in the same hearts. Another limitation is that the model does not consider discrete contributions of epicardial and endocardial tissue layers and their complex interconnections, which appear to have considerable potential importance in AF.14,15 Finally, their modelling does not consider ectopic sources, which may be important even in long-standing persistent AF.20
6. Conclusions
Zahid et al. are to be congratulated for providing this exciting paper, which advances our understanding of the mechanisms determining AF persistence, an important clinical problem. In addition, their work provides important and powerful new tools that will lead to new advances in AF pathophysiology and hopefully to improved clinical management.
Conflict of interest: none declared.
Funding
The study was supported by Canadian Institutes of Health Research (grants 6957, 44365) and Heart and Stroke Foundation of Canada.
References
- 1.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res 2014;114:1453–1468. [DOI] [PubMed] [Google Scholar]
- 2.Nattel S, Guasch E, Savelieva I, Cosio FG, Valverde I, Halperin JL, Conroy JM, Al-Khatib SM, Hess PL, Kirchhof P, De Bono J, Lip GY, Banerjee A, Ruskin J, Blendea D, Camm AJ. Early management of atrial fibrillation to prevent cardiovascular complications. Eur Heart J 2014;35:1448–1456. [DOI] [PubMed] [Google Scholar]
- 3.Nattel S, Andrade J, Macle L, Rivard L, Dyrda K, Mondesert B, Khairy P. New directions in cardiac arrhythmia management: present challenges and future solutions. Can J Cardiol 2014;30(Suppl):S420–S430. [DOI] [PubMed] [Google Scholar]
- 4.Nishida K, Datino T, Macle L, Nattel S. Atrial fibrillation ablation: translating basic mechanistic insights to the patient. J Am Coll Cardiol 2014;64:823–831. [DOI] [PubMed] [Google Scholar]
- 5.Heijman J, Algalarrondo V, Voigt N, Melka J, Wehrens XH, Dobrev D, Nattel S. The value of basic research insights into atrial fibrillation mechanisms as a guide to therapeutic innovation: a critical analysis. Cardiovasc Res 2016;109:467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zahid S, Cochet H, Boyle PM, Schwarz EL, Whyte KN, Vigmond EJ, Dubois R, Hocini M, Haïssaguerre M, Jaïs P, Trayanova NA. Patient-derived models link re-entrant driver localization in atrial fibrillation to fibrosis spatial pattern. Cardiovasc Res 2016;110:443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation 1999;100:87–95. [DOI] [PubMed] [Google Scholar]
- 8.Cha TJ, Ehrlich JR, Zhang L, Shi YF, Tardif JC, Leung TK, Nattel S. Dissociation between ionic remodeling and ability to sustain atrial fibrillation during recovery from experimental congestive heart failure. Circulation 2004;109:412–418. [DOI] [PubMed] [Google Scholar]
- 9.Burstein B, Comtois P, Michael G, Nishida K, Villeneuve L, Yeh YH, Nattel S. Changes in connexin expression and the atrial fibrillation substrate in congestive heart failure. Circ Res 2009;105:1213–1222. [DOI] [PubMed] [Google Scholar]
- 10.Yue L, Xie J, Nattel S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res 2011;89:744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zlochiver S, Muñoz V, Vikstrom KL, Taffet SM, Berenfeld O, Jalife J. Electrotonic myofibroblast-to-myocyte coupling increases propensity to reentrant arrhythmias in two-dimensional cardiac monolayers. Biophys J 2008;95:4469–4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miragoli M, Salvarani N, Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res 2007;101:755–758. [DOI] [PubMed] [Google Scholar]
- 13.McGann C, Akoum N, Patel A, Kholmovski E, Revelo P, Damal K, Wilson B, Cates J, Harrison A, Ranjan R, Burgon NS, Greene T, Kim D, Dibella EV, Parker D, Macleod RS, Marrouche NF. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ Arrhythm Electrophysiol 2014;7:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allessie MA, de Groot NM, Houben RP, Schotten U, Boersma E, Smeets JL, Crijns HJ. Electropathological substrate of long-standing persistent atrial fibrillation in patients with structural heart disease: longitudinal dissociation. Circ Arrhythm Electrophysiol 2010;3:606–615. [DOI] [PubMed] [Google Scholar]
- 15.Verheule S, Tuyls E, Gharaviri A, Hulsmans S, van Hunnik A, Kuiper M, Serroyen J, Zeemering S, Kuijpers NH, Schotten U. Loss of continuity in the thin epicardial layer because of endomysial fibrosis increases the complexity of atrial fibrillatory conduction. Circ Arrhythm Electrophysiol 2013;6:202–211. [DOI] [PubMed] [Google Scholar]
- 16.Martins RP, Kaur K, Hwang E, Ramirez RJ, Willis BC, Filgueiras-Rama D, Ennis SR, Takemoto Y, Ponce-Balbuena D, Zarzoso M, O'Connell RP, Musa H, Guerrero-Serna G, Avula UM, Swartz MF, Bhushal S, Deo M, Pandit SV, Berenfeld O, Jalife J. Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation. Circulation 2014;129:1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Courtemanche M, Ramirez RJ, Nattel S. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol 1998;275(Pt 2):H301–H321. [DOI] [PubMed] [Google Scholar]
- 18.Krummen DE, Bayer JD, Ho J, Ho G, Smetak MR, Clopton P, Trayanova NA, Narayan SM. Mechanisms of human atrial fibrillation initiation: clinical and computational studies of repolarization restitution and activation latency. Circ Arrhythm Electrophysiol. 2012;5:1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vigmond E, Pashaei A, Amraoui S, Cochet H, Hassaguerre M. Percolation as a mechanism to explain atrial fractionated electrograms and reentry in a fibrosis model based on imaging data. Heart Rhythm. 2016; doi:10.1016/j.hrthm.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Kurotobi T, Shimada Y, Kino N, Ito K, Tonomura D, Yano K, Tanaka C, Yoshida M, Tsuchida T, Fukumoto H. Residual arrhythmogenic foci predict recurrence in long-standing persistent atrial fibrillation patients after sinus rhythm restoration ablation. Can J Cardiol 2014;30:1535–1540. [DOI] [PubMed] [Google Scholar]