Summary
Purpose
We established the effects of the antiepileptic drugs (AEDs) carbamazepine (CBZ), topiramate (TPM), and valproic acid (VPA) on the epileptiform activity induced by 4-aminopyridine (4AP) in the rat entorhinal cortex (EC) in an in vitro brain slice preparation.
Methods
Brain slices were obtained from Sprague-Dawley rats (200–250 g). Field and intracellular recordings were made from the EC during bath application of 4AP (50 μM). AEDs, and in some experiments, picrotoxin were bath applied concomitantly.
Results
Prolonged (>3 s), ictal-like epileptiform events were abolished by CBZ (50 μM), TPM (50 μM), and VPA (1 mM), whereas shorter (<3 s) interictal-like discharges continued to occur, even at concentrations up to 4-fold as high. γ-Aminobutyric acid (GABA)A–receptor antagonism changed the 4AP-induced activity into recurrent interictal-like events that were not affected by CBZ or TPM, even at the highest concentrations. To establish whether these findings reflected the temporal features of the epileptiform discharges, we tested CBZ and TPM on 4AP-induced epileptiform activity driven by stimuli delivered at 100-, 10-, and 5-s intervals; these AEDs reduced ictal-like responses to stimuli at 100-s intervals at nearly therapeutic concentrations, but did not influence shorter interictal-like events elicited by stimuli delivered every 10 or 5 s.
Conclusions
We conclude that the AED ability to control epileptiform synchronization in vitro depends mainly on activity-dependent characteristics such as discharge duration. Our data are in keeping with clinical evidence indicating that interictal activity is unaffected by AED levels that are effective to stop seizures.
Keywords: Antiepileptic drugs, 4-Aminopyridine, Entorhinal Cortex, Epilepsy, Hippocampus, Picrotoxin
The in vitro brain slice preparation has been extensively used to study the mechanisms underlying epileptiform synchronization and epileptogenesis (Prince, 1999; Traub et al., 1999), as well as the action of antiepileptic drugs (AEDs) (Schneiderman & Schwartzkroin, 1982; Heinemann et al., 1985; Fueta & Avoli, 1992; Watts & Jefferys, 1993; Zhang et al., 1995). Early work performed in isolated rat hippocampal slices treated with the K+ channel blocker 4-aminopyridine (4AP) has shown in young tissue that several AEDs can abolish ictal-like discharges at concentrations that are unable to influence shorter, interictal-like events (Fueta & Avoli, 1992). This study also demonstrated that AEDs reduced interictal-like activity in adult tissue only at very high doses (Fueta & Avoli, 1992).
Similar findings have been later obtained from combined adult rat hippocampus-entorhinal cortex (EC) slices. Thus, Brückner and Heinemann (2000) have reported that prolonged ictal-like discharges that are initiated by EC networks are blocked by AEDs, whereas interictal-like activity continues to occur under these conditions. In addition, Brückner et al. (1999) have found that recurrent, interictal-like activity generated by EC networks during concomitant application of 4AP and bicuculline [which is known to antagonize γ-aminobutyric acid (GABA)A receptors] is insensitive to AEDs. Because recurrent epileptiform discharges induced by Mg2+-free medium are also unaffected by AEDs at a time when GABAA receptor–mediated inhibition is presumably weakened (Whittington et al., 1995; Zhang et al., 1995), it has been proposed that the inability of AEDs to influence the occurrence of these epileptiform events represents an in vitro model of pharmacoresistance (Zhang et al., 1995; Dreier et al., 1998). These studies also raised the interesting possibility that weakened inhibition may cause refractoriness to pharmacologic treatment in epileptic patients.
The EC plays a role in the initiation of limbic seizures in patients presenting with temporal lobe epilepsy (Rutecki et al., 1989; Spencer & Spencer, 1994; Bartolomei et al., 2001) and in animal models mimicking this disorder (Dreier & Heinemann, 1991; Bragdon et al., 1992; Avoli et al., 1996; Nagao et al., 1996). Hence, we further analyzed here the effects induced by the AEDs carbamazepine (CBZ), topiramate (TPM), and valproate (VPA) on the epileptiform activity generated by rat EC slices superfused with 4AP-containing medium. Our findings demonstrate that at nearly therapeutic doses, all AEDs abolished prolonged (>3 s) ictal-like epileptiform discharges resembling electrographic limbic seizures, but failed to block shorter epileptiform events reminiscent of the interictal activity. In addition, by using repetitive stimulation at different rates in the presence of 4AP, we found evidence indicating that such difference in AED efficacy may mainly reflect an activity-dependent mechanism rather than specific neuronal changes such as a decrease in GABAA receptor–mediated inhibition.
Methods
Rat brain slices were obtained following standard procedures (Avoli et al., 1996; Benini et al., 2003) from male, adult Sprague-Dawley rats (200–250 g, n = 32). Animals were used according to the procedures established by the Canadian Council of Animal Care. All efforts were made to minimize the number of animals used and their suffering. In brief, rats were decapitated under halothane anesthesia, the brain was quickly removed, and a block of brain tissue containing the retrohippocampal region was placed in cold (1–3°C), oxygenated artificial cerebrospinal fluid (ACSF). Horizontal slices (450–500 μm thick) containing the EC were cut using a Vibratome and were transferred into a tissue chamber where they lay at the interface between ACSF and humidified gas (95% O2, 5% CO2) at a temperature of 32–34°C (pH = 7.4). ACSF composition was (in mM): NaCl 124, KCl 2, KH2PO4 1.25, MgSO4 2, CaCl2 2, NaHCO3 26, and glucose 10. Surgical separation of the EC from the hippocampus proper was performed with a knife mounted on a micromanipulator at the beginning of the experiment to minimize the influence exerted by CA3-driven activity on EC epileptiform synchronization (Barbarosie & Avoli, 1997; Benini et al., 2003) (see inset in Fig. 1A).
Figure 1.
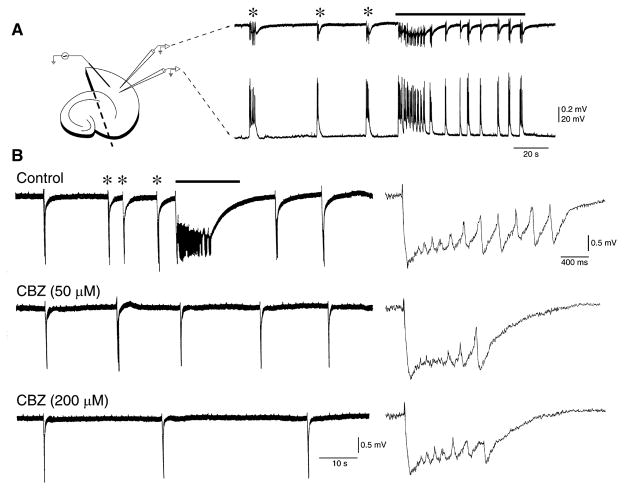
Carbamazepine (CBZ) abolishes epileptiform ictal-like discharges but fails to block interictal-like events. (A) Typical pattern of spontaneous epileptiform activity recorded from the entorhinal cortex (EC) with field and intracellular recordings (inset on the left) during 4-aminopyridine (4AP) application; note the two types of epileptiform discharge: one short-lasting and characterized by depolarizations capped by action potential firing resembling interictal events (asterisks), and the other consisting of prolonged periods of epileptiform synchronization (continuous line) and intracellularly mirrored by long-lasting depolarizations with concurrent action potential discharge, similar to ictal events. (B) Ictal-like discharges (continuous line) are abolished by bath application of low CBZ concentrations (50 μM), whereas interictal-like events (asterisks) continue to occur even in the presence of larger doses (200 μM panel), but at longer intervals. Single interictal-like discharges recorded under the three different experimental conditions are expanded on the right.
4-Aminopyridine (4AP, 50 μM), CBZ (50–200 μM), picrotoxin (PTX, 50 μM), TPM (50–200 μM), and VPA (1–3 mM) were bath applied. Chemicals were acquired from Sigma (St. Louis, MO, U.S.A.), with the exception of topiramate, a kind gift of the R. W. Johnson Pharmaceutical Research Institute (Spring House, PA, U.S.A.). Increasing concentrations of CBZ, TPM, or VPA were applied in a cumulative fashion for at least 30 min for each dose. The doses of AEDs used in our study were 5–30-fold higher than those reported as clinically relevant in patients (see Table 1) but similar to those employed in previous in vitro experiments (Heinemann et al., 1985; Fueta & Avoli, 1992; Brückner et al., 1999; Brückner & Heinemann, 2000). The effects induced by different concentrations of these AEDs were measured after 20–25 min of application.
Table 1.
Serum and cerebrospinal fluid (CSF) concentrations of CBZ, TPM, and VPA. Values for CBZ and TPM were deduced from Rambeck et al. (2006). Note that for TPM only one sample was available. The serum and CSF concentrations for VPA were from Löscher et al. (1988)
| Drug | Serum (μg/ml) | Serum (μM) | CSF (μg/ml) | CSF (μM) |
|---|---|---|---|---|
| CBZ (m.w. 236.26) | 7.7 ± 1.7 | 32.5 ± 7.2 | 2.5 ± 0.6 | 10.5 ± 2.5 |
| TPM (m.w. 339.37) | 3.2 | 9.3 | 2.7 | 8.0 |
| VPA (m.w. 166.19) | 102.9 ± 38.4 | 968.8 ± 361.3 | 9.6 ± 5.2 | 90.4 ± 48.9 |
CBZ, carbamazepine; CSF, cerebrospinal fluid; TPM, topiramate; VPA, valproate.
Values for CBZ and TPM were deduced from Rambeck et al. (2006). Note that for TPM only one sample was available. The serum and CSF concentrations for VPA were from Löscher et al. (1988)
Field potential recordings were made in the EC with ACSF-filled, glass pipettes (resistance = 2–10 MΩ) that were connected to a high-impedance amplifier. Sharp-electrode intracellular recordings were also performed in the EC deep layers with pipettes filled with 3 M K-acetate (tip resistance = 70–120 MΩ). Intracellular signals were fed to a high-impedance amplifier with an internal bridge circuit for intracellular current injection. The resistance compensation was monitored throughout the experiment and adjusted as required. The passive membrane properties of EC neurons included in this study were as follows: (1) resting membrane potential (RMP) = −68.5±1.0 mV (n = 11); (2) apparent input resistance (Ri) from the maximum voltage change in response to a hyperpolarizing current pulse (100–200 ms, <−0.5 nA) = 45.7±4.3 MΩ (n = 9); (3) action potential amplitude (APA) from the baseline = 88.7±2.1 mV (n = 11); and (4) action potential duration (APD) at half-amplitude = 1.3±0.1 ms (n = 11).
Field potential and intracellular signals were displayed on a Gould pen chart recorder and/or fed to a computer interface (Digidata 1322A; Axon Instruments, Foster City, CA, U.S.A.) through which they were acquired and stored using the pClamp 9 software (Axon Instruments). Extra-cellular stimuli (50–150 μs; <300 μA) were delivered through a bipolar stainless steel electrode placed in the EC deep layers. The location of the stimulating and recording electrodes in the brain slices is illustrated in the inset of Fig. 1A.
Throughout this study we arbitrarily termed as “interictal” and “ictal” the synchronous epileptiform events with durations shorter or longer than 3 s, respectively (cf., Traub et al., 1996). Measurements in the text are expressed as mean±standard deviation (SD), and n indicates the number of slices or neurons studied under each specific protocol. Data were compared with one-way analysis of variance (ANOVA) instead of repeated-measures ANOVA because of incomplete series in data sampling. After having established a significant ANOVA, data were further compared by post hoc Dunnett’s t-test. Linear regression analysis was used to analyze the relation between interictal discharge duration and drug effects. Differences were considered statistically significant if p < 0.05.
Results
As reported in previous studies (Avoli et al., 1996; Lopantsev & Avoli, 1998a, 1998b; Brückner & Heinemann, 2000), two types of spontaneous epileptiform discharge were recorded in the EC during bath application of 4AP. The first type consisted of prolonged (up to 40 s, interval of occurrence = 12–165 s) periods of epileptiform synchronization (continuous line in Fig. 1A, B); these ictal events were intracellularly mirrored by long-lasting depolarizations with concurrent action potential discharge (cf. Lopantsev & Avoli, 1998a, 1998b). The second type of spontaneous epileptiform activity was characterized by recurrent interictal discharges (asterisks in Fig. 1A, B) with duration of 0.3–2.3 s and interval of occurrence ranging between 6 and 26.8 s. Interictal discharges recorded in the slices included in this study were characterized by membrane depolarizations capped by action-potential firing (see Lopantsev & Avoli, 1998a, 1998b). Ictal activity occurred in a stable fashion in 31 of 55 slices analyzed in this study under control conditions (i.e., during application of ACSF that contained 4AP), whereas interictal events could be recorded in all experiments.
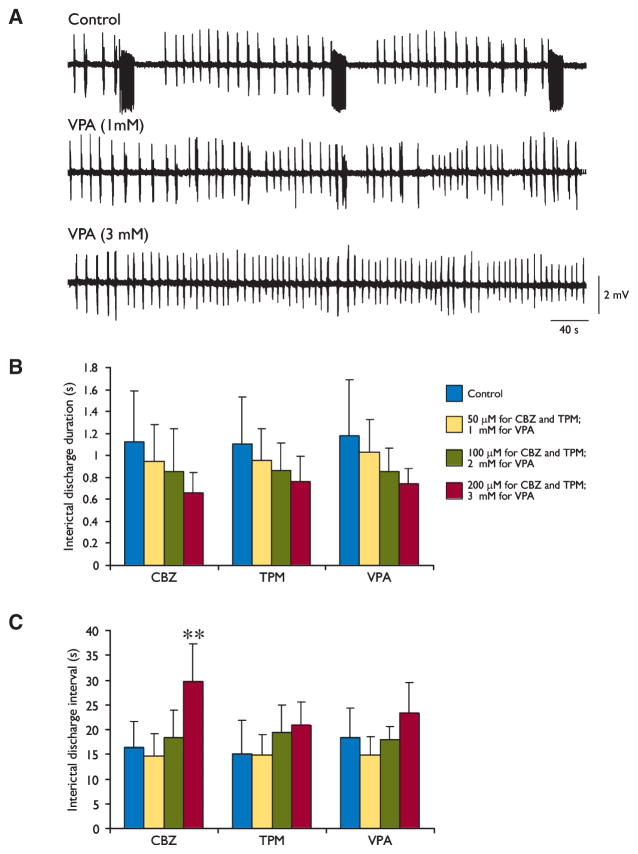
Bath application of CBZ at low concentrations (50 μM; n = 9 slices) abolished 4AP-induced ictal discharges in all experiments, but failed in inducing any consistent change of the interval of occurrence of interictal discharges (Fig. 1B). In addition, interictal activity continued to occur in the presence of larger (100 μM, n = 8 and 200 μM, n = 5) concentrations of CBZ (Fig. 1B), but at longer intervals when the highest dose was tested. These effects, along with those induced by CBZ on interictal discharge duration, are summarized in Fig. 2B, C. It is possible to appreciate there that 50–200 μM CBZ did not cause any significant change in interictal discharge duration (F3,32 = 1.58, p = 0.21), whereas the interval of occurrence of the interictal discharges was increased (F3,29 = 7.14, p < 0.01) by augmenting CBZ concentration to 200 μM.
Figure 2.
Changes induced by valproate (VPA) on the epileptiform activity generated by entorhinal cortex (EC) networks and cumulative effects induced by the three antiepileptic drugs (AEDs) on interictal-like discharges. (A) VPA (1 mM) abolishes ictal-like activity, whereas interictal-like discharges continue to be generated even when this AED concentration is increased to 3 mM. (B, C) Summary of the effects induced by increasing concentrations of carbamazepine (CBZ), topiramate (TPM), and VPA on the duration and interval of occurrence of the interictal discharges induced by 4-aminopyridine (4AP); **p < 0.01, one-way analysis of variance (ANOVA) followed by Dunnett’s test.
Similar findings were obtained by testing the other two AEDs. TPM (50 μM, n = 7; not shown) or VPA (1 mM, n = 6; Fig. 2A) abolished ictal activity without consistently modifying the rate of occurrence, the duration, or the amplitude of the interictal discharges. In addition, larger AED concentrations (TPM: 100 μM, n = 6, and 200 μM, n = 6; VPA: 2 mM, n = 5 and 3 mM, n = 4, Fig. 2A) failed to cause significant changes in either the duration (F3,26 = 1.71, p = 0.19 for TPM; F3,18 = 1.59, p = 0.23 for VPA) or rate of occurrence (F3,24 = 1.99, p = 0.14 for TPM; F3,18 = 2.49, p = 0.09 for VPA) of the interictal events. These data are quantitatively summarized in Fig. 2B, C.
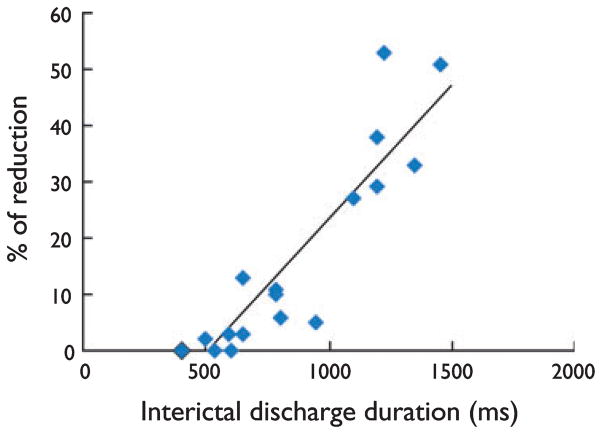
CBZ (50 μM, n = 9 slices), TPM (50 μM, n = 7), and VPA (1 mM, n = 8) also lacked any consistent effect on the rate of occurrence, duration, and amplitude of interictal-like discharges recorded in those slices that generated only this type of epileptiform activity during 4AP application (not shown). However, when this analysis was restricted to slices generating more robust interictal-like events (duration > 0.5 s), AEDs could reduce the length of events lasting longer than 0.7 s. This finding is illustrated in Fig. 3, where we plotted the average length of the interictal-like event discharges generated by different slices versus the normalized length reduction that was seen during application of CBZ-(50 μM, n = 7), TPM-(50 μM, n = 5), or VPA-(1 mM, n = 5) containing medium. Linear regression analysis showed a highly significant relationship (p < 0.01, r2 = 0.83) between the two considered variables.
Figure 3.
Reduction of interictal-like discharge duration by antiepileptic drugs (AEDs) is a function of their length under control conditions. In this plot the average length of interictal-like discharges generated by different slices was computed versus the normalized length reduction seen during application of carbamazepine (CBZ; 50 μM, n = 7), topiramate (TPM; 50 μM, n = 5), and valproate (VPA; 1 mM, n = 5). Note that this analysis was restricted to entorhinal cortex (EC) slices capable of generating interictal-like events with duration > 0.5 s. Linear regression analysis shows a highly significant relationship (p < 0.01, r2 = 0.83) between the two considered variables.
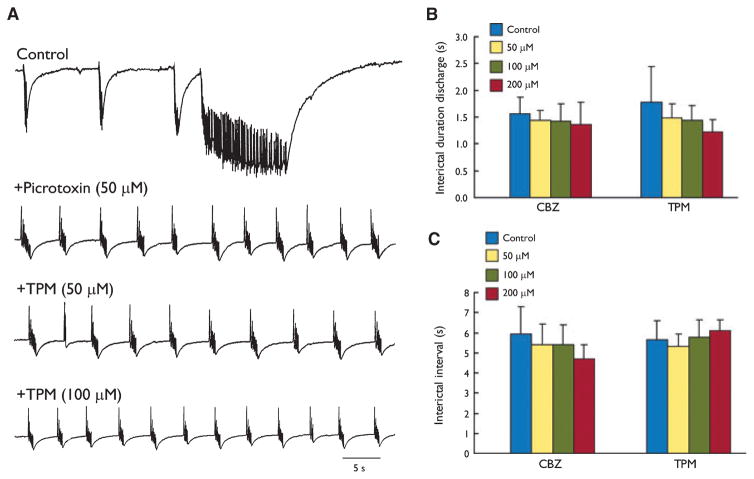
As previously reported (Lopantsev & Avoli, 1998a; Brückner et al., 1999), blockade of GABAA receptor readily changed the typical pattern of 4AP-induced activity (which consists of slow interictal-like along with ictal-like events) into recurrent, robust interictal-like discharges (duration = 1.7±0.5 s; interval of occurrence = 5.8±1.1 s; n = 11 slices) (Fig. 4A). In keeping with the data obtained by Brückner et al. (1999), who reported on the effects induced by phenytoin, CBZ, VPA, and phenobarbital on the epileptiform activity induced by concomitant application of 4AP and bicuculline, we found that recurrent interictal events recorded during GABAA-receptor antagonism by PTX were not influenced by CBZ (50, 100 and 200 μM tested in 5, 6, and 5 slices, respectively; F3,18 = 0.41, p = 0.74 for duration; F3,18 = 1.13, p = 0.36 for interval) or TPM (50, 100, and 200 μM tested in six slices for each dose; F3,16 = 1.65, p = 0.22 for duration; F3,16 = 0.92, p = 0.45 for interval). These results are quantitatively summarized in Fig. 4B, C.
Figure 4.
Effects induced by topiramate (TPM) on recurrent interictal-like discharges seen during application of 4-aminopyridine (4AP) + picrotoxin (PTX). (A) Blockade of γ-aminobutyric acid (GABA)A receptor changes the typical pattern of 4AP-induced slow interictal- and ictal-like events into recurrent interictal-like activity. Note that under these experimental conditions interictal-like events continue to occur during application of TPM increasing concentrations. (B, C) Summary of the effects induced by increasing concentrations of carbamazepine (CBZ) and TPM on the duration and interval of occurrence of the interictal discharges induced by 4AP + PTX. Note that both antiepileptic drugs (AEDs) failed to produce any significant change.
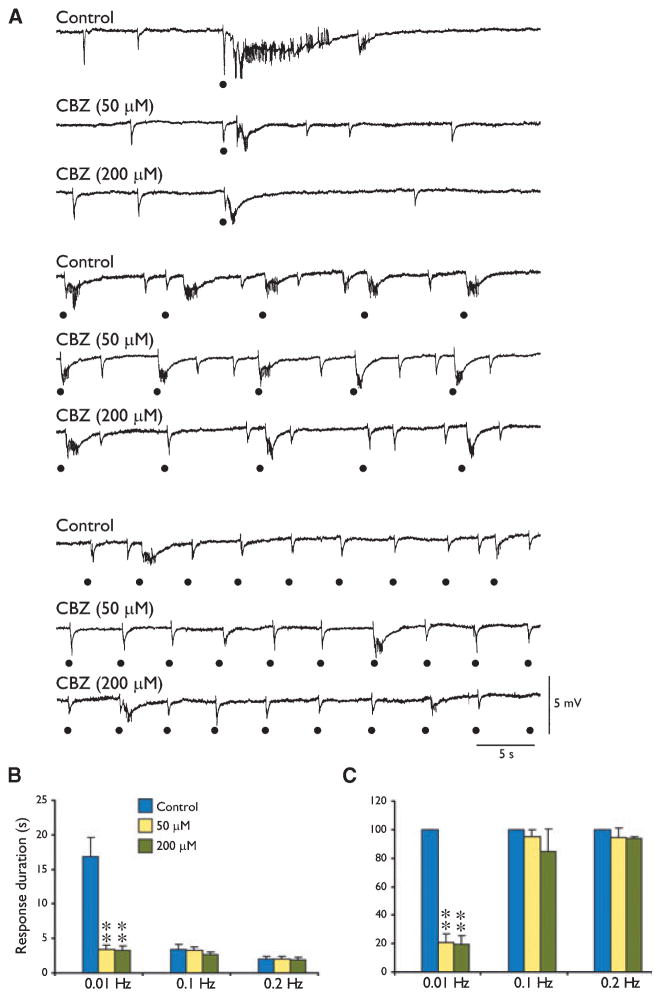
Overall these findings suggest that the ability of AEDs to influence epileptiform synchronization in the EC may depend on the temporal characteristics of the synchronous epileptiform discharges. To further address this aspect we tested the effects induced by CBZ and TPM on the epileptiform events induced by focal electrical stimuli delivered at intervals ranging from 1–100 s. Previous studies from our laboratories have indeed shown that when delivered at intervals of approximately 100 s, electrical stimuli can induce ictal-like responses, whereas at shorter intervals (10 s or less) they elicit shorter, interictal-like events (Barbarosie & Avoli, 1997; Barbarosie et al., 2002; Benini et al., 2003). As illustrated in Fig. 5A and quantified in Fig. 5B, both 50- and 200-μM CBZ reduced (F2,12 = 106.79, p < 0.01) the duration of the long-lasting epileptiform events induced by stimuli delivered every 100 s (i.e., at 0.01 Hz). In contrast, no significant (F2,11 = 1.96, p = 0.19 for 0.1 Hz; F2,11 = 0.25, p = 0.79 for 0.2 Hz) reduction could be observed when CBZ was tested on the epileptiform discharges elicited by stimuli delivered at intervals of 10 or 5 s (i.e., at 0.1 or 0.2 Hz) (Fig. 5B, C).
Figure 5.
Effects induced by carbamazepine (CBZ) on the epileptiform events induced by focal electrical stimuli delivered at 0.01, 0.1, and 0.2 Hz. (A) Field potential recordings from the entorhinal cortex (EC) during application of medium containing 4-aminopyridine (4AP) (Control) show that electrical stimuli delivered at 0.01 Hz induce in the EC an ictal-like response, whereas stimuli at shorter intervals (10 s or less) elicit shorter, interictal-like events. Note that application of 50 and 200 μM CBZ abolishes the long-lasting, ictal-like epileptiform response, whereas consistent changes did not occur when stimuli delivered at 0.1 or 0.2 Hz were used. (B) Summary of the effects induced by CBZ on the duration of the epileptiform events elicited by electrical stimuli delivered at 0.01, 0.1, and 0.2 Hz. (C) Graphs shown in B were normalized to the response duration seen under control conditions. **p < 0.01, one-way analysis of variance (ANOVA) followed by Dunnett’s test.
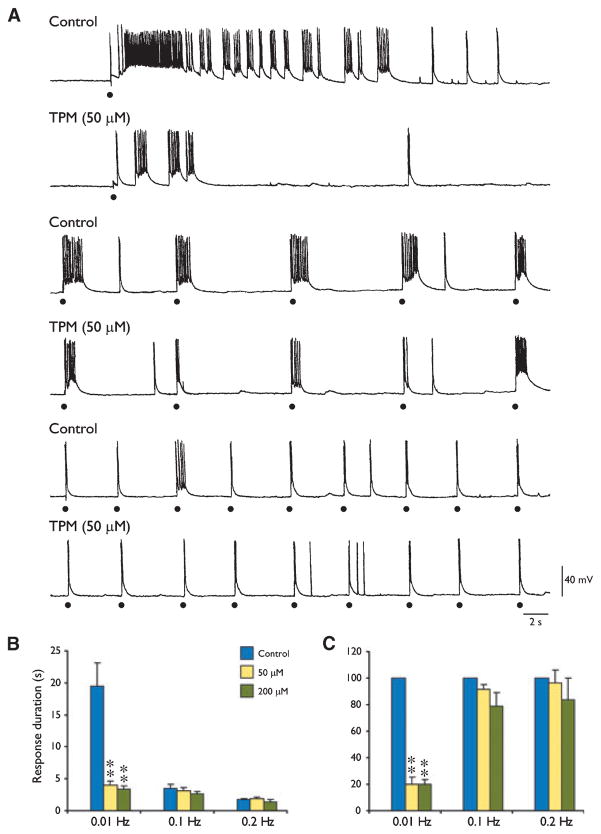
Finally we repeated this type of experiment by analyzing the effects induced by TPM on the stimulus-induced epileptiform events. TPM (50 μM, n = 5) reduced (F2,13 = 77.28, p < 0.01) the duration of the ictal-like responses induced by electrical stimuli delivered at an interval of 100 s, but did not influence the duration of the epileptiform responses elicited by stimuli occurring every 10 s (F2,13 = 3.07, p = 0.08) or 5 s (F2,13 = 3.42, p = 0.06) (Fig. 6A). Similar findings were obtained with larger TPM concentrations (200 μM, n = 5) (not illustrated). When intracellular recordings were used (n = 3), the effects induced by TPM were not associated with any appreciable decrease in the depolarizing envelope underlying the stimulus-induced epileptiform discharge (Fig. 6A). Data obtained from five experiments with TPM doses of 50 and 200 μM are summarized in Fig. 6B, C.
Figure 6.
Effects induced by topiramate (TPM) on the epileptiform events induced by focal electrical stimuli delivered at 0.01, 0.1, and 0.2 Hz. (A) Intracellular recordings from a deep-layer entorhinal cortex (EC) neuron during application of medium containing 4-aminopyridine (4AP) (Control) demonstrate that electrical stimuli delivered at 0.01 Hz induce an ictal-like depolarization, whereas stimuli at shorter intervals (10 s or less) elicit shorter, interictal-like depolarizations. Note that application of TPM (50 μM) abolishes the ictal-like depolarization, whereas no changes are seen when analyzing the responses induced by stimuli delivered at 0.1 or 0.2 Hz. (B) Summary of the effects induced by TPM on the duration of the epileptiform depolarizations elicited by electrical stimuli delivered at 0.01, 0.1, and 0.2 Hz. (C): Graphs shown in B were normalized to the response duration seen under control conditions. **p < 0.01, one-way analysis of variance (ANOVA) followed by Dunnett’s test.
Discussion
The main findings of our study are as follows. First, three well-established AEDs such as CBZ, TPM, and VPA can abolish ictal-like epileptiform discharges generated in vitro by EC networks during application of the convulsant drug 4AP, but are unable to block the ongoing interictal-like activity. Second, these AEDs cannot influence the recurrent interictal-like activity recorded in the EC of brain slices during GABAA-receptor antagonism. Third, two of these AEDs—that is, CBZ and TPM—can reduce the length of long-lasting epileptiform responses resembling ictal-like events induced by stimuli delivered at long intervals during 4AP application, but did not change the duration of shorter epileptiform events similar to the interictal-like activity elicited by more frequent stimulation (i.e., every 10 or 5 s).
As previously reported by us (Avoli et al., 1996; Benini et al., 2003) and other investigators (Brückner et al., 1999; Gutschmidt et al., 1999; Brückner & Heinemann, 2000), bath application of 4AP induced the appearance of spontaneous ictal-like and interictal-like events that were initiated in the EC, and are contributed by glutamatergic and GABAA receptor–mediated conductances (Avoli et al., 2002). Despite similar underlying synaptic mechanisms, however, all AEDs were unable to block interictal-like events but readily abolished ictal-like discharges. In line with these findings, early work performed in isolated rat hippocampal slices treated with 4AP has shown that in young tissue AEDs can abolish ictal-like discharges at concentrations that are unable to influence shorter, interictal-like events (Fueta & Avoli, 1992). This study also demonstrated that AEDs reduced interictal-like activity in the adult hippocampus only at very high doses. Similar findings have been later obtained in combined adult rat hippocampus-EC slices by Brückner and Heinemann (2000).
As reported by Brückner et al. (1999), we have also found here that the recurrent, interictal-like discharges generated by EC networks during concomitant application of 4AP and PTX (which antagonizes GABAA receptors) are insensitive to CBZ, TPM, and VPA. These findings may suggest that GABAA receptor–mediated inhibition must be operational for having AEDs exert their effect. In line with this view, previous studies (Dreier et al., 1998) have shown that AEDs fail in influencing epileptiform discharges induced by Mg2+-free medium at a time when GABAA receptor–mediated inhibition is weakened. Indeed, it has been proposed that this condition may represent an in vitro model of pharmacoresistance. In contrast with this view, we have found that the inability of AEDs to reduce interictal-like activity in vitro depends presumably on the duration of the discharge itself. This conclusion rests on two pieces of evidence. First, inhibition is preserved during application of 4AP at the time when AEDs fail in abolishing interictal activity. Second, by using stimuli delivered at various frequencies we have found that CBZ and TPM are able to reduce the duration of interictal-like events lasting longer than 0.7 s. However, a contribution of weakened inhibition to the lack of effects during AED treatment cannot be excluded since, in our study neither CBZ nor TPM exerted any significant effect on the pattern of recurrent epileptiform activity recorded in the EC during concomitant application of 4AP and PTX. Indeed, these interictal-like discharges had a mean duration of 1.7 s.
The resistance of short interictal-like epileptiform discharges induced in vitro by 4AP is also in line with clinical evidence obtained by investigating the relationship between the electroencephalographic spiking rate and the AED levels (Gotman & Marciani, 1985). These authors found that drug levels that influence seizure occurrence do not influence spiking (i.e., interictal discharge) rate. A more recent study confirmed this evidence and even found a small but significant decrease in interictal spiking by AED withdrawal (Spencer et al., 2008).
Acknowledgments
This study was supported by grants from the Canadian Institutes of Health Research (Grant MT-8109), the FIRB-MIUR (Ministero dell’ Istruzione, Università e Ricerca), the Emilia-Romagna Region (PRIER 2007/09-Programma Università-Regione), and the Pierfranco and Maria Luisa Mariani Foundation (R-06-50). We acknowledge the participation of Dr. V. Lopantsev in preliminary experiments, and we thank Ms. T. Papadopoulos for secretarial assistance.
Footnotes
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure: None of the authors has any conflict of interest to disclose.
References
- Avoli M, Barbarosie M, Lucke A, Nagao T, Lopantsev V, Köhling R. Synchronous GABA-mediated potentials and epileptiform discharges in the rat limbic system in vitro. J Neurosci. 1996;16:3912–3924. doi: 10.1523/JNEUROSCI.16-12-03912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, D’Antuono M, Louvel J, Köhling R, Biagini G, Pumain R, D’Arcangelo G, Tancredi V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol. 2002;68:167–207. doi: 10.1016/s0301-0082(02)00077-1. [DOI] [PubMed] [Google Scholar]
- Barbarosie M, Avoli M. CA3-driven hippocampal-entorhinal loop controls rather than sustains in vitro limbic seizures. J Neurosci. 1997;17:9308–9314. doi: 10.1523/JNEUROSCI.17-23-09308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarosie M, Louvel J, D’Antuono M, Kurcewicz I, Avoli M. Masking synchronous GABA-mediated potentials controls limbic seizures. Epilepsia. 2002;43:1469–1479. doi: 10.1046/j.1528-1157.2002.17402.x. [DOI] [PubMed] [Google Scholar]
- Bartolomei F, Wendling F, Bellanger JJ, Regis J, Chauvel P. Neural networks involving the medial temporal structures in temporal lobe epilepsy. Clin Neurophysiol. 2001;112:1746–1760. doi: 10.1016/s1388-2457(01)00591-0. [DOI] [PubMed] [Google Scholar]
- Benini R, D’Antuono M, Pralong E, Avoli M. Involvement of amygdala networks in epileptiform synchronization in vitro. Neuroscience. 2003;120:75–84. doi: 10.1016/s0306-4522(03)00262-8. [DOI] [PubMed] [Google Scholar]
- Bragdon AC, Kojima H, Wilson WA. Suppression of interictal bursting in hippocampus unleashes seizures in entorhinal cortex: a proepileptic effect of lowering [K+]o and raising [Ca2+]o. Brain Res. 1992;590:128–135. doi: 10.1016/0006-8993(92)91088-v. [DOI] [PubMed] [Google Scholar]
- Brückner C, Stenkamp K, Meierkordb H, Heinemann U. Epileptiform discharges induced by combined application of bicuculline and 4-aminopyridine are resistant to standard anticonvulsants in slices of rats. Neurosci Lett. 1999;268:163–165. doi: 10.1016/s0304-3940(99)00341-9. [DOI] [PubMed] [Google Scholar]
- Brückner C, Heinemann U. Effects of standard anticonvulsant drugs on different patterns of epileptiform discharges induced by 4-aminopyridine in combined entorhinal cortex–hippocampal slices. Brain Res. 2000;859:15–20. doi: 10.1016/s0006-8993(99)02348-3. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Heinemann U. Regional and time dependent variations of low Mg2+ induced epileptiform activity in rat temporal cortex slices. Exp Brain Res. 1991;87:581–596. doi: 10.1007/BF00227083. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Zhang CL, Heinemann U. Phenytoin, phenobarbital, and midazolam fail to stop status epilepticus-like activity induced by low magnesium in rat entorhinal slices, but can prevent its development. Acta Neurol Scand. 1998;98:154–160. doi: 10.1111/j.1600-0404.1998.tb07286.x. [DOI] [PubMed] [Google Scholar]
- Fueta Y, Avoli M. Effects of antiepileptic drugs on 4-aminopyridine-induced epileptiform activity in young and adult rat hippocampus. Epilepsy Res. 1992;12:207–215. doi: 10.1016/0920-1211(92)90075-5. [DOI] [PubMed] [Google Scholar]
- Gotman J, Marciani MG. Electroencephalographic spiking activity, drug levels, and seizure occurrence in epileptic patients. Ann Neurol. 1985;17:597–603. doi: 10.1002/ana.410170612. [DOI] [PubMed] [Google Scholar]
- Gutschmidt KU, Stenkamp K, Buchheim K, Heinemann U, Meierkord H. Anticonvulsant actions of furosemide in vitro. Neuroscience. 1999;91:1471–1481. doi: 10.1016/s0306-4522(98)00700-3. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Franceschetti S, Hamon B, Konnerth A, Yaari Y. Effects of anticonvulsants on spontaneous epileptiform activity which develops in the absence of chemical synaptic transmission in hippocampal slices. Brain Res. 1985;325:349–352. doi: 10.1016/0006-8993(85)90338-5. [DOI] [PubMed] [Google Scholar]
- Lopantsev V, Avoli M. Laminar organization of 4 aminopyridine-induced epileptiform discharges in the rat entorhinal cortex. J Physiol (London) 1998a;509:785–796. doi: 10.1111/j.1469-7793.1998.785bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopantsev V, Avoli M. Participation of GABAA-mediated inhibition in ictal-like discharges in the rat entorhinal cortex. J Neurophysiol. 1998b;79:352–360. doi: 10.1152/jn.1998.79.1.352. [DOI] [PubMed] [Google Scholar]
- Löscher W, Nau H, Siemes H. Penetration of valproate and its active metabolites into cerebrospinal fluid of children with epilepsy. Epilepsia. 1988;29:311–316. doi: 10.1111/j.1528-1157.1988.tb03725.x. [DOI] [PubMed] [Google Scholar]
- Nagao T, Alonso A, Avoli M. Epileptiform activity induced by pilocarpine in the rat hippocampal-entorhinal slice preparation. Neuroscience. 1996;72:399–408. doi: 10.1016/0306-4522(95)00534-x. [DOI] [PubMed] [Google Scholar]
- Prince DA. Epileptogenic neurons and circuits. Adv Neurol. 1999;79:665–684. [PubMed] [Google Scholar]
- Rambeck B, Jürgens UH, May TW, Pannek HW, Behne F, Ebner A, Gorji A, Straub H, Speckmann EJ, Pohlmann-Eden B, Löscher W. Comparison of brain extracellular fluid, brain tissue, cerebrospinal fluid, and serum concentrations of antiepileptic drugs measured intraoperatively in patients with intractable epilepsy. Epilepsia. 2006;47:681–694. doi: 10.1111/j.1528-1167.2006.00504.x. [DOI] [PubMed] [Google Scholar]
- Rutecki PA, Grossman RG, Armstrong D, Irish-Loewen S. Electrophysiological connections between the hippocampus and entorhinal cortex in patients with complex partial seizures. J Neurosurg. 1989;70:667–675. doi: 10.3171/jns.1989.70.5.0667. [DOI] [PubMed] [Google Scholar]
- Schneiderman JH, Schwartzkroin PA. Effects of phenytoin on normal activity and on penicillin-induced bursting in the guinea pig hippocampal slice. Neurology. 1982;32:730–738. doi: 10.1212/wnl.32.7.730. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Spencer DD. Entorhinal-hippocampal interactions in medial temporal lobe epilepsy. Epilepsia. 1994;35:721–727. doi: 10.1111/j.1528-1157.1994.tb02502.x. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Goncharova II, Duckrow RB, Novotny EJ, Zaveri HP. Interictal spikes on intracranial recording: behavior, physiology, and implications. Epilepsia. 2008;49:1881–1892. doi: 10.1111/j.1528-1167.2008.01641.x. [DOI] [PubMed] [Google Scholar]
- Traub RD, Borck C, Colling SB, Jefferys JG. On the structure of ictal events in vitro. Epilepsia. 1996;37:879–891. doi: 10.1111/j.1528-1157.1996.tb00042.x. [DOI] [PubMed] [Google Scholar]
- Traub RD, Jefferys JG, Whittington MA. Functionally relevant and functionally disruptive (epileptic) synchronized oscillations in brain slices. Adv Neurol. 1999;79:709–724. [PubMed] [Google Scholar]
- Watts AE, Jefferys JG. Effects of carbamazepine and baclofen on 4-aminopyridine-induced epileptic activity in rat hippocampal slices. Br J Pharmacol. 1993;108:819–823. doi: 10.1111/j.1476-5381.1993.tb12884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Jefferys JG. Erosion of inhibition contributes to the progression of low magnesium bursts in rat hippocampal slices. J Physiol (London) 1995;486:723–734. doi: 10.1113/jphysiol.1995.sp020848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Dreier JP, Heinemann U. Paroxysmal epileptiform discharges in temporal lobe slices after prolonged exposure to low magnesium are resistant to clinically used anticonvulsants. Epilepsy Res. 1995;20:105–111. doi: 10.1016/0920-1211(94)00067-7. [DOI] [PubMed] [Google Scholar]