Abstract
In biomedicine, scientific literature is a valuable source for knowledge discovery. Mining knowledge from textual data has become an ever important task as the volume of scientific literature is growing unprecedentedly. In this paper, we propose a framework for examining a certain disease based on existing information provided by scientific literature. Disease-related entities that include diseases, drugs, and genes are systematically extracted and analyzed using a three-level network-based approach. A paper-entity network and an entity co-occurrence network (macro-level) are explored and used to construct six entity specific networks (meso-level). Important diseases, drugs, and genes as well as salient entity relations (micro-level) are identified from these networks. Results obtained from the literature-based literature mining can serve to assist clinical applications.
Introduction
Scientific literature is the primary source for scholars to communicate with others as well as the public. Scholars publish papers and present research outcomes in conferences to convey ideas and disseminate knowledge to the community. As online accessibility to scholarly literature is enhanced, the growth rate of scholarly literature is unprecedentedly high. A linear growth of publications has been reported for fields such as bioinformatics [1]. A concern as a result of such proliferations is the lagged consumption of scientific literature. To alleviate this tension, scholars have attempted to apply a variety of text mining techniques, such as information extraction [2], topic modeling [3], and document summarization [4], to systematically distill knowledge from large scientific literature corpora.
In biomedicine, scientific literature, primarily from PubMed [5] ―a free portal to publications and citation in Medline, has been employed in relation to text mining techniques to aid biomedical research. The focus is typically to extract relations among biomedical entities such as protein-disease associations [6], gene relations [7], gene-drug relations [8, 9, 10], gene-disease relations [11, 12], and protein-protein interactions [13, 14]. Al-Mubaid & Singh [6] applied a text mining approach to Medline abstracts to discover protein-disease association and confirmed that literature-based approach is capable of discovering associations between proteins and diseases. In the same vein, Stephens and colleagues [7] proposed a method to detect gene relations from Medline abstracts and highlighted the strength of literature-based methods that is the ability to analyze large volume of data in a limited time. Chang & Altman [8] proposed a method to extract gene-drug relations from literature and showed the effectiveness of a co-occurrence method to extract gene-drug relations in published articles (at the 78% accuracy level). Similarly, Chun and colleagues [11] proposed a system that used a co-occurrence-based machine learning algorithm to automatically extract relations between genes and relations from Medline, and emphasized the importance of gene and disease dictionaries. Temkin & Gilder [13] proposed a method that used context-free grammar to extract protein interactions from unstructured texts. They reported that the proposed method recorded a precision rate of 70% for extracting interactions among proteins, genes and small molecules (PGSM). In addition to relation identification, studies have also focused on extracting entities such as genes [15] and chemical entities [16]. Stapley & Benoit [15] extracted genes from literature by using gene co-occurrence information curated in genomic databases to improve biomedical information retrieval. Grego & Couto [16] applied a semantic similarity validation-based method to enhance the identification of chemical entities. They showed that the method can be used as a complementary method to assist other entity identification methods without redundant entity filtrations. Detailed surveys on biomedical text mining are made available in Cohen & Hersh [17], Zweigenbaum et al., [18] and Simpson and Demner-Fushman [19]. Extracted entities and entity relations can be further analyzed using techniques such as network centrality [20], statistical analysis [21], and citation analysis [22].
It is apparent from these studies that understanding various relations among biomedical entities is a cornerstone, because these entities are better understood by probing into their interactions with others. There is an emerging trend of applying bibliometric techniques to study biomedical entities, coined by the term “Entitymetrics” [23]. In Entitymetrics, entity-driven bibliometrics tackles the problems of knowledge transfer and discovery at three different levels: micro-, meso-, and macro-level. While many aforementioned studies mainly examined the ways of discovering biomedical entities and entity relations from scientific literature, there lacks an integrated research that uses extracted entities and entity relations to facilitate literature-based information discovery. Therefore, the goal of this study is to fill the gap between the techniques of entity and entity relation extraction and the application of these techniques to gain insights into scientific literature.
Specifically, the following two research questions will be investigated: 1) In biomedicine, given a body of scientific literature, what biomedical entities have a higher impact on others and thusly should be further studied? 2) Which pairs of entities have the potential to have meaningful relations for information discovery, entity and entity relation recommendation, and other retrieval and clinical applications? In this sense, our study servers as a bridge that connects prior studies on biomedical text mining with practical applications to assist more focused research through entities and their relations of the highest importance. To achieve this goal, we propose a framework for identifying important diseases, drugs, and genes for a given disease. The framework comprises an entity extraction method and a three-level network-based approach for the analysis of a literature-based dataset.
Cancer is a primary cause of death worldwide, among which, liver cancer is the second leading cause of cancer deaths [24]. As many as 564,000 people are diagnosed with liver cancer each year, and the trend tends to continue for several decades in several developed countries such as the United States [25]. It is known that most liver cancer instances started from other parts of the body and several types of tumors can grow in liver because liver comprises different types of cells [26]. Thus, in this broad scope of literature-based liver cancer study, identifying important entities and relations among entities that are highly relevant to liver cancer is seen as beneficial. In this regard, we apply the proposed methods to a publication dataset to understand the disease using this rich source of scientific literature.
Data and Methods
Data
“Liver cancer” was selected as the seed term to query PubMed. We retrieved 169,774 PubMed records and downloaded them in XML format. We then parsed the downloaded records to extract titles and abstracts for entity extraction by implementing a SAX Parsing module. Our dataset comprises 16,568 entities (S1 File. Entities) and 1,023,204 entity-entity and paper-entity relations (S2 File. Paper-Entity Relations). Table 1 shows the percentage of each type of entities among all 16,568 entities. The process of entity extraction from the downloaded records will be discussed in the method section.
Table 1. Entity types and their percentage.
| Entity Type | Number | Percentage |
|---|---|---|
| Disease | 9,681 | 58.44% |
| Drug | 4,347 | 26.24% |
| Gene | 2,540 | 15.32% |
Methods overview
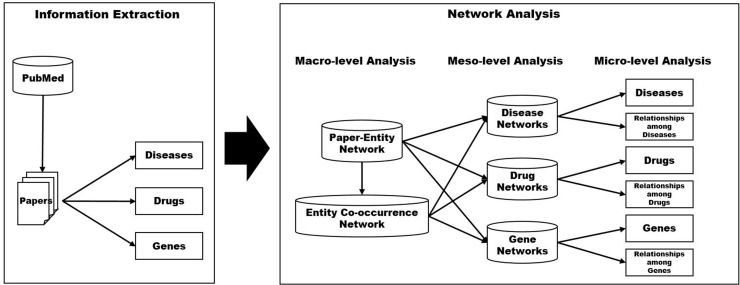
Disease, drug, and gene entities were extracted from articles obtained from PubMed. Extracted entities are used to construct a paper-entity network as well as an entity co-occurrence network. These macro-level networks were further decomposed into three types of meso-level networks (i.e., disease networks, drug networks, and gene networks). These entity specific networks are employed to investigate important diseases, drugs, and genes as well as salient relations within each entity group. Fig 1 shows the schematic diagram for the propose method.
Fig 1. A schematic diagram for the proposed methods.
We explain two main steps of the proposed method, information extraction and network analysis, in the following subsections.
Information Extraction
We implemented an entity extraction module by extending Stanford CoreNLP [27]. Stanford CoreNLP provides a set of natural language processing (NLP) analysis tools which can take English language texts and perform a variety of NLP tasks such as sentence splitting, Part-Of-Speech (POS) tagging, and dependency parsing. The entity extraction module went through the following four steps. The first step is to split a record into sentences. We used the “ssplit” pipe provided in Stanford CoreNLP. The second step is to build three dictionaries for diseases, genes, and drugs. We used the CTD database (http://www.ctdbase.org/) to create the three dictionaries. In total, the drug dictionary comprises 151,729 drug names; the disease dictionary comprises 11,937 disease names; and the gene dictionary comprises 297,514 gene names. The third step is to incorporate PubTator [28] to strengthen the inducted dictionaries. We conducted a preliminary test of extracting bio-entities only with CTD-based dictionaries and found that the quality of entity extraction was not satisfactory. Thus, we decided to add PubTator to further strengthen the dictionaries. PubTator, developed to fulfill two curation tasks—document triage and bio-concept annotation, contains bio-entity annotations for several entities such as chemicals, diseases, genes, mutations, and species. Out of these types, we are only interested in disease, drug, and gene types. Pubtator contains 16,582,474 genes, 26,788,622 diseases, and 24,915,999 drugs. When we merged three CTD dictionaries with three PubTator dictionaries for drug, disease, and gene, we checked if there is a common dictionary entry shared in both dictionaries. If found, we only kept one entry. This duplicate elimination step results in 25,053,123 drug names for the drug dictionary, 26,791,436 disease names for the disease dictionary, and 16,761,566 gene names for the gene dictionary. The fourth step is to match tokenized phrases to the three dictionaries. In this step, we employed the following three sub-steps: tokenization, lemmatization, and normalization.
Tokenization. We used the Stanford PTBTokenizer tokenization technique [29]. PTBTokenizer is designed to be a fast, rule-based tokenizer to conform to the Penn Treebank tokenization conventions [29].
Lemmatization. We used the lemmatization technique which is available in the Stanford CoreNLP package. It provides full morphological analysis for accurate identification of the lemma for each word. Lemmatization is similar to word stemming, but rather than produce a stem of the word, it replaces the suffix to get the normalized word form.
Normalization. We used the string normalization technique to reduce the string variation by case sensitivity and special characters including +, *,;, and _. Strings with uppercase are changed to those with lowercase, and/or the appointed special characters are removed from all the input texts and dictionary data. In the case of the special character ‘-’, it is replaced by whitespace, allowing for the general entity name patterns.
Network Analysis
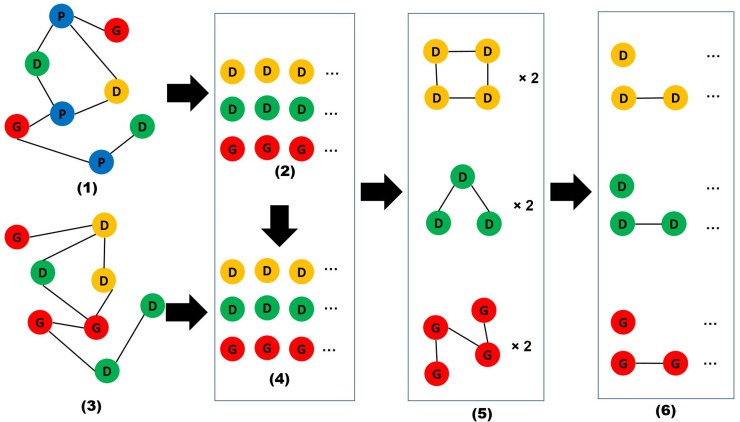
The network analysis comprises six steps (Fig 2): 1) the construction of a paper-entity network; 2) the identification of top entities; 3) the construction of an entity co-occurrence network; 4) the identification of entities highly co-occurred with top entities; 5) the construction of entity specific networks (PageRank- and betweenness-based); and 6) the exploration of entity specific networks. These steps are elaborated in the following paragraphs.
Fig 2. A flow chart of six steps.
A paper-entity network was constructed using the extracted entities. It is a heterogeneous, unweighted network that contains four types of nodes: papers, diseases, drugs, and genes. The network captures relations between papers and entities so that there is an edge if a paper includes an entity (i.e., a disease, drug, or gene). The paper-entity network forms the basis for identifying important entities through topological investigations. Two network-based measures, PageRank and betweenness centrality, were used to identify important entities from this network. PageRank is an algorithm used to rank web pages according to the impact of inlinks [30]. Entities ranked highly by PageRank are those with the highest impact. Betweenness centrality is an indicator of measuring the influence of nodes in terms of the ability to transfer information in a network [31]. Thus, a node with high betweenness centrality means it plays an important role in transferring information to others. In the paper-entity network, entities with a high betweenness centrality play a key role in the whole network by connecting other entities. These two algorithms have been applied to a number of areas to identify important artifacts and actors. For instance, Zhu & Yan [32] applied PageRank to identify important subfield within computer science to understand its knowledge diffusion patterns; Jing & Baluja [33] applied PageRank to retrieve highly relevant images in an image search. Likewise, betweenness centrality was employed to identify important nodes to solve the problem of network control in communication networks [34]; it was also applied to an alliance network to explore novel technologies [35].
A co-occurrence network was then constructed from the paper-entity network. The co-occurrence network is a heterogeneous, weighted network comprises diseases, drugs, and genes. Paper-entity relations were used to calculate co-occurrence values. That is, if two or more entities co-occurred within a paper, the number of co-occurrence was recorded and treated as the weight in the entity co-occurrence network. Co-occurrence networks have been widely studied [36, 37], based on the notion that entities have strong interactions with each other tend to co-occur frequently. Thus, co-occurrence relations are an important feature in examining between-entity relations.
In an entity co-occurrence network, diseases that highly co-occurred with top diseases identified from the paper-entity network were then extracted. Because we have two sets of top diseases identified separately from PageRank and betweenness centrality, two disease specific datasets were collected. Four more datasets (i.e., on drugs and genes) were also constructed separately by using the same method. Thus, each of the six datasets includes top entities and entities that highly co-occurred with these top entities. The six datasets were then used to construct six homogeneous networks (i.e., two disease networks (PageRank-based and betweenness centrality-based), two drug networks, and two gene networks) by reserving the co-occurrence value as link weight. These six networks are the transformed networks of the previous entity co-occurrence network by only including one type of entities as well as a small set of important entities. The entity specific networks are constructed to get a condensed and meaningful view of the seed disease. In each of the six entity networks, we also extracted highly co-occurred entity pairs. Because each entity type is associated with two entity specific networks (PageRank-based and betweenness centrality-based), two sets of pairs within an entity type were identified.
Results
In this section, we sequentially report important diseases, drugs, and entities as well as important pairs of entities in the area of liver cancer research.
Important Entities
Table 2 shows two sets of top 10 disease, drugs, and genes: one based on PageRank and the other based on betweenness. We discuss these important entities in the following three subsections.
Table 2. Top entities highly ranked by PageRank and Betweenness centrality.
| Rank | Disease | Drug | Gene | |||
|---|---|---|---|---|---|---|
| PageRank | Betweenness | PageRank | Betweenness | PageRank | Betweenness | |
| 1 | Tumor | hepatoma | alcohol | gamma-glutamyl | HCC | recombinant human interleukin 2 |
| 2 | hepatocellular carcinoma | Tumor | cisplatin | Tyrosine | AFP | creatine kinase B |
| 3 | Cancer | Cancer | glucose | trastuzumab | p53 | G21 |
| 4 | HCC | autosomal recessive, inherited disorder | oxygen | metallocorrole | CEA | gamma-glutamyl transpeptidase |
| 5 | Hepatoma | intrahepatic and extrahepatic cholangiocarcinoma | tyrosine | glutamyl | albumin | ED2 |
| 6 | Cirrhosis | CRLM and extra hepatic disease | ethanol | [11C]CH3OTf | TACE | CDH2 |
| 7 | Hepatitis | Thyrotoxicosis | 5-FU | 3-methylcholanthrene | insulin | thyroid hormone receptor beta |
| 8 | colorectal cancer | mitochondrial dysfunction | bilirubin | calcium folinate | alpha-fetoprotein | vascular-endothelial growth factor and fibroblast growth factor receptors |
| 9 | liver metastasis | HPV | glutathione | CBD | VEGF | Histone |
| 10 | liver cirrhosis | absence of disease progression, fatty liver | amino acid | diethylnitrosamine | IL-6 | beta-galactosidase |
Diseases
As shown in Table 2, three diseases (i.e., tumor, cancer, and hepatoma) appeared in both lists. Hepatocellular carcinoma, HCC, and hepatoma denote the same concept and so do cirrhosis and liver cirrhosis. Hepatocellular carcinoma is one common type of liver cancer caused by cirrhosis in most cases. Cirrhosis/liver cirrhosis might be caused by hepatitis [38]. Compare to PageRank, betweenness centrality includes more specific terms (i.e., autosomal recessive, inherited disorder, intrahepatic and extrahepatic cholangiocarcinoma, and CRLM and extra hepatic disease) and terms that may not be easily associated with liver cancer such as thyrotoxicosis, mitochondrial dysfunction, and HPV. These diseases’ connections to liver cancer might be the ones that have the potential to be further understood.
Drugs
Unlike diseases, only one drug (i.e., tyrosine) appeared in both lists. Tyrosine or Tyrosine kinase inhibitor (TKI) is a drug used to treat liver cancer by inhibiting Tyrosine kinases which are enzymes used by cells to transmit growing and dividing signals [39, 40]. Trastuzumab is used to treat breast cancer and malignant tumors [41] and calcium folinate is used to reduce side-effects caused by using some anti-cancer medicines [42]. Betweenness centrality ranks chemical compounds highly such as metallocorrole, [11C]CH3OTf, 3-methylcholanthrene, CBD (Cannabidiol), and diethylnitrosamine. We give s brief overview to some important drugs in this section.
Cisplatin: Cisplatin is used to treat various cancers including liver cancer [43].
Glucose: Liver cells are known to produce glucose which helps human maintain healthy blood-sugar levels. If these cells become cancerous, then they lose the ability and this makes tumor cells proliferate [44].
5-FU: 5-fluorouracil is a drug used to treat cancer [45].
Glutathione: Glutathione, also known as gamma-glutamyl, is a substance contained in cells. It is taken to detoxify and prevent heart diseases, various cancers, etc. [46].
Besides these drugs, some basic elements, such as oxygen, amino acid, tyrosine (one of the 22 amino acids) are also highly ranked by PageRank. These elements have the ability to stimulate body functions and repair body tissues.
Genes
Unlike diseases and drugs, two lists in Table 2 do not share any common gene. Because genes are more granular entities than diseases and drugs, they may not exclusively relate to liver cancer. Readers can visit GeneCards (http://www.genecards.org), a human gene database, for more information on these genes.
Network Characteristics of Entity Networks
Top entities shown in Table 2 were used to identity other entities that highly co-occurred with these entities in entity co-occurrence networks. Then, these entities altogether form two disease networks (PageRank-based and betweenness centrality-based), two drug networks, and two gene networks, from which we identified top pairs of diseases, drugs, and genes. Table 3 shows the statistics of each network.
Table 3. Network characteristics for the six entity networks.
| Indicators | Disease | Drug | Gene | |||
|---|---|---|---|---|---|---|
| PageRank | Betweenness | PageRank | Betweenness | PageRank | Betweenness | |
| No. of Nodes | 41 | 55 | 77 | 58 | 67 | 47 |
| No. of Edges | 79 | 64 | 93 | 63 | 86 | 40 |
| Avg. Degree | 3.85 | 2.33 | 2.42 | 2.17 | 2.57 | 1.7 |
| Avg. Weighted Degree | 2210 | 836 | 156 | 46 | 124 | 6 |
| Avg. Path Length | 2.79 | 3.34 | 3.43 | 3.2 | 3.39 | 3.06 |
| Graph Density | 0.096 | 0.043 | 0.032 | 0.038 | 0.039 | 0.037 |
| Modularity | 0.19 | 0.1 | 0.71 | 0.59 | 0.46 | 0.54 |
| No. of Communities | 2 | 7 | 7 | 8 | 6 | 10 |
| Avg. Clustering Coefficient | 0.11 | 0.44 | 0.49 | 0.51 | 0.48 | 0 |
As shown in Table 3, PageRank-based networks have higher average degrees as well as average weighted degrees. This indicates that entities in PageRank-based networks interact more actively with each other. For average path length, each network has a similar average path length (i.e., about 3). All networks are sparse with graph density lower than 0.1. Modularity is used to measure the likelihood that a network can be divided into communities [47]. Disease networks have lower modularity than the drug and gene networks. This is because diseases generally interact with many other diseases. While betweenness centrality-based networks have more communities than PageRank-based networks, the PageRank-based disease network only has two communities, which is much lower than the minimum number of communities of other networks. Betweenness centrality-based gene network recorded an average clustering coefficient of zero. This suggests that there is no triangle in this network, as genes shown in Table 2 (betweenness centrality-based) have rather distinct characteristics.
Salient Pairs of Diseases, Drugs, and Genes
Table 4 shows highly co-occurred pairs of diseases, drugs, and genes. These pairs were divided into three groups based on the number of co-occurrence. We discuss these important entity pairs in the following three subsections.
Table 4. Top 15 entity pairs.
| Range | Rank | Disease | Drug | Gene | |||
|---|---|---|---|---|---|---|---|
| PageRank | Betweenness | PageRank | Betweenness | PageRank | Betweenness | ||
| > = 1000 | 1 | tumor—hepatocellular carcinoma | tumor—hepatocellular carcinoma | cisplatin—doxorubicin | N/A | HCC—AFP | N/A |
| > = 1000 | 2 | tumor—HCC | tumor—HCC | bilirubin—aspartate | N/A | HCC—TACE | N/A |
| > = 1000 | 3 | tumor—liver metastasis | tumor—liver metastasis | cisplatin—5-fluorouracil | N/A | HCC—p53 | N/A |
| > = 1000 | 4 | cirrhosis—hepatitis | tumor—hepatoma | alcohol—ethanol | N/A | HCC—VEGF | N/A |
| > = 1000 | 5 | hepatocellular carcinoma—liver cirrhosis | cancer—hepatocellular carcinoma | oxygen—superoxide | N/A | HCC—alpha-fetoprotein | N/A |
| <1000 and > = 100 | 1 | hepatitis—chronic hepatitis | cancer–HCC | cisplatin—platinum | diethylnitrosamine—phenobarbital | AFP—DCP | N/A |
| <1000 and > = 100 | 2 | cancer—HCC | tumor—metastasis | tyrosine—serine | tyrosine—serine | p53—Bcl-2 | N/A |
| <1000 and > = 100 | 3 | HCC—liver cirrhosis | cancer—liver metastasis | 5-FU—folinic acid | 3-methylcholanthrene—phenobarbital | p53—p21 | N/A |
| <1000 and > = 100 | 4 | HCC—chronic hepatitis | tumor—liver cirrhosis | cisplatin—adriamycin | tyrosine—sorafenib | insulin—glucagon | N/A |
| <1000 and > = 100 | 5 | tumor—metastasis | cancer—colorectal cancer | cisplatin—CDDP | gamma-glutamyl—glutamyl | HCC—IFN | N/A |
| <100 | 1 | cirrhosis—viral hepatitis | hepatoma—hepatitis B | 5-FU—uracil | tyrosine—imatinib | TACE—RFA | gamma-glutamyl transpeptidase—alkaline phosphatase |
| <100 | 2 | hepatoma—hepatitis B | hepatoma—liver cirrhosis | alcohol—aldehyde | tyrosine—threonine | IL-6—IL-10 | gamma-glutamyl transpeptidase—HCC |
| <100 | 3 | liver cirrhosis—liver cancer | hepatoma—liver tumor | glucose—phosphoenolpyruvate | diethylnitrosamine—glutathione | VEGF—CD34 | gamma-glutamyl transpeptidase—AST |
| <100 | 4 | liver metastasis—liver tumor | hepatoma—breast cancer | tyrosine—imatinib | diethylnitrosamine—2-acetylaminofluorene | p53—Bax | histone—HCC |
| <100 | 5 | cirrhosis—hepatitis C | hepatoma—chronic hepatitis | ethanol—PEIT | diethylnitrosamine—glucose | IL-6—TNF-alpha | histone—HDAC |
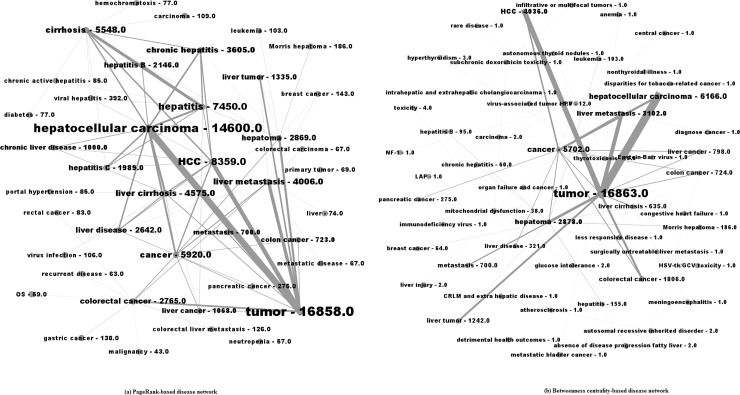
Diseases
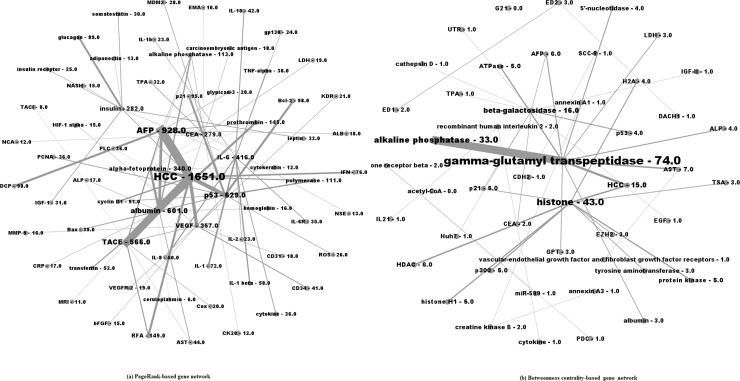
PageRank-based and betweenness centrality-based disease networks are visualized in Fig 3. Node labels are proportional to weighted degree and the width of links are proportional to the number of co-occurrence between two diseases.
Fig 3.
PageRank-based (a) and Betweenness centrality-based (b) disease networks.
The most important entity in Fig 3(A) is tumor. Tumor highly co-occurred with hepatocellular carcinoma, HCC, cancer, and hepatoma. Important diseases in Fig 3(A) are generally the same diseases that are highly ranked by PageRank in Table 2.
Diseases in Fig 3(B) tend to co-occur infrequently with each other, which is in contrast with the PageRank-based disease network. One possible explanation is that top diseases with high betweenness centrality were not studied much in papers; thus, they did not co-occur frequently with other diseases.
Six pairs of diseases (i.e., tumor—hepatocellular carcinoma, tumor–HCC, tumor—liver metastasis, cancer–HCC, tumor–metastasis, and hepatoma—hepatitis B) appeared in both lists. Relations of these diseases are self-explanatory, probably with the exception of “hepatoma-breast cancer”. Recent discoveries have found that breast cancer, similar to cancers such as colon cancer, bladder cancer, and kidney cancer, is one of the cancers that may spread to livers [48].
Drugs
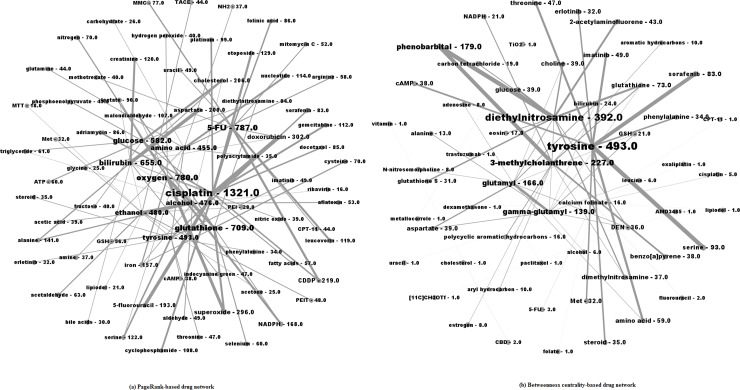
Fig 4 shows two types of drug networks constructed from the paper-entity network and the entity co-occurrence network.
Fig 4.
PageRank-based (a) and Betweenness centrality-based (b) drug networks.
Ten most visible entities shown in Fig 4(A) are exactly the same as top 10 entities ranked by PageRank in Table 2 while the level of visibility is different.
Two important drugs in Fig 4(B) are tyrosine and diethylnitrosamine. Tyrosine, as mentioned in the previous section, is used to treat liver cancer by inhibiting Tyrosine kinases [36]. Diethylnitrosamine, ranked the second, co-occurred 392 times with other drugs. The status of diethylnitrosamine is more apparent in the drug specific network (ranked the second) than in the paper-entity network (ranked the 10th). This finding has supported the need to construct such entity specific networks―by doing so, we are able to gain more granular understanding of the interactivity of entities which may be overlooked in the global network.
Top 15 drug pairs are shown in Table 4. Two pairs (i.e., tyrosine–serine and tyrosine–imatinib) are shown in both PageRank- and betweenness centrality-based lists. Both tyrosine and serine belong to the same group- proteingenic amino acids which are building blocks of proteins [49]. Imatinib is one kind of tyrpsine-kinase inhibitor used for treating cancers. In the list of betweenness centrality, there is no pair occurred more than 100 times.
Relations shown in Table 4 were examined by referencing online resources including WebMD (http://www.webmd.com) and Drugs.com (http://www.drugs.com). These websites provide detailed information on drugs as well as drug interaction checker services. Relations that were mentioned by the two online resources were bold-faced in Table 4. Only two relations (i.e., bilirubin-aspartate and tyrosine-serine) were not confirmed in PageRank-based list while in the betweenness centrality-based list, two relations (i.e., gamma-glutamyl–glutamyl and tyrosine–imatinib) were confirmed. Thus, literature-based approach is a valuable way to assist clinical trials.
Genes
Fig 5 illustrates two gene specific networks constructed from a collection of top genes and genes that highly co-occurred with these genes.
Fig 5.
PageRank-based (a) and Betweenness centrality-based (b) gene networks.
Betweenness centrality-based gene network includes 47 significant genes, which has fewer genes than the PageRank-based one that has 67 genes. One feature of Fig 5(B) is that most genes co-occurred less than five times with other genes. This suggests that they were not widely studied in previous literature and interactions between these genes and liver cancer may need to be further investigated.
Table 4 shows top 15 gene pairs identified from the PageRank- and betweenness centrality-based gene network. All pairs in the list of betweenness centrality occurred less than 50 times. Investigating interactions between diseases and genes may be more difficult than looking into the relations between diseases and diseases/drugs because genes are more granular entities and may actively or latently relate to a slew of diseases or drugs. In this sense, interactions shown in this study can be used to initiate a meaningful research.
To examine gene relations in Table 4, we referenced online resources including BioGRID (http://www.thebiogrid.org), BioGraph (http://www.biograph.be), CTD (http://www.ctdbase.org), and GeneCards (http://www.genecards.org). BioGRID confirmed three relations (i.e., p53-Bcl-2, p53-Bax, and histone-HDAC), BioGraph confirmed one relation (i.e., p53-p21), and CTD confirmed one relation (i.e., insulin-glucagon). Relations that were confirmed by these online resources were bold-faced in Table 4. Unlike diseases and drugs, a number of gene relations in Table 4 were not confirmed by clinical trials. This is probably due to the large volume of genes and their relations that may relate to liver cancer.
Discussion and Conclusions
In this study, we proposed a literature-based approach for identifying disease-related entities that include diseases, drugs, and genes for liver cancer. A series of network-based approaches were applied to identify important entities among the extracted entities. Top diseases, drugs, and genes were identified by two distinct measures and thusly two groups of entities were obtained. One group, formed based on entities that have the highest PageRank scores, includes entities that gained popularity and were widely investigated in literature. Entities included in this group are important in understanding diseases. The other group, formed based on entities that have the highest betweenness centrality, includes entities that played key roles in the whole network in connecting other entities. Entities in this group possibly possess topological importance in studying the given disease. Six entity specific networks were constructed by combining the entity co-occurrence network and the identified top entities to discover salient entity relations. A portion of the discovered entity relations was verified by clinical trials.
Key findings were obtained: 1) PageRank and betweenness centrality are complementary in identifying important entities. As PageRank identifies popular entities while betweenness centrality identifies influential entities, the combinatorial use of the two is a reasonable and effective way to select and examine important entities; 2) the integrative use of global and regional networks effectively identifies global entities as well as entities that are important, but not noticeable in the global topology. Regional networks make it possible to identify important pairs of entities from a large volume of links in global networks; 3) diseases, drugs, and genes present different characteristics in identifying important entities and pairs of entities that relate to liver cancer. Identified diseases and pairs of diseases have the highest familiarity while the interpretation of identified drugs and genes imposes more challenges as shown in the cross-validation of the results with external resources. This implies an increased level of demandingness in bio-entity research as the studied entities become more granular. Thus, similar research in a more detailed level is promising and critical in advancing literature-based biomedical research; and 4) some relations identified by the proposed method have a high consistency with clinical trials (i.e., drug relations) while some does not (i.e., gene relations). Unconfirmed relations do not mean unimportant relations; rather, they stand out among many others because they signify potentially important relations that might be validated in future research. Researchers and practitioners can take the results of literature-based approach as an initiating point of their research. The proposed method can serve to assist clinical trials to identify important entity relations.
This study has some limitations. Links among entities were based on co-occurrence relations. Co-occurrence may not directly demonstrate actual interactions among entities. Because the goal of this study is to propose a supplementary way to discover key biomedical information, the proposed methods should be of value to assist biomedical studies, particularly from the aspect of identifying latent biomedical entities and their relations. Due to the exploratory nature of the study, results have included both known facts that have been proven by others and also hypotheses that need to be verified in future studies. This means there is no standard guideline and test dataset to compare the performance of our approach. Instead, the identification results were verified by consulting facts published in scientific articles and authoritative biomedical resources.
As there will be more scientific literature available in the future, automated knowledge mining from text will become vital. The proposed method is an applicable and effective way to achieve this goal by integrating a series of information extraction and network-based approaches. Future studies may need to focus on other relations among entities by applying advanced statistical and natural language processing techniques, such as side effects, induce, and inhibit relations. As the infrastructures and techniques of processing big data are improving, the discovery of latent knowledge will be accelerated by applying the proposed literature-based approaches to a large volume of scientific literature—we see this as another valuable thread of research.
Supporting Information
(ZIP)
(ZIP)
Acknowledgments
This project was made possible in part by the Institute of Museum and Library Services (Grant Award Number: RE-07-15-0060-15), for the project titled “Building an entity-based research framework to enhance digital services on knowledge discovery and delivery”. In addition, the project was supported partly by the Bio-Synergy Research Project (NRF-2013M3A9C4078138) of the Ministry of Science, ICT and Future Planning through the National Research Foundation.
Data Availability
All relevant data are within the paper and its Supporting Information files: S1 File. Entity table for genes, drugs, and diseases. S2 File. Paper entity association table.
Funding Statement
This project was made possible in part by the Institute of Museum and Library Services (Grant Award Number: RE-07-15-0060-15), for the project titled “Building an entity-based research framework to enhance digital services on knowledge discovery and delivery”. In addition, the project was supported partly by the Bio-Synergy Research Project (NRF-2013M3A9C4078138) of the Ministry of Science, ICT and Future Planning through the National Research Foundation.
References
- 1.Song M, Kim SY. Detecting the knowledge structure of bioinformatics by mining full-text collections. Scientometrics. 2013;96(1):183–201. [Google Scholar]
- 2.Yan E, Zhu Y. Identifying entities from scientific publications: A comparison of vocabulary-and model-based methods. Journal of Informetrics. 2015;9(3):455–65. [Google Scholar]
- 3.Blei DM, Ng AY, Jordan MI. Latent dirichlet allocation. the Journal of machine Learning research. 2003;3:993–1022. [Google Scholar]
- 4.Haghighi A, Vanderwende L, editors. Exploring content models for multi-document summarization. Proceedings of Human Language Technologies: The 2009 Annual Conference of the North American Chapter of the Association for Computational Linguistics; 2009: Association for Computational Linguistics.
- 5.Li X, Chen H, Huang Z, Su H, Martinez JD. Global mapping of gene/protein interactions in PubMed abstracts: A framework and an experiment with P53 interactions. Journal of biomedical informatics. 2007;40(5):453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Mubaid H, Singh RK. A new text mining approach for finding protein-to-disease associations. American Journal of Biochemistry and Biotechnology. 2005;1(3):145–52. [Google Scholar]
- 7.Stephens MJ, Palakal MJ, Mukhopadhyay S, Raje RR, Mostafa J, editors. Detecting gene relations from Medline abstracts. Pacific Symposium on Biocomputing; 2001: World Scientific. [DOI] [PubMed]
- 8.Chang JT, Altman RB. Extracting and characterizing gene–drug relationships from the literature. Pharmacogenetics and Genomics. 2004;14(9):577–86. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Liu M, Zheng WJ, Zhao Z, & Xu H. Ranking gene-drug relationships in biomedical literature using latent dirichlet allocation. Pacific Symposium on Biocomputing. 2012:422 [PMC free article] [PubMed] [Google Scholar]
- 10.Xu R, Wang Q. A semi-supervised approach to extract pharmacogenomics-specific drug–gene pairs from biomedical literature for personalized medicine. Journal of biomedical informatics. 2013:46(4):585–593 10.1016/j.jbi.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun H-W, Tsuruoka Y, Kim J-D, Shiba R, Nagata N, Hishiki T, et al. Extraction of gene-disease relations from Medline using domain dictionaries and machine learning. Pacific Symposium on Biocomputing. 2006:4. [PubMed] [Google Scholar]
- 12.Quan C, Ren F. Gene–disease association extraction by text mining and network analysis. Proceedings of the 5th International Workshop on Health Text Mining and Information Analysis. 2014:54–63.
- 13.Temkin JM, Gilder MR. Extraction of protein interaction information from unstructured text using a context-free grammar. Bioinformatics. 2003;19(16):2046–53. [DOI] [PubMed] [Google Scholar]
- 14.Zhou D, He Y, & Kwoh CK. From biomedical literature to knowledge: mining protein-protein interactions. Computational Intelligence in Biomedicine and Bioinformatics. 2008:397–421 [Google Scholar]
- 15.Stapley BJ, Benoit G, editors. Biobibliometrics: information retrieval and visualization from co-occurrences of gene names in Medline abstracts. Pacific Symposium of Biocomputing; 2000: World Scientific. [DOI] [PubMed]
- 16.Grego T, Couto FM. Enhancement of chemical entity identification in text using semantic similarity validation. PloS one. 2013;8(5):e62984 10.1371/journal.pone.0062984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen AM, Hersh WR. A survey of current work in biomedical text mining. Briefings in bioinformatics. 2005;6(1):57–71. [DOI] [PubMed] [Google Scholar]
- 18.Zweigenbaum P, Demner-Fushman D, Yu H, Cohen KB. Frontiers of biomedical text mining: current progress. Briefings in bioinformatics. 2007;8(5):358–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simpson MS, Demner-Fushman D. Biomedical text mining: a survey of recent progress Mining text data: Springer; 2012. p. 465–517. [Google Scholar]
- 20.Özgür A, Vu T, Erkan G, Radev DR. Identifying gene-disease associations using centrality on a literature mined gene-interaction network. Bioinformatics. 2008;24(13):i277–i85. 10.1093/bioinformatics/btn182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang H, Ding Y, Tang J, Dong X, He B, Qiu J, et al. Finding complex biological relationships in recent PubMed articles using Bio-LDA. PloS one. 2011;6(3):e17243 10.1371/journal.pone.0017243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song M, Han N-G, Kim Y-H, Ding Y, Chambers T. Discovering implicit entity relation with the gene-citation-gene network. PloS one. 2013;8(12):e84639 10.1371/journal.pone.0084639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding Y, Song M, Han J, Yu Q, Yan E, Lin L, et al. Entitymetrics: Measuring the impact of entities. PloS one. 2013;8(8):e71416 10.1371/journal.pone.0071416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cancer [Internet]. World Health Organization; 2015 Feb [cited 2016 Jan 20]. Available from: http://www.who.int/mediacentre/factsheets/fs297/en/
- 25.Bosch F. X., Ribes J., Díaz M., & Cléries R. (2004). Primary liver cancer: worldwide incidence and trends. Gastroenterology, 127(5), S5–S16. [DOI] [PubMed] [Google Scholar]
- 26.Understanding Liver Cancer—the Basics [Internet]. WebMD; [cited 2016 Jan 15]. Available from: http://www.webmd.com/cancer/understanding-liver-cancer-basic-information
- 27.Manning CD, Surdeanu M, Bauer J, Finkel J, Bethard SJ, McClosky D, editors. The Stanford CoreNLP natural language processing toolkit. Proceedings of 52nd Annual Meeting of the Association for Computational Linguistics: System Demonstrations; 2014.
- 28.Wei C-H, Kao H-Y, Lu Z. PubTator: a web-based text mining tool for assisting biocuration. Nucleic acids research. 2013:gkt441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manning, C., Grow, T., Grenager, T., Finkel, J., & Bauer, J. (n.d.). PTBTokenizer. Available from: http://nlp.stanford.edu/software/tokenizer.shtml
- 30.Page L, Brin S, Motwani R, Winograd T. PageRank: Bringing order to the web. Stanford Digital Libraries Working Paper, 1997.
- 31.Freeman LC. A set of measures of centrality based on betweenness. Sociometry. 1977:35–41. [Google Scholar]
- 32.Zhu Y, Yan E. Dynamic subfield analysis of disciplines: an examination of the trading impact and knowledge diffusion patterns of computer science. Scientometrics. 2015;104(1):335–59. [Google Scholar]
- 33.Jing Y, Baluja S, editors. Pagerank for product image search. Proceedings of the 17th international conference on World Wide Web; 2008: ACM.
- 34.Tizghadam A, Leon-Garcia A. Betweenness centrality and resistance distance in communication networks. Network, IEEE. 2010;24(6):10–6. [Google Scholar]
- 35.Gilsing V, Nooteboom B, Vanhaverbeke W, Duysters G, van den Oord A. Network embeddedness and the exploration of novel technologies: Technological distance, betweenness centrality and density. Research Policy. 2008;37(10):1717–31. [Google Scholar]
- 36.Cohen AM, Hersh WR, Dubay C, Spackman K. Using co-occurrence network structure to extract synonymous gene and protein names from MEDLINE abstracts. BMC bioinformatics. 2005;6(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faust K, Sathirapongsasuti JF, Izard J, Segata N, Gevers D, Raes J, et al. Microbial co-occurrence relationships in the human microbiome. PLoS Comput Biol. 2012;8(7):e1002606–e. 10.1371/journal.pcbi.1002606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liver cancer—Hepatocellular carcinoma [Internet]. U.S. National Library of Medicine; 2013 Sep 20 [cited 2015 Jul 1]. Available from: http://www.nlm.nih.gov/medlineplus/ency/article/000280.htm.
- 39.Huynh H. Tyrosine kinase inhibitors to treat liver cancer. Expert opinion on emerging drugs. 2010;15(1):13–26. 10.1517/14728210903571659 [DOI] [PubMed] [Google Scholar]
- 40.Ramadori G, Füzesi L, Grabbe E, Pieler T, Armbrust T. Successful treatment of hepatocellular carcinoma with the tyrosine kinase inhibitor imatinib in a patient with liver cirrhosis. Anti-cancer drugs. 2004;15(4):405–9. [DOI] [PubMed] [Google Scholar]
- 41.Trastuzumab (Injection) [Internet]. National Center for Biotechnology Information; 2015 May 1 [cited 2015 Jul 1]. Available from: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMHT0012500/
- 42.Calcium Folinate [Internet]. National Center for Biotechnology Information; 2005 [cited 2015 Jul 1]. Available from: http://www.medicinenet.com/liver_cancer_hepatocellular_carcinoma/article.htmhttp://pubchem.ncbi.nlm.nih.gov/compound/15150#section=Top
- 43.Cisplatin [Internet]. National Cancer Institute; 2014 Sep 17 [cited 2015 Jul 1]. Available from: http://www.cancer.gov/about-cancer/treatment/drugs/cisplatin
- 44.Wang B, Hsu SH, Frankel W, Ghoshal K, Jacob ST. Stat3‐mediated activation of microRNA‐23a suppresses gluconeogenesis in hepatocellular carcinoma by down‐regulating Glucose‐6‐phosphatase and peroxisome proliferator‐activated receptor gamma, coactivator 1 alpha. Hepatology. 2012;56(1):186–97. 10.1002/hep.25632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nature Reviews Cancer. 2003;3(5):330–8. [DOI] [PubMed] [Google Scholar]
- 46.GLUTATHIONE [Internet]. WebMD; 2009 [cited 2015 Jul 1]. Available from: http://www.webmd.com/vitamins-supplements/ingredientmono-717-glutathione.aspx?activeingredientid=717&activeingredientname=glutathione.
- 47.Blondel VD, Guillaume J- L, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. Journal of Statistical Mechanics: Theory and Experiment. 2008(10):P10008. [Google Scholar]
- 48.Liver Cancer (Hepatocellular Carcinoma) [Internet]. MedicineNet; 2014 Dec 18 [cited 2015 Jul 1]. Available from: http://www.medicinenet.com/liver_cancer_hepatocellular_carcinoma/article.htm
- 49.Ambrogelly A, Palioura S, Söll D. Natural expansion of the genetic code. Nature chemical biology. 2007;3(1):29–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(ZIP)
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files: S1 File. Entity table for genes, drugs, and diseases. S2 File. Paper entity association table.