Abstract
South Asians are 1/4 of the world’s population and have increased susceptibility to central obesity and related cardiometabolic disease. Knowledge of genetic variants affecting risk of central obesity is largely based on genome-wide association studies of common SNPs in Europeans. To evaluate the contribution of DNA sequence variation to the higher levels of central obesity (defined as waist hip ratio adjusted for body mass index, WHR) among South Asians compared to Europeans we carried out: i) a genome-wide association analysis of >6M genetic variants in 10,318 South Asians with focused analysis of population-specific SNPs; ii) an exome-wide association analysis of ~250K SNPs in protein-coding regions in 2,637 South Asians; iii) a comparison of risk allele frequencies and effect sizes of 48 known WHR SNPs in 12,240 South Asians compared to Europeans. In genome-wide analyses, we found no novel associations between common genetic variants and WHR in South Asians at P<5x10-8; variants showing equivocal association with WHR (P<1x10-5) did not replicate at P<0.05 in an independent cohort of South Asians (N = 1,922) or in published, predominantly European meta-analysis data. In the targeted analyses of 122,391 population-specific SNPs we also found no associations with WHR in South Asians at P<0.05 after multiple testing correction. Exome-wide analyses showed no new associations between genetic variants and WHR in South Asians, either individually at P<1.5x10-6 or grouped by gene locus at P<2.5x10−6. At known WHR loci, risk allele frequencies were not higher in South Asians compared to Europeans (P = 0.77), while effect sizes were unexpectedly smaller in South Asians than Europeans (P<5.0x10-8). Our findings argue against an important contribution for population-specific or cosmopolitan genetic variants underlying the increased risk of central obesity in South Asians compared to Europeans.
Introduction
Central obesity is a major risk factor for insulin resistance, type-2 diabetes (T2D), and cardiovascular disease (CVD) [1,2,3,4]. South Asians, who comprise 1/4 of the world’s population, are at high risk of developing central obesity compared to Europeans [5,6,7,8,9], and this is widely considered to underlie their 2–4 fold excess risk of T2D and CVD [10,11,12,13,14]. Central obesity is heritable amongst South Asians [15,16], consistent with an important genetic contribution. Genome-wide association studies (GWAS) predominantly carried out in people of European ancestry have identified genetic loci underlying central obesity [17,18,19,20,21,22]. Few studies have investigated genetic factors underlying central obesity amongst South Asians [23,24].
To investigate whether genetic variation accounts for the increased risk of central obesity amongst South Asians compared to Europeans, we carried out South Asian-specific genome- and exome-wide association analyses and related our results to published European findings. We selected waist hip ratio adjusted for body mass index (WHR) as a measure of central obesity. We focused on population-specific SNPs present amongst South Asians but not Europeans to test whether these influence WHR amongst South Asians. We also targeted cosmopolitan SNPs associated with WHR in previous GWAS, and SNPs in protein coding regions which are considered to have a higher probability of functional relevance.
Materials and Methods
Design
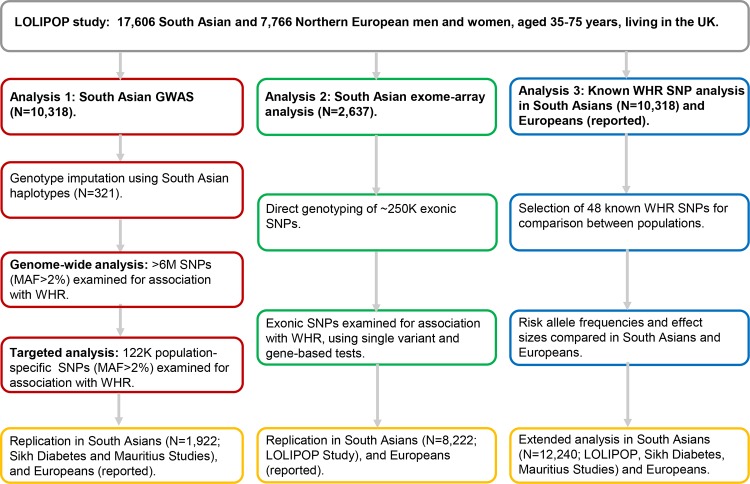
We investigated the contribution of population-specific and cosmopolitan DNA sequence variation to the increased risk of central obesity in South Asians using genome- and exome-wide association analyses. The study design is described in Fig 1.
Fig 1. Study Design.
Population and phenotype
Samples for the discovery analyses were selected from the London Life Sciences Population (LOLIPOP) study, an ongoing population-based cohort of 17,606 South Asian and 7,766 Northern European men and women, aged 35–75 years, recruited from the lists of 58 general practitioners in West London. South Asians were recruited to the study if all 4 grandparents were born in the Indian Subcontinent (countries of India, Pakistan, Sri Lanka or Bangladesh). Data on medical history, current prescribed medication, and cardiovascular risk factors were obtained by a trained research nurse using an interviewer-administered questionnaire. Country of birth of participants, parents, and grandparents were recorded together with language and religion, for assignment of ethnic subgroups. Physical measurements included blood pressure (mean of 3 readings, taken with an Omron 705CP), height, weight, waist and hip circumference. Blood was collected after an 8 hour fast for plasma glucose, total and HDL cholesterol, triglycerides, insulin and high sensitivity C-reactive protein. The research was approved by the West London Research Ethics Committee (reference number: 07/H0712/150), and all participants gave written informed consent. Replication testing was done amongst 1,922 South Asian participants from the Sikh Diabetes and Mauritius Family Studies (S1 Appendix; S1 Table) [25,26,27,28].
Waist hip ratio adjusted for body mass index (WHR) was used as a measure of central obesity. This allows assessment of abdominal fat independent of differences in overall adiposity [19].
Genotyping
Sample selection
We selected 10,318 South Asians for genome-wide association, and 2,637 South Asians for exome-wide association (2,096 individuals were present in both groups). We also carried out genome-wide association in 2,148 Europeans to enable comparison between ethnic groups. Sample selection criteria and characteristics of participants are summarised in S1 Table.
Genome-wide association
Genotyping platforms, calling algorithms, and quality control measures used for genome-wide association are summarised in S2 Table. Hidden relatedness or duplicate samples were sought using identity-by-descent methods implemented in PLINK; samples with evidence for relatedness (pi_hat ≥ 0.5) were identified and one sample was retained. Principal components analysis was carried out in Eigensoft v3.0; eigenvalues inconsistent with either South Asian or European ancestry were removed.
Imputation of unmeasured genotypes was carried out amongst South Asians using sequencing data from 321 South Asian participants in the LOLIPOP study [29], enabling the investigation of population-specific genetic variants. In Europeans, imputation of unmeasured genotypes was carried out using the cosmopolitan 1000 genomes reference panel (phase 1, version 3). All imputation was performed using IMPUTE2; markers with low imputation info score (<0.4), Hardy-Weinberg equilibrium P<1.0x10−6, or minor allele frequency (MAF) <2% were excluded. We assessed the accuracy of our South Asian-specific imputation by comparing imputed genotypes with corresponding direct genotyping results from our exome-wide dataset among overlapping individuals (6,480 variants with MAF >2%). Compared to the exome-wide dataset, mean sensitivity and specificity of the imputed SNPs were 99.41% (0.03) and 99.40% (0.03) respectively. Mean concordance between direct and imputed genotypes was 98.31% (0.04). 6,571,328 SNPs were generated for South Asian-specific genome-wide association analysis.
Exome-wide association
We genotyped 247,870 variants in protein-coding regions among 2,637 South Asians, using the Illumina HumanExome Beadchip and OmniExpressExome (S2 Table). Variants were called using Gencall, followed by post-processing with zCall to improve calling of rare variants. Samples with a SNP call rate <95% were removed, as were SNPs with call rate <97%, MAF <1%, or Hardy-Weinberg equilibrium P<1.0x10−6. 245,496 SNPs passed quality control, of which 35,368 SNPs had MAF >1%.
Statistical analyses
South Asian-specific GWAS
Single variants were examined for association with WHR using linear regression using SNPTEST; covariate adjustments were made for age, sex, BMI, and 10 principal components to control for residual population stratification. SNP-trait associations were examined under additive, dominant and recessive inheritance models. Results were normalised using an inverse normal transformed ranked scale, to enable comparison with reported SNPs shown to be associated with WHR in previous GWAS. Each genotyping platform was analysed separately, and results were combined by fixed effects inverse variance meta-analysis within METAL. Heterogeneity was evaluated using the Cochran's Q statistic. A significance threshold of P<5x10-8 was used for the discovery GWAS.
We refined our approach by focusing on a smaller number of genetic variants from our genome-wide analysis which were population-specific (present in South Asians but not present in Europeans within the 1000 genomes reference panel phase 1, version 3). Amongst the >6 million SNPs investigated in our genome-wide analysis we identified 122,391 South Asian-specific SNPs with MAF >2% which we examined for association with WHR. For the analyses of population-specific genetic variants, statistical significance was inferred at P<0.05 after Bonferroni correction for the number of SNPs tested, thus enhancing study power compared to genome-wide association.
South Asian-specific exome-wide association
Exonic variants were examined for association with WHR using linear regression and an additive genetic model within RAREMETALWORKER, with adjustments for age, sex, and 10 principal components of ancestry. Results for each genotyping platform were analysed separately and combined by meta-analysis within METAL. Common, low-frequency, and rare variants were also grouped by genetic locus for gene-based association analysis within RAREMETAL (CMC, Madsen-Browning, Variable Threshold, and SKAT methods). We selected significance thresholds of P<1.5x10-6 and P<2.5x10−6 for the single variant and gene-based analyses respectively (Bonferroni corrected).
Replication analysis
We carried out replication testing of all genetic variants showing suggestive association with WHR amongst South Asians in genome-wide (P<1x10-5) and exome-wide (P<1x10-3) analyses. Genome-wide results were tested for replication in an independent cohort of South Asians (N = 1,922; S1 and S2 Tables; S1 Appendix) [25,26,27], and in published, predominantly European meta-analysis data from the Giant Consortium (2012–2014 release) [20]. For our exome-wide results we performed replication testing among independent samples from our discovery South Asian GWAS analysis (N = 8,222). A replication significance threshold of P<0.05 was selected.
Population comparisons
We carried out a literature search to identify previously reported SNPs associated with central obesity in GWAS at P<5x10-8. We found 38 SNPs associated with WHR in men and women [19,22], and 11 SNPs associated in women alone [20,22].
We selected 48 of the 49 known WHR SNPs for comparison between South Asians and Europeans. One SNP, rs7801581 in HOXA11, was not available within our South Asian sample. We analysed the 48 known WHR SNPs for association with WHR in South Asians at P<0.05. We examined for systematic differences in risk allele frequencies and in effect sizes between South Asian participants in LOLIPOP and reported European results using the sign test; assessing whether the observed South Asian results, categorised individually as higher or lower than the European reference, differed significantly from the binomial probability distribution (expected value of 0.5). The contribution of known genetic variants to the excess in WHR among South Asians compared to Europeans in the LOLIPOP study was quantified by multivariate linear regression. To extend our analysis, we examined the 48 WHR SNPs in an independent cohort of South Asians (N = 1,922), and used meta-analysis to combine the results with our findings among South Asian participants in LOLIPOP.
Study power
Our South Asian GWAS has 80% power to identify single variants explaining 0.40% of the variation in WHR among 10,318 South Asians at P<5x10-8 (S3A Table); power to detect variants with effects equivalent to the average effect size of reported WHR variants is <1% at genome-wide significance. In our exome-array analysis we have 80% power to detect variants explaining 1.0% of the variation in WHR among 2,637 South Asians at P<1.5x10-6 (S3A Table). For our population comparison of previously reported WHR SNPs from GWAS, we estimate between 10 and 84% power to replicate individual variants at P<0.05 among 10,318 South Asians (S3B Table; or 15 and 91% among 12,240 South Asians, combined discovery and replication cohorts).
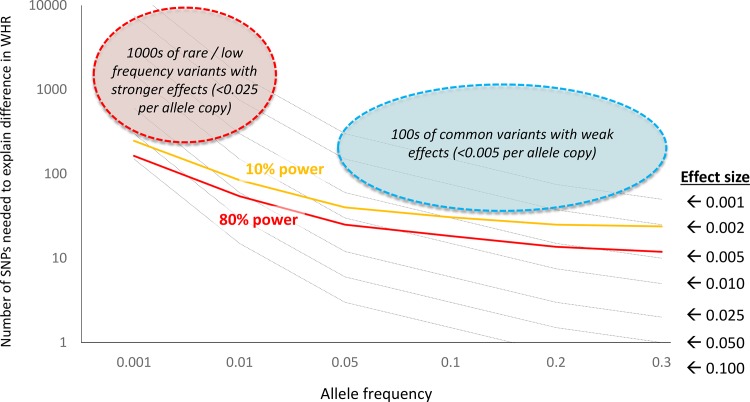
To enhance study power we carried out targeted analyses of South Asian-specific SNPs. We simulated the numbers of population-specific SNPs that would be required to explain the increase in WHR amongst South Asians compared to Europeans as a function of allele frequency and effect size (Fig 2). Amongst 10,318 South Asians we have >80% power to detect common, population-specific variants associated with WHR with an effect size of >0.005 per allele copy (untransformed β value). At this effect size there would need to be in excess of >20 common, population-specific common genetic variants associated with WHR amongst South Asians (Fig 2).
Fig 2. Contribution of population-specific genetic variants to increased WHR amongst South Asians compared to Europeans.
Results are shown as the number of SNPs needed to fully explain the difference in WHR between the populations across a range of effect sizes (per allele copy, dashed black lines). Superimposed are lines showing the power (10% power, orange line; 80% power, red line) of the current study to detect common and low frequency genetic variants associated with WHR amongst South Asians. Results show that our study sample size is sufficient to identify common variants with effect size >0.005 per allele copy, and rare / infrequent variants with effect size >0.025 (untransformed β values). At these effect sizes, there would need to be 10s to 100s of population-specific genetic variants to explain increased WHR amongst South Asians. At effect sizes smaller than those identifiable in the current study, there would need to be 100s of common variants or 1000s of rare / low frequency variants that are population-specific and associated with WHR, to account for increased WHR amongst South Asians.
Results
Population characteristics
The clinical characteristics of the 25,372 South Asian and European participants of the LOLIPOP Study are shown in Table 1. Compared to Europeans, South Asians have higher waist hip ratio (0.94 (0.08) v. 0.91 (0.08); P<0.001) despite similar body mass index (BMI) (27.4 (4.6) v. 27.5 (5.2); P = 0.23). South Asians also have a higher prevalence of T2D and CVD, higher fasting glucose, insulin, HOMA-IR, HbA1c, and triglycerides, with lower HDL-cholesterol compared to Europeans. Waist hip ratio remained higher amongst South Asians compared to Europeans after adjustment for differences in age, gender, and BMI (Table 2).
Table 1. Characteristics of participants in the LOLIPOP Study.
| South Asians | Europeans | P value | |
|---|---|---|---|
| n | 17,606 | 7,766 | |
| Age (yrs) | 50.5 (11.2) | 52.3 (11.6) | 8.3E-29 |
| Male (%) | 61 | 60 | 1.7E-02 |
| Coronary heart disease (%) | 11 | 6 | 1.1E-32 |
| Type-2 diabetes (%) | 19 | 7 | 9.4E-106 |
| Hypertension (%) | 29 | 20 | 1.1E-47 |
| Treated type-2 diabetes (%) | 13 | 4 | 3.2E-90 |
| Treated hypertension (%) | 28 | 19 | 5.6E-45 |
| Treated dyslipidaemia (%) | 20 | 13 | 6.5E-34 |
| Body mass index (kg/m2) | 27.4 (4.6) | 27.5 (5.2) | 2.3E-01 |
| Waist hip ratio | 0.94 (0.08) | 0.91 (0.08) | 3.9E-154 |
| Systolic BP (mmHg) | 131 (19) | 131 (19) | 6.2E-01 |
| Diastolic BP (mmHg) | 80 (11) | 79 (11) | 2.5E-12 |
| Cholesterol (mmol/L) | 5.2 (1.1) | 5.4 (1.1) | 4.9E-45 |
| LDL cholesterol (mmol/L) | 3.2 (0.9) | 3.3 (0.9) | 4.3E-28 |
| HDL cholesterol (mmol/L) | 1.3 (0.3) | 1.4 (0.4) | 4.0E-186 |
| Triglycerides (mmol/L) | 1.7 (1.2) | 1.5 (1.1) | 7.7E-32 |
| Glucose (mmol/L) | 5.8 (2.0) | 5.4 (1.5) | 3.8E-65 |
| Insulin (mU/L) | 13.1 (12.2) | 9.9 (10.1) | 7.6E-85 |
| HbA1c | 6.0 (1.3) | 5.5 (0.9) | 3.0E-289 |
| HOMA-IR | 3.6 (4.3) | 2.6 (3.7) | 1.0E-63 |
Results are presented as mean (standard deviation), or % for categorical variables; comparisons using student t- and chi-squared tests.
Table 2. Multivariate regression analysis of waist hip ratio in South Asian compared to European participants in the LOLIPOP Study.
| Adjustments made | WHR difference (95% CI) South Asians vs. Europeans | P value |
|---|---|---|
| Age, Sex | 0.030 (0.027–0.034) | 6.3E-68 |
| Age, Sex + BMI | 0.038 (0.035–0.041) | 2.3E-132 |
| Age, Sex + BMI + 37 WHR SNPs | 0.038 (0.034–0.042) | 6.0E-76 |
| Age, Sex + BMI + 37 WHR SNPs + 11 additional WHR SNPs | 0.037 (0.033–0.042) | 3.1E-61 |
37 WHR SNPs–previously reported associations with WHR in men and women (P<5x10-8); 11 additional WHR SNPs–previously reported associations with WHR in women alone (P<5x10-8).
South Asian-specific GWAS
Genome-wide analysis
There were no genetic variants (MAF >2%) associated with WHR at genome-wide significance under additive, dominant, or recessive inheritance models (S4 Table and S1 Fig). Comparisons of the distributions for observed and expected association results revealed little evidence for test statistic inflation (Lambdas additive = 1.025, dominant = 1.015, recessive = 1.015, S2 Fig). Variants showing equivocal association with WHR under an additive model (5x10-8<P<1x10-5) did not replicate at P<0.05, either in an independent cohort of South Asians (N = 1,922) or in published, predominantly European data for the Giant Consortium (S4 Table).
Population-specific SNPs
We examined population-specific genetic variants for association with WHR in South Asians, adopting 4 MAF thresholds: i. >2% (N = 122,391); ii. >5% (N = 38,639); iii. >10% (N = 7,349); and iv. >20% (N = 596). None of these population-specific genetic variants were found to be associated with WHR in South Asians at P<0.05 after Bonferroni correction for the number of SNPs tested in their respective allele frequency window (S5 Table). In addition, the observed distributions of association statistics did not deviate from the expected null distributions arguing against enrichment for association with WHR amongst the population-specific SNPs tested (Lambda = 1.022, S3 Fig).
South Asian-specific exome-wide association
None of the examined variants (MAF >1%) were found to be associated with WHR in South Asians at P<1.5x10-6 (S6 Table and S4 Fig), and we found no evidence of deviation of the observed distribution of the test statistics from that expected under the null hypothesis (Lambda = 0.996, S5 Fig). In replication testing of equivocal variants (1.5x10-6<P<1x10-3), only the rs17778003 SNP in ZFAT showed evidence of association with WHR at P<0.05, in South Asians (P = 7.2x10-3) but not in Europeans (P = 0.58) (S6 Table). We found no genes associated with WHR at Bonferroni genome-wide significance threshold of P<2.5x10−6 using gene-based association analyses (S7 Table).
Comparison of known genetic variants
We examined 37 known WHR SNPs for association with variation in WHR amongst South Asian men and women participating in the LOLIPOP study. Only 4 achieved nominal significance (rs6556301 near FGFR4, P = 6.3x10-03; rs984222 in TBX15, P = 6.6x10-3; rs1011731 in DNM3, P = 1.8x10-2; rs12608504 near JUND, P = 2.7x10-02; Table 3). For the 11 SNPs known to be associated with WHR in women alone, 2 had a nominally significant effect in South Asian women (rs4684854 near PPARG, P = 1.3x10-2; rs1534696 in SNX10, P = 2.7x10-3; Table 3).
Table 3. Comparison of 48 known WHR SNPs in South Asians and Europeans.
| Marker Name | Nearest Gene | E/A | Ref | EAF | β (SEM) | P value | n | Dir | Power | EAF | β (SEM) | P value | n |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SOUTH ASIANS COMBINED LOLIPOP | EUROPEANS COMBINED REPORTED | ||||||||||||
| rs9491696 | RSPO3 | G/C | (19) | 0.49 | -0.011 (0.012) | 3.5E-01 | 10,318 | - | 84% | 0.48 | 0.042 | 1.8E-40 | 113,582 |
| rs6905288 | VEGFA | A/G | (19) | 0.81 | 0.014 (0.018) | 4.2E-01 | 10,318 | + | 71% | 0.56 | 0.036 | 5.9E-25 | 95,430 |
| rs984222 | TBX15-WARS2 | G/C | (19) | 0.48 | 0.032 (0.012) | 6.6E-03 | 10,318 | + | 64% | 0.64 | 0.034 | 8.7E-25 | 109,623 |
| rs1055144 | NFE2L3 | T/C | (19) | 0.39 | 0.018 (0.012) | 1.3E-01 | 10,318 | + | 63% | 0.21 | 0.04 | 1.0E-24 | 113,636 |
| rs10195252 | GRB14 | T/C | (19) | 0.72 | -0.005 (0.013) | 7.0E-01 | 10,318 | - | 63% | 0.6 | 0.033 | 2.1E-24 | 102,449 |
| rs4846567 | LYPLAL1 | G/T | (19) | 0.76 | 0.008 (0.014) | 5.6E-01 | 10,318 | + | 57% | 0.72 | 0.034 | 6.9E-21 | 91,820 |
| rs1011731 | DNM3-PIGC | G/A | (19) | 0.44 | 0.028 (0.012) | 1.8E-02 | 10,318 | + | 50% | 0.43 | 0.028 | 9.5E-18 | 92,018 |
| rs718314 | ITPR2-SSPN | G/A | (19) | 0.24 | 0.003 (0.014) | 8.1E-01 | 10,318 | + | 46% | 0.26 | 0.03 | 1.1E-17 | 107,503 |
| rs1294421 | LY86 | G/T | (19) | 0.45 | -0.007 (0.039) | 8.5E-01 | 10,318 | - | 49% | 0.61 | 0.028 | 1.8E-17 | 102,189 |
| rs1443512 | HOXC13 | A/C | (19) | 0.32 | 0.002 (0.013) | 9.0E-01 | 10,318 | + | 47% | 0.24 | 0.031 | 6.4E-17 | 112,353 |
| rs4823006 | ZNRF3-KREMEN1 | A/G | (19) | 0.44 | -0.009 (0.012) | 4.8E-01 | 10,318 | - | 36% | 0.57 | 0.023 | 1.1E-11 | 93,911 |
| rs6784615 | NISCH-STAB1 | T/C | (19) | 0.96 | -0.037 (0.03) | 2.1E-01 | 10,318 | - | 30% | 0.94 | 0.043 | 3.8E-10 | 109,028 |
| rs6861681 | CPEB4 | A/G | (19) | 0.13 | 0.007 (0.017) | 6.7E-01 | 10,318 | + | 31% | 0.34 | 0.022 | 1.9E-09 | 85,722 |
| rs6795735 | ADAMTS9 | C/T | (19) | 0.28 | -0.002 (0.014) | 8.6E-01 | 10,318 | - | 41% | 0.59 | 0.025 | 1.1E+13 | 84,480 |
| rs4765219 | CCDC92 | C/A | (22) | 0.79 | 0.005 (0.015) | 7.2E-01 | 10,005 | + | 47% | 0.67 | 0.028 (0.004) | 1.6E-15 | 209,807 |
| rs979012 | BMP2 | T/C | (22) | 0.44 | 0.017 (0.012) | 1.7E-01 | 10,005 | + | 45% | 0.34 | 0.027 (0.004) | 3.3E-14 | 209,941 |
| rs17451107 | LEKR1 | T/C | (22) | 0.73 | 0.010 (0.014) | 4.9E-01 | 9,504 | + | 45% | 0.61 | 0.026 (0.004) | 1.1E-12 | 207,795 |
| rs4081724 | CEBPA | G/A | (22) | 0.96 | 0.031 (0.031) | 3.1E-01 | 9,505 | + | 43% | 0.85 | 0.035 (0.005) | 7.4E-12 | 207,418 |
| rs4646404 | PEMT | G/A | (22) | 0.87 | -0.031 (0.019) | 1.0E-01 | 9505 | - | 45% | 0.67 | 0.027 (0.004) | 1.4E-11 | 198,196 |
| rs12679556 | MSC | G/T | (22) | 0.44 | 0.021 (0.012) | 9.0E-02 | 10,005 | + | 39% | 0.25 | 0.027 (0.004) | 2.1E-11 | 203,826 |
| rs7759742 | BTNL2 | A/T | (22) | 0.58 | -0.005 (0.013) | 7.0E-01 | 9,504 | - | 38% | 0.51 | 0.023 (0.003) | 4.4E-11 | 208,263 |
| rs6090583 | EYA2 | A/G | (22) | 0.54 | -0.015 (0.013) | 2.5E-01 | 9,505 | - | 35% | 0.48 | 0.022 (0.003) | 6.2E-11 | 209,435 |
| rs1440372 | SMAD6 | C/T | (22) | 0.74 | 0.009 (0.014) | 5.3E-01 | 10,005 | + | 35% | 0.71 | 0.024 (0.004) | 1.1E-10 | 207,447 |
| rs7801581 | HOXA11 | T/C | (22) | NA | NA | NA | NA | NA | NA | 0.24 | 0.027 (0.004) | 3.7E-10 | 195,215 |
| rs1569135 | CALCRL | A/G | (22) | 0.43 | 0.010 (0.012) | 4.0E-01 | 10,005 | + | 32% | 0.53 | 0.021 (0.003) | 5.6E-10 | 209,906 |
| rs905938 | DCST2 | T/C | (22) | 0.85 | 0.003 (0.017) | 8.6E-01 | 9,505 | + | 35% | 0.74 | 0.025 (0.004) | 7.3E-10 | 207,867 |
| rs12608504 | JUND | A/G | (22) | 0.33 | 0.028 (0.013) | 2.7E-02 | 10,005 | + | 33% | 0.36 | 0.022 (0.004) | 8.8E-10 | 209,990 |
| rs8042543 | KLF13 | C/T | (22) | 0.79 | 0.017 (0.016) | 2.7E-01 | 9,505 | + | 34% | 0.78 | 0.026 (0.004) | 1.2E-09 | 208,255 |
| rs1385167 | MEIS1 | G/A | (22) | 0.18 | 0.019 (0.016) | 2.2E-01 | 10,005 | + | 31% | 0.15 | 0.029 (0.005) | 1.9E-09 | 206,619 |
| rs10919388 | GORAB | C/A | (22) | 0.75 | 0.003 (0.014) | 8.3E-01 | 9,505 | + | 34% | 0.72 | 0.024 (0.004) | 3.2E-09 | 181,049 |
| rs10804591 | PLXND1 | A/C | (22) | 0.65 | 0.005 (0.013) | 6.7E-01 | 10,005 | + | 30% | 0.80 | 0.025 (0.004) | 6.6E-09 | 209,921 |
| rs8030605 | RFX7 | A/G | (22) | 0.26 | -0.007 (0.014) | 6.0E-01 | 10,005 | - | 32% | 0.14 | 0.030 (0.005) | 8.8E-09 | 208,374 |
| rs10991437 | ABCA1 | A/C | (22) | 0.09 | 0.003 (0.021) | 8.9E-01 | 10,005 | + | 28% | 0.11 | 0.031 (0.005) | 1.0E-08 | 209,941 |
| rs224333 | GDF5 | G/A | (22) | 0.44 | 0.012 (0.013) | 3.2E-01 | 10,004 | + | 29% | 0.62 | 0.020 (0.004) | 2.6E-08 | 208,025 |
| rs6556301 | FGFR4 | T/G | (22) | 0.41 | 0.034 (0.013) | 6.3E-03 | 9,505 | + | 33% | 0.36 | 0.022 (0.004) | 2.6E-08 | 178,874 |
| rs303084 | SPATA5-FGF2 | A/G | (22) | 0.73 | 0.019 (0.014) | 1.6E-01 | 10,005 | + | 26% | 0.80 | 0.023 (0.004) | 3.9E-08 | 209,941 |
| rs9991328 | FAM13A | T/C | (22) | 0.58 | 0.002 (0.012) | 8.7E-01 | 10,005 | + | 28% | 0.49 | 0.019 (0.003) | 4.5E-08 | 209,925 |
| rs11231693 | MACROD1-VEGFB | A/G | (22) | 0.03 | 0.041 (0.042) | 3.2E-01 | 9,505 | + | 29% | 0.06 | 0.041 (0.008) | 4.5E-08 | 198,072 |
| 27 / 37 | |||||||||||||
| SOUTH ASIANS WOMEN LOLIPOP | EUROPEANS WOMEN REPORTED | ||||||||||||
| rs4684854 | PPARG | C/G | (20) | 0.61 | 0.088 (0.035) | 1.3E-02 | 1,463 | + | 17% | 0.43 | 0.037 | 4.2E-14 | 96,472 |
| rs10478424 | HSD17B4 | A/T | (20) | 0.73 | -0.026 (0.039) | 5.1E-01 | 1,463 | - | 14% | 0.78 | 0.039 | 3.5E-09 | 73,066 |
| rs7830933 | NKX2-6 | A/G | (22) | 0.73 | 0.073 (0.041) | 7.3E-02 | 1,443 | + | 13% | 0.77 | 0.037 (0.005) | 1.2E-12 | 116,567 |
| rs9687846 | MAP3K1 | A/G | (22) | 0.17 | -0.018 (0.047) | 7.1E-01 | 1,443 | - | 14% | 0.19 | 0.041 (0.006) | 3.8E-12 | 115,897 |
| rs2925979 | CMIP | T/C | (22) | 0.30 | 0.051 (0.038) | 1.7E-01 | 1,443 | + | 12% | 0.31 | 0.032 (0.005) | 3.4E-11 | 115,431 |
| rs12454712 | BCL2 | T/C | (22) | 0.45 | 0.043 (0.034) | 2.1E-01 | 1,443 | + | 15% | 0.61 | 0.035 (0.006) | 1.1E-09 | 96,182 |
| rs8066985 | KCNJ2 | A/G | (22) | 0.43 | 0.006 (0.034) | 8.7E-01 | 1,443 | + | 10% | 0.50 | 0.026 (0.005) | 4.0E-09 | 116,683 |
| rs7917772 | SFXN2 | A/G | (22) | 0.40 | 0.031 (0.036) | 3.8E-01 | 1,443 | + | 11% | 0.62 | 0.027 (0.005) | 5.5E-09 | 116,514 |
| rs1776897 | HMGA1 | G/T | (22) | 0.05 | 0.055 (0.077) | 4.7E-01 | 1,443 | + | 12% | 0.08 | 0.052 (0.009) | 6.8E-09 | 100,516 |
| rs3805389 | NMU | A/G | (22) | 0.26 | -0.019 (0.039) | 6.3E-01 | 1,443 | - | 10% | 0.28 | 0.027 (0.005) | 4.6E-08 | 116,226 |
| rs1534696 | SNX10 | C/A | (22) | 0.42 | -0.087 (0.039) | 2.7E-02 | 1,443 | - | 11% | 0.44 | 0.027 (0.005) | 5.4E-08 | 111,643 |
| 7 / 11 | |||||||||||||
Abbreviations: E/A–effect and alternative alleles; EAF—effect allele frequencies; β (SEM)—β coefficients (standard error of mean) per change of WHR-increasing allele on WHR (adjusted for BMI, inverse normal transformed ranked scale); P value—for association with WHR; Dir—direction of effect allele compared to reported European results.
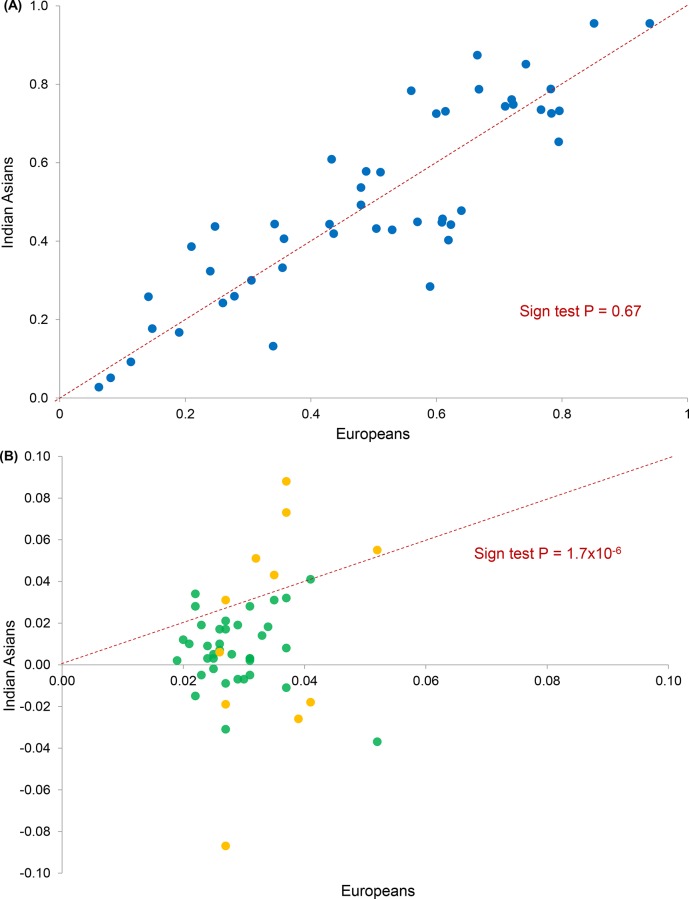
We compared overall effect allele frequencies and effect sizes for these 48 SNPs between South Asian participants in the LOLIPOP Study and reported European results. Effect allele frequencies were similar between the two groups (sign test P = 0.67, Fig 3A). Effect sizes for WHR were consistently lower in South Asians compared to Europeans (sign test P<1.7x10-6, Fig 3B). Inclusion of results from a further 1,922 independent South Asians did not increase the number of SNPs achieving nominal significance (S8 Table), and did not alter comparisons of effect allele frequencies or effect sizes between populations (sign test P = 0.77 and P<5.0x10-8 respectively, S6A and S6B Fig).
Fig 3. Known WHR SNPs.
(A) Comparison of risk allele frequencies in South Asians (N = 10,318) and Europeans (reported). (B) Comparison of effect sizes (β WHR) in South Asians and Europeans. Green–men and women combined (37 SNPs in South Asians (N = 10,318) and Europeans (reported)); orange–women alone (11 SNPs in South Asians (N = 1,463) and Europeans (reported)).
Together the 48 established WHR SNPs explained 0.7% of variation in WHR, equivalent to 2.5% of the reported ~27% heritability [16], among South Asians (LOLIPOP Study participants, men and women combined). When included in multivariate regression analyses examining the differences in WHR between the two populations, these variants did not account for any of the excess risk of central obesity observed in South Asians compared to Europeans (Table 2).
Discussion and Conclusion
Central obesity, characterised by accumulation of excess body fat in an abdominal distribution, is a leading risk factor for T2D, CVD, and premature mortality [1,2,3,4,30,31]. Central obesity is more prevalent amongst South Asians compared to Europeans [5,6,7,8,9], but the mechanisms underlying this are not well understood. Family studies show that central obesity is heritable in South Asians [15,16]. Current knowledge of genetic loci influencing central obesity risk is however largely based on the study of common variants using GWAS among Europeans [17,18,19,20,21,22].
We carried out a GWAS of WHR in South Asians, using imputation of population-specific and cosmopolitan genetic variants identified through next-generation sequencing in 321 South Asians [29]. We found that none of the >6 million evaluated variants were associated with variation in WHR among 10,318 South Asians at genome-wide significance. We then carried out a targeted analysis of population-specific SNPs to test whether these might account for increased WHR amongst South Asians. Despite focus on a smaller number of genetic variants, enhancing study power compared to genome-wide association, we again found no genetic variants associated with WHR in South Asians. In parallel we carried out an exome-array analysis among 2,637 South Asians to provide more comprehensive characterisation of genetic variation in protein coding regions, which are considered to have a higher probability of functional relevance. We found a single variant, rs17778003 in ZFAT, showing weak association with WHR in South Asians, but no novel associations were identified at P<1.5x10-6. Further we found no evidence of genes showing enrichment for common, low-frequency or rare protein-coding variants underlying WHR in South Asians.
We modelled the number of population-specific SNPs required to explain the increase in WHR amongst South Asians compared to Europeans as a function of allele frequency and effect size, as well as study power to detect these variations. Our simulations show we are well powered to identify variants with modest phenotypic effects in our sample of 10,318 South Asians (Fig 2). At these effect sizes there would need to be upwards of 20 population-specific genetic variants associated with WHR amongst South Asians. In contrast we find none. We therefore conclude that a small number of common, population-specific genetic variants with modest effect size do not account for increased WHR amongst South Asians. Indeed, if increased WHR amongst South Asians is the result population-specific genetic variants, this is a polygenic disorder comprising very many genetic variants (>20) that are uncommon (MAF <2%) and / or have small effect size (<0.005 increase in WHR per allele copy).
Differences in risk allele frequencies and effect sizes at known adiposity loci, including FTO and MC4R, have been observed between South Asians and Europeans [23,32]. We compared reported SNPs at 48 established WHR loci in South Asians and in Europeans to investigate whether these variants underlie the excess risk of central obesity observed in South Asians. When compared with published European results, we observed limited evidence for replication at known WHR SNPs among South Asians; only 34 of 48 WHR loci showed directionally consistent effects on WHR in South Asians. When all 48 known WHR variants were examined together we observed no systematic differences in risk allele frequencies, and consistently smaller effect sizes, in South Asians compared to Europeans. Combined these variants accounted for <1% of phenotypic variation in WHR among South Asians, and did not contribute to the excess of WHR observed in South Asians compared to Europeans. The reasons for the lack of replication of known WHR SNPs in South Asians are not known but may include: (i) genetic loci with European-specific effects; (ii) stronger relationships between tag and causal SNPs in Europeans because of differences in haplotype structure; (iii) inflated effect sizes in the European discovery sample due to winner's curse; (iv) greater phenotypic heterogeneity in South Asians, who have smaller stature but greater central adiposity than Europeans, with reduced power to detect SNP-trait associations. Nevertheless, our findings robustly demonstrate that known WHR SNPs do not account for the increased risk of central obesity amongst South Asians compared to Europeans.
Mechanisms underlying central obesity risk among South Asians remain unclear. We have not excluded the possibility of a genetic contribution through multiple common variants with small effects, rare variants with larger phenotypic effects, or structural variations that are inadequately captured using current analytic platforms. Environmental influences have an important role in central adiposity [1,33,34,35,36,37,38], and may contribute to increased risk of central obesity in South Asians. For example differences in lifestyle, such as lower levels of physical activity [39,40,41,42], and higher levels of total calorie, refined starch and saturated fat intake [43,44,45,46], have been reported in South Asians compared to Europeans. Similarly, the prevalence of low birth weight, which is associated with accelerated childhood weight gain [47,48,49] and future development of central obesity and metabolic disturbance [50,51,52,53,54,55], is higher in South Asians than Europeans [56,57,58,59,60,61]. Another explanation is that population-specific epigenomic modifications [62,63,64,65], which regulate gene expression and phenotypic variation without change in DNA sequence [66,67], contribute to central obesity predisposition amongst South Asians. These modifications in DNA methylation, histone modification, and chromatin remodelling, can be transmitted through the germline and modified by environmental exposures [66,68,69,70,71], providing a compelling putative mechanism for unexplained phenotypic variation. Future initiatives combining new, more targeted analysis strategies and larger sample sizes will be required to elucidate the relative roles of these genomic and environmental factors in the excess of central obesity among South Asians.
Supporting Information
(DOCX)
(A) Additive inheritance model. (B) Dominant inheritance model. (C) Recessive inheritance model.
(TIF)
(A) Additive inheritance model (Lambda = 1.025). (B) Dominant inheritance model (Lambda = 1.015). (C) Recessive inheritance model (Lambda = 1.015).
(TIF)
Relationship of observed association statistics with those expected under the null distribution (MAF>2%, Lambda = 1.022).
(TIF)
(TIF)
(TIF)
(A) Comparison of risk allele frequencies in South Asians, extended analysis (N = 12,240) and Europeans (reported). (B) Comparison of effect sizes (β WHR) in South Asians and Europeans, extended analysis. Green–men and women combined (37 SNPs in South Asians (N = 12,240) and Europeans (reported)); orange–women alone (11 SNPs in South Asians (N = 2,363) and Europeans (reported)).
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The LOLIPOP study is supported by the National Institute for Health Research (NIHR) Comprehensive Biomedical Research Centre Imperial College Healthcare NHS Trust, the British Heart Foundation (SP/04/002), the Medical Research Council (G0601966,G0700931), the Wellcome Trust (084723/Z/08/Z), and the NIHR (RP-PG-0407-10371). The work was carried out in part at the NIHR/Wellcome Trust Imperial Clinical Research Facility. The Sikh Diabetes Study is supported by National Institute of Health grants KO1TW006087, funded by the Fogarty International Center, R01DK082766, funded by National Institute of Diabetes and Digestive and Kidney Diseases, and a seed grant from University of Oklahoma Health Sciences Center, Oklahoma City, USA. The Mauritius Family Study is supported by the Mauritius Ministry of Health and Quality of Life, Australian Government National Health and Medical Research Council NHMRC project grant numbers 1020285 and 1037916, the Victorian Government’s OIS Program, and partly funded by US National Institutes of Health Grant DK-25446. We thank the participants and research staff who made the study possible.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The LOLIPOP study is supported by the National Institute for Health Research Comprehensive Biomedical Research Centre Imperial College Healthcare NHS Trust, the British Heart Foundation (SP/04/002, https://www.bhf.org.uk/), the Medical Research Council (G0601966,G0700931, http://www.mrc.ac.uk/), the Wellcome Trust (084723/Z/08/Z, http://www.wellcome.ac.uk/), and the National Institute for Health Research (RP-PG-0407-10371, http://www.nihr.ac.uk/research/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Despres JP, Lemieux I (2006) Abdominal obesity and metabolic syndrome. Nature 444: 881–887. [DOI] [PubMed] [Google Scholar]
- 2.Langenberg C, Sharp SJ, Schulze MB, Rolandsson O, Overvad K, Forouhi NG, et al. (2012) Long-term risk of incident type 2 diabetes and measures of overall and regional obesity: the EPIC-InterAct case-cohort study. PLoS medicine 9: e1001230 10.1371/journal.pmed.1001230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Koning L, Merchant AT, Pogue J, Anand SS (2007) Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. European heart journal 28: 850–856. [DOI] [PubMed] [Google Scholar]
- 4.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. (2005) Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet 366: 1640–1649. [DOI] [PubMed] [Google Scholar]
- 5.Misra A, Khurana L (2011) Obesity-related non-communicable diseases: South Asians vs White Caucasians. International Journal of Obesity 35: 167–187. 10.1038/ijo.2010.135 [DOI] [PubMed] [Google Scholar]
- 6.Kohli S, Sniderman AD, Tchernof A, Lear SA (2010) Ethnic-specific differences in abdominal subcutaneous adipose tissue compartments. Obesity 18: 2177–2183. 10.1038/oby.2010.94 [DOI] [PubMed] [Google Scholar]
- 7.Tillin T, Forouhi NG, McKeigue PM, Chaturvedi N (2012) Southall And Brent REvisited: Cohort profile of SABRE, a UK population-based comparison of cardiovascular disease and diabetes in people of European, Indian Asian and African Caribbean origins. International journal of epidemiology 41: 33–42. 10.1093/ije/dyq175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron AJ, Sicree RA, Zimmet PZ, Alberti KG, Tonkin AM, Balkau B, et al. (2010) Cut-points for waist circumference in Europids and South Asians. Obesity 18: 2039–2046. 10.1038/oby.2009.455 [DOI] [PubMed] [Google Scholar]
- 9.McKeigue PM, Pierpoint T, Ferrie JE, Marmot MG (1992) Relationship of glucose intolerance and hyperinsulinaemia to body fat pattern in south Asians and Europeans. Diabetologia 35: 785–791. [DOI] [PubMed] [Google Scholar]
- 10.McKeigue PM, Shah B, Marmot MG (1991) Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet 337: 382–386. [DOI] [PubMed] [Google Scholar]
- 11.Zaninotto P, Mindell J, Hirani V (2007) Prevalence of cardiovascular risk factors among ethnic groups: results from the Health Surveys for England. Atherosclerosis 195: e48–57. [DOI] [PubMed] [Google Scholar]
- 12.Wild SH, Fischbacher C, Brock A, Griffiths C, Bhopal R (2007) Mortality from all causes and circulatory disease by country of birth in England and Wales 2001–2003. Journal of public health 29: 191–198. [DOI] [PubMed] [Google Scholar]
- 13.Barnett AH, Dixon AN, Bellary S, Hanif MW, O'Hare JP, Raymond NT, et al. (2006) Type 2 diabetes and cardiovascular risk in the UK south Asian community. Diabetologia 49: 2234–2246. [DOI] [PubMed] [Google Scholar]
- 14.HSE (2004) Health Survery for England: Health of ethnic minorities.
- 15.Davey G, Ramachandran A, Snehalatha C, Hitman GA, McKeigue PM (2000) Familial aggregation of central obesity in Southern Indians. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity 24: 1523–1527. [DOI] [PubMed] [Google Scholar]
- 16.Zabaneh D, Chambers JC, Elliott P, Scott J, Balding DJ, Kooner JS (2009) Heritability and genetic correlations of insulin resistance and component phenotypes in Asian Indian families using a multivariate analysis. Diabetologia 52: 2585–2589. 10.1007/s00125-009-1504-7 [DOI] [PubMed] [Google Scholar]
- 17.Lindgren CM, Heid IM, Randall JC, Lamina C, Steinthorsdottir V, Qi L, et al. (2009) Genome-wide association scan meta-analysis identifies three Loci influencing adiposity and fat distribution. PLoS genetics 5: e1000508 10.1371/journal.pgen.1000508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heard-Costa NL, Zillikens MC, Monda KL, Johansson A, Harris TB, Fu M, et al. (2009) NRXN3 is a novel locus for waist circumference: a genome-wide association study from the CHARGE Consortium. PLoS genetics 5: e1000539 10.1371/journal.pgen.1000539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heid IM, Jackson AU, Randall JC, Winkler TW, Qi L, Steinthorsdottir V, et al. (2010) Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nature genetics 42: 949–960. 10.1038/ng.685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Randall JC, Winkler TW, Kutalik Z, Berndt SI, Jackson AU, Monda KL, et al. (2013) Sex-stratified genome-wide association studies including 270,000 individuals show sexual dimorphism in genetic loci for anthropometric traits. PLoS genetics 9: e1003500 10.1371/journal.pgen.1003500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox CS, Liu Y, White CC, Feitosa M, Smith AV, Heard-Costa N, et al. (2012) Genome-wide association for abdominal subcutaneous and visceral adipose reveals a novel locus for visceral fat in women. PLoS genetics 8: e1002695 10.1371/journal.pgen.1002695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shungin D, Winkler TW, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi R, et al. (2015) New genetic loci link adipose and insulin biology to body fat distribution. Nature 518: 187–196. 10.1038/nature14132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chambers JC, Elliott P, Zabaneh D, Zhang W, Li Y, Froguel P, et al. (2008) Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nature genetics 40: 716–718. 10.1038/ng.156 [DOI] [PubMed] [Google Scholar]
- 24.Zabaneh D, Balding DJ (2010) A genome-wide association study of the metabolic syndrome in Indian Asian men. PLoS One 5: e11961 10.1371/journal.pone.0011961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanghera DK, Bhatti JS, Bhatti GK, Ralhan SK, Wander GS, Singh JR, et al. (2006) The Khatri Sikh Diabetes Study (SDS): study design, methodology, sample collection, and initial results. Human biology 78: 43–63. [DOI] [PubMed] [Google Scholar]
- 26.Soderberg S, Zimmet P, Tuomilehto J, de Courten M, Dowse GK, Chitson P, et al. (2004) High incidence of type 2 diabetes and increasing conversion rates from impaired fasting glucose and impaired glucose tolerance to diabetes in Mauritius. Journal of internal medicine 256: 37–47. [DOI] [PubMed] [Google Scholar]
- 27.Soderberg S, Zimmet P, Tuomilehto J, de Courten M, Dowse GK, Chitson P, et al. (2005) Increasing prevalence of Type 2 diabetes mellitus in all ethnic groups in Mauritius. Diabetic medicine: a journal of the British Diabetic Association 22: 61–68. [DOI] [PubMed] [Google Scholar]
- 28.Jowett JB, Diego VP, Kotea N, Kowlessur S, Chitson P, Dyer TD, et al. (2009) Genetic influences on type 2 diabetes and metabolic syndrome related quantitative traits in Mauritius. Twin research and human genetics: the official journal of the International Society for Twin Studies 12: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chambers JC, Abbott J, Zhang W, Turro E, Scott WR, Tan ST, et al. (2014) The South Asian genome. PLoS One 9: e102645 10.1371/journal.pone.0102645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, et al. (2008) General and abdominal adiposity and risk of death in Europe. The New England journal of medicine 359: 2105–2120. 10.1056/NEJMoa0801891 [DOI] [PubMed] [Google Scholar]
- 31.Boggs DA, Rosenberg L, Cozier YC, Wise LA, Coogan PF, Ruiz-Narvaez EA, et al. (2011) General and abdominal obesity and risk of death among black women. The New England journal of medicine 365: 901–908. 10.1056/NEJMoa1104119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Kilpelainen TO, Liu C, Zhu J, Liu Y, Hu C, et al. (2012) Association of genetic variation in FTO with risk of obesity and type 2 diabetes with data from 96,551 East and South Asians. Diabetologia 55: 981–995. 10.1007/s00125-011-2370-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schleinitz D, Bottcher Y, Bluher M, Kovacs P (2014) The genetics of fat distribution. Diabetologia 57: 1276–1286. 10.1007/s00125-014-3214-z [DOI] [PubMed] [Google Scholar]
- 34.Murakami K, McCaffrey TA, Livingstone MB (2013) Associations of dietary glycaemic index and glycaemic load with food and nutrient intake and general and central obesity in British adults. The British journal of nutrition 110: 2047–2057. 10.1017/S0007114513001414 [DOI] [PubMed] [Google Scholar]
- 35.Bradlee ML, Singer MR, Qureshi MM, Moore LL (2010) Food group intake and central obesity among children and adolescents in the Third National Health and Nutrition Examination Survey (NHANES III). Public health nutrition 13: 797–805. 10.1017/S1368980009991546 [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Beydoun MA (2009) Meat consumption is associated with obesity and central obesity among US adults. International Journal of Obesity 33: 621–628. 10.1038/ijo.2009.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brandhagen M, Forslund HB, Lissner L, Winkvist A, Lindroos AK, Carlsson LMS, et al. (2012) Alcohol and macronutrient intake patterns are related to general and central adiposity. European journal of clinical nutrition 66: 305–313. 10.1038/ejcn.2011.189 [DOI] [PubMed] [Google Scholar]
- 38.Kondoh T, Takase H, Yamaguchi TF, Ochiai R, Katashima M, Katsuragi Y, et al. (2014) Association of dietary factors with abdominal subcutaneous and visceral adiposity in Japanese men. Obesity research & clinical practice 8: e16–25. [DOI] [PubMed] [Google Scholar]
- 39.Hayes L, White M, Unwin N, Bhopal R, Fischbacher C, Harland J, et al. (2002) Patterns of physical activity and relationship with risk markers for cardiovascular disease and diabetes in Indian, Pakistani, Bangladeshi and European adults in a UK population. Journal of public health medicine 24: 170–178. [DOI] [PubMed] [Google Scholar]
- 40.Fischbacher CM, Hunt S, Alexander L (2004) How physically active are South Asians in the United Kingdom? A literature review. Journal of public health 26: 250–258. [DOI] [PubMed] [Google Scholar]
- 41.(1994) UK Prospective Diabetes Study. XII: Differences between Asian, Afro-Caribbean and white Caucasian type 2 diabetic patients at diagnosis of diabetes. UK Prospective Diabetes Study Group. Diabetic medicine: a journal of the British Diabetic Association 11: 670–677. [PubMed] [Google Scholar]
- 42.Dhawan J, Bray CL (1997) Asian Indians, coronary artery disease, and physical exercise. Heart 78: 550–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Misra A, Khurana L, Isharwal S, Bhardwaj S (2009) South Asian diets and insulin resistance. The British journal of nutrition 101: 465–473. 10.1017/S0007114508073649 [DOI] [PubMed] [Google Scholar]
- 44.Merchant AT, Anand SS, Kelemen LE, Vuksan V, Jacobs R, Davis B, et al. (2007) Carbohydrate intake and HDL in a multiethnic population. The American Journal of Clinical Nutrition 85: 225–230. [DOI] [PubMed] [Google Scholar]
- 45.Donin AS, Nightingale CM, Owen CG, Rudnicka AR, McNamara MC, Prynne CJ, et al. (2010) Nutritional composition of the diets of South Asian, black African-Caribbean and white European children in the United Kingdom: the Child Heart and Health Study in England (CHASE). The British journal of nutrition 104: 276–285. 10.1017/S000711451000070X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donin AS, Nightingale CM, Owen CG, Rudnicka AR, McNamara MC, Prynne CJ, et al. (2010) Ethnic differences in blood lipids and dietary intake between UK children of black African, black Caribbean, South Asian, and white European origin: the Child Heart and Health Study in England (CHASE). The American Journal of Clinical Nutrition 92: 776–783. 10.3945/ajcn.2010.29533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meas T (2010) Fetal origins of insulin resistance and the metabolic syndrome: a key role for adipose tissue? Diabetes & metabolism 36: 11–20. [DOI] [PubMed] [Google Scholar]
- 48.Amador-Licona N, Martinez-Cordero C, Guizar-Mendoza JM, Malacara JM, Hernandez J, Alcala JF (2007) Catch-up growth in infants born small for gestational age—a longitudinal study. Journal of pediatric endocrinology & metabolism: JPEM 20: 379–386. [DOI] [PubMed] [Google Scholar]
- 49.Mericq V, Ong KK, Bazaes R, Pena V, Avila A, Salazar T, et al. (2005) Longitudinal changes in insulin sensitivity and secretion from birth to age three years in small- and appropriate-for-gestational-age children. Diabetologia 48: 2609–2614. [DOI] [PubMed] [Google Scholar]
- 50.Oken E, Gillman MW (2003) Fetal origins of obesity. Obesity research 11: 496–506. [DOI] [PubMed] [Google Scholar]
- 51.Rogers I (2003) The influence of birthweight and intrauterine environment on adiposity and fat distribution in later life. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity 27: 755–777. [DOI] [PubMed] [Google Scholar]
- 52.Lindsay RS, Dabelea D, Roumain J, Hanson RL, Bennett PH, Knowler WC (2000) Type 2 diabetes and low birth weight: the role of paternal inheritance in the association of low birth weight and diabetes. Diabetes 49: 445–449. [DOI] [PubMed] [Google Scholar]
- 53.Martinson ML, McLanahan S, Brooks-Gunn J (2015) Variation in child body mass index patterns by race/ethnicity and maternal nativity status in the United States and England. Maternal and child health journal 19: 373–380. 10.1007/s10995-014-1519-7 [DOI] [PubMed] [Google Scholar]
- 54.Ramadhani MK, Grobbee DE, Bots ML, Castro Cabezas M, Vos LE, Oren A, et al. (2006) Lower birth weight predicts metabolic syndrome in young adults: the Atherosclerosis Risk in Young Adults (ARYA)-study. Atherosclerosis 184: 21–27. [DOI] [PubMed] [Google Scholar]
- 55.Iniguez G, Ong K, Bazaes R, Avila A, Salazar T, Dunger D, et al. (2006) Longitudinal changes in insulin-like growth factor-I, insulin sensitivity, and secretion from birth to age three years in small-for-gestational-age children. The Journal of clinical endocrinology and metabolism 91: 4645–4649. [DOI] [PubMed] [Google Scholar]
- 56.WHO (2004) World Health Organisation. Low birthweight.Country, regional and global estimates.
- 57.Wells JC, Sharp G, Steer PJ, Leon DA (2013) Paternal and maternal influences on differences in birth weight between Europeans and Indians born in the UK. PLoS One 8: e61116 10.1371/journal.pone.0061116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Veena SR, Kumaran K, Swarnagowri MN, Jayakumar MN, Leary SD, Stein CE, et al. (2004) Intergenerational effects on size at birth in South India. Paediatric and perinatal epidemiology 18: 361–370. [DOI] [PubMed] [Google Scholar]
- 59.Leon DA, Moser KA (2012) Low birth weight persists in South Asian babies born in England and Wales regardless of maternal country of birth. Slow pace of acculturation, physiological constraint or both? Analysis of routine data. Journal of epidemiology and community health 66: 544–551. 10.1136/jech.2010.112516 [DOI] [PubMed] [Google Scholar]
- 60.Veena SR, Geetha S, Leary SD, Saperia J, Fisher DJ, Kumaran K, et al. (2007) Relationships of maternal and paternal birthweights to features of the metabolic syndrome in adult offspring: an inter-generational study in South India. Diabetologia 50: 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harding S, Rosato MG, Cruickshank JK (2004) Lack of change in birthweights of infants by generational status among Indian, Pakistani, Bangladeshi, Black Caribbean, and Black African mothers in a British cohort study. International journal of epidemiology 33: 1279–1285. [DOI] [PubMed] [Google Scholar]
- 62.Fraser HB, Lam LL, Neumann SM, Kobor MS (2012) Population-specificity of human DNA methylation. Genome biology 13: R8 10.1186/gb-2012-13-2-r8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moen EL, Zhang X, Mu W, Delaney SM, Wing C, McQuade J, et al. (2013) Genome-wide variation of cytosine modifications between European and African populations and the implications for complex traits. Genetics 194: 987–996. 10.1534/genetics.113.151381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barfield RT, Almli LM, Kilaru V, Smith AK, Mercer KB, Duncan R, et al. (2014) Accounting for population stratification in DNA methylation studies. Genetic epidemiology 38: 231–241. 10.1002/gepi.21789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Heyn H, Moran S, Hernando-Herraez I, Sayols S, Gomez A, Sandoval J, et al. (2013) DNA methylation contributes to natural human variation. Genome research 23: 1363–1372. 10.1101/gr.154187.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petronis A (2010) Epigenetics as a unifying principle in the aetiology of complex traits and diseases. Nature 465: 721–727. 10.1038/nature09230 [DOI] [PubMed] [Google Scholar]
- 67.Feinberg AP (2007) Phenotypic plasticity and the epigenetics of human disease. Nature 447: 433–440. [DOI] [PubMed] [Google Scholar]
- 68.Feil R, Fraga MF (2011) Epigenetics and the environment: emerging patterns and implications. Nature reviews Genetics 13: 97–109. [DOI] [PubMed] [Google Scholar]
- 69.Johnstone SE, Baylin SB (2010) Stress and the epigenetic landscape: a link to the pathobiology of human diseases? Nature reviews Genetics 11: 806–812. 10.1038/nrg2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jirtle RL, Skinner MK (2007) Environmental epigenomics and disease susceptibility. Nature reviews Genetics 8: 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Portela A, Esteller M (2010) Epigenetic modifications and human disease. Nature biotechnology 28: 1057–1068. 10.1038/nbt.1685 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(A) Additive inheritance model. (B) Dominant inheritance model. (C) Recessive inheritance model.
(TIF)
(A) Additive inheritance model (Lambda = 1.025). (B) Dominant inheritance model (Lambda = 1.015). (C) Recessive inheritance model (Lambda = 1.015).
(TIF)
Relationship of observed association statistics with those expected under the null distribution (MAF>2%, Lambda = 1.022).
(TIF)
(TIF)
(TIF)
(A) Comparison of risk allele frequencies in South Asians, extended analysis (N = 12,240) and Europeans (reported). (B) Comparison of effect sizes (β WHR) in South Asians and Europeans, extended analysis. Green–men and women combined (37 SNPs in South Asians (N = 12,240) and Europeans (reported)); orange–women alone (11 SNPs in South Asians (N = 2,363) and Europeans (reported)).
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.