Abstract
Objective
The Roma minority represents the largest ethnic group in Central and South-East European countries. Data regarding the mortality in Roma hemodialysis subjects are limited. We evaluated the 3 year mortality of ESRD Roma patients treated with hemodialysis (HD).
Study Design and Setting
Our prospective cohort study included 600 ESRD patients on HD therapy recruited from 7 HD centers, from the main geographical regions of Romania. The median age of the patients was 56 (19) years, 332 (55.3%) being males, 51 (8.5%) having Roma ethnicity.
Results
Roma ESRD patients initiate dialysis at a younger age, 47.8 years vs. 52.3 years (P = 0.017), present higher serum albumin (P = 0.013) and higher serum phosphate levels (P = 0.021). In the Roma group, the overall 3 year mortality was higher when compared to Caucasians (33.3% vs. 24.8%). The multivariate survival analysis revealed that being of Roma ethnicity is an independent risk factor for mortality (HR = 1.74; 95% CI = 1.04–2.91; P = 0.035).
Conclusions
Roma patients with ESRD initiate HD therapy at a younger age as compared to Caucasians. They have a higher 3 year mortality rate and are dying at a younger age. Roma ethnicity represents an independent risk factor for mortality in our cohort.
Introduction
Recent epidemiology data have shown that the Roma minority represents the largest ethnic group in Central and South-East European countries (up to7-9% of the population) [1].
Several studies showed a higher prevalence of chronic diseases (hypertension, diabetes, chronic respiratory disease, obesity, metabolic syndrome) in the Roma minority in comparison to the general population [2, 3, 4]. These findings, along with genetic, social, cultural and economic contributors, may explain the 20% prevalence of chronic kidney disease (CKD) in this ethnic group [4], with a relative risk of progressing to end stage renal disease (ESRD) 1.34 times higher comparing to the general population [5]. However, there is no data about the outcomes of the Roma minority with ESRD undergoing HD therapy. According to our knowledge this is the first multicenter study to address this issue.
Subjects and Methods
In this prospective observational study we included all 600 ESRD patients under chronic HD therapy on 1st of November 2010 (332 men and 268 women) in 7 Romanian centers. The cohort was followed-up until 31st of December 2013, or until death. No patient was lost to follow-up.
At inclusion, patient’s data were obtained from their medical records. Dialysis vintage was defined as the time between the first day of dialysis treatment and the study entry date. A routine, complete hematologic and biochemical panel of analysis was performed according to the protocols of the Romanian Ministry of Health (assessment of anemia, inflammatory status, CKD-MBD, liver disease markers and hepatitis virus infection). Patients were treated with high flux, high surface, polysulfone (Xevonta) filters (not reused) and ready-to-use dialysis fluid (B. Braun acidic bicarbonate HD concentrate). Anemia and CKD-MBD were treated according to the KDIGO guidelines [6, 7].
Ethnicity was established based on patient’s self-identification and the appraisal of the nephrologist.
Outcome analysis
Survival time was calculated from the study entry date (1st November 2010) to the date of death or the administrative end of the study (31st of December 2013). We assessed the association between ethnicity and all-cause mortality using Cox-proportional hazard models in which patients remained at risk until death or the end of the study. In the adjusted analysis of survival differences, the following covariates were included: ethnicity (Roma vs. Caucasian), patient’s age, HD duration, body mass index (BMI), hemoglobin, vitamin D levels, history of coronary artery disease, peripheral vascular disease, stroke, hepatitis virus infection and type 2 diabetes mellitus (DM).
Statistical analysis
Data were collected and analyzed using the SPSS v.17 software suite (SPSS Inc. Chicago, IL, USA) and are presented as mean ± standard deviations for continuous variables with Gaussian distribution, median (interquartile range) for continuous variables without Gaussian distribution, or percentages for categorical variables. Survival was analyzed with Hazard Ratio (HR) method and presented using Kaplan-Meier diagrams.
To assess the significance of the differences between groups, the Student t-test (means, Gaussian populations), Mann-Whitney-U test (medians, non-Gaussian populations), Chi-square (proportions) and log-rank test (differences between survival curves and hazard ratio) were used. Continuous variable distributions were tested for normality using the Shapiro-Wilk test, and the Levene’s test for equality of variances. For evaluating the involvement of more confounding factors in time-related risk, Cox proportional-hazards models were constructed. The acceptance of a predictor in the equation was performed according to a backward Wald principle, having an entry probability threshold of 0.05 respectively removal probability threshold at 0.10. A p value of <0.05 was considered as the threshold for statistical significance.
The baseline characteristics of the cohort are presented in Table 1.
Table 1. The baseline characteristics of the studied cohort.
| Studied parameter | Value |
|---|---|
| Roma (%) | 51 (8.5%) |
| Men (%) | 332 (55.3%) |
| Age—median (IQR) | 56 (19) years |
| Time from first HD session–median (IQR) | 2.7 (5) years |
| Weekly HD time–median (IQR) | 12 (3) hours |
| eKt/V–mean ± SD | 1.41 ± 0.31 |
| Filtering surface–median (IQR) | 1.5 (0.3) m2 |
| Blood flow–median (IQR) | 300 (40) ml/min |
| BMI–mean ± SD | 25.5 ± 4.7 kg/m2 |
| Coronary artery disease (%) | 375 (62.5%) |
| Peripheral vascular disease (%) | 150 (25.0%) |
| Stroke (%) | 98 (16.3%) |
| Type 2 Diabetes Mellitus (%) | 92 (15.3%) |
| Hepatitis B virus infection (%) | 57 (9.5%) |
| Hepatitis C virus infection (%) | 164 (27.3%) |
BMI–body mass index
Ethics statement
The study was approved by the Ethical Committee of BBraun Avitum Ltd Romania and the Ethical Committee for Human Research of the University of Medicine and Pharmacy Timisoara. Every patient provided written informed consent before enrollment. The study have been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Results
The studied group was divided in two subgroups in respect to their ethnicity: Caucasian patients (n = 549) respectively Roma patients (n = 51) and compared accordingly. (Tables 2 and 3)
Table 2. Comparison of the studied parameters between Roma and Caucasian dialysis patients.
| Parameter | Caucasian (n = 549) | Roma (n = 51) | p |
|---|---|---|---|
| Age (years)a | 56 [19] | 53 [19] | 0.008 * |
| Dialysis duration (years)a | 2.7 [5] | 2.5 [5.5] | 0.760 |
| Age at the time of HD initiation (years)a | 52.0 [19.85] | 47.8 [16.6] | 0.017 |
| Weekly dialysis time (hours)a | 12 [0] | 12 [2] | 0.755 |
| eKtVb | 1.40 ± 0.31 | 1.41 ± 0.31 | 0.859 |
| Filtering surface (m2)a | 1.8 [0.3] | 1.5 [0.3] | 0.187 |
| Blood flow (mL/min)a | 300 [40] | 300 [5] | 0.737 |
| BMI (kg/m2) b | 25.4 ± 4.7 | 26.4 ± 6.6 | 0.275 |
| Hemoglobin (g/dL) b | 11.2 ± 1.6 | 11.4 ± 1.3 | 0.356 |
| Ferritin (ng/mL)a | 633.4 [512.3] | 624.7 [475.5] | 0.199 |
| TSAT (%) a | 18.7 [31.2] | 18.7 [27.3] | 0.726 |
| hsCRP (mg/dL)a | 1.8 [5.2] | 1.5 [5.0] | 0.780 |
| Albumin (g/dL)b | 4.3 ± 0.6 | 4.5 ± 0.6 | 0.013 * |
| Ca (mg/dL)b | 8.5 ± 0.9 | 8.4 ± 0.9 | 0.116 |
| PO4 (mg/dl)b | 5.6 ± 1.7 | 6.3 ± 2.0 | 0.021 * |
| HCO3 (mEq/L)b | 19.4 ± 4.3 | 20.2 ± 5.6 | 0.302 |
| iPTH (pg/mL) a | 386.5 [655.0] | 568.0 [439.1] | 0.158 |
| Cholecalciferol (ng/mL)a | 19.9 [21.6] | 20.9 [15.5] | 0.294 |
| ALP (UI/L) a | 95.0 [63.0] | 115.5 [67.8] | 0.027 * |
*Differences are significant
a Distributions are not Gaussian. Data is presented as median and [interquartile range]
b Data is presented as mean±SD
BMI–body mass index, ALP—alkaline phosphatase, TSAT- transferrin saturation, hsCRP-high sensitive C-reactive protein, iPTH—intact parathyroid hormone
Table 3. The prevalence of co-morbidities in dialysis patients stratified by ethnicity.
| Parameter | Caucasian (n = 549) | Roma (n = 51) | p |
|---|---|---|---|
| Coronary artery disease | 342 (62.3%) | 33 (64.7%) | 0.734 |
| Peripheral vascular disease | 139 (25.4%) | 11 (21.6%) | 0.549 |
| History of stroke | 94 (17.11%) | 4 (7.8%) | 0.057 |
| Type 2 Diabetes Mellitus | 85 (15.5%) | 7 (13.7%) | 0.739 |
| Hepatitis B virus infection | 50 (9.1%) | 7 (13.7%) | 0.284 |
| Hepatitis C virus infection | 152 (27.7%) | 12 (23.5%) | 0.519 |
Data are presented as number of cases and (percentage from the total of the subgroup)
The Roma group had a significantly lower median age (53 years vs. 56 years, P = 0.008) having at the same time a similar duration of chronic HD (2.5 years vs. 2.7 years, P = 0.76). Also, Roma patients initiated maintenance HD at a significantly younger age (median age 47.8 [16.6] years vs. 52.0 [19.85] years, P = 0.017), leading to the conclusion that Roma ethnicity is associated with an earlier onset of end-stage renal disease.
Compared to the control group, in the Roma ethnicity group there were significantly higher mean PO4 levels (6.3 vs. 5.6 mg/dL, P = 0.021), alkaline phosphatase (115.5 vs. 95.0 U/L, P = 0.027) and serum albumin (4.5 vs. 4.3 mg/dL, P = 0.013). There were no other significant differences between the two groups. (Table 2).
Although the Roma ethnicity group included significantly younger patients than those included in the control group, there were no differences regarding the prevalence of comorbidities between the two groups. Treatment prescriptions with iron, erythropoietin, vitamin D, vitamin D receptor activator, and phosphate binders did not significantly differ in the two groups (data not shown).
During the 3 years of follow-up, 153 deaths were recorded, representing 25.5% of the enrolled patients. In the Roma group the overall mortality was higher compared to Caucasians (33.3% vs. 24.8%). The difference in the median of ages between the two groups, (the Roma group being younger by 3 years), suggests that Roma dialysis patients have a poorer outcome during dialysis therapy and are dying at a younger age compared to Caucasians.
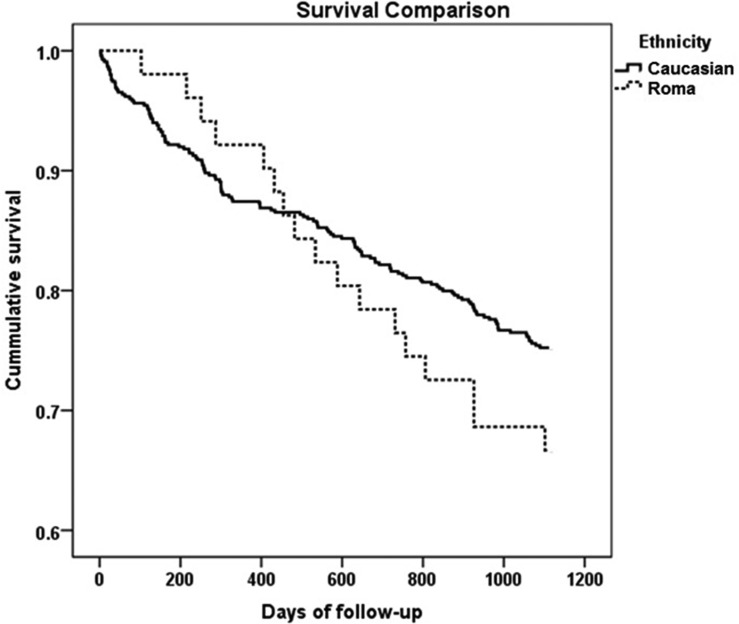
An interesting observation is the crossing of the non-adjusted survival curves in the 424th day of follow-up, with a more pronounced mortality in the Roma ethnic group after this moment. (Fig 1).
Fig 1. Survival analysis in dialysis patients: Roma vs. Caucasian.
In order to assess the role of multiple factors in the hazard of mortality and, also, in order to analyze the mortality in an adjusted, standardized manner, we created a multivariate, stepwise, Wald backward, Cox-regression model, with the following variables considered for the backward algorithm: ethnicity (Roma vs. Caucasian), patient’s age, HD duration, BMI, hemoglobin, vitamin D levels, PO4, albumin, history of coronary artery disease, peripheral vascular disease, stroke, hepatic virus infections and type 2 diabetes. According to the backward Wald method and by defining an entry probability threshold of 0.05, respectively removal probability threshold at 0.10, the final equation was obtained after 7 removal steps. The final predictors accepted in the hazard equation were ethnicity, age, BMI, vitamin D levels, coronary artery disease and peripheral vascular disease. The excluded co-variates proved to be neither significant, nor marginally significant, having a p value for hazard ratio higher than 0.10.
According to our model Roma ethnicity (HR = 1.74; P = 0.035), higher age (HR = 1.03; P = 0.001) and the presence of coronary artery disease (HR = 1.67; P = 0.022) were associated with an increase in the risk of mortality in dialysis patients, while a higher value of vitamin D levels at baseline proved to have a significant protective impact (HR = 0.98; P = 0.017). In our model a higher body mass index also proved to have a protective action; however, it was only marginally significant (HR = 0.97; P = 0.068). The details of the multivariate Cox regression analysis are presented in Table 4.
Table 4. The results of multivariate Cox proportional hazards analysis.
| Predictor | HR (95% CI) | p |
|---|---|---|
| Roma ethnicity | 1.74 (1.04–2.91) | 0.035 |
| Age (per one year increase) | 1.02 (1.01–1.04) | 0.001 |
| BMI (per one kg/m2 increase) | 0.97 (0.93–1.01) | 0.068 |
| Cholecalciferol (per one ng/mL increase) | 0.98 (0.97–0.99) | 0.017 |
| Coronary artery disease | 1.67 (1.08–2.58) | 0.022 |
| Peripheral vascular disease | 1.38 (0.96–1.97) | 0.081 |
Our results suggest that after adjusting for the studied confounding factors, Roma ethnicity represents an important and significant risk factor for mortality in dialysis patients.
Since the survival curves were crossed at the middle of the studied period and thus the proportional hazards hypothesis may be biased [8, 9], we created two separate Cox models, one for the short time and another one for the long term survival (the second one, starting from the moment which the two curves are crossing– 424 days of follow-up).
According to these new models, the significant predictors for the short-term survival (424 days of follow-up) were age (HR = 1.021; p = 0.045) and peripheral vascular disease (HR = 1.686; p = 0.046) while BMI (HR = 0.952; p = 0.085) and the presence of coronary artery disease (HR = 1.903; p = 0.073) were only marginally significant (Table 5).
Table 5. The results of multivariate Cox proportional hazards analysis for the short-term survival (424 days).
| Predictor | HR (95% CI) | p |
|---|---|---|
| Roma ethnicity | 1.00 (0.40–2.52) | 0.996 |
| Age (per one year increase) | 1.02 (1.00–1.04) | 0.045 |
| BMI (per one kg/m2 increase) | 0.95 (0.90–1.01) | 0.085 |
| Cholecalciferol (per one ng/mL increase) | 0.99 (0.97–1.01) | 0.321 |
| Coronary artery disease | 1.91 (0.94–3.85) | 0.073 |
| Peripheral vascular disease | 1.69 (1.01–2.82) | 0.046 |
For the long-term survival, as defined the survival from day 425 to the end of the follow-up period (day 1125), we observed that Roma ethnicity (HR = 2.53; p = 0.004), age (HR = 1.02; p = 0.02) and cholecalciferol (HR = 0.98; p = 0.03) were significant predictors for mortality (Table 6).
Table 6. The results of multivariate Cox proportional hazards analysis for the long-term survival (425 to 1125 days).
| Predictor | HR (95% CI) | p |
|---|---|---|
| Roma ethnicity | 2.52 (1.34–4.76) | 0.004 |
| Age (per one year increase) | 1.02 (1.01–1.04) | 0.020 |
| BMI (per one kg/m2 increase) | 0.98 (0.94–1.03) | 0.581 |
| Cholecalciferol (per one ng/mL increase) | 0.98 (0.96–0.99) | 0.030 |
| Coronary artery disease | 1.47 (0.84–2.57) | 0.178 |
| Peripheral vascular disease | 1.18 (0.71–1.96) | 0.525 |
Discussion
Data regarding the survival of Roma people with ESRD on maintenance HD are lacking. For the first time, we assessed the 3 year survival rate of Roma ethnicity from a large cohort of HD patients. Our data showed that the prevalence of Roma ethnics among HD patients is high. Roma peoples are initiating chronic HD at a younger age than Caucasians and are presenting more frequently higher phosphate levels. They are at a higher risk of dying at a younger age. Furthermore, in the long-term survival analysis, Roma ethnicity represents per se, a significant, independent risk factor for mortality in HD patients.
In our cohort the prevalence of Roma patients is high (8.5%), though, according to the 2011 Census, in our country, Roma ethnic minority represents only 3.3% of the population [10]. Similar findings have been reported by Kolvek et al. in one Slovakian ESRD cohort, the authors showing that the relative risk of ESRD for Roma was 1.34 times higher as compared to the majority population [5]. Also, epidemiological studies regarding racial/ethnic disparities in CKD revealed that the prevalence of ESRD is greater for minorities in the population of United States of America (USA) [11]. Thus, as compared to Caucasians, a 4-fold higher race-specific risk for developing ESRD was identified among African-Americans and a 1.5-fold higher risk in Hispanics [12].
According to our data, Roma patients with CKD initiated HD therapy at a significantly younger age compared to Caucasians, as the African-Americans in the USA [12]. Racial/ethnic minority groups are shown to have a more accelerated CKD progression [11, 13, 14] but the biological, physiological and socio-cultural differences which account for the faster decline of renal function remain unclear [15, 16, 17, 18].
The racial disparity in CKD could be related to the hereditary gene polymorphisms, to the variances in mineral bone disorder, nutritional status, dietary intake, inflammatory and oxidative status profile or in body composition [13]. Some psycho-social, cultural and economic particularities may also intervene: lack of health insurance and limited health care access, unemployment, cultural conflicts, poor communication abilities, poor education, high illiteracy level, social marginalization, poor living conditions, lack of ability to adapt to social rules, distrust in institutions or in the medical system. All these create a barrier leading to a late referral to a nephrologist, delayed diagnosis, low adherence to medical recommendations even if the access to the health care system is granted [15, 19, 20, 21, 22].
The data focusing on CKD in the Roma ethnicity are limited to few studies. Thus, scarce reports have shown a higher prevalence of primary renal diseases among Roma children, a higher frequency of nephropathies with a lower eGFR in Roma women, and a higher relative risk of ESRD (RR = 1.34) for Roma people as compared to Caucasian counterparts [23, 24, 5]. The average age of Roma ethnicity at HD initiation or renal transplantation is significantly lower than in the Caucasians [5, 25]. Our results are consistent with those presented above: high prevalence in the HD treated ESRD population and lower median age at the initiation of HD therapy in the Roma ethnic group.
The higher risk of CKD in Roma minority and the faster progression to ESRD seem to be associated with the similar disparities reported in African-Americans and/or Hispanics, but with some particularities. Giving the Indian origin of this population, specific genetic factors could be associated with the higher risk of CKD [26]. Recently, this minority has changed its traditional lifestyle, distinguished by a high level of physical activity and low energy diet to a more inactive lifestyle with an excess of caloric intake [2]. Thus, Roma people are at risk of developing obesity, insulin resistance, albuminuria, diabetes mellitus and cardiovascular disease [2]. Higher prevalence of unhealthy behaviors such as smoking, alcohol consumption has also been reported in these people [27]. Lower socio-economic conditions, marginalization, insufficient integration may be important contributors [4, 5, 22, 24, 27, 28]. Several studies have shown that chronic diseases in Roma people occur 5–20 times more frequently than in the general population and are associated with social exclusion, unfavorable housing conditions, inadequate nutrition and low education level [1, 29, 30].
In the last decade racial differences have been described in the regulation of bone and mineral metabolism in patients with ESRD [31, 32, 33, 34]. African-American HD patients present higher levels of plasma PTH, serum phosphorus and alkaline phosphatase as compared to Caucasians, and are more likely to exhibit high bone turnover abnormalities [31, 32, 34]. These findings have been attributed to a higher skeletal resistance to PTH or enhanced activation of a calcium-sensing receptor in the parathyroid gland [35]. In our cohort serum phosphorus and total alkaline phosphatase levels were significantly higher in Roma people than in Caucasians, though the dialysis efficiency (assessed as eKt/V) and treatment prescriptions with phosphate-binders, paricalcitol or active vitamin D were similar. The results are consistent with those described in the US African-Americans even if the genetic background is totally different, so, maybe other factors could be involved in our findings, for example lower adherence to diet recommendations. Roma patients presented increased serum levels of both albumin and phosphate as compared with their controls in this cohort, suggesting a diet with a higher protein content [36, 37, 38]. In HD patients with albumin levels above 3.5g/dl, the increase of phosphate concentration is associated with an increased risk of mortality [38, 39].
According to the results of the Global Burden of Disease 2013 study, CKD is a non-communicable cause of premature death, with a 90% increase over the last years [40]. The life expectancy of patients with ESRD treated with dialysis continues to be lower than for many cancer patients [12, 41]. Thus, a 5-year adjusted survival rate for all RRT patients in Europe was 59.7% and for the USA less than 35% [15, 41]. African-Americans and Hispanics in the USA seem to have a 13% to 45% survival-advantage under dialysis therapy compared to Caucasians [19]. Related to these findings, a survival paradox was described in these ethnic groups highlighting the survival advantage of HD therapy in contrast to the higher mortality rates in the general population [19, 42, 43, 44]. Various explanations have been hypothesized: genetic polymorphism, increased rates of major risk factors such as arterial hypertension and diabetes mellitus, racial differences in nutritional status, inflammation or mineral bone disease, differential sensitivity to dialysis, disparities in socioeconomic status affecting access to care or differences in mental coping mechanisms [20, 31, 45, 46].
The survival paradox does not apply to the Roma minority. Data regarding the mortality of Roma patients treated with HD are scarce and we are not aware of any other similar research. In our cohort of HD patients, Roma ethnicity represents an independent risk factor for mortality in long term survival analysis (HR = 2.52, P = 0.004). Moreover, Roma dialysis patients are dying at a younger age compared to Caucasians. In our study, the survival analysis revealed an interesting fact: Roma people presented a similar survival rate in the first 424 days of the study period, and a higher mortality rate after this date, as compared to Caucasian patients. It is possible that, at the initiation of HD therapy, Roma patients, being younger and healthier, had an initial better survival. Later on, they developed more severe complications at a higher rate which increased their mortality.
In the Roma minority from the general population or with kidney transplants the results seem to be similar. Jackson et al reported a life expectancy gap of 5–15 years in Roma people compared with the general population [47]. Furthermore, in a Hungarian adult kidney transplantation cohort of 1063 transplanted patients, 5.64% were Roma subjects and Roma ethnicity was independently associated with a 77% higher risk (HR = 1.77) of all-cause mortality [25].
The lower socio-economic status with subsequent limited access to health care system, lower adherence to medical treatment prescriptions and specific genetic background related to their North-Indian origin could be considered potential causes of the poorer outcome of the Roma population [2, 26, 48].
In Romania some of those explanations are not valid. Our country has a long-standing universal access to health care with a single, state managed financing system, generating a higher degree of homogeneity of quality of care and higher equity. Even under these conditions, the Roma population continues to experience a higher mortality rate.
Our study has several strengths: the large size of the studied cohort, recruited from one of the East European countries where Roma people represent the second-largest ethnic minority; a follow-up period of 3 years and the fact that it is the first study investigating Roma patients survival on HD therapy.
In our opinion the study has some limitations also. Firstly, we analyzed the risk of mortality by performing an extensive multivariate Cox regression analysis, but, as with all observational studies, we cannot exclude the possibility of residual confounding factors. Secondly, the severity of comorbidities could not be assessed. Finally some variables, such as education, income or hospitalization rate were not available in the patient’s medical records though they could have influenced the outcome.
Conclusions
In conclusion, this study demonstrates that Roma people experience a higher prevalence of ESRD requiring dialysis. In the Roma minority group, patients initiate HD at a younger age, suggesting that CKD progression rate in the pre-dialysis period is higher in this ethnicity. No survival paradox could be evidenced in the Roma minority. According to our study, Roma patients with CKD treated with HD are at an increased risk of death and are dying at a younger age compared to their Caucasians counterparts. Further research is required to establish the causes of these findings.
Acknowledgments
We thank MD. Carina Daniela Andrei, MD. Szofia Ivacson, MD. Petru Craciun, MD. Carmen Elena Anton, MD. Violeta Roman, MD. Suzana Berca for their invaluable help in performing this research. Also we express our gratitude to BBraun Avitum Ltd.
Data Availability
All relevant data are within the paper.
Funding Statement
This research project was funded by an internal grant from the University of Medicine and Pharmacy ‘‘Victor Babes’’ Timisoara, PIII-C2-PCFI-2015/2016, www.umft.ro. AS received the funding. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pappa E, Chatzikonstantinidou S, Chalkiopoulos G, Papadopoulos A, Niakas D. Health-Related Quality of Life of the Roma in Greece: The Role of Socio-Economic Characteristics and Housing Conditions. Int J Environ Res Public Health. 2015;12:6669–81. 10.3390/ijerph120606669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vozarova de Courten B, de Courten M, Hanson RL, Zahorakova A, Egyenes HP, Tataranni PA, et al. Higher prevalence of type 2 diabetes, metabolic syndrome and cardiovascular diseases in gypsies than in non-gypsies in Slovakia. Diabetes Res Clin Pract. 2003;62:95–103 [DOI] [PubMed] [Google Scholar]
- 3.Bogdanović D, Nikić D, Petrović B, Kocić B, Jovanović J, Nikolić M, et al. Mortality of Roma population in Serbia, 2002–2005. Croat Med J. 2007;48:720–6. [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas JD, Doucette MM, Thomas DC, Stoeckle JD. Disease, lifestyle, and consanguinity in 58 American Gypsies. Lancet. 1987;2:377–9 [DOI] [PubMed] [Google Scholar]
- 5.Kolvek G, Rosicova K, Rosenberger J, Podracka L, Stewart RE, Nagyova I, et al. End-stage renal disease among Roma and non-Roma: Roma are at risk. Int J Public Health. 2012;57:751–4. 10.1007/s00038-012-0365-x [DOI] [PubMed] [Google Scholar]
- 6.KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kid Int Suppl, 2012, 2:279–335 [Google Scholar]
- 7.KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kid Int, 2009, 76 (Supplement 113s), Supp S1–S130 [DOI] [PubMed] [Google Scholar]
- 8.Li H, Han D, Hou Y, Chen H, Chen Z. Statistical inference methods for two crossing survival curves: a comparison of methods. PLoS One. 2015;10(1):e0116774] 10.1371/journal.pone.0116774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Logan BR, Klein JP, Zhang MJ. Comparing treatments in the presence of crossing survival curves: an application to bone marrow transplantation. Biometrics. 2008;64(3):733–40. 10.1111/j.1541-0420.2007.00975.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.„Romanian 2011 census" (PDF) (in Romanian). http://www.recensamantromania.ro/wp-content/uploads/2013/07/REZULTATE-DEFINITIVE-RPL_2011.pdf
- 11.Garcia-Garcia G, Jha V; World Kidney Day Steering Committee. CKD in disadvantaged populations. Can J Kidney Health Dis. 2015;2:18 10.1186/s40697-015-0050-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Ishani A, et al. US Renal Data System 2013 Annual Data Report. Am J Kidney Dis. 2014;63(1 Suppl):A7 10.1053/j.ajkd.2013.11.001 [DOI] [PubMed] [Google Scholar]
- 13.Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, et al. APOL1 risk variants,race, and progression of chronic kidney disease. N Engl J Med. 2013;369:2183–96 10.1056/NEJMoa1310345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crews DC, Liu Y, Boulware LE. Disparities in the burden, outcomes, and care of chronic kidney disease. Curr Opin Nephrol Hypertens. 2014;23:298–305. 10.1097/01.mnh.0000444822.25991.f6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicholas SB, Kalantar-Zadeh K, Norris KC. Racial disparities in kidney disease outcomes. Semin Nephrol. 2013;33:409–15. 10.1016/j.semnephrol.2013.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crews DC, Charles RF, Evans MK, Zonderman AB, Powe NR. Poverty, race, and CKD in a racially and socioeconomically diverse urban population. Am J Kidney Dis.2010;55:992–1000. 10.1053/j.ajkd.2009.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vart P, Gansevoort RT, Coresh J, Reijneveld SA, Bültmann U. Socioeconomic measures and CKD in the United States and The Netherlands. Clin J Am Soc Nephrol.2013;8:1685–93. 10.2215/CJN.12521212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vart P, Reijneveld SA, Bültmann U, Gansevoort RT. Added value of screening for CKD among the elderly or persons with low socioeconomic status. Clin J Am Soc Nephrol. 2015;10:562–70. 10.2215/CJN.09030914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalantar-Zadeh K, Kovesdy CP, Norris KC. Racial survival paradox of dialysis patients: robust and resilient. Am J Kidney Dis. 2012;60:182–5 10.1053/j.ajkd.2012.02.321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lertdumrongluk P, Kovesdy CP, Norris KC, Kalantar-Zadeh K. Nutritional and inflammatory axis of racial survival disparities. Semin Dial. 2013;26:36–9. 10.1111/sdi.12025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi AI, Weekley CC, Chen SC, Li S, Tamura MK, Norris KC, et al. Association of educational attainment with chronic disease and mortality: the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2011;58:228–34. 10.1053/j.ajkd.2011.02.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plantinga L, Howard VJ, Judd S, Muntner P, Tanner R, Rizk D, et al. Association of duration of residence in the southeastern United States with chronic kidney disease may differ by race: the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort study. Int J Health Geogr. 2013;12:17 10.1186/1476-072X-12-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolvek G, Podracka L, Rosenberger J, Stewart RE, van Dijk JP, Reijneveld SA. Kidney diseases in Roma and non-Roma children from eastern Slovakia: are Roma children more at risk? Int J Public Health. 2014;59:1023–6. 10.1007/s00038-014-0609-z [DOI] [PubMed] [Google Scholar]
- 24.Rosenberger J, Majerníková M, Jarcuska P, Pella D, Mareková M, Gecková AM, et al. Higher prevalence of nephropathy in young Roma females compared with non-Roma females. Cent Eur J Public Health. 2014;22 Suppl:S28–31. [DOI] [PubMed] [Google Scholar]
- 25.Molnar MZ, Langer RM, Remport A, Czira ME, Rajczy K, Kalantar-Zadeh K, et al. Roma ethnicity and clinical outcomes in kidney transplant recipients. Int Urol Nephrol. 2012;44:945–54 10.1007/s11255-011-0088-6 [DOI] [PubMed] [Google Scholar]
- 26.Tripathi G, Sharma RK, Baburaj VP, Sankhwar SN, Jafar T, Agrawal S. Genetic risk factors for renal failure among north Indian ESRD patients. Clin Biochem.2008;41:525–31 10.1016/j.clinbiochem.2008.01.009 [DOI] [PubMed] [Google Scholar]
- 27.Cook B, Wayne GF, Valentine A, Lessios A, Yeh E. Revisiting the evidence on health and health care disparities among the Roma: a systematic review 2003–2012. Int J Public Health. 2013;58:885–911. 10.1007/s00038-013-0518-6 [DOI] [PubMed] [Google Scholar]
- 28.Hurtado A, Johnson RJ. Hygiene hypothesis and prevalence of glomerulonephritis. Kidney Int Suppl. 2005;(97):S62–7 [DOI] [PubMed] [Google Scholar]
- 29.Michos A, Terzidis A, Kalampoki V, Pantelakis K, Spanos T, Petridou ET. Seroprevalence and risk factors for hepatitis A, B, and C among Roma and non-Roma children in a deprived area of Athens, Greece. J Med Virol. 2008;80:791–7 10.1002/jmv.21134 [DOI] [PubMed] [Google Scholar]
- 30.Sivic S, Huremovic A, Djerzic H. Social exclusion as a determining health factor of the Roma population. Med Arch. 2013;67:60–2 [DOI] [PubMed] [Google Scholar]
- 31.Kalantar-Zadeh K, Miller JE, Kovesdy CP, Mehrotra R, Lukowsky LR, Streja E, et al. Impact of race on hyperparathyroidism, mineral disarrays, administered vitamin D mimetic, and survival in hemodialysis patients. J Bone Miner Res. 2010;25:2724–34. Erratum in: J Bone Miner Res. 2011;26:439. 10.1002/jbmr.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malluche HH, Mawad HW, Monier-Faugere MC. Renal osteodystrophy in the first decade of the new millennium: analysis of 630 bone biopsies in black and white patients. J Bone Miner Res. 2011;26:1368–76. Erratum in: J Bone Miner Res. 2011;26:2793 10.1002/jbmr.309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scialla JJ, Parekh RS, Eustace JA, Astor BC, Plantinga L, Jaar BG, et al. Race, Mineral Homeostasis and Mortality in Patients with End-Stage Renal Disease on Dialysis. Am J Nephrol. 2015; 20;42:25–34 10.1159/000438999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jorgetti V, dos Reis LM, Ott SM. Ethnic differences in bone and mineral metabolism in healthy people and patients with CKD. Kidney Int. 2014;85:1283–9. 10.1038/ki.2013.443 [DOI] [PubMed] [Google Scholar]
- 35.Kato A. Racial differences in secondary hyperparathyroidism. Nephrourol Mon. 2013;5:932 10.5812/numonthly.9449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sridhar NR, Josyula S. Hypoalbuminemia in hemodialyzed end stage renal disease patients: risk factors and relationships—a 2 year single center study. BMC Nephrol. 2013;14:242 10.1186/1471-2369-14-242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaysen GA, Greene T, Daugirdas JT, Kimmel PL, Schulman GW, Toto RD, et al. Longitudinal and cross-sectional effects of C-reactive protein, equilibrated normalized protein catabolic rate, and serum bicarbonate on creatinine and albumin levels in dialysis patients. Am J Kidney Dis. 2003;42:1200–11 [DOI] [PubMed] [Google Scholar]
- 38.Noori N, Kalantar-Zadeh K, Kovesdy CP, Bross R, Benner D, Kopple JD. Association of dietary phosphorus intake and phosphorus to protein ratio with mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:683–92 10.2215/CJN.08601209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zitt E, Lamina C, Sturm G, Knoll F, Lins F, Freistätter O, et al. Interaction of time-varying albumin and phosphorus on mortality in incident dialysis patients. Clin J Am Soc Nephrol. 2011;6:2650–6. 10.2215/CJN.03780411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pippias M, Stel VS, Abad Diez JM, Afentakis N, Herrero-Calvo JA, Arias M, et al. Renal replacement therapy in Europe: a summary of the 2012 ERA-EDTA Registry Annual Report. Clin Kidney J. 2015;8:248–61. 10.1093/ckj/sfv014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rhee CM, Lertdumrongluk P, Streja E, Park J, Moradi H, Lau WL, et al. Impact of age, race and ethnicity on dialysis patient survival and kidney transplantation disparities. Am J Nephrol. 2014;39:183–94 10.1159/000358497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan G, Norris KC, Yu AJ, Ma JZ, Greene T, Yu W, et al. The relationship of age, race, and ethnicity with survival in dialysis patients. Clin J Am Soc Nephrol. 2013;8:953–61 10.2215/CJN.09180912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drew DA, Tighiouart H, Scott T, Kantor A, Fan L, Artusi C, et al. Asymmetric dimethylarginine, race, and mortality in hemodialysis patients. Clin J Am Soc Nephrol. 2014;9:1426–33. 10.2215/CJN.00770114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Streja E, Molnar MZ, Kovesdy CP. Race, age, and mortality among patients undergoing dialysis. JAMA. 2011;306:2215. [DOI] [PubMed] [Google Scholar]
- 46.Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int. 2005;68:914–24. [DOI] [PubMed] [Google Scholar]
- 47.Jackson Y, Tabin JP, Hourton G, Bodenmann P. [Roma populations and health]. Rev Med Suisse. 2015;11:735–9. [PubMed] [Google Scholar]
- 48.Vokó Z, Csépe P, Németh R, Kósa K, Kósa Z, Széles G, et al. Does socioeconomic status fully mediate the effect of ethnicity on the health of Roma people in Hungary? J Epidemiol Community Health. 2009;63:455–60. 10.1136/jech.2008.079715 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.