Abstract
Objective
The existing screening batteries assessing multiple neuropsychological functions are not specific to amyotrophic lateral sclerosis (ALS) patients and are limited to their physical dysfunctions, whereas category cognitive tests are too time-consuming to assess all the domains. The Edinburgh Cognitive and Behavioural ALS Screen (ECAS) was recently developed as a fast and easy cognitive screening tool specifically designed for patients. The purpose of the study was to validate the effectiveness of the Chinese version in Chinese ALS populations.
Methods
Eighty-four ALS patients and 84 age-, gender- and education-matched healthy controls were included in this cross-sectional study. All the participants took the ECAS, Mini-Mental State Examination (MMSE) and Frontal Assessment Battery (FAB). Primary caregivers of patients were interviewed for behavioural and psychiatric changes.
Results
Significant differences were noted in language (p = 0.01), fluency, executive function, ALS-specific functions, and ECAS total score (p<0.01) between ALS patients and controls. The cut-off value of the total ECAS score was 81.92. Cognitive impairment was observed in 35.71% of patients, and 27.38% exhibited behavioural abnormalities. The ECAS total score had a medium correlation with education year. Memory was more easily impaired in the lower education group, whereas verbal fluency and language function tended to be preserved in the higher education group. The average time of ECAS was only 18 minutes.
Conclusion
The Chinese version of the ECAS is the first screening battery assessing multiple neuropsychological functions specially designed for the ALS population in China, which provides an effective and rapid tool to screen cognitive and behavioural impairments.
Introduction
Amyotrophic lateral sclerosis (ALS) is a multiple-system neurodegenerative disease, and its extra-motor disorders have received increasing concern in recent years. Frontotemporal dementia (FTD) has been known to overlap with ALS. Strong et al. proposed the concept of ALS cognitive impairment (ALS-ci) and behaviour impairment (ALS-bi) [1]. Researchers subsequently found that 10 to 60% of patients had the diagnosis of ALS-ci [2]. Executive difficulty is a typical ALS cognitive domain of which we are familiar [3]. However, non-executive cognitive dysfunctions are also present in ALS [4–6]. These dysfunctions include language, memory and some cases of psychiatric change [7–11]. Thus, different scales are necessary to conduct a comprehensive assessment. Some groups have already undergone screening [12,13], and some measurements have been created [14–20]. However, a single domain cannot reflect the entire profile of cognitive and behavioural change. It is too time-consuming to assess all the domains. Thus, a screening battery assessing multiple neuropsychological functions is needed. Unfortunately, classical measurements, such as the Mini-Mental State Examination (MMSE) [21] and Montreal Cognitive Assessment (MOCA) [22], are not sensitive for the ALS population and have significant limitations for those with physical dysfunctions.
The Edinburgh Cognitive and Behavioural ALS Screen (ECAS) [23] is a brief assessment tool that was designed specifically for ALS patients. The screen includes ALS-specific functions (language, verbal fluency and executive functions) and ALS-non-specific functions (memory and visuospatial functions). Furthermore, primary caregivers completed a questionnaire for five-domain characteristic behavioural changes of FTD and three psychotic questions. The ECAS provides a rapid measurement of cognitive and behavioural functions for ALS, which is not influenced by physical disorders and reflects the severity and nature of the disease. Abrahams’s group later validated the ECAS against a gold standard extensive neuropsychology assessment and demonstrated that the ECAS was a screening tool with high sensitivity and specificity characteristic ALS impairments [24]. The German/Swiss-German version also demonstrated that the ECAS was a fast and easy cognitive screening instrument that was sensitive for ALS-specific dysfunctions and behavioural changes [25].
Regarding the Chinese population, cognitive changes are obviously different between cultures and languages [26]. However, limited assessments have been made in Chinese ALS patients. Yuan et al. investigated the cognition function in 22 early phase patients [27]. Wei et al. [28] screened 145 patients using the MMSE and the revised Addenbrooke’s Cognitive Examination (ACE-R). Cui et al. [29] conducted a neuropsychological investigation between ALS and progressive muscular atrophy (PMA) patients. However, the measurement tools used were time-consuming and potentially restricted by physical dysfunction. Thus, a rapid assessment tool designed especially for Chinese ALS patients is urgent.
The purpose of this research is to validate the Chinese translation of the ECAS as an effective measurement for the Chinese ALS population.
Materials and Methods
Participants
We enrolled 84 patients who met the ALS revised El Escorial criteria in the Peking University Third Hospital in 2015 [30]. We excluded patients with the clinical diagnosis of dementia according to ICD-10 criteria as well as those who were illiterate and could not write. As controls, we enrolled 84 healthy people without central nerve system disease who were literate and had the ability to write. No significant differences were noted between patients and healthy controls in terms of age, gender and education (Table 1).
Table 1. Characteristics of participants: ALS patients and healthy controls.
| Characteristics | ALS patients | Healthy controls | |
|---|---|---|---|
| Age (years) | 55.07(10.74)33-80 | 54.83(10.98)31-79 | |
| Gender | Female | 26 | 27 |
| Male | 58 | 57 | |
| Years of education | 11.45(3.44)6-19 | 11.51(3.36)6-19 | |
| Duration of illness (months) | 15.81(14.53)10-78 | ||
| ALSFRS-R | 41.33(4.64)23-48 | ||
| Onset of disease | Bulbar | 16(19.05%) | |
| Cervical | 49(58.33%) | ||
| Thoracic | 3(3.57%) | ||
| Lumbar | 16(19.05%) | ||
| Diagnostic level | Definite | 21(25.00%) | |
| Probable | 25(29.76%) | ||
| Lab-supported probable | 26(30.95%) | ||
| Possible | 12(14.29%) | ||
| Bulbar involved | 41(48.81%) | ||
| Ventilation | 1(1.19%) | ||
| PEG | 0 |
Mean (SD) and range are reported for age, gender, year of education, duration of illness, ALSFRS-R. Patient numbers (percentage) are reported for onset of disease, diagnostic level, bulbar involved, ventilation and PEG. There are no significant differences between patients and healthy controls in terms of age, gender and education.ALSFRS-R: Revised ALS Functional Rating Scale. PEG: Percutaneous Endoscopic Gastrostomy.
Primary caregivers of all the patients were interviewed for behavioural and psychiatric changes.
All of the interviews were performed by clinical neurologists. The total interview lasted approximately 25 to 50 minutes, depending on the physical state of the participants.
This study was approved by the Research Ethics Committee of Peking University Third Hospital. All patients were included after informed written consent obtained from patients or their guardians and controls were informed written consent by themselves, as set forth by the Declaration of Helsinki. The consent procedure was approved by ethics committees.
The Edinburgh Cognitive and Behavioural ALS Screen (ECAS) Chinese version
We translated the Chinese version from the ECAS English version after the authors granted permission, and performed a back-translation by another clinical doctor of neurology who never read the original English version before. Although there were language and cultural differences, we attempted to maintain consistency with the original document. The ECAS Chinese version still contained ALS-specific functions, including language, fluency and executive sections, and ALS-non-specific functions, including memory and visuospatial sections. In addition, five domains of FTD and three domains of psychotic changes were included to interview caregivers in a behavioural screen.
The amendment in the Chinese version compared to the original English version is listed as Table 2. During the translation, two pictures in the “naming” section and the story in the “memory” section were altered according to Chinese culture. The characters chosen for the “spelling” section followed these principles: 1) different structures: left-right structure (跨kua4, 增zeng1, 秽hui4, 短duan3, 颁ban1), top-down structure (管guan3, 焚fen2, 藏cang2), and surrounding structure (廉lian2, 固gu4, 闻wen2, 囵lun2); 2) different component: one part’s pronunciation is the same as the character (夸kua-跨kua, 官guan-管guan, 古gu-固gu, 仑lun-囵lun); one part’s pronunciation is similar to the character but not the same: (曾ceng-增zeng, 岁sui-秽hui, 臧zang-藏cang, 兼jian-廉lian); the character gets its meaning from two parts but not the pronunciation [闻(门door-耳ear-闻hear), 颁(分give-页head-颁give), 焚(木wood-火fire-焚burn), 短(矢measurement unit-豆bean pod-短short)]; 3) frequency of character: high frequency (管0.6194, 增0.3473, 固0.2008, 藏0.1217), middle frequency (短0.02063, 闻0.0089, 跨0.00376, 廉0.00149, 颁0.00144), low frequency (秽0.00044, 焚0.00044, 囵0.00006). The number was the frequency of characters according to the Modern Chinese Frequency Dictionary [31]. Verbal fluency scores were normalized according to the performance of 40 healthy subjects in the preliminary experiment (see Abrahams et al. [23] and guidelines published https://www.era.lib.ed.ac.uk/handle/1842/6592). “发fa1, 开kai1” were chosen in the Chinese version. For the “alternation” section, we chose twelve well-known Chinese zodiac signs. However, except for the two examples of “1-rat, 2-cattle law”, only 10 animals remained. Thus, the total score for this part could only be 10. An additional 2 scores were added to the “sentence completion” section. Thus, the total score for the executive function was 48.
Table 2. Amendments in the Chinese version compared to the original English version.
| Section | English version | Score | Chinese version | Score |
|---|---|---|---|---|
| Naming | Scorpion and fox | 8 | Change into centipede and owl | 8 |
| Comprehension | Do not change | 8 | Do not change | 8 |
| Spelling | English words | 12 | Chinese words | 12 |
| Verbal fluency | Letter "T" and "S" | 24 | Character "fa" and "kai" | 24 |
| Reverse digit span | Do not change | 12 | Do not change | 12 |
| Alternation | letters | 12 | Change into Chinese zodiac signs | 10 |
| Sentence completion | 6 English sentences | 12 | 7 Chinese sentences | 14 |
| Social cognition | Do not change | 12 | Do not change | 12 |
| Immediate recall | English story | 10 | Chinese story | 10 |
| Delayed retention | English story | 10 | Chinese story | 10 |
| Numberlocation | English story | 4 | Chinese story | 4 |
| Dot counting | Do not change | 4 | Do not change | 4 |
| Cube counting | Do not change | 4 | Do not change | 4 |
| Verbal fluency | Do not change | 4 | Do not change | 4 |
| Language | 28 | 28 | ||
| Verbal fluency | 24 | 24 | ||
| Executive | 48 | 48 | ||
| Memory | 24 | 24 | ||
| Visuospatial | 12 | 12 | ||
| ALS-specific functions | 100 | 100 | ||
| ALS-non-specific functions | 36 | 36 | ||
| Total ECAS score | 136 | 136 |
The table shows a comparison of each single section and score between the Chinese version and English version.
The ECAS Chinese version is available from the author.
Other neuropsychological tests
In addition to the Chinese version of the ECAS, we also chose a general cognitive scale, the MMSE [21] and Frontal Assessment Battery (FAB) [32], which is specifically used to assess frontal functions.
Statistics
Kolmogorov-Smirnov test was used for analyzing normal distribution. To compare clinical features (age and education), score of each section between patients and controls, total ECAS score between different sex and whether bulbar involved, two sided t-test was used if the variables were normally distributed and Mann Whitney U-test was used if not. One-way ANOVA was used to compare total ECAS score between different diagnostic levels and sites of onset. Categorical variable (sex between patients and controls) was compared using a chi-square test. To analyse relationship between the scores of ECAS and other neuropsychological tests, ECAS total score and clinical features (education, age, ALSFRS-R and duration of illness), correlation coefficients were determined via Pearson correlation analysis when the data were continuous, normally distributed and had a linear relationship. Otherwise, Spearman correlation analysis was performed. Cut-off scores were defined as two standard deviations below the mean of the healthy controls according to the work of Abrahams[23]. A Cronbach’s alpha test was used to evaluate reliability. A threshold of p<0.05 was used for statistical inference. Statistical analysis was performed using SPSS 18.0 statistical software.
Results
Comparison between patients and controls
We identified significant differences in language, fluency, executive function, ALS-specific functions, and the total ECAS score between ALS patients and controls, whereas no differences in memory, visuospatial and ALS-non-specific functions were noted. However memory (p = 0.07) and ALS-non-specific functions (p = 0.06) seem to have trend to be significant. The mean time of the ECAS was only 18 minutes (Table 3).
Table 3. Performances in cognitive scores and ECAS time of ALS patients and healthy controls.
| Characteristics | ALS patients | Healthy controls | p |
|---|---|---|---|
| Language | 20.01(3.94)9-28 | 21.71(3.06)13-27 | 0.01 |
| Fluency | 15.28(6.13)0-24 | 19.44(3.98)2-24 | <0.01* |
| Executive | 25.17(8.34)7-46 | 37.53(6.26)22-47 | <0.01* |
| ALS-specific functions | 60.46(13.68)30-92 | 78.68(9.85)49-96 | <0.01* |
| Memory | 14.01(4.80)0-23 | 15.60(3.89)2-23 | 0.07 |
| Visuospatial | 11.83(0.65)8-12 | 11.96(0.24)10-12 | 0.14 |
| ALS-non-specific functions | 25.85(4.94)12-35 | 27.56(3.93)14-35 | 0.06 |
| Total ECAS score | 86.31(16.51)50-122 | 106.24(12.16)68-128 | <0.01* |
| ECAS time | 18.13(3.85)11-30 | 18.77(3.21)13-28 | 0.09 |
Mean (SD) and range are reported forperformances in cognitive scores.
*p< 0.01.
ECAS cut-off score and frequency of patients with cognitive impairments
The executive function was the most common domain, with approximately half of patients performing below the cut-off score, followed by fluency (20.24%) and language (15.48%). Approximately one-third of patients had cognitive impairments according to the total ECAS score (Table 4).
Table 4. Abnormal cut-off scores and frequency of patients with cognitive impairments.
| Characteristics | Cut-off score | Patient No. | Percent |
|---|---|---|---|
| Total ECAS score | 81.92 | 30 | 35.71 |
| ALS-specific functions | 58.98 | 36 | 42.86 |
| ALS-non-specific functions | 19.70 | 7 | 8.33 |
| Executive | 25.00 | 39 | 46.43 |
| Fluency | 11.48 | 17 | 20.24 |
| Language | 15.59 | 13 | 15.48 |
| Memory | 7.82 | 7 | 8.33 |
| Visuospatial | 11.48 | 6 | 7.14 |
Cut-off score: Mean-2SD.
ECAS and other neuropsychological tests
Medium correlations were noted between the total ECAS score and FAB score as well as the scores of ALS-specific functions and FAB. Slight correlations were noted between the MMSE score and total ECAS score, ALS-specific functions score and separate ALS-non-specific functions score. No relationship was noted between the scores of ALS-non-specific functions and FAB (Table 5).
Table 5. Correlations between the scores of ECAS and other neuropsychological tests.
| Characteristics | r | p |
|---|---|---|
| Total ECAS score& MMSE | 0.48 | <0.01* |
| Total ECAS score & FAB | 0.52 | <0.01* |
| ALS-specific functions & MMSE | 0.42 | <0.01* |
| ALS-specific functions & FAB | 0.55 | <0.01* |
| ALS-non-specific functions & MMSE | 0.45 | <0.01* |
| ALS-non-specific functions & FAB | 0.29 | 0.01 |
r>0.8 implies high range correlation, 0.5<r<0.8implies middle range correlation, 0.3<r<0.5implies low range correlation, r<0.3 implies no relation.
*p< 0.01.
ECAS and clinical features
The total ECAS score and education year exhibited a moderate correlation (r = 0.61, p<0.01). However, no obvious relationship was noted between the total ECAS score and age (r = -0.23, p = 0.04), ALSFRS-R (r = 0.28, p = 0.01). Although p<0.05 here, r<0.3 implied no relationship for correlation analysis. There was also no correlation between total ECAS score and duration of illness (r = 0.07, p = 0.54). No significant differences were found in the total ECAS scores between different sexes, diagnostic levels, sites of onset, and bulbar involvement (p>0.05).
ECAS and education
Given that years of education and the total ECAS score were related, we divided the participants into a higher education group (>12 years) and a lower education group (≤12 years). No differences in age and gender were noted between the patients and controls of these two groups (p>0.05).
Interestingly, in the lower education group, significant differences were noted in memory (z = -1.99, p = 0.04) and ALS-non-specific functions (z = -2.15, p = 0.03). Language (t = -3.21, p<0.01), verbal fluency (z = -5.24, p<0.01), executive (t = -9.55, p<0.01), ALS-specific function (t = -9.54, p<0.01), and total ECAS score (t = -8.84, p<0.01) still differed between patients and controls; however, the visuospatial function did not (z = -1.50, p = 0.13).
However, in the higher education group, no differences were observed in language (t = -1.16, p = 0.25) and verbal fluency (t = -1.23, p = 0.23). Differences were noted in executive (t = -5.04, p<0.01), ALS-specific function (t = -4.46, p<0.01), and the total ECAS score (t = -4.31, p<0.01), whereas memory (z = -0.82, p = 0.41), visuospatial (z = 0.00, p = 1.00) and ALS-non-specific functions (z = -0.78, p = 0.44) did not differ.
Frequency of patients with behavioural impairments
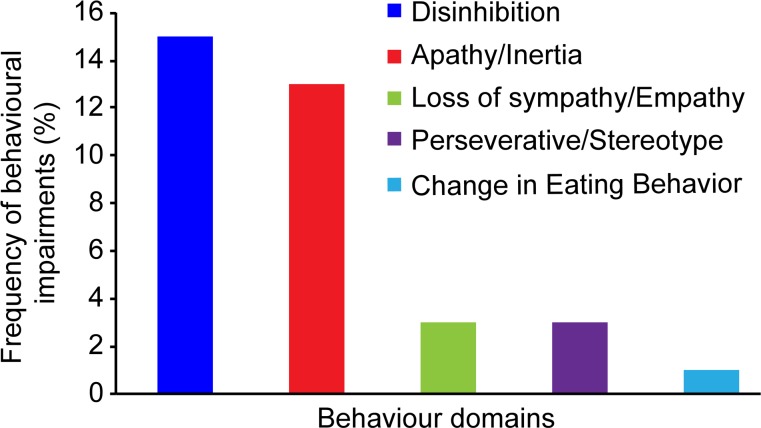
The percentage of each domain is presented in Fig 1. One domain of behavioural impairment was observed in 14.28% of patients. In addition, 11.90% of patients had two domains, and 1.19% had three. Thus, 27.38% of patients exhibited behavioural abnormalities.
Fig 1. Behaviour Domains: frequency of behavioral impairments.
15 patients had disinhibition, 13 patients had apathy/inertia, 3 patients had loss of sympathy/empathy, 3 patients had perseverative/stereotype, and 1 patient had change in eating behavior.
Reliability
The Cronbach’s alpha coefficient of the Chinese ECAS version was 0.74, which was acceptable for exploratory research.
Discussion
Comparison between patients and controls
A significant difference in the total ECAS score was noted between patients and controls, indicating that this metric is potentially an index for cognitive assessment. We anticipated differences in the language, verbal fluency, executive function, and ALS-specific functions, whereas language was not as obvious as the others. Although language impairment was reported to be more common than executive function [10], no ALS language study has been specifically performed with the Chinese population. Spelling might be the largest difference between Chinese and English. Recent research has demonstrated that orthographic skills and morphological awareness were important contributors to Chinese reading and spelling development and that memory of these character stroke orders was important for character spelling in Chinese [33]. However, these functions might not be as sensitive as the executive function in ALS patients. However, the results provided a clue for further study of ALS language disorders in the Chinese population. Memory has also been shown to be affected in recent years [8,11]. Although the p values for memory and ALS-non-specific functions were >0.05, we noticed that it approached significance. It is worth mentioning that the average time for the ECAS was only approximately 18 minutes, which made it possible to use the Chinese version of the ECAS in the clinic as a routine test.
ECAS cut-off score and frequency of patients with cognitive impairments
The cut-off value of the total ECAS score was 81.92 according to the method of Abrahams; 35.71% of patients performed below the value and were thought to have cognitive impairment. This percentage is consistent with previously reported data [2] and is slightly higher than 30.34%, which was observed by another Chinese group using ACE-R[28]. Notably, approximately half of patients exhibited executive impairment, which may cause the highly abnormal proportion of ALS-specific functions. According to unpublished data from our group, 20.0% of patients had this disorder when we screened 110 ALS patients and 96 controls for their executive functions [34]. Abnormality on more than two distinct tests that were sensitive to executive functions was used for diagnosing according to Strong’s criteria[1] in this screen. However, in the ECAS Chinese version, the executive category included digital span, alternation, sentence completion, and social recognition. Overlapping these four parts might enhance the sensitivity of the tool.
ECAS and other neuropsychological tests
As FAB was designed especially for assessing executive function and ECAS is more focused on executive function, it is easy to understand why correlations existed between the total ECAS score, ALS-specific functions score and FAB score. The MMSE is a general scale that contains different domains of cognition. Thus, this screen could have a relationship with the total ECAS score, ALS-specific functions and ALS-non-specific functions score. However, the correlations might not be as strong as FAB score given lacking pertinence. ALS-non-specific functions included memory and visuospatial, whereas FAB was used for executive function. Thus, the scores of these tests were not closely related. Given that all the results could be explained, this might enhance the reliability of the Chinese version of the ECAS.
ECAS and clinical features
We anticipated that education level would be correlated with the total ECAS score. However, gender [35] and age [25] were also thought to be important influential factors. One explanation might be that some other variants affect cognitive function, such as occupation and social economic status. However, we didn’t consider these factors in our research.
ECAS and education
Interestingly, memory was more easily impaired in the lower education group, whereas verbal fluency and language function tended to be preserved in the higher education group. A cross-sectional and longitudinal study revealed that memory decline was faster in less-educated people [36].In the ALS population, this phenomenon might be similar. There was substantial evidence of an association between higher educational levels and a decreased incidence of dementia in various populations and studies. The main hypothesis was that clinical manifestations of cognitive disorders related to brain lesions were delayed in more highly educated people [37]. The preservation of brain lesions might minimize the differences in verbal fluency and language function in ALS patients. However, this hypothesis must be proven. As a general scale, ECAS is advantageous in testing different profiles of ALS cognitive impairments and could help to further explore the nature of the disease.
Frequency of patients with behavioural impairments
The proportion of behavioural disorders was 27.38%. This result is slightly higher than our group’s previous study, which reported 20.9% with cut-off values from the Frontal Behavioural Inventory (FBI) and Neuropsychiatric Inventory (NPI) scores [34]. Only one patient exhibited impairment in three domains, which met the clinical features of FTD according to Neary’s criteria [38]. However, during the study period, there were another four ALS/FTD patients who had impairment in at least three behavioural domains. Since they had severe cognitive dysfunction, interfere with daily life and had abnormal MRI, fulfilling the dementia criteria of ICD-10, they were excluded from the 84 ALS patients when performing the cognitive test. If these four patients had been added, the FTD percentage would have reached 5.68%, which was in the range of 5 to 15% according to the literature [2,39].
Reliability
In the Chinese ECAS version, the Cronbach’s alpha coefficient was 0.74. As an exploratory research, this was acceptable (a coefficient of 0.7–0.8 is considered good to excellent). The value was 0.77 in the English version.
Conclusion
The Chinese ECAS version is the first general scale specifically designed for the Chinese ALS population. This test is an effective and rapid tool to screen cognitive and behavioural impairments in ALS patients. The Chinese ECAS may be more sensitive for assessing executive function and can help to further detect the nature of the disease. However, limitations of this research should be noted, including the lack of information about occupation, social economic status and inventories to evaluate a patient’s mood. Additionally, the Chinese ECAS version requires further validation regarding sensitivity and specificity.
Acknowledgments
The authors would like to thank all the ALS patients and their caregivers for participating in this research. The authors also thank Professors Sharon Abrahams and Thomas H. Bak for providing guidance during the translation and performance testing. We would like to thank Professors Albert C. Ludolph and Dorothee Lule for their advice during the results analysis.
Data Availability
All relevant data are within the paper.
Funding Statement
The study was supported by the National Key Clinical Specialist Construction Program of China.
References
- 1.Strong MJ, Grace GM, Freedman M, Lomen-Hoerth C, Woolley S, Goldstein LH, et al. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2009;10: 131–146. [DOI] [PubMed] [Google Scholar]
- 2.Ringholz GM, Appel SH, Bradshaw M, Cooke NA, Mosnik DM, Schulz PE. Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology. 2005;65: 586–590. [DOI] [PubMed] [Google Scholar]
- 3.Abrahams S, Leigh PN, Harvey A, Vythelingum GN, Grise D, Goldstein LH. Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS). Neuropsychologia. 2000;38: 734–747. [DOI] [PubMed] [Google Scholar]
- 4.Phukan J, Elamin M, Bede P, Jordan N, Gallagher L, Byrne S, et al. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. J Neurol Neurosurg Psychiatry. 2012;83: 102–108. 10.1136/jnnp-2011-300188 [DOI] [PubMed] [Google Scholar]
- 5.Goldstein LH, Abrahams S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: nature of impairment and implications for assessment. Lancet Neurol. 2013;12: 368–380. 10.1016/S1474-4422(13)70026-7 [DOI] [PubMed] [Google Scholar]
- 6.Abrahams S. Executive dysfunction in ALS is not the whole story. J Neurol Neurosurg Psychiatry. 2013;84: 474–475. 10.1136/jnnp-2012-303851 [DOI] [PubMed] [Google Scholar]
- 7.Ash S, Olm C, McMillan CT, Boller A, Irwin DJ, McCluskey L, et al. Deficits in sentence expression in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16: 31–39. 10.3109/21678421.2014.974617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Machts J, Bittner V, Kasper E, Schuster C, Prudlo J, Abdulla S, et al. Memory deficits in amyotrophic lateral sclerosis are not exclusively caused by executive dysfunction: a comparative neuropsychological study of amnestic mild cognitive impairment. BMC Neurosci. 2014;15: 83 10.1186/1471-2202-15-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mioshi E, Caga J, Lillo P, Hsieh S, Ramsey E, Devenney E, et al. Neuropsychiatric changes precede classic motor symptoms in ALS and do not affect survival. Neurology. 2014;82: 149–155. 10.1212/WNL.0000000000000023 [DOI] [PubMed] [Google Scholar]
- 10.Taylor LJ, Brown RG, Tsermentseli S, Al-Chalabi A, Shaw CE, Ellis CM, et al. Is language impairment more common than executive dysfunction in amyotrophic lateral sclerosis? J Neurol Neurosurg Psychiatry. 2013;84: 494–498. 10.1136/jnnp-2012-303526 [DOI] [PubMed] [Google Scholar]
- 11.Mantovan MC, Baggio L, Dalla BG, Smith P, Pegoraro E, Soraru' G, et al. Memory deficits and retrieval processes in ALS. Eur J Neurol. 2003;10: 221–227. [DOI] [PubMed] [Google Scholar]
- 12.Floris G, Borghero G, Chio A, Secchi L, Cannas A, Sardu C, et al. Cognitive screening in patients with amyotrophic lateral sclerosis in early stages. Amyotroph Lateral Scler. 2012;13: 95–101. 10.3109/17482968.2011.605453 [DOI] [PubMed] [Google Scholar]
- 13.Osborne RA, Sekhon R, Johnston W, Kalra S. Screening for frontal lobe and general cognitive impairment in patients with amyotrophic lateral sclerosis. J Neurol Sci. 2014;336: 191–196. 10.1016/j.jns.2013.10.038 [DOI] [PubMed] [Google Scholar]
- 14.Lange F, Vogts MB, Seer C, Furkotter S, Abdulla S, Dengler R, et al. Impaired set-shifting in amyotrophic lateral sclerosis: an event-related potential study of executive function. Neuropsychology. 2015. [DOI] [PubMed] [Google Scholar]
- 15.Evans J, Olm C, McCluskey L, Elman L, Boller A, Moran E, et al. Impaired cognitive flexibility in amyotrophic lateral sclerosis. Cogn Behav Neurol. 2015;28: 17–26. 10.1097/WNN.0000000000000049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji Y, Wei L, Chui D, Wang K, Fan D. Prospective memory tasks: a more sensitive method for screening cognitive impairment in ALS? BMC Neurol. 2012;12: 142 10.1186/1471-2377-12-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woolley SC, York MK, Moore DH, Strutt AM, Murphy J, Schulz PE, et al. Detecting frontotemporal dysfunction in ALS: utility of the ALS Cognitive Behavioural Screen (ALS-CBS). Amyotroph Lateral Scler. 2010;11: 303–311. 10.3109/17482961003727954 [DOI] [PubMed] [Google Scholar]
- 18.Gordon PH, Wang Y, Doorish C, Lewis M, Battista V, Mitsumoto H, et al. A screening assessment of cognitive impairment in patients with ALS. Amyotroph Lateral Scler. 2007;8: 362–365. [DOI] [PubMed] [Google Scholar]
- 19.Simmons Z, Felgoise SH, Bremer BA, Walsh SM, Hufford DJ, Bromberg MB, et al. The ALSSQOL: balancing physical and nonphysical factors in assessing quality of life in ALS. Neurology. 2006;67: 1659–1664. [DOI] [PubMed] [Google Scholar]
- 20.van der Hulst EJ, Bak TH, Abrahams S. Impaired affective and cognitive theory of mind and behavioural change in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2014;86: 1208–1215. 10.1136/jnnp-2014-309290 [DOI] [PubMed] [Google Scholar]
- 21.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48: 314–318. [PubMed] [Google Scholar]
- 22.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53: 695–699. [DOI] [PubMed] [Google Scholar]
- 23.Abrahams S, Newton J, Niven E, Foley J, Bak TH. Screening for cognition and behaviour changes in ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15: 9–14. 10.3109/21678421.2013.805784 [DOI] [PubMed] [Google Scholar]
- 24.Niven E, Newton J, Foley J, Colville S, Swingler R, Chandran S, et al. Validation of the Edinburgh Cognitive and Behavioural Amyotrophic Lateral Sclerosis Screen (ECAS): A cognitive tool for motor disorders. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16: 172–179. 10.3109/21678421.2015.1030430 [DOI] [PubMed] [Google Scholar]
- 25.Lule D, Burkhardt C, Abdulla S, Bohm S, Kollewe K, Uttner I, et al. The Edinburgh Cognitive and Behavioural Amyotrophic Lateral Sclerosis Screen: a cross-sectional comparison of established screening tools in a German-Swiss population. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16: 16–23. 10.3109/21678421.2014.959451 [DOI] [PubMed] [Google Scholar]
- 26.Tran CD, Arredondo MM, Yoshida H. Differential effects of bilingualism and culture on early attention: a longitudinal study in the U.S., Argentina, and Vietnam. Front Psychol. 2015;6: 795 10.3389/fpsyg.2015.00795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan Q, Jia J. Reduced cognitive function in Chinese patients with early amyotrophic lateral sclerosis and associated factors. Int J Neurosci. 2010;120: 641–646. 10.3109/00207454.2010.506585 [DOI] [PubMed] [Google Scholar]
- 28.Wei Q, Chen X, Zheng Z, Huang R, Guo X, Cao B, et al. Screening for cognitive impairment in a Chinese ALS population. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16: 40–45. 10.3109/21678421.2014.966311 [DOI] [PubMed] [Google Scholar]
- 29.Cui B, Cui L, Liu M, Li X, Ma J, Fang J, et al. Neuropsychological investigation in chinese patients with progressive muscular atrophy. PLOS ONE. 2015;10: e128883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1: 293–299. [DOI] [PubMed] [Google Scholar]
- 31.Modern Chinese Frequency Dictionary: Beijing Language Institure Press, 1986. [Google Scholar]
- 32.Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55: 1621–1626. [DOI] [PubMed] [Google Scholar]
- 33.Yeung PS, Ho CS, Chan DW, Chung KK. Modeling the relationships between cognitive-linguistic skills and writing in Chinese among elementary grades students. Read Writ. 2013;26: 1195–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, Fan D. Screen of cognitive and behavioural changes in Chinese Amytrophic Lateral Sclerosis patients. 2013.
- 35.Palmieri A, Mento G, Calvo V, Querin G, D'Ascenzo C, Volpato C, et al. Female gender doubles executive dysfunction risk in ALS: a case-control study in 165 patients. J Neurol Neurosurg Psychiatry. 2015;86: 574–579. 10.1136/jnnp-2014-307654 [DOI] [PubMed] [Google Scholar]
- 36.Schmand B, Smit J, Lindeboom J, Smits C, Hooijer C, Jonker C, et al. Low education is a genuine risk factor for accelerated memory decline and dementia. J Clin Epidemiol. 1997;50: 1025–1033. [DOI] [PubMed] [Google Scholar]
- 37.Dufouil C, Alperovitch A, Tzourio C. Influence of education on the relationship between white matter lesions and cognition. Neurology. 2003;60: 831–836. [DOI] [PubMed] [Google Scholar]
- 38.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51: 1546–1554. [DOI] [PubMed] [Google Scholar]
- 39.Bak TH, Hodges JR. Motor neurone disease, dementia and aphasia: coincidence, co-occurrence or continuum? J Neurol. 2001;248: 260–270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.