Abstract
Extracts from twelve samples of propolis collected from different regions of Libya were tested for their activity against Trypanosoma brucei, Leishmania donovani, Plasmodium falciparum, Crithidia fasciculata and Mycobacterium marinum and the cytotoxicity of the extracts was tested against mammalian cells. All the extracts were active to some degree against all of the protozoa and the mycobacterium, exhibiting a range of EC50 values between 1.65 and 53.6 μg/ml. The toxicity against mammalian cell lines was only moderate; the most active extract against the protozoan species, P2, displayed an IC50 value of 53.2 μg/ml. The extracts were profiled by using liquid chromatography coupled to high resolution mass spectrometry. The data sets were extracted using m/z Mine and the accurate masses of the features extracted were searched against the Dictionary of Natural Products (DNP). A principal component analysis (PCA) model was constructed which, in combination with hierarchical cluster analysis (HCA), divided the samples into five groups. The outlying groups had different sets of dominant compounds in the extracts, which could be characterised by their elemental composition. Orthogonal partial least squares (OPLS) analysis was used to link the activity of each extract against the different micro-organisms to particular components in the extracts.
Introduction
Bees collect propolis from plants and use it to coat the inside surfaces of the hive in order to maintain a sterile environment. A wide variety of plant species are used by bees as a source for propolis production, leading to a wide chemical diversity [1]. Even within a fairly limited geographical region such as the UK propolis composition varies substantially [2]. Bees are subject to infection by a range of micro-organisms and these include the protozoal Crithidia species, and the Nosema species that were originally classified as protozoa but have now been reclassified as fungi. It has been found that N. ceranae and N. apis infections are widespread in Scottish beehives [3]. The best-characterised Crithidia parasite that infects bees is Crithidia bombi, which infects bumble bees [4]. In a recent publication it was found that Crithidia mellificae and Nosema ceranae infections are associated with winter mortality in European bees [5]. Thus it would seem logical that selection pressure would drive bees to collect phytochemicals that are effective against protozoa and other micro-organisms that could infect the hive [6, 7]. Crithidia, which are classified as lower Trypanosomatidae, which are very prevalent in the infection of invertebrates, are closely related to the human pathogens of the genera Leishmania and Trypanosoma [8]. Since propolis is collected by bees for the specific purpose of providing phytochemical protection against pathogens, there is a strong likelihood of finding highly active antimicrobials in it which might be effective in treating humans [9]. Moreover, the fact that propolis permeates the environment of the beehive makes it likely that it would not be particularly toxic to other multicellular organisms. Libya covers an area of over 1,759,540 km2 and the Libyan Desert, which constitutes approximately 90% of Libya, is one of the most arid places on earth. Oases can be found scattered throughout Libya, the most important of which are Ghadames and El-Kufra. The northern regions enjoy a milder Mediterranean climate. Most of the commercial beekeepers are located in an agricultural belt that extends to about 30 km from the coast [10, 11]. Table A in S1 File summarises the main plants in Libya from which bees are known to collect nectar. The current work follows from our earlier work on a sample of propolis collected from the East of Libya, from which four known compounds with activity against T. brucei and L. donovani were isolated [12]. The samples studied in this paper represent a larger variety of habitats and climates. The aim of the study was to continue our chemical mapping of the composition of African propolis and carry out anti-parasitic screens in search of high activity samples which might be useful in treating human parasitic infections.
Materials and Methods
Materials
Absolute ethanol, HPLC grade acetonitrile, methanol, formic acid and Acrodisc syringe filters were obtained from Fisher Scientific (Loughborough, UK). Chloroform and dimethyl sulphoxide (DMSO) were obtained from Sigma Aldrich, Dorset, UK. HPLC grade Water was produced in-house using a Milli Q system (Millipore, UK).
Animals
Age matched inbred BALB/c female mice (20–25 g) in-house bred were used in studies at Strathclyde University. Animal studies were carried out with local ethical approval and had UK Home Office approval (Project license PPL 60/4334).
Propolis samples
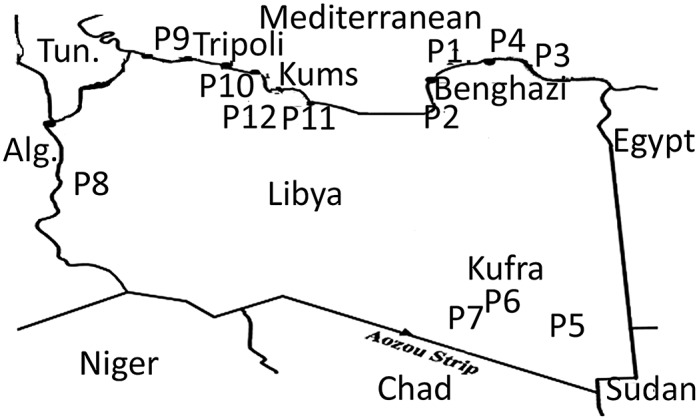
Twelve propolis samples were collected from different Libyan localities: Tukra (Al`Aquriyah,) 70 km East of Benghazi (32° 31’ N, 20° 34’ E) (P1); Qaminis 53km South of Benghazi (31° 39’ N, 20° 00’ E) (P2); Bayda East of Benghazi (32° 45’ N, 21° 44’ E) (P3); Quba East of Benghazi (32° 46’ N, 22° 15’ E) (P4); three samples from Kufra in South East Libya (24° 15’ N, 23° 18’ E) (P5, P6 and P7); Ghadames South West Libya (30° 8’ N, 9° 30’ E) (P8); Tripoli North West Libya (32° 54’ N, 13° 11’ E) (P9); Khaser Khiar 80 km East of Tripoli (32° 45’ N, 13° 43’ E) (P10) and two samples from Khumas 120 km East of Tripoli (32° 38’ N, 14° 15’ E) (P11, P12) (Fig 1). The samples were collected between December 2012 and March 2014. The physical properties of the samples are summarised in Table B in S1 File. The samples were collected by scraping the propolis sample off the top of the hive using a spatula and collecting in a clean tray.
Fig 1. Libyan map showing the collection points Libyan Propolis samples P1 (Alagoria), P2 (Gaminis), P3 (Byda), P4 (Quba), P5,P6, P7 (Kufra), P8(Ghadames), P9 (Tripoli), P10 (Khasr Khiar), P11, P12 (Khumas).
Sample Extraction
A sample of approximately 20 g of each propolis sample was extracted by sonication in 100 mL of absolute ethanol for 60 min (Clifton ultrasonic bath, Fisher Scientific, Loughborough, UK), after which the extract was filtered and re-extracted twice more with 100 mL of ethanol, filtering each time (Whatman grade 1 filter paper, Fisher Scientific, Loughborough, UK). The extracts were combined, and the solvent was evaporated using a rotary evaporator (Buchi, VWR, Leicestershire, UK), and the residue weighed.
Anti-parasitic Assays
Anti-trypanosomal assay
Testing was carried out against a standard drug-sensitive T. brucei clone, Lister strain 427 (s427) [13, 14], and the results were expressed as EC50 values based on three replicates at each concentration. The assay is based on viable cells metabolizing the blue non-fluorescent dye resazurin to resorufin, which is pink and fluorescent. The assays were performed using serial dilutions in white opaque plastic 96-well plates (F Cell Star, Greiner Bio-one GmbH, Frickenhausen, Germany), with each compound or mixture double diluted over 2 rows of the plate (i.e. 23 double dilutions and a no-drug control well), facilitating an optimally-defined EC50 value after plotting of the reading to a sigmoid curve with variable slope (GraphPad Prism 5.0). The seeding density at the start of the assay was 2×104 cells/well, and the cells were exposed for 48 h to the test compounds, at 37°C/5% CO2, before the addition of the resazurin dye and a further incubation of 24 h under the same conditions. Fluorescence was determined in a FLUOstar Optima (BMG Labtech, Ortenberg, Germany) at wavelengths of 544 nm and 620 nm for excitation and emission, respectively.
Anti-leishmanial assay
Intraperitoneal macrophages were recovered from the peritoneal cavity of BALB/c mice 3 days after intraperitoneal injection with 1mL 3% w/v aqueous sterile starch solution. The mice were then euthanized, and 3mL of incomplete medium (RPMI-1640, 100 μg/mL penicillin–streptomycin and 200 mM L-glutamine) was injected into the peritoneal cavity. The macrophage-containing medium was then removed and collected, and the resulting cell suspension centrifuged at 3000 × g for 5 min and then re-suspended in 10mL complete medium (in complete RPMI-1640 supplemented with 10% heat inactivated fetal calf serum (FCS) [v/v]). The cells were then used in antileishmanial assays. Bone marrow was then harvested from the femurs of each mouse by flushing out the removed bone with 5ml of bone marrow medium (Dulbecco’s modified Eagle’s medium, 20% heat-inactivated fetal calf serum (FCS) [v/v], 30% L-Cell solution [v/v], 100μg/mL penicillin–streptomycin and 200mM L-glutamine). The cell suspension was added to sterile petri dishes (one petri dish/mouse) and incubated for 7 days at 37°C in an atmosphere of 5% CO2:95%air. The medium was removed from the plate, and 7mL TrypLE Express was added to detach the bone marrow-derived macrophages. The resulting suspension of bone marrow-derived macrophages was collected, pelleted by centrifugation and re-suspended in 10mL of incomplete medium and then used in anti-leishmanial assays. The number of live macrophages per millilitre was determined microscopically using a haemocytometer, by mixing a cell sample with 1:1 trypan blue (20 μL) and viewing at ×10 magnification. In all cases, cell viability was >95%. Cells (0.5 × 105 in 200 μL complete medium) were added to the appropriate wells of a 96- well tissue culture plate and incubated for 24 h at 37°C in an atmosphere of 5% CO2:95% air. Cells were then infected with L. donovani luciferase-expressing promastigotes, produced at the University of Strathclyde using strain MHOM/ET/67:LV82, using a 20:1 parasite/host cell ratio. The plate was incubated as before for 24 h. The medium was removed from each well and replaced with 200 μL complete medium (control, n = 6) or various concentrations of the one of the extracts (diluted in 4% DSMO v/v in complete medium, n = 3) or amphotericin B solution (4–0.02 μg/mL). The plate was incubated as before for 72 h, the medium was then removed, and 150 μL of luciferin solution (150 μg/mL luciferin in complete RPMI-1640) was added to each well. The bioluminescence intensity (BLI) emitted per well was determined using the IVIS® imaging system (Caliper Life Sciences, Runcorn, UK) [12, 15]. The suppression in bioluminescent signal for each test sample was compared with the mean control value. The mean IC50 value was then calculated for each sample by Probit analysis. Data were analysed using MINITAB® software version 16.1.1 supplied by Minitab Ltd. Coventry, UK, and an Anderson–Darling test was used to establish if the data were normally distributed. Parametric data were analysed using a Student’s unpaired t-test or by one-way analysis of variance dependent on the number of treatments/experiments, and significance was confirmed by a Fisher test. A Mann–Whitney or Kruskal– Wallis test was used to analyse data that did not have a normal distribution. Results were considered statistically significant at a p-value of <0.05.
Anti-Mycobacterium marinum assay
The anti-bacterial bioassays against Mycobacterium marinum (ATCC.BAA535) were performed in 96-well microtitre plates using a modification of the well-established Alamar Blue™ method [16, 17]. M. marinum was inoculated on to a Columbia agar with chocolated horse blood slope (Fisher Scientific, UK) and incubated at 31°C for 5 days. A loopful of the 5 day old M.marinum culture was transferred to a sterile universal container containing 10 ml saline plus (425–600μm) glass beads (Sigma Aldrich, Dorset, UK). The bacterial suspension was mixed vigorously and allowed to settle, an aliquot of the bacterial suspension was transferred to a tube containing saline, and the turbidity was matched to that of a 0.5 McFarland standard (1.5x108 CFUs/ml) and then diluted with MHB (Cation Adjusted Mueller Hinton Broth, TREK Diagnostic Systems Ltd. UK) to 1.5x 107 CFUs/ml and then 1:1 in the assay microplate to give a final concentration of 0.75 x 107 CFUs/ml. The assay microplate was incubated at 31°C for 6 days, after which 10% Alamar Blue™ was added and the incubation continued for a further 24 h. Fluorescence was determined using a Wallac Victor 2 microplate reader (Excitation 560nm Emission 590nm) (Perkin Elmer, Waltham MA, USA). The samples were tested in duplicate over a concentration range of 100–0.19μg/ml and negative and positive controls were included containing 1–0.0019% DMSO and 100–0.78 μg/ml gentamycin respectively
Anti-Plasmodium falciparum assays
Activity against P. falciparum (3D7, The Netherlands) was determined as described previously [18, 19]. Synchronous ring stage parasites were seeded and incubated in triplicate into 96 well plates at 0.5% parasitemia and 2.5% haematocrit, using hypoxanthine free RPMI 1640 (Sigma Aldrich, Dorset, UK) medium, containing 0.5% [v/v] AlbuMAX II (Life technologies, Paisley, UK), 2 mM L-glutamine (Sigma Aldrich, Dorset, UK) and increasing concentrations of each compound (0.1 to 200 μg/mL and no drug control; final DMSO concentration < 0.5% v/v). Increasing concentrations of chloroquine (Sigma Aldrich, Dorset, UK) were used as a positive control (0.05 to 100 nM and no drug control). Parasites were cultured for 48 h before 5 μCi/mL [3H]-hypoxanthine (American Radiolabeled Chemicals, Saint Louis MO, USA) was added to each well to be then incubated for an additional 24 h before being frozen at -20°C. After thawing, plates were harvested onto filter mats with a Harvester 96™ Mach III (TomTec, Hamden CT, USA) and [3H]-hypoxanthine incorporation determined by scintillation counting using a Wallac 1450 MicroBeta Trilux counter (Perkin Elmer, Waltham MA, USA).
Anti-Crithidia fasciculata assays
C. fasciculata (ATCC50083) was grown in RPMI 1640 medium supplemented with L-glutamine and 10% v/v heat inactivated foetal bovine serum for 24 h with shaking prior to use [20]. These cells were then used to inoculate wells of a 96 well plate with 1 x 105 cells per well in 100μl of medium. Stock extracts were prepared in DMSO for each concentration so that there was a constant percentage of DMSO per well (2.5% v/v). The absorbance of plates was determined at 620nm (T0) using a Bio Rad xMark Microplate Spectrophotometer (Hemel Hempstead, UK) and plates and these were then incubated for 48 h at 25°C. The absorbance of the wells was then determined again at 620nm (T48). For compounds showing no change in absorbance (T48-T0) terminal subculture was performed and growth determined by absorbance @620nm and by microscopy. Pentamidine was included as a control drug in all assays but it shows variable activity against C. fasciculata [21] and thus menadione was used as an additional control drug.
Cell Toxicity Assay
The U937 cells (from the European Collection of Cell Cultures Cat. No. 85011440, supplied by Sigma-Aldrich, Dorset, UK).were grown until approximately 70–80% confluence before plating at 1x105cells/ml in a 96 well plate. The cell plates were then incubated overnight at 37°C, 5% CO2. Samples were prepared on a dilution plate in normal cell culture media respective to the cell line used. For initial testing, samples were added to the cells at a range of different concentrations in order to determine the IC50 value for each sample. Samples were serially diluted 1 in 2 from 200μg/ml to 1.56μg/ml. Following the addition of the extracts, the cell plates were incubated for 24 h at 37°C and then resazurin solution was added to a final concentration of 10% (v/v). The cell plates were incubated at 37°C in the dark for 4 h and 24 h before the fluorescence reading (560nm excitation, 590nm emission) was recorded on a Spectramax Plate Reader (Molecular Devices, CA, USA). Each sample was tested in triplicate and the results are expressed as cell viability as a percentage of the cell only control.
Liquid Chromatography High Resolution Mass Spectroscopy LC-HRMS
A sample of the ethanolic extract of each crude sample (1 mg), was dissolved in methanol (1 mL) and analysed by LC–MS. The separation was performed on an ACE C18 column (150 × 3mm, 3 μm) from HiChrom, Reading, UK with 0.1% v/v formic acid in water as mobile phase A and 0.1% (v/v) formic acid in acetonitrile as mobile phase B, at a flow rate of 0.300 mL/min using a gradient as follows: 0–15 min linear gradient from 30% to 50% of B, 15–25 min 50% of B, 25–40 min linear gradient from 50% to 80% of B, 40–50 min 80% of B, 50–51 min increasing to 100% B, 51–59 min at 100% of B with the flow rate increasing to 500 μl/min, 60–70 min 30% of B. The Accela HPLC system was interfaced to and Orbitrap Exactive mass spectrometer (Thermo Fisher, Hemel Hempstead, UK) used in ESI positive negative ion switching mode with needle voltages of +4.5kV and -4.0kV in positive and negative modes respectively. Sheath and drying gas flows were set at 50 and 17 arbitrary units respectively the heated capillary temperature was 275°C. In addition, data dependent MSn fragmentation [19] was carried out by using collision induced dissociation (CID) at 35 V on a LTQ-Orbitrap mass spectrometer combined with a Surveyor HPLC system using the chromatographic method outlined above.
Software and Data processing
MZMine 2.10 [22] was used for LC-HRMS data processing. The procedure and the settings were the same as described in our previous study [23]. The generated peak lists from both ESI positive and negative modes were imported separately into SIMCA-P 14 (Umetrics, Sweden) for Principal Component Analysis (PCA). The data was Pareto scaled and log transformed prior to PCA modelling. The first 500 LC-HRMS features from each sample were selected based on the mean peak area and putatively identified by searching for the accurate masses against the Dictionary of Natural Products (DNP 2013 version) [24]. The raw data files are publically accessible at: https://pure.strath.ac.uk/portal/en/datasets/search.html with a DOI: 10.15129/0b549ed7-de92-4389-8fa0-a36549a3553b.
Results
Propolis Samples Cluster Partly According to Geographic Origin
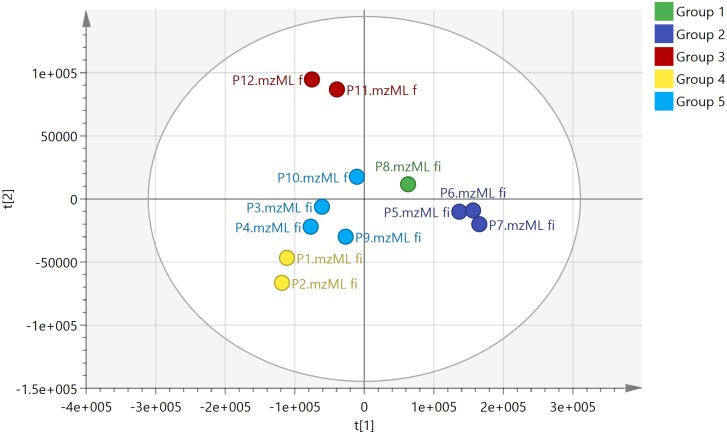
In order to get an overview of the differences in the chemical composition of the different propolis samples PCA was used. This method reduces the hundreds of variables (chemical compounds) in the samples to two principle components using the covariance within the data, essentially mapping the samples according to how close they are in composition. Fig 2 shows a PCA based on the 300 features with the highest mean peak areas across the 12 samples selected by m/z mine from the negative ion data which included 30020 features. The R2X score for the data was 0.689 indicating that 68.9% of the variation in the data was explained by the first two principal components. Hierarchical cluster analysis (HCA) was used to divide the samples into 5 groups. Only samples P5, P6 and P7 from the SE of the country gave a distinct group and they were grouped close to the sample from the SW (P8). The samples from the coast did not divide according to longitude and the two groups P3, P4, P9, P10 and P11, P12 are composed of samples from the E and W. Although P10 was collected from a site close to P11 and P12 it seems to be quite different in composition. Table 1 lists the ten most important variables (VIPs) used in the PCA classification of the samples for each group [25]. Samples P1 and P2 were similar in composition and three diterpenes and a lignan were previously isolated from sample P2 in our earlier study [12]. However, in the PCA model shown in Fig 2 the most important variables for the classification of the samples are not the diterpenes isolated previously but unknown compounds with m/z values in negative ion mode at m/z 325.145 and m/z 341.140. All masses deviated by < 2 ppm from the proposed elemental composition but, as can be seen in Table 1 the DNP often has many isomeric possibilities matching the elemental compositions of the VIPS. A compound with m/z 373.27 in negative ion mode has the highest importance for locating P5, P6 and P7 and is present in smaller amounts in the other samples. Samples P11 and P12 from the West also have clear marker compounds whereas the weightings of the VIPs in samples P3, P4, P9 and P10 are weak, indicating that these samples have an average composition. Data extraction of the positive ion data yielded 6363 features of which the top 500 by mean intensity were selected for PCA. The groupings obtained were similar to those obtained with the negative ion data (Fig A in S1 File).
Fig 2. PCA with HCA based on the 300 most intense features obtained in negative ion mode for the 12 propolis samples R2X 0.689, Q2 0.48.
Table 1. The top 10 VIPs, composed of negative ion masses measured to within 2 ppm of that of the proposed elemental compositions responsible locating the groups shown in Fig 2.
| m/z | Rt (min) | Molecular formula | Isomers in DNP | VIP |
|---|---|---|---|---|
| P1/P2 | ||||
| 325.145 | 24.925.0 | C20H22O4 | 109 | 10.1 |
| 341.140 | 21.4 | C20H22O5 | 188 | 8.2 |
| 595.168 | 3.3 | C27H32O15 | 52 | 3.5 |
| 329.067 | 11.1 | C17H14O7 | 163 | 3.5 |
| 325.145 | 10.1 | C20H22O4 | 109 | 2.8 |
| 331.155 | 17.7 | C19H24O5 | 106 | 2.7 |
| 341.140 | 13.6 | C20H22O5 | 188 | 2.6 |
| 341.103 | 10.5 | C19H18O6 | 127 | 2.5 |
| 421.093 | 14.2 | C23H18O8 | 16 | 2.4 |
| 357.135 | 29.0 | C20H22O6 | 236 | 2.2 |
| 301.217 | 43.6 | C20H30O2 | 598 | 2.0 |
| 381.192 | 8.2 | C20H30O7 | 184 | 2.0 |
| P5/P6/P7 | ||||
| 373.275 | 52.6 | C24H38O3 | 45 | 13.0 |
| 401.306 | 56.4 | C26H42O3 | 27 | 10.1 |
| 375.291 | 57.4 | C24H40O3 | 27 | 9.3 |
| 369.244 | 48.8 | C24H34O3 | 11 | 7.1 |
| 385.239 | 36.8 | C24H34O4 | 45 | 5.7 |
| 345.244 | 50.0 | C22H34O3 | 127 | 5.0 |
| 387.254 | 49.1 | C24H36O4 | 51 | 4.8 |
| 347.259 | 52.9 | C22H36O3 | 114 | 4.6 |
| 361.275 | 54.9 | C23H38O3 | 24 | 4.2 |
| 371.260 | 50.3 | C24H36O3 | 21 | 3.6 |
| P11/P12 | ||||
| 289.108 | 10.6 | C16H18O5 | 81 | 13.5 |
| 333.171 | 7.4 | C19H26O5 | 94 | 12.7 |
| 247.098 | 6.0 | C14H16O4 | 108 | 8.6 |
| 333.171 | 8.1 | C19H26O5 | 81 | 8.2 |
| 587.339 | 32.4 | C37H48O6 | 3 | 7.7 |
| 645.308 | 19.5 | C38H46O9 | 8 | 7.7 |
| 373.166 | 15.3 | C21H26O6 | 107 | 7.7 |
| 331.155 | 8.6 | C19H24O5 | 93 | 7.2 |
| 313.145 | 15.2 | C19H22O4 | 117 | 6.4 |
| 349.166 | 6.6 | C19H26O6 | 102 | 6.1 |
| P3/P4/P9/P10 | ||||
| 619.438 | 47.9 | C40H60O5 | 1 | 1.5 |
| 347.187 | 19.5 | C20H28O5 | 531 | 1.2 |
| 763.551 | 57.9 | C48H76O7 | 1 | 1.0 |
| 707.474 | 9.1 | C40H68O10 | 5 | 0.9 |
| 763.551 | 53.6 | C48H76O7 | 1 | 0.8 |
| 369.301 | 47.9 | C22H42O4 | 8 | 0.7 |
| 397.223 | 12.4 | C21H34O7 | 26 | 0.7 |
| 333.207 | 14.0 | C20H30O4 | 776 | 0.6 |
| 379.213 | 20.0 | C21H32O6 | 52 | 0.6 |
| 187.098 | 6.0 | C9H16O4 | 31 | 0.5 |
| P8 | ||||
| 401.306 | 56.4 | C26H42O3 | 27 | 4.2 |
| 345.244 | 50.0 | C22H34O3 | 127 | 4.2 |
| 371.26 | 50.3 | C24H36O3 | 21 | 4.1 |
| 375.291 | 57.4 | C24H40O3 | 27 | 3.7 |
| 369.244 | 48.8 | C24H34O3 | 11 | 3.4 |
| 255.066 | 15.6 | C15H12O4 | 145 | 3.2 |
| 347.259 | 52.9 | C22H36O3 | 114 | 3.1 |
| 373.275 | 52.6 | C24H38O3 | 45 | 2.9 |
| 375.291 | 55.6 | C24H40O3 | 27 | 2.6 |
| 397.275 | 50.8 | C26H38O3 | 23 | 2.1 |
The twelve propolis sample extracts were tested for their activity against P. falciparum, T. brucei, L. donovani, C. fasciculata and M. marinum. In addition cellular toxicity assays were carried out using mammalian cells.
Anti-parasitic Activity
Activity of propolis extracts against P. falciparum
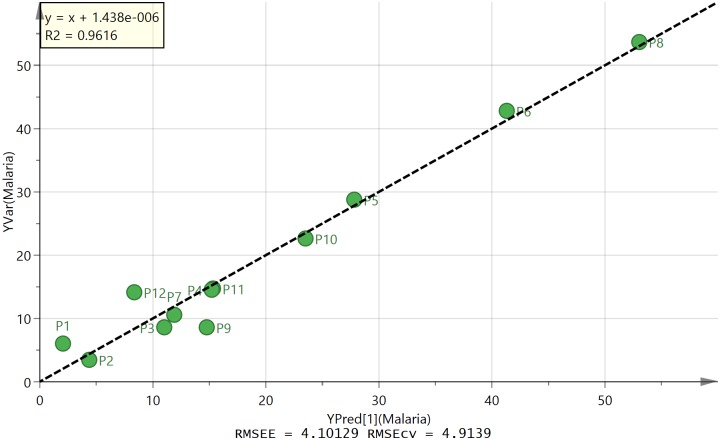
Fig 3 shows an OPLS plot for the observed activity of the extracts against P. falciparum shown in Table 2 constructed using 5 of the 300 variables used to produce Fig 1 by systematically discarding the variables with less impact on the model. The correlation between observed and predicted activity is very good with all the samples falling on the line. Table 3 shows the five most important variables contributing to the high activity of sample P2. From the loadings plot the greatest activity was associated with compound D which is abundant in samples P1 and P2. As can be seen from Fig B S1 File, the more active samples have a greater abundance of compound D. However, sample P11 is more active than would be predicted from levels of compound D and the activity appears to be based on a combination of the five marker compounds. Compound A seems to be associated with lower activity but not always since it is high in P7 which has relatively high activity. MS2 and MS3 spectra were obtained for the marker compounds and are described below. The MS2 and MS3 spectra for these compounds are shown in Figs C-L in S1 File.
Fig 3. OPLS plot of observed against predicted activity against P. falciparum based on five compounds (A-E).
Table 2. Activity of samples P1-P12 against P.falciparum (n = 3).
| EC50 (μg/mL) | |||||
|---|---|---|---|---|---|
| Compound | 1 | 2 | 3 | Mean | SEM |
| P1 | 5.9 | 6.0 | 6.2 | 6.1 | 0.10 |
| P2 | 5.3 | 2.3 | 2.6 | 3.4 | 0.96 |
| P3 | 7.8 | 9.6 | 8.4 | 8.6 | 0.52 |
| P4 | 14.5 | 13.7 | 15.4 | 14.5 | 0.48 |
| P5 | 26.8 | 32.2 | 27.2 | 28.7 | 1.8 |
| P6 | 40.7 | 44.1 | 43.6 | 42.8 | 1.0 |
| P7 | 6.6 | 13.3 | 12.1 | 10.6 | 2.1 |
| P8 | 46.7 | 50.3 | 63.8 | 53.6 | 5.22 |
| P9 | 7.0 | 9.8 | 9.2 | 8.7 | 0.84 |
| P10 | 23.1 | 20.0 | 24.9 | 22.7 | 1.43 |
| P11 | 14.9 | 14.9 | 14.2 | 14.7 | 0.23 |
| P12 | 14.6 | 14.9 | 13.1 | 14.2 | 0.57 |
| Chloroquine (nM) | 7.4 | 7.6 | 7.5 | 7.5 | 0.07 |
Table 3. Most important variables determining the activity of P2 in anti-protozoal and anti-microbial tests and important variables determining cellular toxicity based on sample P8 which was the most cytotoxic sample.
| [M-H]- | Rt (min) | Molecular formula | Compound |
|---|---|---|---|
| P.falciparum | |||
| 373.275 | 52.6 | C24H38O3 | Compound A |
| 347.259 | 52.9 | C22H36O3 | Compound B |
| 345.244 | 50.0 | C22H34O3 | Compound C |
| 341.14 | 21.4 | C20H22O5 | Compound D |
| 301.217 | 43.6 | C20H30O2 | Compound E |
| T. brucei | |||
| 373.275 | 52.6 | C24H38O3 | Compound A |
| 329.067 | 13.1 | C17H14O7 | Compound F |
| 325.145 | 25.0 | C20H22O4 | Compound G |
| 301.217 | 43.6 | C20H30O2 | Compound E |
| L.donovani | |||
| 373.275 | 54.6 | C24H38O3 | Compound A |
| 325.145 | 25.0 | C20H22O4 | Compound D |
| 341.14 | 13.6 | C20H22O5 | Compound H |
| 341.103 | 10.5 | C19H18O6 | Compound I |
| C. fasciculata | |||
| 329.067 | 13.1 | C17H14O7 | Compound F |
| 325.145 | 25.0 | C20H22O4 | Compound G |
| 369.301 | 47.9 | C22H42O4 | Compound J |
| M. marinum | |||
| 341.14 | 21.4 | C20H22O5 | Compound D |
| 325.145 | 25.0 | C20H22O4 | Compound G |
| 289.108 | 10.6 | C16H18O5 | Compound K |
| 369.301 | 47.9 | C22H42O4 | Compound J |
| U937 Cells | |||
| 373.275 | 52.6 | C24H38O3 | Compound A |
| 341.14 | 21.4 | C20H22O5 | Compound D |
| 325.145 | 25.0 | C20H22O4 | Compound G |
| 397.275 | 50.8 | C26H38O3 | Compound L |
Compound A C24H38O3, 45 isomers in DNP.
MS2 m/z 329.2850 (100) (C23H37O). MS3 (329.2850) No fragmentation at the energy used. Not much information can be derived from the mass spectra since the base peak formed in MS2 does not fragment.
Compound B C22H36O3, 114 isomers in DNP
MS2 m/z 303.2689 (100) (C21H35O). MS3 (303.2689) No fragmentation. Not much information can be derived from the mass spectra since the base peak formed in MS2 does not fragment.
Compound C C22H34O3, 127 isomers in DNP
MS2 m/z 301.2550 (100) (C21H33O). MS3 (301.2550) No fragmentation. Not much information can be derived from the mass spectra since the base peak formed in MS2 does not fragment.
Compound D C20H22O5, 189 isomers in DNP
MS2 323.1284 (100) (C20H19O4) 313.1287 (C19H21O4) 311.1287 (C19H19O4) 242.0584 (C14H10O4)
MS3 (311.1287) 216.0429 (C12H8O4) 188.0479 (C11H8O3) 144.0581 (C10H8O)
The ion at m/z 144.0581 is an important diagnostic fragment since it corresponds to naphthol and the ion at 188.0479 corresponds to a hydroxylated naphthoic acid. The ion at m/z 216.0429 has an additional CO suggesting a carbonyl is also substituted onto a hydroxynaphthoic acid and this fragment would arise from the molecular ion via the loss of a hydroxylated C8H13 hydrocarbon chain. It was not possible to correlate this information to any structure in the literature.
Compound E C20H30O2, 598 isomers in DNP
MS2 220.1470 (100) (C14H20O2), 205.1235 (C13H17O2)
MS3 (220.1470) 205.1235 (100) (C13H17O2)
Not much structural information is revealed from the fragments produced.
Activity of propolis extracts against T. brucei
Fig M in S1 File shows an OPLS model based on four compounds correlating strongly with activity against T. brucei (Table C in S1 File). Two of these were compounds A and E which were also important in the activity against P. falciparum. Compounds F and G are discussed below.
Compound F C17H14O7, 163 isomers in DNP
MS2 m/z 314.0660(100) (C16H10O4) m/z 299.0196 (14.3) (C15H7O7)
MS3 (299.0196) m/z 271.0246 (100) (C14H7O6) m/z 255.0299 (6.3) (C14H7O5)
The structure could be related to dimethylquercetin which occurs in temperate propolis. However, the diagnostic fragments which usually arise from cleavage of the C ring in flavonoids were not identified [26].
Compound G C20H22O4, 109 isomers in the DNP
MS2 m/z 242.0584 (6.1) (C14H10O4) m/z 216.0427 (44.8) (C12H8O4) m/z 188.0477 (65.4) (C11H8O3) m/z 144.0581 (5) (C10H8O)
MS3 (188.0477) m/z 144.0581 (100) (C10H8O)
This compound is related to compound D but lacks the hydroxyl group in the side chain and thus appears to be a substituted hydroxy naphthoic acid.
Activity of propolis extracts against L. donovani
Only 9 out of 12 propolis samples could be fitted into and OPLS model (Fig N and table D in S1 File). Compounds A and D were important to the model and two additional compounds H and I were also important and are discussed below.
Compound H C20H22O5, 189 isomers in DNP
MS2 m/z 271.0973 (100) (C16H15O4) m/z 242.0584 (12.0) (C14H10O4) m/z 216.0429 (10.8) (C12H8O4) m/z 188.0479 (14.2) (C11H8O3) m/z 144.0581 (0.8) (C10H8O)
MS3 (271.0973) 242.0584 (100) (C14H10O4) 216.0429 (30.0) (C12H8O4) 188.0479 (46.0) (C11H8O3) 144.0581 (1.8) (C10H8O)
Compound H is an isomer of compound D and has very similar mass spectrum, and thus is clearly structurally related to compound D.
Compound I C19H18O6 Isomers in DNP 128
MS2 m/z 323.0923 (19.6) (C19H15O5) m/z 311.0921 (52.8) (C14H10O4) m/z 293.0818 (36.4) (C18H13O4) m/z 265.0479 (10.7) (C17H13O3) m/z 176.0478 (84.2) (C10H8O3)
MS3 (m/z 176.0478) m/z 147.0452 (100) (C9H7O2)
Compound I is most probably closely related to the lignan sesamin previously characterised in Libyan propolis [12] but lacks one of the methylene groups, having a catechol structure in one of the aromatic rings rather than a methylene dioxy group.
Activity of propolis extracts against C. fasciculata
The activity against C. fasciculata (Table E in S1 File) correlated strongly with three compounds in an OPLS model (Fig O in S1 File). Compounds F and G, which were important in other models of activity, also correlated with high activity; compound J correlated with low activity. Compound J is a relatively minor peak and did not afford a clear MS2 spectrum.
Activity of propolis against M. marinum
The samples were tested against M.marinum in order to determine whether or not any observed activity against a mycobacterium might associate with different components in the samples. M.marinum is of interest since is genetically the closest mycobacterium to Mycobacterium tuberculosis [27]. An OPLS model based on four components (Fig P in S1 File) gave a good fit to the activity against M. marinum (Table F in S1 File). Again compounds D and G were responsible for high activity while compounds J and K correlated with low activity.
Toxicity of propolis against mammalian cells (U937)
The toxicity of the propolis extracts was tested against mammalian cells (Table G in S1 File). For three of the samples, P9, P11, P12 there was no significant toxicity up to 100 μg/ml and thus they were excluded from the OPLS model (Fig Q in S1 File). The most toxic sample was P8 which gave an IC50 value of 34.1 μg/ml. Of the samples showing toxicity below 100 μg/ml P2 was the least toxic. The main compounds responsible for the toxicity of the samples were compound A and compound L. From the similar elemental compositions it seemed possible that compound A and compound L might be related. The mass spectrum of compound L is discussed below.
Compound L C26H38O3, 23 isomers in DNP.
MS2 m/z 353.2867 (100) (C25H37O). MS3 (m/z 353.2867) 351.2715 (100) (C25H35O) m/z 337.2557 (15.7) (C24H33O3), m/z 323.2400 (2.9) (C23H31O), m/z 309.2243 (5.9) (C22H29O), m/z 295.2084 (7.3) (C21H27O), m/z 281.1929 (6.3) (C20H25O), m/z 267.1771 (5.9) (C19H23O), m/z 253.1613 (5.6) (C18H21O), m/z 239.1451 (5.5) (C17H19O), m/z 225.1299 (3.4) (C16H17O) m/z 133.0667 (0.8) (C9H9O), 119.0511 (2.3) (C8H7O), 107.0509 (2.2) (C7H7O).
MS3 suggested a phenol substituted with a 17 carbon chain containing four units of unsaturation. The compound also contains a carboxylic acid shown by the loss of CO2 in the MS2 spectrum. The structure is consistent with an anacardic acid, these compounds are found in cashew oil [28]. On closer examination of the MS3 spectrum of compound A it was also observed that very small ions corresponding at m/z 119.0511 and 107.0509 could be observed. Thus it seems likely that compound A is also an anacardic acid with substituted with a 17 carbon chain with two units of unsaturation. Looking at the marker compounds in Table 1 all but one of the top 10 VIPs for sample 8, the most toxic sample, have elemental compositions that would fit anacardic acids substituted with varying alkyl chains. Sample P8 is from the SW of the country from an oasis area with a very dry climate thus there is nothing to suggest that cashew trees might grow in this area, however, pistachio trees (Pistacia vera) are cultivated in Libya and these contain anacardic acids [29]. A closely related series of alkylated phenols was recently observed in Cameroonian propolis [30] and were thought to originate from the Anacardiaceae family of plants. Anacardic acids have also been observed in propolis from Oman and Brazil [31, 32]. Anacardic acids have been shown to exhibit cytotoxicity [33] and their high levels in P8 would explain why it is the most cytotoxic sample. The samples from the other oasis area in the SE of the country P5/6/7 also contain anacardic acids and are relatively cytotoxic.
Concluding Remarks
Evidence is mounting that propolis protects bee hives against microbial infection [6, 7, 34–37]. With the problems of colony collapse affecting bee hives in many parts of the world a better understanding of propolis is of great importance. The chemical composition of propolis could potentially reveal a great deal about the interaction between the bee and its environment. What is not known is whether or not bees through selection pressure have targeted plants producing resins with the desired biological properties or it just happens that the plant resins which are suitable the coating of hives just happen to have antimicrobial properties. Strong anti-microbial properties are not universal and in a survey of anti-bacterial activity of propolis from various parts of the world it was found that many samples from Sub-Saharan Africa did not have anti-bacterial properties [38] against the eight types of bacteria studied. In the current case the samples from the South of Libya were less active against protozoa but did exhibit more cytotoxicity. Is this variation just random because the plant sources are varied or is it that the bees face different environmental pressures in different regions? Considering protozoa specifically it is known that these infect insects [8] and it is also known that trypanosomatids occur in plant latexes and in fruits [39]. Thus plants also have an interest in defence against infection against protozoa and it might be expected that some plant resins would have anti-protozoal properties but obviously not all as judged from the current survey. Again a question which arises regarding whether or not plants from certain environments are more likely to face pressure from protozoal infection? The same might be true of bacterial infection and we concluded in our earlier study that propolis from tropical areas with high rain fall and warm temperatures has the highest anti-microbial activity [38]. Thus since nature is so interconnected it might be that bees for instance in an environment where plants do not face pressure from protozoal attack also are not susceptible to this pressure. Protozoal infection might not occur in the dry areas in the South of Libya. However, propolis is still collected by bees in these areas and this might simply be for its properties as a mechanical barrier rather to ward off infection. The propolis from the South of Libya is more cytotoxic and from the plant’s point of view this might be simply to make it unpalatable to animals. Finally there is little doubt the discovery of new anti-protozoal compounds is particularly important. There has been little development of new anti-protozoal drugs for many decades, resistance to the existing treatments has become a problem and the treatments that are used are quite toxic and often have poor bioavailability and have to be given by injection [40, 41]. Although there is a resistance to the notion of using extracts as treatments bees appear to exert a degree of quality control as judged similar activity for samples P1 and P2. Thus could propolis extracts have a role in treating these diseases at low cost and in the process encourage bee keeping?
Supporting Information
Fig A PCA separation of propolis samples according to positive ion MS data.
Fig B Abundance of compound D according to chromatographic peak area in the 12 Libyan propolis samples.
Fig C Compound A MS2 and MS3 spectra obtained with a collision energy of 35V.
Fig D Compound B MS2 and MS3 spectra obtained with a collision energy of 35V.
Fig E Compound C MS2 and MS3 spectra obtained with a collision energy of 35V.
Fig F Compound D MS2 and MS3 spectra obtained with a collision energy of 35V.
Fig G Compound E MS2 and MS3 spectra obtained with a collision energy of 35V.
Fig H Compound F MS2 and MS3 spectra obtained with a collision energy of 35V.
Fig I Compound G MS2 and MS3 spectra obtained with a collision energy of 35V.
Fig J Compound H MS2 and MS3 spectra obtained with a collision energy of 35V.
Fig K Compound I MS2 and MS3 spectra obtained with a collision energy of 35V.
Fig L Compound L MS2 and MS3 spectra obtained with a collision energy of 35V.
Fig M OPLS model of the activity of Libyan propolis samples against T.brucei based on four compounds. P3 was omitted in order to improve the fit of the model.
Fig N OPLS plot of observed against predicted activity of propolis samples against L.donovani. Samples P3, P6 and P11 were omitted in order to improve the fit of the model.
Fig O OPLS plot of observed against predicted activity of propolis samples against C. fasciculata. Sample P3 was omitted in order to improve the fit of the model.
Fig P OPLS plot of observed against predicted activity of propolis samples against M.marinum.
Fig Q OPLS plot of observed against predicted activity of propolis samples against cells. Samples P3 and P12 were omitted in order to improve the fit of the model.
Table A Main plants visited by bees in Libya and their flowering period
Table B The physical properties of the Libyan propolis samples.
Table C Anti-trypanosomal activity of samples P1-P12 against T.brucei (s427) (n = 3).
Table D IC values obtained for P1-12 against L. donovani amastigotes (n = 3).
Table E EC50 and EC90 values μg/ml (n = 4) obtained for propolis extracts against C. fasciculata.
Table F MIC values for P1-P12 tested against against M. marinum (n = 2, values identical for the replicates).
Table G Cytotoxicity for P1-9 and P11 measured against U937 cells.
(DOCX)
Acknowledgments
We thank the Libyan Government for funding Weam Siheri’s PhD studies.
Data Availability
The raw data files are publically accessible at: https://pure.strath.ac.uk/portal/en/datasets/search.html with a DOI: 10.15129/0b549ed7-de92-4389-8fa0-a36549a3553b.
Funding Statement
Weam Siheri’s PhD studies were funded by the Libyan Government. JF is employed by BeeVital. The specific role of this author is outlined in the Author Contributions section. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Righi A, Negri G, Salatino A (2013) Comparative chemistry of propolis from eight brazilian localities. Evidence-Based Comp Alt Med. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saleh K, Zhang T, Fearnley J, Watson DG. A Comparison of the Constituents of Propolis from Different Regions of the United Kingdom by Liquid Chromatography-high Resolution Mass Spectrometry Using a Metabolomics Approach. Current Metabolomics. 5: 42–53. [Google Scholar]
- 3.Bollan KA, Hothersall JD, Moffat C, Durkacz J, Saranzewa N, Wright GA, et al. (2013) The microsporidian parasites Nosema ceranae and Nosema apis are widespread in honeybee (Apis mellifera) colonies across Scotland. Parasitology research 112: 751–759. 10.1007/s00436-012-3195-0 [DOI] [PubMed] [Google Scholar]
- 4.Schlüns H, Sadd BM, Schmid-Hempel P, Crozier RH (2010) Infection with the trypanosome Crithidia bombi and expression of immune-related genes in the bumblebee Bombus terrestris. Dev Comp Immunol 34: 705–709. 10.1016/j.dci.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 5.Ravoet J, Maharramov J, Meeus I, De Smet L, Wenseleers T, Smagghe G, et al. (2013) Comprehensive bee pathogen screening in Belgium reveals Crithidia mellificae as a new contributory factor to winter mortality. PLoS One 8: e72443 10.1371/journal.pone.0072443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simone-Finstrom M, Spivak M (2010) Propolis and bee health: the natural history and significance of resin use by honey bees. Apidologie 41: 295–311. [Google Scholar]
- 7.Simone-Finstrom MD, Spivak M (2012) Increased resin collection after parasite challenge: a case of self-medication in honey bees. PLoS One 7: e34601 10.1371/journal.pone.0034601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGhee RB, Cosgrove WB (1980) Biology and physiology of the lower Trypanosomatidae. Microbiol Rev. 44: 140–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salomao K, de Souza EM, Henriques-Pons A, Barbosa HS, de Castro SL (2011) Brazilian green propolis: effects in vitro and in vivo on Trypanosoma cruzi. Evidence-Based Comp Alt Med. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaibi T, Fuchs S, Moritz RF (2009) Morphological study of Honeybees (Apis mellifera) from Libya. Apidologie 40: 97–105. [Google Scholar]
- 11.Keshlaf M. 2014. "Beekeeping in Libya. Int. J. Biol. Vet. Agric. Food Eng. 8. [Google Scholar]
- 12.Siheri W, Igoli JO, Gray AI, Nasciemento TG, Zhang T, Fearnley J, et al. (2014) The Isolation of Antiprotozoal Compounds from Libyan Propolis. Phytotherapy Research 28: 1756–1760. 10.1002/ptr.5194 [DOI] [PubMed] [Google Scholar]
- 13.de Koning HP, MacLeod A, Barrett MP, Cover B, Jarvis SM (2000) Further evidence for a link between melarsoprol resistance and P2 transporter function in African trypanosomes. Mol Biochem Parasitol 106: 181–185. [DOI] [PubMed] [Google Scholar]
- 14.Omar RM, Igoli J, Gray AI, Ebiloma GU, Clements C, Fearnley J, et al. (2016) Chemical characterisation of Nigerian red propolis and its biological activity against Trypanosoma brucei. Phytochem Anal. 27:107–115. 10.1002/pca.2605 [DOI] [PubMed] [Google Scholar]
- 15.Alsaadi M, Italia JL, Mullen A, Kumar MR, Candlish A, Williams R, et al. (2012) The efficacy of aerosol treatment with non-ionic surfactant vesicles containing amphotericin B in rodent models of leishmaniasis and pulmonary aspergillosis infection. Journal of controlled release 160: 685–691. 10.1016/j.jconrel.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 16.Almutairi S, Edrada-Ebel R, Fearnley J, Igoli JO, Alotaibi W, Clements CJ, et al. (2014) Isolation of diterpenes and flavonoids from a new type of propolis from Saudi Arabia. Phytochemistry Letters 10: 160–163. [Google Scholar]
- 17.Franzblau SG, Witzig RS, McLaughlin JC, Torres P, Madico G, Hernandez A, et al. (1998) Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. Journal of clinical microbiology 36: 362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fidock DA, Nomura T, Wellems TE (1998) Cycloguanil and Its Parent Compound Proguanil Demonstrate Distinct Activities against Plasmodium falciparum Malaria Parasites Transformed with Human Dihydrofolate Reductase. Mol Pharmacol 54: 1140–1147. [DOI] [PubMed] [Google Scholar]
- 19.Laine LM, Biddau M, Byron O, Muller S (2015) Biochemical and structural characterization of the apicoplast dihydrolipoamide dehydrogenase of Plasmodium falciparum. Bioscience Rep. 35: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alcolea PJ, Alonso A, García-Tabares F, Toraño A, Larraga V (2014) An Insight into the Proteome of Crithidia fasciculata Choanomastigotes as a Comparative Approach to Axenic Growth, Peanut Lectin Agglutination and Differentiation of Leishmania spp. Promastigotes. PloS one 9: e113837 10.1371/journal.pone.0113837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacchi C, Lambros C, Goldberg B, Hutner S, De Carvalho G (1974) Susceptibility of an insect Leptomonas and Crithidia fasciculata to several established antitrypanosomatid agents. Antimicrobial Agents Chemother. 6: 785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pluskal T, Castillo S, Villar-Briones A, Orešič M (2010) MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform 11: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang T, Omar R, Siheri W, Al Mutairi S, Clements C, Fearnley J, et al. (2014) Chromatographic analysis with different detectors in the chemical characterisation and dereplication of African propolis. Talanta 120: 181–190. 10.1016/j.talanta.2013.11.094 [DOI] [PubMed] [Google Scholar]
- 24.Buckingham J (1993). Dictionary of Natural Products. CRC Press; ISBN 9780412466205. [Google Scholar]
- 25.Galindo-Prieto B, Eriksson L, Trygg J (2014) Variable influence on projection (VIP) for orthogonal projections to latent structures (OPLS). J Chemometrics 28: 623–632. [Google Scholar]
- 26.Hughes RJ, Croley TR, Metcalfe CD, March RE (2001) A tandem mass spectrometric study of selected characteristic flavonoids. Int J Mass Spectrom 210: 371–385. [Google Scholar]
- 27.Tobin DM, Ramakrishnan L (2008) Comparative pathogenesis of Mycobacterium marinum and Mycobacterium tuberculosis. Cellular Microbiol 10: 1027–1039. [DOI] [PubMed] [Google Scholar]
- 28.Kubo I, Muroi H, Himejima M, Yamagiwa Y, Mera H, Tokushima K, et al. (1993) Structure-antibacterial activity relationships of anacardic acids. Journal of Agricultural and Food Chemistry 41: 1016–1019. [Google Scholar]
- 29.Khadem S, Marles RJ (2010) Monocyclic phenolic acids; hydroxy-and polyhydroxybenzoic acids: occurrence and recent bioactivity studies. Molecules 15: 7985–8005. 10.3390/molecules15117985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kardar M, Zhang T, Coxon G, Watson D, Fearnley J, Seidel V (2014) Characterisation of triterpenes and new phenolic lipids in Cameroonian propolis. Phytochemistry 106: 156–163. 10.1016/j.phytochem.2014.07.016 [DOI] [PubMed] [Google Scholar]
- 31.Silva M, De Lima S, Oliveira E, Lopes J, Chaves M, Reis F, et al. (2008) Anacardic acid derivatives from Brazilian propolis and their antibacterial activity. Eclética Química 33: 53–58. [Google Scholar]
- 32.Popova M, Dimitrova R, Al-Lawati HT, Tsvetkova I, Najdenski H, Bankova V (2013) Omani propolis: chemical profiling, antibacterial activity and new propolis plant sources. Chemistry Central Journal 7: 158 10.1186/1752-153X-7-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kubo I, Ochi M, Vieira PC, Komatsu S (1993) Antitumor agents from the cashew (Anacardium occidentale) apple juice. J Agric Food Chem 41: 1012–1015. [Google Scholar]
- 34.Wilson M, Brinkman D, Spivak M, Gardner G, Cohen J (2015) Regional variation in composition and antimicrobial activity of US propolis against Paenibacillus larvae and Ascosphaera apis. J Invert Pathol 124: 44–50. [DOI] [PubMed] [Google Scholar]
- 35.Borba RS, Klyczek KK, Mogen KL, Spivak M (2015) Seasonal benefits of a natural propolis envelope to honey bee immunity and colony health. J Exp Biol 218: 3689–3699. 10.1242/jeb.127324 [DOI] [PubMed] [Google Scholar]
- 36.Nicodemo D, Malheiros EB, De Jong D, Couto RHN (2014) Increased brood viability and longer lifespan of honeybees selected for propolis production. Apidologie 45: 269–275. [Google Scholar]
- 37.Bilikova K, Popova M, Trusheva B, Bankova V (2013) New anti-Paenibacillus larvae substances purified from propolis. Apidologie 44: 278–285. [Google Scholar]
- 38.Seidel V, Peyfoon E, Watson DG, Fearnley J (2008) Comparative study of the antibacterial activity of propolis from different geographical and climatic zones. Phytother Res 22: 1256–1263. 10.1002/ptr.2480 [DOI] [PubMed] [Google Scholar]
- 39.Camargo EP (1999) Phytomonas and other trypanosomatid parasites of plants and fruit. Advances Parasitol 42: 29–112. [DOI] [PubMed] [Google Scholar]
- 40.Trouiller P, Olliaro P, Torreele E, Orbinski J, Laing R, Ford N (2002) Drug development for neglected diseases: a deficient market and a public-health policy failure. The Lancet 359: 2188–2194. [DOI] [PubMed] [Google Scholar]
- 41.Moran M (2005) A breakthrough in R&D for neglected diseases: new ways to get the drugs we need. PLoS Med 2: 828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig A PCA separation of propolis samples according to positive ion MS data.
Fig B Abundance of compound D according to chromatographic peak area in the 12 Libyan propolis samples.
Fig C Compound A MS2 and MS3 spectra obtained with a collision energy of 35V.
Fig D Compound B MS2 and MS3 spectra obtained with a collision energy of 35V.
Fig E Compound C MS2 and MS3 spectra obtained with a collision energy of 35V.
Fig F Compound D MS2 and MS3 spectra obtained with a collision energy of 35V.
Fig G Compound E MS2 and MS3 spectra obtained with a collision energy of 35V.
Fig H Compound F MS2 and MS3 spectra obtained with a collision energy of 35V.
Fig I Compound G MS2 and MS3 spectra obtained with a collision energy of 35V.
Fig J Compound H MS2 and MS3 spectra obtained with a collision energy of 35V.
Fig K Compound I MS2 and MS3 spectra obtained with a collision energy of 35V.
Fig L Compound L MS2 and MS3 spectra obtained with a collision energy of 35V.
Fig M OPLS model of the activity of Libyan propolis samples against T.brucei based on four compounds. P3 was omitted in order to improve the fit of the model.
Fig N OPLS plot of observed against predicted activity of propolis samples against L.donovani. Samples P3, P6 and P11 were omitted in order to improve the fit of the model.
Fig O OPLS plot of observed against predicted activity of propolis samples against C. fasciculata. Sample P3 was omitted in order to improve the fit of the model.
Fig P OPLS plot of observed against predicted activity of propolis samples against M.marinum.
Fig Q OPLS plot of observed against predicted activity of propolis samples against cells. Samples P3 and P12 were omitted in order to improve the fit of the model.
Table A Main plants visited by bees in Libya and their flowering period
Table B The physical properties of the Libyan propolis samples.
Table C Anti-trypanosomal activity of samples P1-P12 against T.brucei (s427) (n = 3).
Table D IC values obtained for P1-12 against L. donovani amastigotes (n = 3).
Table E EC50 and EC90 values μg/ml (n = 4) obtained for propolis extracts against C. fasciculata.
Table F MIC values for P1-P12 tested against against M. marinum (n = 2, values identical for the replicates).
Table G Cytotoxicity for P1-9 and P11 measured against U937 cells.
(DOCX)
Data Availability Statement
The raw data files are publically accessible at: https://pure.strath.ac.uk/portal/en/datasets/search.html with a DOI: 10.15129/0b549ed7-de92-4389-8fa0-a36549a3553b.