Abstract
Population-based evidence of the relative risk of cancer among heart, kidney, and liver transplant recipients from Asia is lacking. The Taiwan National Health Insurance Research Database was used to conduct a population-based cohort study of transplant recipients (n = 5396), comprising 801 heart, 2847 kidney, and 1748 liver transplant recipients between 2001 and 2012. Standardized incidence ratios and Cox regression models were used. Compared with the general population, the risk of cancer increased 3.8-fold after heart transplantation, 4.1-fold after kidney transplantation and 4.6-fold after liver transplantation. Cancer occurrence showed considerable variation according to transplanted organs. The most common cancers in all transplant patients were cancers of the head and neck, liver, bladder, and kidney and non-Hodgkin lymphoma. Male recipients had an increased risk of cancers of the head and neck and liver, and female kidney recipients had a significant risk of bladder and kidney cancer. The adjusted hazard ratio for any cancer in all recipients was higher in liver transplant recipients compared with that in heart transplant recipients (hazard ratio = 1.5, P = .04). Cancer occurrence varied considerably and posttransplant cancer screening should be performed routinely according to transplanted organ and sex.
Introduction
Heart, kidney, and liver transplantation are standard procedures for patients with end-stage organ disease. In Taiwan, transplant recipients have excellent outcomes, with 1-year survival rates of 78%–96%. [1] However, cancer incidence is increased in these recipients because of immunosuppressive therapy, medication (analgesic abuse and certain herbal preparations), and viral infections (Epstein–Barr virus [EBV] and hepatitis C and B). Western studies have shown an overall increase in the risk of cancer of 2–10-fold in heart transplant recipients, 2–3-fold in liver transplant recipients, and 2–6-fold in kidney transplant recipients compared with that in the general population. [2–5]
Nevertheless, few population-based studies have been conducted in Asia and are limited mostly to kidney transplantation.[6, 7] The comparison of cancer incidence among recipients of different transplanted organs can clarify the pattern of post transplantation cancer etiology. These evidence-based results can also guide the development of strategies for cancer prevention and benefit high-risk recipients by minimizing the cancer risk.
In Taiwan, the variation in cancer occurrence among different transplanted organs is unclear. Therefore, we estimated the incidence of cancer in heart, kidney, and liver transplant recipients from 2001 to 2012 using the Taiwan National Health Insurance Research Database (NHIRD).
Methods
Study population
The 1995 National Health Insurance Act established the National Health Insurance (NHI) program, which is a mandatory single-payer system with the principle of equal access to all health care services. At the end of June 2014, 23 508 577 people (99.9% of Taiwan’s population) were enrolled in the program, and 93% of hospitals and clinics were contracted with the NHI. People with catastrophic illnesses are exempt from copayments to ensure that costly treatment does not impede them from receiving the necessary medical services. Malignant neoplasms and follow-up treatment after kidney, heart, lung, liver, or bone marrow transplant are recognized as catastrophic illnesses in the NHI program. Health care providers are not reimbursed if their submitted medical service claims violate insurance regulations after review and auditing by the National Health Insurance Administration (NHIA). Each year, the NHIA collects data including registration files and original claims data for reimbursement from the NHI program and sorts this information into data files. These deidentified data are sent to the National Health Research Institutes (NHRI) to generate the NHIRD. This study was exempted from institutional review board approval according to the regulations. After customized screenings, the inpatient and outpatient data of heart, kidney, and liver recipients between 2001 and 2012 were extracted from the NHIRD and catastrophic illness dataset.
Data analysis
Recipients who had received transplantation before 2001 or multiorgan transplantation between 2001 and 2012 and those who were diagnosed with cancer before transplantation were excluded from this study. Cancers were classified according to International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 140–208. We also excluded patients who developed cancer within the first 30 days after transplantation. The exception was Kaposi sarcoma, because the incidence rate in the general population is unavailable. Patients were followed until death, subsequent transplantation, their most recent medical record, or the end of 2012—whichever came first. Cancer incidence rates in transplant recipients were compared with Taiwanese general population by using the standardized incidence ratio (SIR). The SIR was defined as the ratio of the observed number of new cases of cancers to the expected number in the general population. The expected number of incident cases was calculated by multiplying the age- and sex-specific incidence rates in the general population obtained from the Taiwan National Cancer Registry for 2001–2011 and the numbers of person-years. The 95% confidence intervals (CIs) for SIRs were calculated by assuming that the observed cancer cases followed a Poisson distribution. We performed additional analyses of the 5 most common cancers with significantly elevated SIRs in all transplanted patients: cancers of the head and neck, bladder, kidney, and liver and non-Hodgkin lymphoma (NHL). The Cox regression model was used to calculate the effects of covariates on the long-term risk of cancer. Risk factors included the recipient’s sex and transplanted organ, both of which were categorical variables. Patient age at transplantation and the calendar year of transplantation were included as continuous variables. Sex, age, calendar year of transplantation, and transplanted organ were used to examine the independent effects on the risk of selected cancers after transplantation. The same analysis was performed separately to investigate the risk of the 5 most common cancers. P values < 0.05 were considered statistically significant. Statistical analyses were performed with the SPSS statistical software, version 18.0 (SPSS, Inc., Chicago, IL, USA).
Results
Current statistics
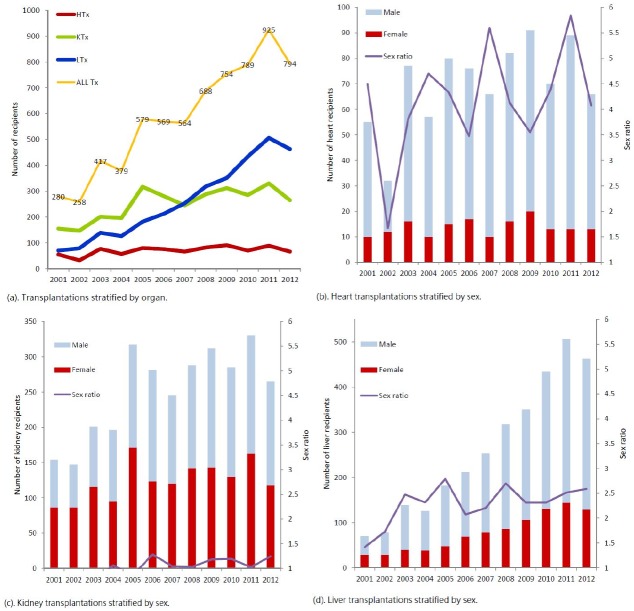
From 2001 to 2012, 6995 transplantations were performed in Taiwan, according to the NHIRD (Fig 1). On average, 70 heart, 252 kidney, and 261 liver transplantations were performed annually. The number of heart transplantations remained constant, and the number of liver transplantations increased substantively during the 12-year period. Most of the heart and liver transplant recipients were men (male:female ratio = 3.8 and 2.2, respectively). An equal number of male and female patients received kidney transplants.
Fig 1. Heart, kidney, and liver transplantations performed during the study period.
(a). Transplantations stratified by the organ. (b), (c), and (d). Heart, kidney, and liver transplantations stratified by sex. The purple line indicates the male:female ratio.
Study cohort
After the exclusion of patients with previous transplants, multiorgan transplants, and cancer diagnosis before transplantation or within 30 days of transplantation, the cohort contained 5396 transplant recipients, comprising 801 (14.8%) heart, 2847 (52.8%) kidney, and 1748 (32.4%) liver transplant recipients (Table 1). The recipients were followed for a total of 22 050 person-years (median individual follow-up duration: 3.4 y). The female recipients had a higher follow-up duration than did the male recipients (median follow-up duration: 3.9 vs. 3.1 y), and the kidney transplant recipients had the highest median follow-up duration of 4.3 years among the organ transplant groups. A total of 326 patients (18.6%) underwent liver transplantation before the age of 20 years; 55.7% (n = 3005) of all transplants occurred in the age group of 40–59.
Table 1. Characterisstics of recipients of heart, kidney and liver transplants in Taiwan between 2001 and 2012.
| Heart | Kidney | Liver | ALL | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | PY | % | No. | % | PY | % | No. | % | PY | % | No. | % | PY | % | |
| Total | 801 | 2808.51 | 2847 | 13369.24 | 1748 | 5879.44 | 5396 | 22057.19 | ||||||||
| Sex | ||||||||||||||||
| Male | 653 | 81.52 | 2225.88 | 79.25 | 1435 | 50.40 | 6386.62 | 47.77 | 1139 | 65.16 | 3735.61 | 63.54 | 3227 | 59.80 | 12348.10 | 55.98 |
| Female | 148 | 18.48 | 582.63 | 20.75 | 1412 | 49.60 | 6982.62 | 52.23 | 609 | 34.84 | 2143.83 | 36.46 | 2169 | 40.20 | 9709.09 | 44.02 |
| Age at transplant, y | ||||||||||||||||
| 0–9 | 14 | 1.75 | 50.83 | 1.81 | 17 | 0.60 | 65.35 | 0.49 | 269 | 15.39 | 1417.96 | 24.12 | 300 | 5.56 | 1534.14 | 6.96 |
| 10–19 | 43 | 5.37 | 163.82 | 5.85 | 113 | 3.97 | 589.75 | 4.41 | 57 | 3.26 | 207.85 | 3.54 | 213 | 3.95 | 961.42 | 4.36 |
| 20–29 | 58 | 7.24 | 223.93 | 7.99 | 321 | 11.28 | 1847.57 | 13.82 | 47 | 2.69 | 155.60 | 2.65 | 426 | 7.89 | 2227.10 | 10.10 |
| 30–39 | 110 | 13.73 | 437.51 | 15.62 | 612 | 21.50 | 3143.68 | 23.51 | 122 | 6.98 | 424.66 | 7.22 | 844 | 15.64 | 4005.85 | 18.17 |
| 40–49 | 173 | 21.60 | 619.76 | 22.13 | 849 | 29.82 | 4182.45 | 31.28 | 426 | 24.37 | 1438.48 | 24.47 | 1448 | 26.83 | 6240.70 | 28.30 |
| 50–59 | 253 | 31.59 | 855.51 | 30.54 | 718 | 25.22 | 2872.62 | 21.48 | 586 | 33.52 | 1680.04 | 28.57 | 1557 | 28.85 | 5408.17 | 24.52 |
| ≥ 60 | 150 | 18.73 | 449.77 | 16.06 | 217 | 7.62 | 669.99 | 5.01 | 241 | 13.79 | 554.86 | 9.44 | 608 | 11.27 | 1674.62 | 7.59 |
Abbreviations: PY, person years.
Cancer risk according to the transplanted organ in comparison with the general population
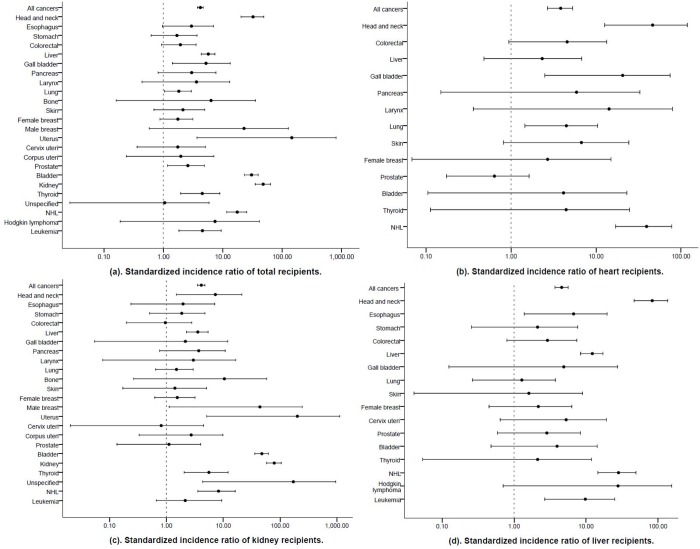
Of the 5396 transplant recipients, 310 developed malignancies during the follow-up, corresponding to a 4-fold higher cancer risk compared with that of the general population (SIR = 4.2, 95% CI 3.8–4.7). Compared with the general population, the risk of any cancer was 3-fold higher in the heart recipients (SIR = 3.8, 95% CI 2.7–5.3) and 4-fold higher in the kidney (SIR = 4.1, 95% CI 3.5–4.8) and liver (SIR = 4.6, 95% CI 3.7–5.6) recipients (Table 2, Fig 2).
Table 2. Standardized incidence ratios (SIRs) of cancer sites among heart, kidney, and liver between 2001 and 2012, NHIRD.
| Heart | Kidney | Liver | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer site | ICD-9-CM | O | E | SIR | O | E | SIR | O | E | SIR | O | E | SIR |
| All cancers | 140–208 | 36 | 9.38 | 3.84 | 184 | 44.66 | 4.12 | 90 | 19.64 | 4.58 | 310 | 73.66 | 4.21 |
| Head and neck | 140–149 | 4 | 0.09 | 46.32 | 3 | 0.41 | 7.28 | 15 | 0.18 | 82.85 | 22 | 0.68 | 32.38 |
| Esophagus | 150 | 2 | 1.02 | 1.96 | 3 | 0.45 | 6.70 | 5 | 1.68 | 2.98 | |||
| Stomach | 151 | 4 | 2.15 | 1.86 | 2 | 0.94 | 2.12 | 6 | 3.54 | 1.69 | |||
| Colorectal | 153–154 | 3 | 0.66 | 4.57 | 3 | 3.13 | 0.96 | 4 | 1.38 | 2.91 | 10 | 5.16 | 1.94 |
| Liver | 155 | 3 | 1.29 | 2.32 | 22 | 6.16 | 3.57 | 33 | 2.71 | 12.20 | 58 | 10.15 | 5.71 |
| Gall bladder | 156 | 2 | 0.10 | 20.58 | 1 | 0.46 | 2.16 | 1 | 0.20 | 4.90 | 4 | 0.76 | 5.23 |
| Pancreas | 157 | 1 | 0.17 | 5.90 | 3 | 0.81 | 3.71 | 4 | 1.33 | 3.00 | |||
| Larynx | 161 | 1 | 0.07 | 14.23 | 1 | 0.34 | 2.98 | 2 | 0.55 | 3.62 | |||
| Lung | 162 | 5 | 1.12 | 4.48 | 8 | 5.33 | 1.50 | 3 | 2.34 | 1.28 | 16 | 8.78 | 1.82 |
| Bone | 170 | 1 | 0.10 | 10.47 | 1 | 0.16 | 6.35 | ||||||
| Skin | 173 | 2 | 0.30 | 6.73 | 2 | 1.42 | 1.41 | 1 | 0.62 | 1.60 | 5 | 2.34 | 2.14 |
| Female breast | 174 | 1 | 0.37 | 2.69 | 7 | 4.51 | 1.55 | 3 | 1.38 | 2.17 | 11 | 6.26 | 1.76 |
| Male breast | 175 | 1 | 0.02 | 44.16 | 1 | 0.04 | 22.84 | ||||||
| Uterus | 179 | 1 | 0.00 | 201.97 | 1 | 0.01 | 145.45 | ||||||
| Cervix uteri | 180 | 1 | 1.24 | 0.81 | 2 | 0.38 | 5.29 | 3 | 1.72 | 1.75 | |||
| Corpus uteri | 182 | 2 | 0.73 | 2.73 | 2 | 1.02 | 1.97 | ||||||
| Prostate | 185 | 4 | 6.28 | 0.64 | 2 | 1.80 | 1.11 | 3 | 1.05 | 2.85 | 9 | 3.48 | 2.58 |
| Bladder | 188 | 1 | 0.24 | 4.15 | 55 | 1.15 | 47.78 | 2 | 0.51 | 3.95 | 58 | 1.90 | 30.56 |
| Kidney | 189 | 47 | 0.59 | 79.04 | 47 | 0.98 | 47.94 | ||||||
| Thyroid | 193 | 1 | 0.22 | 4.45 | 6 | 1.07 | 5.59 | 1 | 0.47 | 2.12 | 8 | 1.77 | 4.52 |
| Unspecified | 199 | 1 | 0.01 | 171.41 | 1 | 0.95 | 1.05 | ||||||
| NHL | 200, 202, 203 | 8 | 0.20 | 39.33 | 8 | 0.97 | 8.26 | 12 | 0.43 | 28.21 | 28 | 1.60 | 17.54 |
| Hodgkin lymphoma | 201 | 1 | 0.04 | 27.79 | 1 | 0.14 | 7.40 | ||||||
| Leukemia | 204–208 | 3 | 1.40 | 2.14 | 4 | 0.41 | 9.76 | 7 | 1.54 | 4.55 | |||
Abbreviations: O, observed numbers of cases; E, expected numbers of cases.
Fig 2. Risk of cancer among Taiwanese recipients.
(a). Cancer risk for total recipients. (b), (c), and (d). Cancer risk for heart, kidney, and liver recipients.
The estimated risks of head and neck cancer and NHL were substantially increased among all the recipients, regardless of transplantation type. The risk of liver cancer was significantly elevated in both kidney (SIR = 3.6, 95% CI 2.2–5.4) and liver (SIR = 12.2, 95% CI 8.4–17.1) transplant recipients, and 3 incident cases of liver cancer were observed in the heart recipients.
The cancer risk according to the transplanted organ varied. The risks of gallbladder (SIR = 20.6, 95% CI 2.5–74.3) and lung cancer (SIR = 4.5, 95% CI 1.5–10.5) were significantly increased only in the heart recipients. Three incident cases of male breast cancer, uterine cancer, and an unspecified cancer occurred in the kidney recipients. The thyroid cancer (SIR = 5.6, 95% CI 2.1–12.2) risk was significantly elevated only in the kidney recipients. The risks of bladder (SIR = 47.8, 95% CI 36.0–62.2) and kidney cancer (SIR = 79.0, 95% CI 58.1–105.1) were observed in kidney recipients. Esophageal cancer and leukemia were also observed in the kidney and liver recipients; however, both risks were significantly increased in only the liver recipients (SIR = 6.7, 95% CI 1.4–19.6 and SIR = 9.8, 95% CI 2.7–25.0, respectively).
Cancer risk comparison
Table 3 presents the risk of selected cancers for the transplant recipients. The table presents estimated hazard ratios (HRs) for the occurrence of head and neck, liver, bladder, and kidney cancer; NHL; and all cancers.
Table 3. Risk factors for selected cancer in transplanted recipients.
| Head and neck | Liver | Bladder | Kidney1 | NHL | ALL | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | p-value | HR | p-value | HR | p-value | HR | p-value | HR | p-value | HR | p-value | |
| Age at transplant (years) | 1.03 | 0.07 | 1.05 | <0.001 | 1.06 | <0.001 | 1.04 | <0.001 | 0.97 | <0.01 | 1.04 | <0.001 |
| Sex | ||||||||||||
| Male | 1 | (ref) | 1 | (ref) | 1 | (ref) | 1 | (ref) | 1 | (ref) | 1 | (ref) |
| Female | 0.18 | 0.02 | 0.53 | 0.03 | 2.29 | <0.01 | 3.52 | <0.001 | 0.70 | 0.40 | 1.01 | 0.95 |
| Calendar year of transplantation | 1.24 | 0.06 | 0.90 | 0.03 | 0.94 | 0.23 | 0.87 | 0.02 | 0.86 | 0.04 | 0.95 | 0.03 |
| Transplanted organ | ||||||||||||
| Heart | 1 | (ref) | 1 | (ref) | 1 | (ref) | 1 | (ref) | 1 | (ref) | ||
| Kidney | 0.25 | 0.07 | 2.38 | 0.16 | 11.90 | 0.01 | 0.23 | <0.01 | 1.31 | 0.15 | ||
| Liver | 2.13 | 0.18 | 7.05 | <0.01 | 1.08 | 0.95 | 0.55 | 0.23 | 1.52 | 0.04 | ||
HR = hazard ratio.
1The kidney cancers were all contributed from kidney transplantations and the model was adjusted for age at transplant (years), sex and calendar year of transplantation.
The results of the Cox regression model indicated that older age at transplantation, early transplantation, and liver transplantation were risk factors for any cancer. For liver, bladder, and kidney cancer, the hazard of occurrence increased with age; however, younger recipients had an increased risk of NHL. Male recipients had an increased risk of cancers of the head and neck and the liver; however, female recipients had a significant risk of bladder and kidney cancer, though they had undergone kidney transplantation. Patients had a significantly lower risk of liver and kidney cancer and NHL in the current year. Recipients of heart transplants and those with transplantation at a younger age or early transplantation had an increased risk of NHL.
Discussion
This Taiwanese, population-based cohort of more than 5000 organ transplant recipients, who were followed for 12 years, enabled the determination of variations in cancer risk according to the transplanted organ, age at transplantation, and sex.
Previous Asian population-based studies comparing the risk of cancers between organ transplant recipients and the general population have focused on recipients of specific organ transplants,[6, 8–10] with few studies having compared the risk of cancer in heart, kidney, and liver recipients. Compared with the general population, the risk of cancer increased 3.8-fold after heart transplantation, 4.1-fold after kidney transplantation, and 4.6-fold after liver transplantation, and this risk increased by an average of 4.2-fold in all transplant recipients. The most common cancers after transplantations in Taiwan were cancers of the head and neck, liver, bladder, and kidney and NHL. Male recipients had an increased risk of cancers of the head and neck and the liver; however, female kidney recipients had a significant risk of bladder and kidney cancer.
Our study presents population-based evidence of a significantly higher risk of any cancer in liver recipients than in both heart and kidney recipients after adjustment for age at transplantation, sex, and transplant year.
We have observed increased risks of NHL and cancers of the head and neck, gallbladder, and lung in heart recipients and confirmed prior population-based evidence of increased risks of lip and oral cancer among heart transplant recipients, [2, 3, 5, 11] with an SIR of 46.3 for cancers of the head and neck compared with the general Taiwan population. Viral infection, immunosuppressive therapy, or drug side effects may increase the likelihood of oral ulcers or tongue edema in transplant recipients.[12–16]
Our data of lung cancer and NHL in the heart transplant recipients was consistent with western studies.[2, 3, 5, 11, 17] Smoking is associated with lung cancer[18]; however, it does not play a significant role in the incidence of lung cancer among Spanish patients. [19] The risk of NHL among transplant recipients is related to the aggressiveness of the immunosuppressive regimen.[20] EBV infection plays a crucial role in posttransplant lymphoproliferative disorder in pediatric heart transplant patients,[21, 22] and an EBV-negative serostatus is associated with an increased risk of NHL in heart transplant patients (HR = 3.6, 95% CI 1.1–11.3; P = .031).[23] We observed a 20-fold increased risk of gallbladder cancer in the heart transplant recipients. The risk of gallbladder cancer has been rising among kidney transplant recipients, [9, 24, 25]but similar to general population in US.[26]
We also confirmed liver transplantation was associated with an increased risk of NHL, [3–5, 11, 17, 27, 28] leukemia, and cancer of the head and neck,[3, 5, 11, 28] esophagus,[28] and liver.[3, 17] Alcohol-related cirrhosis in patients with a history of tobacco abuse before liver transplantation may cause the development of head and neck and esophageal cancer.[28–30] The current study demonstrated that in liver recipients, older age and being male were risk factors for liver cancer; this finding was consistent with US registry data.[31] Among the liver recipients, the risk of NHL was virtually unaffected by EBV serostatus; however, it was much higher than that observed in EBV-positive kidney or heart transplant recipients.[23] Because of the scarcity of leukemia after liver transplantation, cases, including donor-derived acute myeloid leukemia, pediatric patient, therapy-related chronic myelomonocytic leukemia, and hepatitis B virus-associated cirrhosis, have been reported. [32–36] An early research has shown parvovirus B19 may be relevant in the pathogenesis of acute leukemia.[37] Nevertheless, the risk of leukemia after liver transplantation remains unclear.
Our results of kidney transplantation concur with prior population-based evidence on the risks of NHL and cancers of the kidney, bladder, and thyroid[3, 5, 6, 24] but not cancers of the head and neck [3, 6] or liver.[24] We found that female kidney recipients had a higher SIR for bladder cancer (SIR male = 20.7 and SIR female = 113.2; data not shown), supporting previous Asian researches.[6, 7, 9, 10] The binding of female hormones to their receptors exhibits rapid bladder cancer progression in women[38–40]; however, few Western studies have examined the reproducibility of sex differences in bladder cancer after kidney transplantation. [41] Additional studies are warranted to clarify such associations. Female kidney recipients have a higher risk of urinary tract infections than do male recipients. [42] Papillary thyroid carcinoma accounts for more than 88% of all thyroid cancers in Taiwan. [43] Viral infections have been associated with thyroid cancer progression. [44–46] The high incidence of thyroid disorders among kidney recipients necessitates that cancer-related examinations be performed routinely in patients with viral infections and renal disorders. [47, 48]
In Taiwan, liver and oral cancers account for the third and sixth highest incidences of cancer in the general population, and the incidence is particularly high in men. [43] The prevalence is high in patients with chronic hepatitis B virus (HBV) infections (13.7%). [49] Current alcohol consumption, smoking, and self-medication are factors that are correlated to the high cancer prevalence in adults with HBV or hepatitis C virus infections. [50] Heavy alcohol consumption significantly increased the risk of hepatocellular carcinoma in patients with HBV-related cirrhosis. [51] Betel quid use (with or without tobacco addition) has been confirmed as a major independent risk factor for oral and oropharyngeal cancers. [52] Betel quid chewing was observed in 90% of patients with oral cancer. [53] In a cross-sectional study, the prevalences of alcohol consumption, betel quid chewing, and smoking in men were 53.2%, 32.7%, and 50.2%, respectively. [54] Hepatitis virus infections, betel quid chewing, alcohol consumption, and smoking may partially explain the increased risk of liver and oral cancers among male recipients.
Skin cancer after transplantation has been the major concern identified in western studies.[3, 5, 11, 55] This was not observed in our study. Continual sun exposure from childhood is a risk factor for squamous cell carcinoma (SCC); however, the risk varies according to sex. [56] In arseniasis-endemic areas, standardised mortality ratios are significantly high for liver, lung, and bladder cancers. Thus, cutaneous SCC and basal cell carcinoma (BCC) are more strongly associated with arsenic exposure than with sun exposure. [57, 58] Because skin cancer accounts for the 10th highest incidence of cancer in the general population in Taiwan [43] and a study conducted in southern reported no incidence of SCC or BCC after renal transplantation, [59] genetic factors can influence the ethnic variance observed in the occurrence of skin cancer.
Calendar year of transplantation had been reported as protective factors[11, 55], risk factors[2, 5] and non-significant factors[3, 4]. Because these results published from different countries, transplant organs, periods of calendar year of transplantations and cancers, the impact of calendar year of transplantation remains uncertain. Therefore, the association of age and calendar year of transplantation also needs further studies to clarify.
Though this 12-year study period might not sufficient for certain cancer occur, [60, 61] cancers of the head and neck, liver, bladder, and kidney and non-Hodgkin lymphoma after transplantation in Taiwan alert health professionals to the importance of cancer-related screening and behavior risk factors during follow up.
Reviews by the NHIA and government regulation ensure the quality of the NHIRD, which covers almost the entire population of Taiwan. Furthermore, because of the demanding nature of immunosuppressive therapy and copayments are waived for patients with catastrophic illnesses, it is unlikely that transplant recipients would travel abroad or that our estimates would be biased by loss to follow-up. However, patients traveling abroad for transplantation may have led to our study underestimating the cancer incidence rates. By the nature of NHIRD, the lack information of behavior risk factors, donor–recipient individual and matching data [62–66] and cancers arising from the native organs or the transplant limits the elaboration of this research.
In conclusion, this Asian population-based study showed an elevated cancer risk among transplant recipients. Cancer occurrence varied considerably and posttransplant cancer screening should be performed routinely according to transplanted organ and sex. Our findings encourage further studies on oncogenic mechanisms of organ transplantation in the future.
Acknowledgments
We are grateful to the editor and reviewers for comments that have led to a better version of this paper.
Data Availability
These deidentified data in the study are sent from National Health Insurance Administration (http://www.nhi.gov.tw/) and generated by National Health Research Institutes (http://nhird.nhri.org.tw/index1.php).
Funding Statement
This work was funded by National Science Council (MOST 103-2314-B-016 -022 -MY3) and partially by Tri-service General Hospital (TSGH-C103-028-013, TSGH-C104-031 and TSGH-C105-007-S01), Taiwan. This study is based in part on data from the National Health Insurance Research Database provided by the National Health Insurance Administration, Ministry of Health and Welfare and managed by National Health Research Institutes (Registered number NHIRD-103-164). The interpretation and conclusions contained herein do not represent those of National Health Insurance Administration, Ministry of Health and Welfare or National Health Research Institutes. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ministry of Health and Welfare TROC. Survival of organ transplantation 2014 [cited 2015 April 1]. Available: http://www.mohw.gov.tw/cht/ministry/DM2_P.aspx?f_list_no=7&fod_list_no=4556&doc_no=44105.
- 2.Jiang Y, Villeneuve PJ, Wielgosz A, Schaubel DE, Fenton SS, Mao Y. The incidence of cancer in a population-based cohort of Canadian heart transplant recipients. Am J Transplant. 2010;10(3):637–45. 10.1111/j.1600-6143.2009.02973.x . [DOI] [PubMed] [Google Scholar]
- 3.Krynitz B, Edgren G, Lindelof B, Baecklund E, Brattstrom C, Wilczek H, et al. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008—a Swedish population-based study. Int J Cancer. 2013;132(6):1429–38. 10.1002/ijc.27765 . [DOI] [PubMed] [Google Scholar]
- 4.Jiang Y, Villeneuve PJ, Fenton SS, Schaubel DE, Lilly L, Mao Y. Liver transplantation and subsequent risk of cancer: findings from a Canadian cohort study. Liver Transpl. 2008;14(11):1588–97. 10.1002/lt.21554 . [DOI] [PubMed] [Google Scholar]
- 5.Collett D, Mumford L, Banner NR, Neuberger J, Watson C. Comparison of the incidence of malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. 2010;10(8):1889–96. 10.1111/j.1600-6143.2010.03181.x . [DOI] [PubMed] [Google Scholar]
- 6.Cheung CY, Lam MF, Chu KH, Chow KM, Tsang KY, Yuen SK, et al. Malignancies after kidney transplantation: Hong Kong renal registry. Am J Transplant. 2012;12(11):3039–46. 10.1111/j.1600-6143.2012.04209.x . [DOI] [PubMed] [Google Scholar]
- 7.Li WH, Chen YJ, Tseng WC, Lin MW, Chen TJ, Chu SY, et al. Malignancies after renal transplantation in Taiwan: a nationwide population-based study. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2012;27(2):833–9. 10.1093/ndt/gfr277 . [DOI] [PubMed] [Google Scholar]
- 8.Imao T, Ichimaru N, Takahara S, Kokado Y, Okumi M, Imamura R, et al. Risk factors for malignancy in Japanese renal transplant recipients. Cancer. 2007;109(10):2109–15. 10.1002/cncr.22636 . [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Kim SO, Han DJ, Park SK. Post-transplant malignancy: a burdensome complication in renal allograft recipients in Korea. Clinical transplantation. 2014;28(4):434–42. 10.1111/ctr.12328 . [DOI] [PubMed] [Google Scholar]
- 10.Hwang JC, Jiang MY, Lu YH, Weng SF. Sex difference for urologic malignancy risk in uremic patients after kidney transplantation: a population-based study. Transplantation. 2015;99(4):818–22. 10.1097/TP.0000000000000406 . [DOI] [PubMed] [Google Scholar]
- 11.Na R, Grulich AE, Meagher NS, McCaughan GW, Keogh AM, Vajdic CM. Comparison of de novo cancer incidence in Australian liver, heart and lung transplant recipients. Am J Transplant. 2013;13(1):174–83. 10.1111/j.1600-6143.2012.04302.x . [DOI] [PubMed] [Google Scholar]
- 12.Gulec AT, Haberal M. Lip and oral mucosal lesions in 100 renal transplant recipients. Journal of the American Academy of Dermatology. 2010;62(1):96–101. 10.1016/j.jaad.2009.06.022 . [DOI] [PubMed] [Google Scholar]
- 13.Mainville GN, Marsh WL, Allen CM. Oral ulceration associated with concurrent herpes simplex virus, cytomegalovirus, and Epstein-Barr virus infection in an immunocompromised patient. Oral surgery, oral medicine, oral pathology and oral radiology. 2015;119(6):e306–14. 10.1016/j.oooo.2014.10.019 . [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Pintor RM, Hernandez G, de Arriba L, Morales JM, Jimenez C, de Andres A. Oral ulcers during the course of cytomegalovirus infection in renal transplant recipients. Transplantation proceedings. 2009;41(6):2419–21. 10.1016/j.transproceed.2009.06.053 . [DOI] [PubMed] [Google Scholar]
- 15.Stallone G, Infante B, Di Paolo S, Schena A, Grandaliano G, Gesualdo L, et al. Sirolimus and angiotensin-converting enzyme inhibitors together induce tongue oedema in renal transplant recipients. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2004;19(11):2906–8. 10.1093/ndt/gfh352 . [DOI] [PubMed] [Google Scholar]
- 16.Sheen TS, Tsai CC, Ko JY, Chang YL, Hsu MM. Undifferentiated carcinoma of the major salivary glands. Cancer. 1997;80(3):357–63. . [PubMed] [Google Scholar]
- 17.Engels EA, Pfeiffer RM, Fraumeni JF Jr., Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. Jama. 2011;306(17):1891–901. 10.1001/jama.2011.1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Groot P, Munden RF. Lung cancer epidemiology, risk factors, and prevention. Radiologic clinics of North America. 2012;50(5):863–76. 10.1016/j.rcl.2012.06.006 . [DOI] [PubMed] [Google Scholar]
- 19.Crespo-Leiro MG, Villa-Arranz A, Manito-Lorite N, Paniagua-Martin MJ, Rabago G, Almenar-Bonet L, et al. Lung cancer after heart transplantation: results from a large multicenter registry. Am J Transplant. 2011;11(5):1035–40. 10.1111/j.1600-6143.2011.03515.x . [DOI] [PubMed] [Google Scholar]
- 20.Opelz G, Henderson R. Incidence of non-Hodgkin lymphoma in kidney and heart transplant recipients. Lancet. 1993;342(8886–8887):1514–6. . [DOI] [PubMed] [Google Scholar]
- 21.Schubert S, Abdul-Khaliq H, Lehmkuhl HB, Yegitbasi M, Reinke P, Kebelmann-Betzig C, et al. Diagnosis and treatment of post-transplantation lymphoproliferative disorder in pediatric heart transplant patients. Pediatric transplantation. 2009;13(1):54–62. 10.1111/j.1399-3046.2008.00969.x . [DOI] [PubMed] [Google Scholar]
- 22.Schubert S, Renner C, Hammer M, Abdul-Khaliq H, Lehmkuhl HB, Berger F, et al. Relationship of immunosuppression to Epstein-Barr viral load and lymphoproliferative disease in pediatric heart transplant patients. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2008;27(1):100–5. 10.1016/j.healun.2007.09.027 . [DOI] [PubMed] [Google Scholar]
- 23.Opelz G, Daniel V, Naujokat C, Dohler B. Epidemiology of pretransplant EBV and CMV serostatus in relation to posttransplant non-Hodgkin lymphoma. Transplantation. 2009;88(8):962–7. 10.1097/TP.0b013e3181b9692d . [DOI] [PubMed] [Google Scholar]
- 24.Villeneuve PJ, Schaubel DE, Fenton SS, Shepherd FA, Jiang Y, Mao Y. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant. 2007;7(4):941–8. 10.1111/j.1600-6143.2007.01736.x . [DOI] [PubMed] [Google Scholar]
- 25.Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation. Jama. 2006;296(23):2823–31. 10.1001/jama.296.23.2823 . [DOI] [PubMed] [Google Scholar]
- 26.Koshiol J, Pawlish K, Goodman MT, McGlynn KA, Engels EA. Risk of hepatobiliary cancer after solid organ transplant in the United States. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2014;12(9):1541–9 e3 10.1016/j.cgh.2013.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aberg F, Pukkala E, Hockerstedt K, Sankila R, Isoniemi H. Risk of malignant neoplasms after liver transplantation: a population-based study. Liver Transpl. 2008;14(10):1428–36. 10.1002/lt.21475 . [DOI] [PubMed] [Google Scholar]
- 28.Baccarani U, Piselli P, Serraino D, Adani GL, Lorenzin D, Gambato M, et al. Comparison of de novo tumours after liver transplantation with incidence rates from Italian cancer registries. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2010;42(1):55–60. 10.1016/j.dld.2009.04.017 . [DOI] [PubMed] [Google Scholar]
- 29.Kenngott S, Gerbes AL, Schauer R, Bilzer M. Rapid development of esophageal squamous cell carcinoma after liver transplantation for alcohol-induced cirrhosis. Transplant international: official journal of the European Society for Organ Transplantation. 2003;16(9):639–41. 10.1007/s00147-003-0600-8 . [DOI] [PubMed] [Google Scholar]
- 30.Jimenez-Romero C, Justo-Alonso I, Cambra-Molero F, Calvo-Pulido J, Garcia-Sesma A, Abradelo-Usera M, et al. Incidence, risk factors and outcome of de novo tumors in liver transplant recipients focusing on alcoholic cirrhosis. World journal of hepatology. 2015;7(7):942–53. 10.4254/wjh.v7.i7.942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann CJ, Subramanian AK, Cameron AM, Engels EA. Incidence and risk factors for hepatocellular carcinoma after solid organ transplantation. Transplantation. 2008;86(6):784–90. 10.1097/TP.0b013e3181837761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Subklewe M, Nagy M, Schoch C, Jenisch S, Siebert R, Gesk S, et al. Extramedullary manifestation of a donor-derived acute myeloid leukemia in a liver transplant patient. Leukemia. 2004;18(12):2050–3. 10.1038/sj.leu.2403498 . [DOI] [PubMed] [Google Scholar]
- 33.Doti CA, Gondolesi GE, Sheiner PA, Emre S, Miller CM, Aledort LM. Leukemia after liver transplant. Transplantation. 2001;72(10):1643–6. Epub 2001/12/01. . [DOI] [PubMed] [Google Scholar]
- 34.Akar Ozkan E, Ozdemir BH, Yilmaz Akcay E, Ok Atilgan A, Haberal M. T-cell acute lymphoblastic leukemia after liver transplant. Experimental and clinical transplantation: official journal of the Middle East Society for Organ Transplantation. 2014;12 Suppl 1:139–41. Epub 2014/03/19. . [PubMed] [Google Scholar]
- 35.Ahmed F, Osman N, Lucas F, Neff G, Smolarek T, Bennett JM, et al. Therapy related CMML: a case report and review of the literature. International journal of hematology. 2009;89(5):699–703. 10.1007/s12185-009-0318-1 . [DOI] [PubMed] [Google Scholar]
- 36.Cho YU, Chi HS, Park CJ, Seo EJ, Lee JH, Lee JH, et al. Two cases of post-liver transplant acute myeloid leukemia in Korean adults: review of bibliographies and comparison with post-renal transplant acute myeloid leukemia. Annals of hematology. 2008;87(6):513–4. 10.1007/s00277-008-0458-z . [DOI] [PubMed] [Google Scholar]
- 37.Kerr JR, Barah F, Cunniffe VS, Smith J, Vallely PJ, Will AM, et al. Association of acute parvovirus B19 infection with new onset of acute lymphoblastic and myeloblastic leukaemia. Journal of clinical pathology. 2003;56(11):873–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaidya A, Soloway MS, Hawke C, Tiguert R, Civantos F. De novo muscle invasive bladder cancer: is there a change in trend? The Journal of urology. 2001;165(1):47–50; discussion 10.1097/00005392-200101000-00012 . [DOI] [PubMed] [Google Scholar]
- 39.Shen SS, Smith CL, Hsieh JT, Yu J, Kim IY, Jian W, et al. Expression of estrogen receptors-alpha and -beta in bladder cancer cell lines and human bladder tumor tissue. Cancer. 2006;106(12):2610–6. 10.1002/cncr.21945 . [DOI] [PubMed] [Google Scholar]
- 40.Hsu I, Vitkus S, Da J, Yeh S. Role of oestrogen receptors in bladder cancer development. Nature reviews Urology. 2013;10(6):317–26. 10.1038/nrurol.2013.53 . [DOI] [PubMed] [Google Scholar]
- 41.Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4(6):905–13. 10.1111/j.1600-6143.2004.00450.x . [DOI] [PubMed] [Google Scholar]
- 42.Ariza-Heredia EJ, Beam EN, Lesnick TG, Cosio FG, Kremers WK, Razonable RR. Impact of urinary tract infection on allograft function after kidney transplantation. Clinical transplantation. 2014;28(6):683–90. 10.1111/ctr.12366 . [DOI] [PubMed] [Google Scholar]
- 43.Health Promotion Administration MoHaW, Taiwan (R.O.C.). Cancer registry annual report, 2011 2014 [cited 2015 March 8]. Available: http://www.hpa.gov.tw/BHPNet/Web/Stat/StatisticsShow.aspx?No=201404160001.
- 44.Adamson LA, Fowler LJ, Clare-Salzler MJ, Hobbs JA. Parvovirus B19 infection in Hashimoto's thyroiditis, papillary thyroid carcinoma, and anaplastic thyroid carcinoma. Thyroid: official journal of the American Thyroid Association. 2011;21(4):411–7. 10.1089/thy.2010.0307 . [DOI] [PubMed] [Google Scholar]
- 45.Shimakage M, Kawahara K, Sasagawa T, Inoue H, Yutsudo M, Yoshida A, et al. Expression of Epstein-Barr virus in thyroid carcinoma correlates with tumor progression. Human pathology. 2003;34(11):1170–7. . [DOI] [PubMed] [Google Scholar]
- 46.Wang JH, Zhang WP, Liu HX, Wang D, Li YF, Wang WQ, et al. Detection of human parvovirus B19 in papillary thyroid carcinoma. British journal of cancer. 2008;98(3):611–8. 10.1038/sj.bjc.6604196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gungor O, Celik A, Kebapcilar L, Karaoglu O, Ersan S, Atilla K, et al. Incidence of thyroid dysfunction and thyroid cancer in renal transplant recipients: a single center experience. Renal failure. 2010;32(2):167–71. 10.3109/08860220903541119 . [DOI] [PubMed] [Google Scholar]
- 48.Halilcevic A, Hodzic E, Mesic E, Trnacevic S. Incidence of subclinical hypothyroidism in renal transplant patients. Materia socio-medica. 2015;27(2):108–11. 10.5455/msm.2015.27.4-108-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen CL, Yang JY, Lin SF, Sun CA, Bai CH, You SL, et al. Slow decline of hepatitis B burden in general population: Results from a population-based survey and longitudinal follow-up study in Taiwan. Journal of hepatology. 2015;63(2):354–63. 10.1016/j.jhep.2015.03.013 . [DOI] [PubMed] [Google Scholar]
- 50.Fan JY, Huang TJ, Jane SW, Chen MY. Prevention of Liver Cancer Through the Early Detection of Risk-related Behavior Among Hepatitis B or C Carriers. Cancer nursing. 2015;38(3):169–76. 10.1097/NCC.0000000000000153 . [DOI] [PubMed] [Google Scholar]
- 51.Lin CW, Lin CC, Mo LR, Chang CY, Perng DS, Hsu CC, et al. Heavy alcohol consumption increases the incidence of hepatocellular carcinoma in hepatitis B virus-related cirrhosis. Journal of hepatology. 2013;58(4):730–5. 10.1016/j.jhep.2012.11.045 . [DOI] [PubMed] [Google Scholar]
- 52.Guha N, Warnakulasuriya S, Vlaanderen J, Straif K. Betel quid chewing and the risk of oral and oropharyngeal cancers: a meta-analysis with implications for cancer control. Int J Cancer. 2014;135(6):1433–43. 10.1002/ijc.28643 . [DOI] [PubMed] [Google Scholar]
- 53.Kao SY, Lim E. An overview of detection and screening of oral cancer in Taiwan. The Chinese journal of dental research: the official journal of the Scientific Section of the Chinese Stomatological Association. 2015;18(1):7–12. . [PubMed] [Google Scholar]
- 54.Guo SE, Huang TJ, Huang JC, Lin MS, Hong RM, Chang CH, et al. Alcohol, betel-nut and cigarette consumption are negatively associated with health promoting behaviors in Taiwan: a cross-sectional study. BMC public health. 2013;13:257 10.1186/1471-2458-13-257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crespo-Leiro MG, Alonso-Pulpon L, Vazquez de Prada JA, Almenar L, Arizon JM, Brossa V, et al. Malignancy after heart transplantation: incidence, prognosis and risk factors. Am J Transplant. 2008;8(5):1031–9. 10.1111/j.1600-6143.2008.02196.x . [DOI] [PubMed] [Google Scholar]
- 56.Chen YC, Christiani DC, Su HJ, Hsueh YM, Smith TJ, Ryan LM, et al. Early-life or lifetime sun exposure, sun reaction, and the risk of squamous cell carcinoma in an Asian population. Cancer causes & control: CCC. 2010;21(5):771–6. 10.1007/s10552-010-9505-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng PS, Weng SF, Chiang CH, Lai FJ. Relationship between arsenic-containing drinking water and skin cancers in the arseniasis endemic areas in Taiwan. The Journal of dermatology. 2015. 10.1111/1346-8138.13058 . [DOI] [PubMed] [Google Scholar]
- 58.Chung CJ, Huang YL, Huang YK, Wu MM, Chen SY, Hsueh YM, et al. Urinary arsenic profiles and the risks of cancer mortality: a population-based 20-year follow-up study in arseniasis-endemic areas in Taiwan. Environmental research. 2013;122:25–30. 10.1016/j.envres.2012.11.007 . [DOI] [PubMed] [Google Scholar]
- 59.Feng WW, Wang TN, Chen HC, Ho JC, Ko YC. Malignancies after renal transplantation in southern Taiwan: experience in one centre. BJU international. 2007;99(4):825–9. 10.1111/j.1464-410X.2006.06645.x . [DOI] [PubMed] [Google Scholar]
- 60.Chockalingam R, Downing C, Tyring SK. Cutaneous Squamous Cell Carcinomas in Organ Transplant Recipients. Journal of clinical medicine. 2015;4(6):1229–39. 10.3390/jcm4061229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rashtak S, Dierkhising RA, Kremers WK, Peters SG, Cassivi SD, Otley CC. Incidence and risk factors for skin cancer following lung transplantation. Journal of the American Academy of Dermatology. 2015;72(1):92–8. 10.1016/j.jaad.2014.09.010 . [DOI] [PubMed] [Google Scholar]
- 62.Lentine KL, Axelrod D, Klein C, Simpkins C, Xiao H, Schnitzler MA, et al. Early clinical complications after ABO-incompatible live-donor kidney transplantation: a national study of Medicare-insured recipients. Transplantation. 2014;98(1):54–65. 10.1097/TP.0000000000000029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tan JC, Kim JP, Chertow GM, Grumet FC, Desai M. Donor-recipient sex mismatch in kidney transplantation. Gender medicine. 2012;9(5):335–47 e2 10.1016/j.genm.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bergenfeldt H, Hoglund P, Andersson B, Radegran G, Ohlsson M, Nilsson J. ABO-identical blood group matching has no survival benefit for AB heart transplant recipients. The Annals of thoracic surgery. 2015;99(3):762–8. 10.1016/j.athoracsur.2014.10.031 . [DOI] [PubMed] [Google Scholar]
- 65.Jawitz OK, J N G, Yuh DD, Bonde P. Impact of ABO compatibility on outcomes after heart transplantation in a national cohort during the past decade. The Journal of thoracic and cardiovascular surgery. 2013;146(5):1239–45; discussion 45–6. 10.1016/j.jtcvs.2013.06.040 . [DOI] [PubMed] [Google Scholar]
- 66.Khush KK, Kubo JT, Desai M. Influence of donor and recipient sex mismatch on heart transplant outcomes: analysis of the International Society for Heart and Lung Transplantation Registry. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2012;31(5):459–66. 10.1016/j.healun.2012.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
These deidentified data in the study are sent from National Health Insurance Administration (http://www.nhi.gov.tw/) and generated by National Health Research Institutes (http://nhird.nhri.org.tw/index1.php).