Abstract
The outer membrane of Gram-negative bacteria presents an effective barrier that restricts the release of proteins from the cell. Virtually all extracellular proteins of Gram-negative bacteria are exported by specialized systems requiring the action of several gene products. We have constructed a tripartite fusion consisting of (i) the signal sequence and first nine N-terminal amino acids of the mature major Escherichia coli lipoprotein, (ii) amino acids 46-159 of the outer membrane protein OmpA, and (iii) the complete mature beta-lactamase (EC 3.5.2.6) sequence. This protein had an enzymatically active beta-lactamase and was found predominantly in the outer membrane. Immunofluorescence microscopy, the accessibility of the fusion protein to externally added proteases, and the rates of hydrolysis of nitrocefin and penicillin G by whole cells demonstrated that a substantial fraction (20-30%) of the beta-lactamase domain of the fusion protein was exposed on the external surface of E. coli. In cells grown at 24 degrees C the localization of beta-lactamase on the cell surface was almost quantitative (greater than 80% of the enzymatically active protein was exposed to the extracellular fluid) as determined by nitrocefin and penicillin G hydrolysis and trypsin accessibility. These results demonstrated that a soluble protein, beta-lactamase, can be transported through--and become anchored on--the outer membrane by fusion to the proper targeting and localization signals.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agterberg M., Adriaanse H., van Bruggen A., Karperien M., Tommassen J. Outer-membrane PhoE protein of Escherichia coli K-12 as an exposure vector: possibilities and limitations. Gene. 1990 Mar 30;88(1):37–45. doi: 10.1016/0378-1119(90)90057-x. [DOI] [PubMed] [Google Scholar]
- Barbas C. F., 3rd, Kang A. S., Lerner R. A., Benkovic S. J. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7978–7982. doi: 10.1073/pnas.88.18.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch D., Leunissen J., Verbakel J., de Jong M., van Erp H., Tommassen J. Periplasmic accumulation of truncated forms of outer-membrane PhoE protein of Escherichia coli K-12. J Mol Biol. 1986 Jun 5;189(3):449–455. doi: 10.1016/0022-2836(86)90316-5. [DOI] [PubMed] [Google Scholar]
- Bosch D., Scholten M., Verhagen C., Tommassen J. The role of the carboxy-terminal membrane-spanning fragment in the biogenesis of Escherichia coli K12 outer membrane protein PhoE. Mol Gen Genet. 1989 Mar;216(1):144–148. doi: 10.1007/BF00332243. [DOI] [PubMed] [Google Scholar]
- Bowden G. A., Paredes A. M., Georgiou G. Structure and morphology of protein inclusion bodies in Escherichia coli. Biotechnology (N Y) 1991 Aug;9(8):725–730. doi: 10.1038/nbt0891-725. [DOI] [PubMed] [Google Scholar]
- Charbit A., Boulain J. C., Ryter A., Hofnung M. Probing the topology of a bacterial membrane protein by genetic insertion of a foreign epitope; expression at the cell surface. EMBO J. 1986 Nov;5(11):3029–3037. doi: 10.1002/j.1460-2075.1986.tb04602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbit A., Molla A., Saurin W., Hofnung M. Versatility of a vector for expressing foreign polypeptides at the surface of gram-negative bacteria. Gene. 1988 Oct 15;70(1):181–189. doi: 10.1016/0378-1119(88)90116-3. [DOI] [PubMed] [Google Scholar]
- Charbit A., Ronco J., Michel V., Werts C., Hofnung M. Permissive sites and topology of an outer membrane protein with a reporter epitope. J Bacteriol. 1991 Jan;173(1):262–275. doi: 10.1128/jb.173.1.262-275.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Reid G. K., Campana A. Export of hybrid proteins FhuA'-'LacZ and FhuA'-'PhoA to the cell envelope of Escherichia coli K-12. J Bacteriol. 1988 May;170(5):2267–2275. doi: 10.1128/jb.170.5.2267-2275.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filloux A., Bally M., Ball G., Akrim M., Tommassen J., Lazdunski A. Protein secretion in gram-negative bacteria: transport across the outer membrane involves common mechanisms in different bacteria. EMBO J. 1990 Dec;9(13):4323–4329. doi: 10.1002/j.1460-2075.1990.tb07881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudl R., Braun G., Hindennach I., Henning U. Lethal mutations in the structural gene of an outer membrane protein (OmpA) of Escherichia coli K12. Mol Gen Genet. 1985;201(1):76–81. doi: 10.1007/BF00397989. [DOI] [PubMed] [Google Scholar]
- Freudl R. Insertion of peptides into cell-surface-exposed areas of the Escherichia coli OmpA protein does not interfere with export and membrane assembly. Gene. 1989 Oct 30;82(2):229–236. doi: 10.1016/0378-1119(89)90048-6. [DOI] [PubMed] [Google Scholar]
- Freudl R., Schwarz H., Klose M., Movva N. R., Henning U. The nature of information, required for export and sorting, present within the outer membrane protein OmpA of Escherichia coli K-12. EMBO J. 1985 Dec 16;4(13A):3593–3598. doi: 10.1002/j.1460-2075.1985.tb04122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghrayeb J., Inouye M. Nine amino acid residues at the NH2-terminal of lipoprotein are sufficient for its modification, processing, and localization in the outer membrane of Escherichia coli. J Biol Chem. 1984 Jan 10;259(1):463–467. [PubMed] [Google Scholar]
- Klose M., Jähnig F., Hindennach I., Henning U. Restoration of membrane incorporation of an Escherichia coli outer membrane protein (OmpA) defective in membrane insertion. J Biol Chem. 1989 Dec 25;264(36):21842–21847. [PubMed] [Google Scholar]
- Klose M., MacIntyre S., Schwarz H., Henning U. The influence of amino substitutions within the mature part of an Escherichia coli outer membrane protein (OmpA) on assembly of the polypeptide into its membrane. J Biol Chem. 1988 Sep 15;263(26):13297–13302. [PubMed] [Google Scholar]
- Klose M., Schwarz H., MacIntyre S., Freudl R., Eschbach M. L., Henning U. Internal deletions in the gene for an Escherichia coli outer membrane protein define an area possibly important for recognition of the outer membrane by this polypeptide. J Biol Chem. 1988 Sep 15;263(26):13291–13296. [PubMed] [Google Scholar]
- Kornacker M. G., Pugsley A. P. Molecular characterization of pulA and its product, pullulanase, a secreted enzyme of Klebsiella pneumoniae UNF5023. Mol Microbiol. 1990 Jan;4(1):73–85. doi: 10.1111/j.1365-2958.1990.tb02016.x. [DOI] [PubMed] [Google Scholar]
- Kornacker M. G., Pugsley A. P. The normally periplasmic enzyme beta-lactamase is specifically and efficiently translocated through the Escherichia coli outer membrane when it is fused to the cell-surface enzyme pullulanase. Mol Microbiol. 1990 Jul;4(7):1101–1109. doi: 10.1111/j.1365-2958.1990.tb00684.x. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- McCafferty J., Griffiths A. D., Winter G., Chiswell D. J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990 Dec 6;348(6301):552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- Morona R., Krämer C., Henning U. Bacteriophage receptor area of outer membrane protein OmpA of Escherichia coli K-12. J Bacteriol. 1985 Nov;164(2):539–543. doi: 10.1128/jb.164.2.539-543.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C. K., Kalve V. I., Klebba P. E. Surface topology of the Escherichia coli K-12 ferric enterobactin receptor. J Bacteriol. 1990 May;172(5):2736–2746. doi: 10.1128/jb.172.5.2736-2746.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C. K., Klebba P. E. Export of FepA::PhoA fusion proteins to the outer membrane of Escherichia coli K-12. J Bacteriol. 1989 Nov;171(11):5894–5900. doi: 10.1128/jb.171.11.5894-5900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton S. M., Jacob C. O., Stocker B. A. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science. 1989 Apr 7;244(4900):70–72. doi: 10.1126/science.2468182. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Normark S. Sensitivity of Escherichia coli to various beta-lactams is determined by the interplay of outer membrane permeability and degradation by periplasmic beta-lactamases: a quantitative predictive treatment. Mol Microbiol. 1987 Jul;1(1):29–36. doi: 10.1111/j.1365-2958.1987.tb00523.x. [DOI] [PubMed] [Google Scholar]
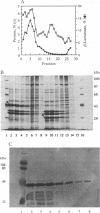
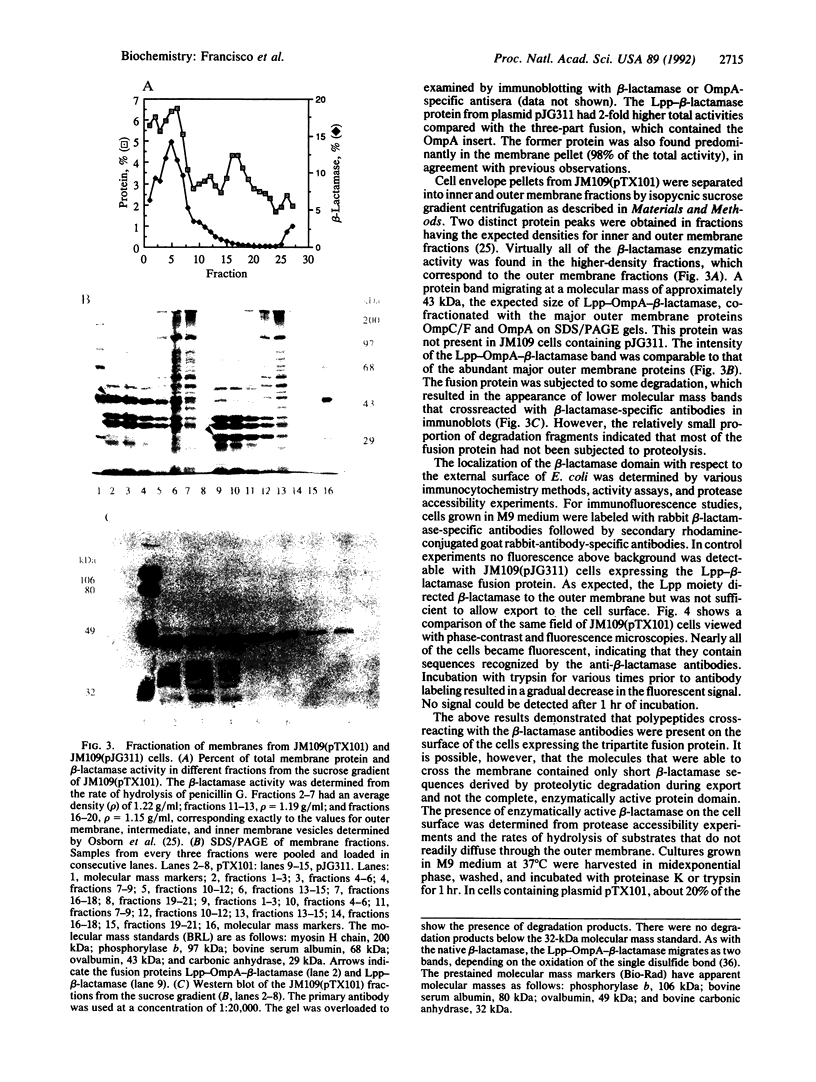
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Pollitt S., Zalkin H. Role of primary structure and disulfide bond formation in beta-lactamase secretion. J Bacteriol. 1983 Jan;153(1):27–32. doi: 10.1128/jb.153.1.27-32.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Kornacker M. G. Secretion of the cell surface lipoprotein pullulanase in Escherichia coli. Cooperation or competition between the specific secretion pathway and the lipoprotein sorting pathway. J Biol Chem. 1991 Jul 25;266(21):13640–13645. [PubMed] [Google Scholar]
- Samuni A. A direct spectrophotometric assay and determination of Michaelis constants for the beta-lactamase reaction. Anal Biochem. 1975 Jan;63(1):17–26. doi: 10.1016/0003-2697(75)90185-2. [DOI] [PubMed] [Google Scholar]
- Sigal I. S., DeGrado W. F., Thomas B. J., Petteway S. R., Jr Purification and properties of thiol beta-lactamase. A mutant of pBR322 beta-lactamase in which the active site serine has been replaced with cysteine. J Biol Chem. 1984 Apr 25;259(8):5327–5332. [PubMed] [Google Scholar]
- Struyvé M., Moons M., Tommassen J. Carboxy-terminal phenylalanine is essential for the correct assembly of a bacterial outer membrane protein. J Mol Biol. 1991 Mar 5;218(1):141–148. doi: 10.1016/0022-2836(91)90880-f. [DOI] [PubMed] [Google Scholar]
- Thiry G., Clippe A., Scarcez T., Petre J. Cloning of DNA sequences encoding foreign peptides and their expression in the K88 pili. Appl Environ Microbiol. 1989 Apr;55(4):984–993. doi: 10.1128/aem.55.4.984-993.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H., Jähnig F. Models for the structure of outer-membrane proteins of Escherichia coli derived from raman spectroscopy and prediction methods. J Mol Biol. 1986 Jul 20;190(2):191–199. doi: 10.1016/0022-2836(86)90292-5. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Yu F., Inouye M. A single amino acid determinant of the membrane localization of lipoproteins in E. coli. Cell. 1988 May 6;53(3):423–432. doi: 10.1016/0092-8674(88)90162-6. [DOI] [PubMed] [Google Scholar]