Abstract
Purpose of review
Neurosteroids are a family of compounds synthesized directly in the brain by transforming cholesterol into pregnenolone, which is then converted to compounds such as allopregnanolone and allotetrahydrodeoxycorticosterone. In view of their ability to modulate neurotransmission, neurosteroids may influence the clinical course of epileptic disorders. In this review, we highlight two emerging properties of neurosteroids, that is, their anticonvulsant and antiepileptogenic activities.
Recent findings
It has been shown that fluctuations in neurosteroid synthesis, such as those seen in response to stress or during the ovarian cycle, determine an increase in seizure threshold. Moreover, increased neurosteroid synthesis, presumably occurring in glial cells during epileptogenesis, delays the appearance of recurrent spontaneous seizures in an animal model of temporal lobe epilepsy; such an effect may be due to augmented tonic γ-aminobutyric acid type A receptor-mediated inhibition. Finally, clinical trials with ganaxolone, an allopregnanolone analogue, have demonstrated beneficial effects in pharmacoresistant epileptic patients, whereas finasteride – which interferes with neurosteroid synthesis – facilitates seizures in catamenial epilepsy.
Summary
The overall evidence suggests that neurosteroids may represent a novel therapeutic strategy in epileptic disorders and a future perspective to control epileptogenicity.
Keywords: epilepsy, epileptogenesis, γ-aminobutyric acid type A receptor, glia, neurosteroids
Introduction
Neurosteroids are currently under clinical evaluation for their potential therapeutic use in epileptic disorders. Clinical trials have demonstrated anticonvulsant effects for ganaxolone, an analogue of the neurosteroid allopregnanolone, in pharmacoresistant epileptic patients [1]. Moreover, Herzog and Frye [2] have reported of a patient affected by catamenial epilepsy, whose seizures were controlled by progesterone administration, but were exacerbated by treatment with finasteride, an inhibitor of allopregnanolone and allotetrahydrodeoxycorticosterone (THDOC) synthesis [3]. The anticonvulsant effect of progesterone is mediated by its nongenomic actions (i.e. independent of progesterone receptor expression), as administration of progesterone maintains powerful antiseizure effects in progesterone receptor-knockout mice [4]. The mechanism underlying these clinical effects is presumably based on the ability of allopregnanolone (a progesterone metabolite) and THDOC to modulate γ-aminobutyric acid type A (GABAA) receptor-mediated transmission as these molecules can increase both tonic and phasic inhibition [5]. The GABAA receptor-related anticonvulsant effects of neurosteroids are further supported by experimental evidence obtained from animal models [5–8]. Furthermore, recent findings have substantiated the role of neuroactive steroids in catamenial epilepsy [9•,10•]. Finally, neurosteroids appear to be involved in temporal lobe epilepsy (TLE) as suggested by their ability to delay the establishment of this chronic condition following pilocarpine-induced status epilepticus in rats [11,12]. Here, we will address these aspects by reviewing recently available information on both clinical and experimental findings and by presenting unpublished data obtained in our laboratories.
Neurosteroids as modulators of neuronal excitability
Neurosteroids are synthesized after the conversion of cholesterol to pregnenolone by the cytochrome P450 cholesterol side chain cleavage (P450scc) enzyme [3]. Pregnenolone is then turned into 17α-hydroxypregnenolone or into progesterone, which are precursors of a cascade of diverse steroid derivatives that are in turn able to interact with various neurotransmitters. Neurosteroids are in fact capable of interacting with GABAA, N-methyl-D-aspartate (NMDA), glycine, and opioid σ1 receptors (reviewed by [13]).
Two classes of neurosteroids can be distinguished, depending on their metabolism by addition of sulfate residues, conferring these compounds different modulatory properties on neuronal excitability. Whereas substances belonging to the family of nonsulfated neurosteroids, such as allopregnanolone and THDOC, enhance GABAA receptor function (as described in the next paragraph, but see also [14•]), sulfated neurosteroids present with more complex properties. Overall, this class of neurosteroids appears to increase neuronal excitability, acting as negative GABAA receptor modulators and/or by enhancing glutamatergic activity. However, several investigations have provided evidence for a negative modulatory effect of sulfated neurosteroids on glutamate-mediated neurotransmission. Glutamate receptor subunit composition appears to be a key factor influencing the modulatory effect of neurosteroids [15] and further investigation is needed to address the pathophysiological relevance of such variable effects in the context of epileptic disorders. These puzzling observations have been recently reviewed [16,17•].
Given the well known role of GABAergic inhibition in epileptic disorders, the remaining review will focus on the effects induced by neurosteroids on GABAA receptor function. The interaction of nonsulfated neurosteroids with GABAA receptors exhibits concentration-dependent mechanisms of action [5]: in the nanomolar range (e.g. during stress and oestrus), they act as modulators of GABAA receptors, whereas at micromolar concentrations (as those physiologically observed during parturition), they can directly open GABAA channels. It has been suggested that neuroactive steroids may potentiate GABAA currents via interaction with the α1 subunit, whereas direct activation of the GABAA receptor relies upon the interaction with both α1 and β2 subunits [18].
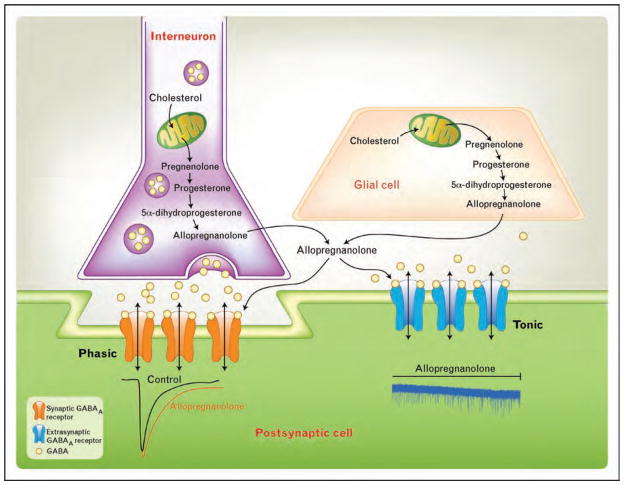
As shown in Fig. 1, GABAA receptor activation can generate two types of current, depending on their location and subunit composition: synaptic receptors give rise to phasic ‘transient’ currents in response to GABA release from synaptic vesicles, whereas extrasynaptic and perisynaptic receptors respond to low levels of ambient GABA by generating a tonic ‘always on’ current [19]. The tonic GABAergic current is largely contributed by α5 and δ-subunit-containing GABAA receptors [20]. Interestingly, mice lacking the GABAA δ-subunit present with an attenuated response to neurosteroids [21], a finding consistent with the view that tonic rather than phasic inhibition may represent the preferential target for neurosteroid modulation (see [22•] for review).
Figure 1.
Neuroactive steroids and their actions on GABAergic inhibition
γ-Aminobutyric acid (GABA) released from an interneuron interacts with GABAA receptors at synaptic (orange) and extrasynaptic (blue) locations, generating phasic and tonic inhibitory currents, respectively. The schematic drawings below the two receptors illustrate the corresponding effect of allopregnanolone on GABAA-mediated currents, showing an increase in the decay time constant of synaptic events and an increase in the inhibitory tone as revealed by the downward shift in the holding current.
Neurosteroids and epileptic seizures
Catamenial exacerbation of epileptic seizures provides compelling evidence of the involvement of steroids in this chronic neurological disorder, a phenomenon explained by the influence on GABAA receptor plasticity exerted by fluctuations in steroid production and their conversion in neurosteroids (see for review [23•]). However, the view that neuroactive steroids may exert a protective role against seizures has been recently challenged by the emergence of opposite effects that these compounds exhibit when studying absence seizures. Although progesterone appears to improve catamenial epilepsy [10•], its derivative allopregnanolone promotes spike-and-wave discharges in Wistar Albino Glaxo rats of Rijswijk (WAG/Rij), a model of absence seizures [24]. Moreover, a role for allopregnanolone in disinhibiting neuronal networks has recently emerged based on experimental data obtained from seizure-prone rats [25]. The following paragraphs will specifically address the effect of neurosteroids as studied in different epilepsy models and will focus on the possible clinical implications of these findings.
Stress and seizures
Stress is among the most frequent seizure precipitating conditions reported by epileptic patients [26], a phenomenon that may be related to corticosterone secretion [27•]. However, the possibility that stressing stimuli may also exert a protective action against epileptic seizures has emerged over the past decades (see [28•] for extensive review). This effect may rest on the conversion of steroid hormones into neuroactive steroids.
Selye [29,30] was the first to describe the anesthetic and anticonvulsant properties of progesterone, another stress-related steroid. More recently, the anticonvulsant effect associated with stress has been ascribed to the conversion of deoxycorticosterone in neuroactive metabolites such as THDOC [31]. The role of stress in restraining seizure activity has recently been reappraised by Verleye et al. [32••] in a mouse model of anxiety. In this study, Balb/cByJ mice exposed to a short immobilization stress exhibited a lower seizure threshold in response to GABAA receptor blockade when neurosteroid synthesis was limited by treatment with finasteride. This effect was antagonized by allopregnanolone, whereas progesterone was ineffective, suggesting that only neurosteroids are able to modulate seizures by potentiating GABAA receptor function at physiological concentrations.
Catamenial epilepsy
Finasteride has been reported to increase seizure activity in a patient affected by catamenial complex partial seizures, which were instead controlled by progesterone [2], suggesting that modulation of neurosteroid pathway can play a role in controlling seizures, at least when hormonal dependence is present. Further support to this view has been recently provided by experimental evidence obtained from a rodent model of catamenial epilepsy, in which progesterone withdrawal or finasteride treatment has been shown to exacerbate pentylenetetrazol-induced and pilocarpine-induced seizures [10•].
The anticonvulsive effect related with physiological fluctuations in neurosteroids during the ovarian cycle has been identified by analyzing the changes in δ-GABAA receptor subunit in mice in which seizure threshold was assessed by kainic acid treatment [33]. It was found that the tonic GABAA receptor-mediated current doubled in amplitude during late diestrus in dentate gyrus granule cells. This change was mirrored by a 43% increase in hippocampal δ-GABAA subunit, and the two findings were related with fluctuations in progesterone plasma levels. Consistent with the findings obtained in the dentate gyrus, mice injected with kainic acid presented with a doubled latency to seizure appearance during diestrus (i.e. when progesterone level peaks) and the average seizure duration was much shorter. All these phenomena were abolished by treating mice with a δ-GABAA subunit antisense mRNA, which reduced the expression of this GABAA subunit by 36% and the tonic current by 76%. Thus, when associated with increased neurosteroid availability occurring during the ovarian cycle, variations in δ-GABAA subunit decrease seizure susceptibility. Overall, these findings point at neurosteroids replacement therapy as a novel therapeutic approach for the treatment of catamenial epilepsy [10•].
Temporal lobe epilepsy
Different isoforms of 5α-reductase and 3α-hydroxysteroid dehydrogenase – which are both involved in the synthesis of allopregnanolone and THDOC – have been identified in the hippocampus and cerebral cortex of patients affected by refractory TLE [34]. The mRNA levels for these enzymes were similar to those found in brain tumor specimens obtained from nonepileptic individuals. However, the mRNA for 3α-hydroxysteroid dehydrogenase isoform 2 was higher in the hippocampus than in the temporal neocortex of TLE patients. Interestingly, allopregnanolone serum levels were found to be significantly decreased in male, but not in female TLE patients compared with healthy controls.
Further support to the view that neurosteroids are involved in TLE comes from evidence obtained from animal models. Studies on pilocarpine-treated mice have shown a 50% decrease in δ-subunit expression of GABAA receptor in the dentate gyrus molecular layer 30 days after status epilepticus. This change was accompanied by loss of efficacy in reducing granule cells excitability by THDOC [35]. However, experiments on pilocarpine-treated rats have revealed only a transient loss (24–48 h after status epilepticus) of the ability of allopregnanolone to modulate GABAA receptor-mediated currents in the dentate gyrus [36]. A more recent study has demonstrated a compensatory increase in GABAA γ2-subunit, so that tonic inhibition was substantially preserved in the dentate gyrus of epileptic mice [37], though the efficacy of THDOC in modulating of the tonic GABAergic current was decreased. In a different model of status epilepticus induction, obtained by continuous hippocampal electrical stimulation, neurons recorded from epileptic rat brain slices were found to be insensitive to low (10–30 nmol/l) but responsive to high (100 nmol/l) allopregnanolone concentrations [38], thus explaining the inconsistencies found in mice [35,37] and rats [36] treated with pilocarpine.
Overall, these findings suggest that the ability of neurosteroids to potentiate GABAA-mediated currents is lost after status epilepticus in chronic epileptic animals, whereas the site for direct receptor activation is preserved and could enhance GABAergic transmission. It must, however, be emphasized that the implication of experimental data on neurosteroids and TLE for clinical practice remains unclear.
Absence seizures
A more complex role is played by neurosteroids in primary generalized epileptic disorders such as absence seizures. The hallmark of generalized absence epilepsy is the generation of spike-and-wave discharges, which are largely contributed by GABAergic mechanisms involved in thalamocortical interaction [39].
Both allopregnanolone and pregnenolone sulfate [intra-peritoneally (i.p.) injected] promote, in a dose-dependent manner, spike-and-wave discharges in WAG/Rij rats [24]. Consistently, a recent study by Pisu et al. [40••] described an increase in allopregnanolone and THDOC along with overexpression of α4 and δ-GABAA subunits in this rodent model of absence seizures. However, Citraro et al. [41] had previously reported in the same model that the effects induced on generalized spike-and-wave activity by local neurosteroid microinjection are both dose-dependent and region-specific. Moreover, these investigators found opposite effects when comparing allopregnanolone with pregnenolone sulfate, which, as summarized above, depend on the resulting interaction with both GABAA and glutamate receptor function. These findings highlight the variability of neurosteroid action in absence seizures and indicate that caution must be taken when considering neurosteroid treatment for this type of epilepsy.
Neurosteroids and epileptogenesis
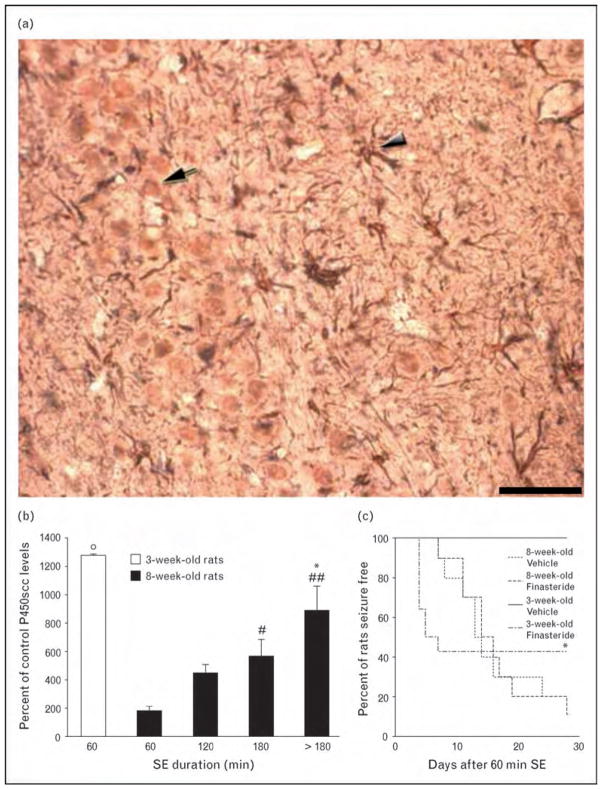
The P450scc enzyme is found in neurons, oligodendrocytes and astrocytes [3], and in activated microglial cells [12] (Fig. 2a). Thus, neurosteroid levels in brain tissue that has been hit by status epilepticus could be altered as a consequence of neuronal damage as well as of glial cell activation. To assess how these changes could affect epileptogenesis, we have recently studied P450scc immunoreactivity after pilocarpine-induced status epilepticus in adult rats [11]. We have found a highly significant increase in P450scc both in neurons and glial cells. However, the neuron-specific changes were limited to the first few days after status epilepticus, whereas those in glial cells were long-lasting and approximately equivalent to the latent period preceding the appearance of spontaneous recurrent seizures. In addition, by varying the duration of the initial status epilepticus induced by pilocarpine, we discovered a clear correlation between the extent of P450scc induction and the duration of the latent period, which was significantly longer in rats exposed to at least 180 min of continuous seizures compared with others exposed to shorter status epilepticus [12] (Fig. 2b). It should also be emphasized that young, 3-week-old rats exposed to short (60 min) status epilepticus present with a more pronounced induction of P450scc than that seen in adult animals (Fig. 2b), and in fact, contrary to adult animals, young rats rarely present with stage V seizures during the chronic epileptic period (Fig. 2c) [42].
Figure 2.
Induction of neurosteroids after status epilepticus modulates epileptogenesis
(a) Triple immunostaining with antibodies against the neuron-specific nuclear protein (NeuN, arrow), the glial fibrillary acidic protein (GFAP) expressed in astrocytes (arrowhead), and the cholesterol side chain cleavage enzyme associated with the cytochrome P450 (P450scc). Methods were previously detailed (ref. [12]); scale bar is 50 μm. (b) Quantification of P450scc immunoreactivity in astrocytes of the CA3 region in young (3-week-old) and adult (8-week-old) rats exposed to variable duration of status epilepticus, obtained by injecting pilocarpine (ref. [42]). *P <0.05 vs. 120 min status epilepticus; #P <0.05, ##P <0.01 vs. 60 min status epilepticus, adult rats; ∘P <0.05 vs. adult rats; one-way analysis of variance (ANOVA) and the Tukey’s test. (c) Finasteride (100 mg/kg subcutaneously, injected from the 4th up to the 28th day after status epilepticus induction) significantly (*P <0.05 vs. vehicle-treated young rats, log rank test) anticipated the onset of seizure activity in young rats. SE, status epilepticus.
The role of neurosteroids in delaying seizure onset in the pilocarpine model has been further tested by treating rats exposed to status epilepticus with finasteride, a procedure that could anticipate the appearance of stage V seizures in rats experiencing at least 180 min of status epilepticus, but not in those experiencing 90 min only [12]. In addition, we compared the effects of finasteride in adult (8-week-old) and young (3-week-old) rats exposed to 60 min of status epilepticus. Again, finasteride was ineffective in altering the latent period in adult rats, in which P450scc is scarcely induced by such a short exposure to status epilepticus (Fig. 2c). On the contrary, seizure manifestation was anticipated in approximately 50% of young rats. Therefore, these findings suggest that neurosteroid synthesis is related to the extent of P450scc induction in glial cells consequent to status epilepticus.
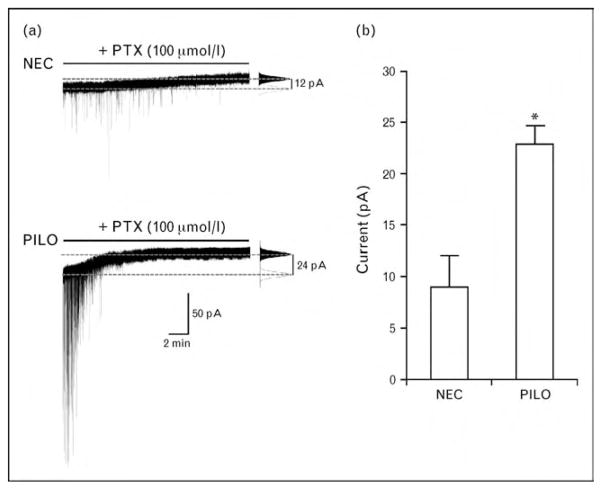
A high neurosteroid synthesis can influence epileptogenesis presumably by providing GABAA receptor activation, whereas low neurosteroid levels are unable to potentiate GABAA receptor transmission in epileptic rats [38]. Consistent with this hypothesis, GABAA-mediated inhibition should be enhanced during the period immediately following the induction of prolonged status epilepticus. Remarkably, ongoing investigations in our laboratories suggest that tonic GABAA current is more pronounced in rat pyramidal-like subicular neurons as early as 3 days following a 2 h long pilocarpine-induced status epilepticus (Fig. 3). These findings may be particularly relevant to better understand the dynamic interplay between epileptogenesis and neurosteroid–GABA interactions and may represent a focal point for future investigation on the mechanisms underlying the antiepileptogenic action of neuroactive steroids.
Figure 3.
GABAergic tonic inhibition is enhanced in the rat subiculum after pilocarpine-induced status epilepticus
(a) Patch clamp recordings (Vm = −70 mV) of subicular pyramidal-like neurons obtained from a nonepileptic control (NEC) and a pilocarpine-treated (PILO) rat, 3 days after induction of status epilepticus lasting for 2 h. In symmetric chloride condition, tonic GABAergic activity is revealed by a positive shift in the holding current during application of the GABAA receptor blocker picrotoxin (PTX, 100 μmol/l). On the right of each trace, all-point histograms indicate the normalized amount of tonic current generated during control condition (white) and after application of PTX (black), as measured at the corresponding dashed gray lines. (b) Pyramidal-like subicular neurons present with enhanced tonic current as early as 3 days after pilocarpine-induced status epilepticus (NEC: 9.06 ± 3.02 pA; pilocarpine: 22.97 ± 1.78 pA; n =7 and 5, respectively; P =0.003, unpaired t-test. Data are expressed as mean ± SEM).
Conclusion
The involvement of endogenous neurosteroids and their synthetic analogues as modulators of neuronal excitability in the context of epileptic disorders still remains under investigation, due to the variability of their influence on neurotransmission. In fact, the modulatory effect of these neuroactive compounds depends on their class (e.g. sulfated vs. nonsulfated), the neurotransmitter receptor subunit composition, and the pathophysiological mechanisms underlying specific epileptic disorders. The literature summarized here clearly indicates that the ability of neurosteroids to modulate neuronal excitability resides primarily in the enhancement of GABAergic inhibitory tone. This physiological characteristic may, however, yield opposite effects, depending on the contribution of GABAergic mechanisms to epileptiform synchronization that are specific to different epileptic syndromes. In rodent models of TLE, glia-derived neurosteroids have proved to exert antiepileptogenic actions, an intriguing finding that let us foresee the possibility of employing neurosteroids or their analogues to prevent the development of a chronic epileptic condition in high-risk patients. This is particularly relevant in epileptology, as there are, to date, no pharmacological agents capable of stopping epileptogenesis.
Acknowledgments
Original work reported in this review was supported by the Pierfranco and Maria Luisa Mariani Foundation (R-06-50), the Emilia-Romagna Region (PRIER 2007/09, Region-University Program, Grant 1232), the Canadian Institutes of Health Research (Grant MT 8109), the Savoy Foundation and Epilepsy Canada.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 198–199).
- 1.Nohria V, Giller E. Ganaxolone. Neurotherapeutics. 2007;4:102–105. doi: 10.1016/j.nurt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herzog AG, Frye CA. Seizure exacerbation associated with inhibition of progesterone metabolism. Ann Neurol. 2003;53:390–391. doi: 10.1002/ana.10508. [DOI] [PubMed] [Google Scholar]
- 3.Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- 4.Reddy DS, Castaneda DC, O’Malley BW, Rogawski MA. Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Ther. 2004;310:230–239. doi: 10.1124/jpet.104.065268. [DOI] [PubMed] [Google Scholar]
- 5.Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 6.Belelli D, Bolger MB, Gee KW. Anticonvulsant profile of the progesterone metabolite 5α-pregnan-3α-ol-20-one. Eur J Pharmacol. 1989;166:325–329. doi: 10.1016/0014-2999(89)90077-0. [DOI] [PubMed] [Google Scholar]
- 7.Kokate TG, Cohen AL, Karp E, Rogawski MA. Neuroactive steroids protect against pilocarpine- and kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology. 1996;35:1049–1056. doi: 10.1016/s0028-3908(96)00021-4. [DOI] [PubMed] [Google Scholar]
- 8.Rogawski MA, Reddy DS. Neurosteroids: endogenous modulators of seizure susceptibility. In: Rho JM, Sankar R, Cavazos JE, editors. Epilepsy: scientific foundations of clinical treatment. New York: Marcel Dekker; 2004. pp. 319–355. [Google Scholar]
- 9•.Guille C, Spencer S, Cavus I, Epperson CN. The role of sex steroids in catamenial epilepsy and premenstrual dysphoric disorder: implications for diagnosis and treatment. Epilepsy Behav. 2008;13:12–24. doi: 10.1016/j.yebeh.2008.02.004. This article focuses on preclinical and clinical findings on the allopregnanolone-mediated actions of estradiol and progesterone and their relevance to catamenial epilepsy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Reddy DS, Rogawski MA. Neurosteroid replacement therapy for catamenial epilepsy. Neurotherapeutics. 2009;6:392–401. doi: 10.1016/j.nurt.2009.01.006. This paper provides new experimental evidence in support of neurosteroid treatment for catamenial epilepsy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biagini G, Baldelli E, Longo D, et al. Endogenous neurosteroids modulate epileptogenesis in a model of temporal lobe epilepsy. Exp Neurol. 2006;201:519–524. doi: 10.1016/j.expneurol.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 12.Biagini G, Longo D, Baldelli E, et al. Neurosteroids and epileptogenesis in the pilocarpine model: evidence for a relationship between P450scc induction and length of the latent period. Epilepsia. 2009;50(Suppl 1):53–58. doi: 10.1111/j.1528-1167.2008.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddy DS. Pharmacology of endogenous neuroactive steroids. Crit Rev Neurobiol. 2003;15:197–234. doi: 10.1615/critrevneurobiol.v15.i34.20. [DOI] [PubMed] [Google Scholar]
- 14•.Mody I. Extrasynaptic GABAA receptors in the crosshair of hormones and ethanol. Neurochem Int. 2008;52:60–64. doi: 10.1016/j.neuint.2007.07.010. This paper discusses the mechanisms of modulation of tonic GABAergic inhibition by neurosteroids, ethanol and hormonal fluctuations, specifically focusing on δ-subunit-containing GABAA receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malayev A, Gibbs TT, Farb DH. Inhibition of the NMDA response by pregnenolone sulphate reveals subtype selective modulation of NMDA receptors by sulphated steroids. Br J Pharmacol. 2002;135:901–909. doi: 10.1038/sj.bjp.0704543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibbs TT, Russek SJ, Farb DH. Sulfated steroids as endogenous neuromodulators. Pharmacol Biochem Behav. 2006;84:555–567. doi: 10.1016/j.pbb.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 17•.Sedlácek M, Korínek M, Petrovic M, et al. Neurosteroid modulation of ionotropic glutamate receptors and excitatory synaptic transmission. Physiol Res. 2008;57(Suppl 3):S49–S57. doi: 10.33549/physiolres.931600. This comprehensive review sheds more light on the complex effects of neurosteroids on ionotropic glutamatergic neurotransmission. [DOI] [PubMed] [Google Scholar]
- 18.Hosie AM, Wilkins ME, da Silva HMA, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 19.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 20.Glykys J, Mann EO, Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mihalek RM, Banerjee PK, Korpi ER, et al. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci USA. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Lambert JJ, Cooper MA, Simmons RD, et al. Neurosteroids: endogenous allosteric modulators of GABAA receptors. Psychoneuroendocrinology. 2009;34(Suppl 1):S48–S58. doi: 10.1016/j.psyneuen.2009.08.009. This paper is a fundamental reading encompassing several aspects of GABAergic modulation by neurosteroids, including plastic changes induced in GABAA receptor subunit composition and recent advances in neurosteroid-binding site(s) [DOI] [PubMed] [Google Scholar]
- 23•.Maguire J, Mody I. Steroid hormone fluctuations and GABAAR plasticity. Psychoneuroendocrinology. 2009;34(Suppl 1):S84–S90. doi: 10.1016/j.psyneuen.2009.06.019. This paper adds fundamental insights into the involvement of neurosteroids in GABAA receptor plasticity as related to hormonal fluctuations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Budziszewska B, Van Luijtelaar G, Coenen AM, et al. Effects of neurosteroids on spike-wave discharges in the genetic epileptic WAG/Rij rat. Epilepsy Res. 1999;33:23–29. doi: 10.1016/s0920-1211(98)00067-9. [DOI] [PubMed] [Google Scholar]
- 25.Schwabe K, Gavrilovici C, McIntyre DC, Poulter MO. Neurosteroids exhibit differential effects on mIPSCs recorded from normal and seizure prone rats. J Neurophysiol. 2005;94:2171–2181. doi: 10.1152/jn.01233.2004. [DOI] [PubMed] [Google Scholar]
- 26.Sperling MR, Schilling CA, Glosser D, et al. Self-perception of seizure precipitants and their relation to anxiety level, depression, and health locus of control in epilepsy. Seizure. 2008;17:302–307. doi: 10.1016/j.seizure.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 27•.Joëls M. Functional actions of corticosteroids in the hippocampus. Eur J Pharmacol. 2008;583:312–321. doi: 10.1016/j.ejphar.2007.11.064. This paper provides a comprehensive review of the long-term consequences of chronic exposure to corticosteroids on hippocampal function during stressing conditions such as epileptic seizures and describes possible mechanisms involved in cognitive impairment and psychiatric disturbances as those seen in epileptic patients. [DOI] [PubMed] [Google Scholar]
- 28•.Joëls M. Stress, the hippocampus, and epilepsy. Epilepsia. 2009;50:586–597. doi: 10.1111/j.1528-1167.2008.01902.x. This paper sheds further light on the interaction between stress and epilepsy and proposes a novel therapeutic approach to epilepsy based on antiglucocorticoid treatment. [DOI] [PubMed] [Google Scholar]
- 29.Selye H. On the hormonal activity of a steroid compound. Science. 1941;94:94. doi: 10.1126/science.94.2430.94. [DOI] [PubMed] [Google Scholar]
- 30.Selye H. Resistance to picrotoxin poisoning induced by catatoxic steroids. Agents Actions. 1970;1:133–135. doi: 10.1007/BF01982399. [DOI] [PubMed] [Google Scholar]
- 31.Reddy DS, Rogawski MA. Stress-induced deoxycorticosterone-derived neurosteroids modulate GABAA receptor function and seizure susceptibility. J Neurosci. 2002;22:3795–3805. doi: 10.1523/JNEUROSCI.22-09-03795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32••.Verleye M, Heulard I, Gillardin JM. Investigation of the anticonvulsive effect of acute immobilization stress in anxious Balb/cByJ mice using GABAA-related mechanistic probes. Psychopharmacology. 2008;197:523–534. doi: 10.1007/s00213-007-1066-7. This paper provides evidence that the involvement of neurosteroids in the anticonvulsant role of stress specifically involves GABAA receptors. The authors performed a complete setting of experiments in which they tested the effects of finasteride, PK11195 (which blocks the cholesterol transport in mitochondria by inhibiting the peripheral benzodiazepine receptor), etifoxine (which is a nonbenzodiazepine anxiolytic able to increase neurosteroid synthesis), progesterone, and allopregnanolone. [DOI] [PubMed] [Google Scholar]
- 33.Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- 34.Stoffel-Wagner B, Watzka M, Steckelbroeck S, et al. Allopregnanolone serum levels and expression of 5α-reductase and 3α-hydroxysteroid dehydrogenase isoforms in hippocampal and temporal cortex of patients with epilepsy. Epilepsy Res. 2003;54:11–19. doi: 10.1016/s0920-1211(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 35.Peng Z, Huang CS, Stell BM, et al. Altered expression of the δ subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leroy C, Poisbeau P, Keller AF, Nehlig A. Pharmacological plasticity of GABAA receptors at dentate gyrus synapses in a rat model of temporal lobe epilepsy. J Physiol (London) 2004;557:473–487. doi: 10.1113/jphysiol.2003.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang N, Wei W, Mody I, Houser CR. Altered localization of GABAA receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J Neurosci. 2007;27:7520–7531. doi: 10.1523/JNEUROSCI.1555-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun C, Mtchedlishvili Z, Erisir A, Kapur J. Diminished neurosteroid sensitivity of synaptic inhibition and altered location of the α4 subunit of GABAA receptors in an animal model of epilepsy. J Neurosci. 2007;27:12641–12650. doi: 10.1523/JNEUROSCI.4141-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crunelli V, Leresche N. Childhood absence epilepsy: genes, channels, neurons and networks. Nat Rev Neurosci. 2002;3:371–382. doi: 10.1038/nrn811. [DOI] [PubMed] [Google Scholar]
- 40••.Pisu MG, Mostallino MC, Dore R, et al. Neuroactive steroids and GABAA receptor plasticity in the brain of the WAG/Rij rat, a model of absence epilepsy. J Neurochem. 2008;106:2502–2514. doi: 10.1111/j.1471-4159.2008.05538.x. This paper provides evidence for age-related changes in neurosteroids associated with the expression of specific GABAA receptor subunits that may contribute to the manifestation and progression of absence epilepsy. [DOI] [PubMed] [Google Scholar]
- 41.Citraro R, Russo E, Di Paola ED, et al. Effects of some neurosteroids injected into some brain areas of WAG/Rij rats, an animal model of generalized absence epilepsy. Neuropharmacology. 2006;50:1059–1071. doi: 10.1016/j.neuropharm.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 42.Biagini G, Baldelli E, Longo D, et al. Proepileptic influence of a focal vascular lesion affecting entorhinal cortex-CA3 connections after status epilepticus. J Neuropathol Exp Neurol. 2008;67:687–701. doi: 10.1097/NEN.0b013e318181b8ae. [DOI] [PMC free article] [PubMed] [Google Scholar]