Abstract
The absence of fragile X mental retardation protein results in the fragile X syndrome (FXS), a common form of mental retardation associated with attention deficit, autistic behavior, and epileptic seizures. The phenotype of FXS is reproduced in fragile X mental retardation 1 (fmr1) knockout (KO) mice that have region-specific altered expression of some γ-aminobutyric acid (GABAA) receptor subunits. However, little is known about the characteristics of GABAergic inhibition in the subiculum of these animals. We employed patch-clamp recordings from subicular pyramidal cells in an in vitro slice preparation. In addition, semiquantitative polymerase chain reaction and western blot experiments were performed on subiculum obtained from wild-type (WT) and KO mice. We found that tonic GABAA currents were downregulated in fmr1 KO compared with WT neurons, whereas no significant differences were observed in phasic GABAA currents. Molecular biology analysis revealed that the tonic GABAA receptor subunits α5 and δ were underexpressed in the fmr1 KO mouse subiculum compared with WT. Because the subiculum plays a role in both cognitive functions and epileptic disorders, we propose that altered tonic inhibition in this structure contributes to the behavioral deficits and epileptic activity seen in FXS patients. This conclusion is in line with evidence implicating tonic GABAA inhibition in learning and memory.
Keywords: fragile X, GABA, patch-clamp, subiculum, tonic inhibition
Introduction
Fragile X syndrome (FXS) is a common form of mental retardation, and it is caused by a trinucleotide expansion in the fragile X mental retardation 1 (fmr1) gene that prevents the expression of the encoded protein, called fragile X mental retardation protein (FMRP) (Oostra and Chiurazzi 2001). The absence of FMRP causes morphological and functional changes of synapses in FXS patients and in animal models such as the fmr1 knockout (KO) mouse (Bakker et al. 1994; Antar and Bassell 2003). Major symptoms of FXS in humans are mental retardation, attention deficit, hyperactivity, autistic behaviors, and epileptic seizures. Most of this neurological phenotype is reproduced in fmr1 KO mice that present with increased locomotor activity, reduced habituation in an open field, learning deficits, and increased seizure susceptibility (Bakker and Oostra 2003).
FMRP is widely expressed in the brain, and its absence is expected to disrupt the synthesis and/or the subcellular localization of several proteins (Miyashiro et al. 2003; Todd et al. 2003). It is therefore not surprising that several neurotransmitter systems are altered in FXS. In line with this view, studies of fmr1 KO mice have identified: 1) alterations in metabotropic glutamate receptor signaling which result in exaggerated protein synthesis–dependent long-term depression of synaptic transmission in hippocampus (Huber et al. 2002; Antar et al. 2004; Aschrafi et al. 2005); 2) reduced GluR1 expression along with decreased long-term potentiation of synaptic transmission in cerebral cortex and amygdala of fmr1 KO mice (Li et al. 2002; Zhao et al. 2005); and 3) altered responses of subicular neuronal networks to cholinergic stimulation, presumably because of impaired γ-aminobutyric acid (GABAA) receptor–mediated inhibition (D’Antuono et al. 2003). Underexpression of GABAA receptor subunits has also been identified in a structure-specific manner in fmr1 KO mice. In particular, reduction in the expression of α1, α3 and α4, β1 and β2, γ1 and γ2, and δ subunits has been observed at an mRNA level (D’Hulst et al. 2006; Gantois et al. 2006) and of β subunit at protein level (El Idrissi et al. 2005).
As recently proposed by D’Hulst and Kooy (2007), the altered composition of GABAA receptors may have functional consequences that relate to the behavioral and neurological phenotype of FXS including, beyond epilepsy, anxiety, depression, sleep disorders, and learning and memory deficits. However, little is known regarding the function of GABAA receptor–mediated inhibition in FXS. The subiculum plays an essential role in cognitive functions such as spatial encoding (Sharp and Green 1994) and retrieval of short-term memories (Gabrieli et al. 1997). In addition, it has been demonstrated that the subiculum works under GABAergic control and can be the focus of epilepsy in condition of altered functionality of GABAA receptors (Cohen et al. 2002; Benini and Avoli 2005; Wozny et al. 2005). In the present study, we use electrophysiological and molecular biology techniques to address whether phasic and tonic GABAA currents are modified in the subiculum of fmr1 KO mice.
Materials and Methods
Mice
C57BL/6 adult male mice (Charles River Canada, Saint-Constant, Quebec, Canada) were used as control group. C57BL/6J-Fmr1tm1Cgr fragile X mice were originally obtained from Jackson Laboratories (Bar Harbor, ME) and used to create the fmr1 KO mouse line in our animal facility. The experimental procedures were in accordance with the guidelines established by the Canadian Council of Animal Care. All efforts were made to minimize the number of animals used and their suffering.
Patch-Clamp Experiments
In all, 4- to 24-weeks-old mice (mean = 53.26 day-old in wild type [WT] and 62.44 day-old in fmr1 KO) were anaesthetized with ketamine–xylazine solution (150 μg–10 μg/g intraperitoneally). The descending aorta was clamped and intracardiac perfusion with ice-cold cutting solution (see below for composition) was applied. Animals were then decapitated and their brain quickly removed and placed in ice-cold cutting solution. Horizontal slices (300 μm) were cut with a VT1000S vibratome (Leica, Nussloch, Germany). The cutting solution contained (in millimolar): sucrose 206, KCl 3.5, MgSO4 2, NaH2PO4 1.25, CaCl2 1, MgCl2 1, NaHCO3 26, glucose 10, ascorbic acid 0.4, and kynurenic acid 1. Slices were kept at 33 °C in the cutting solution for 30 min and then transferred into a holding chamber at room temperature with standard artificial cerebrospinal fluid (ACSF) and kept for at least 1 h before recording. Standard ACSF composition was (in millimolar): NaCl 124, KCl 3.5, MgSO4 2, NaH2PO4 1.25, CaCl2 2, NaHCO3 26, and glucose 10.
Individual slices were transferred into a submersion recording chamber, series 20 model RC-27L (Warner Instruments LLC, Hamden, CT) and continuously perfused (about 40 ml/h) with standard ACSF. Voltage-clamp experiments were performed at room temperature using a Multiclamp 700A amplifier (Molecular Devices, Palo Alto, CA). After Giga-seal formation and patch rupturing, capacitance currents were minimized using the amplifier circuitry. Signals were acquired at a sampling frequency of 10 KHz using pClamp 8.2 software (Molecular Devices). The electrodes (2–4 MΩ resistance) were prepared from borosilicate glass capillaries (1.5 mm outer diameter × 0.86 mm inner diameter; Harvard Apparatus, Holliston, MA) using a P-97 model puller (Sutter Instruments, Novato, CA) and filled with a solution containing (in millimolar): KCl 120, ethyleneglycol-bis(2-aminoethylether)-N,N,N′,N′-tetra acetic acid 10, 4-(2-hydroxyethyl)-1-piperazineethane-sulfonic acid 10, MgCl2 2, CaCl2 1, ATP-Na2 2, GTP-Na3 0.4, phosphocreatine-Na2 20, and creatine phosphokinase 20 U/ml. Contaminant excitatory postsynaptic currents were blocked by applying (RS)-3-(2-carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP, 10 μM) and 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX, 10 μM), or kynurenic acid (2 mM). The (2S)-3-[[(1S)-1-(3,4 dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl) phosphinic acid (CGP 55845, 4 μM) was added to the bath perfusion to block GABAB activity. All compounds have been purchased from Sigma (St Louis, MO) with exception for CPP, CNQX, CGP 55845, and SR 95531 (gabazine) which were obtained from Tocris (Ellisville, MO).
Whole-cell recordings were made from subicular pyramidal neurons visually identified by infrared video camera (model CCD100, DAGE-MTI, Michigan City, IN) using a 60X water immersion objective mounted on an upright microscope (model Eclipse E600FN, Nikon Canada, Montreal, Quebec, Canada), specifically designed for electrophysiological recordings. Access resistance was monitored throughout the experiments by applying brief 5-mV depolarizing steps. Series resistance was typically <20 MΩ and not significantly different between WT and fmr1 KO tissue (WT: Rs = 10.88 ± 2.53 MΩ, n = 18; fmr1 KO: Rs = 14.72 ± 1.86 MΩ, n = 35). When access resistance changed by >20%, cells were discarded. Membrane capacitance was not different between the 2 experimental groups (WT: Cm = 23.54 ± 2.42 pF, n = 19; fmr1 KO: Cm = 21.37 ± 1.30 pF, n = 39). Holding potential of −70 mV was kept during experiments. The following spontaneous inhibitory postsynaptic currents (sIPSCs) parameters were measured: peak amplitude, charge transferred, peak time, half decay time, and interevent time (see Supplementary Materials for details). For kinetic analysis, only single sIPSC events were used from the recording traces, while multipeak sIPSCs were excluded. In all, 20–60 peaks in each experimental condition have been measured from samples of 10–20 s traces randomly chosen.
Molecular Biology Experiments
Tissue samples for real-time reverse transcription–polymerase chain reaction (RT-PCR) and for western blot studies were obtained from subiculum of WT and fmr1 KO mice. For methodological details, see Supplemental Materials.
Data Analysis
Results were expressed as mean ± standard error of the mean. The n indicates number of neurons for patch data and number of animals for molecular biology studies. For patch data, off-line analysis was performed using Clampfit 9 (Molecular Devices) and Origin 7 pro (Microcal Software, Northampton, MA) softwares. Statistical comparison was made between WT and fmr1 KO tissue. Student’s t-test for unpaired data was used and P < 0.05 was consider statistically significant. For real-time RT-PCR, Student’s t-test was performed to verify whether R values were different from 1. We also checked that R values obtained with glyceraldehyde 3-phosphate-dehydrogenase (GAPDH) were not significantly different from R values obtained with TATA box binding protein (TBP) as housekeeping genes (n.s. in Fig. 3). For western blot, the same test was used to determine whether the densitometric ratios (DRs) fmr1 KO/WT were significantly different from 1.
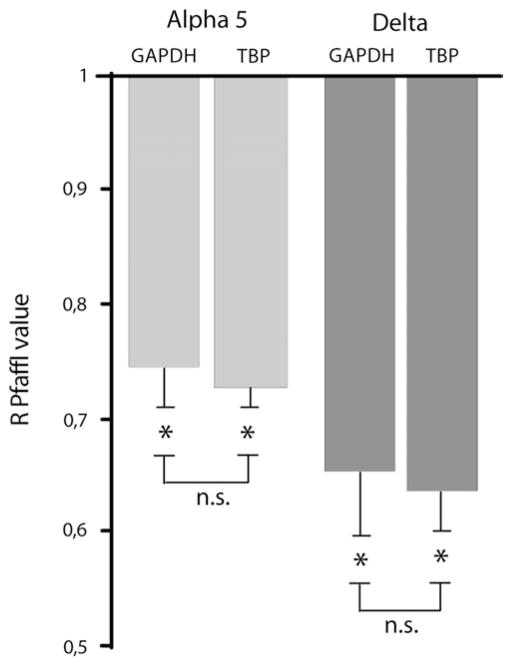
Figure 3.
The mRNA of α5 and δ subunits is underexpressed in subiculum of fmr1 KO mice. R Pfaffl values (Pfaffl 2001) indicating underexpression of the levels of expression of mRNA for α5 (light gray bars) and δ (dark gray bars) subunits in fmr1 KO relative to WT. Data obtained using GAPDH and TBP as housekeeping genes were not significantly different (n.s.).
Results
Phasic GABAA Currents Are Unchanged in fmr1 KO Subicular Tissue
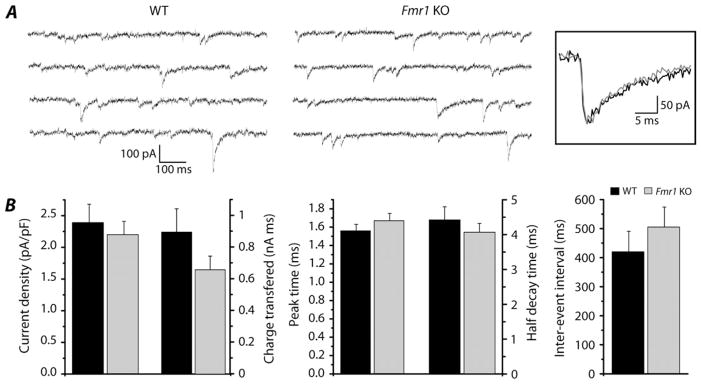
Patch-clamp experiments in whole-cell configuration were performed from subicular pyramidal neurons in brain slices obtained from adult WT and fmr1 KO mice, and sIPSCs were recorded (Fig. 1A). We did not find any difference in current density (WT: 2.39 ± 0.29 pA/pF, n = 16; fmr1 KO: 2.20 ± 0.21 pA/pF, n = 39), charge transferred (WT: 893.65 ± 148.01 pA ms, n = 16; fmr1 KO: 656.14 ± 86.26 pA ms, n = 39), and interevent interval (WT: 420.40 ± 70.18 ms, n = 16; fmr1 KO: 505.09 ± 69.10 ms, n = 39) (Fig. 1B). Peak time (WT: 1.56 ± 0.07 ms, n = 16; fmr1 KO: 1.67 ± 0.08 ms, n = 39) and half decay time (WT: 4.40 ± 0.38 ms, n = 16; fmr1 KO: 4.05 ± 0.25 ms, n = 39) were also similar in WT and fmr1 KO (Figs 1A inset and B). The sIPSCs disappeared upon application of GABAA receptor blockers, such as gabazine 1 μM (n = 8 fmr1 KO; data not shown) or picrotoxin 100 μM (PTX; n = 9 WT and 16 fmr1 KO; Fig. 2A).
Figure 1.
Phasic component of GABAergic current is not altered in subiculum of fmr1 KO mice. (A) The sIPSC events recorded from WT (left) and fmr1 KO mice (right) during voltage-clamp experiments in presence of blockers for the glutamatergic and GABAB receptors. Traces with higher frequency of events than mean values were chosen for figure purpose. Holding potential was −70 mV. In the inset single events from WT (black trace) and fmr1 KO (gray trace) are overlapped to show that time constants are not changed in the 2 groups. (B) Histograms reveal no statistical difference in current density, charge transferred, peak time, half decay time, and interevent interval between WT (black bars) and fmr1 KO (gray bars) tissues.
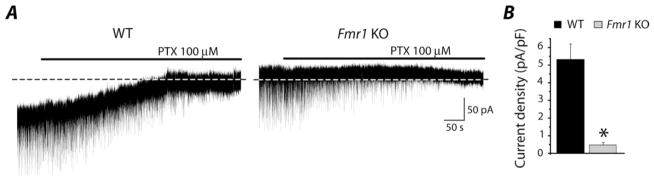
Figure 2.
The tonic component of GABAergic current is downregulated in fmr1 KO subicular neurons. (A) Traces recorded in voltage-clamp from WT (left) and fmr1 KO (right) subicular neurons (holding potential −70 mV; glutamatergic and GABAB blockers in the bath). Application of PTX induces the disappearance of synaptic events and the shift of the holding current presumably due to the block of phasic and tonic GABAergic components, respectively. Note that the shift of the holding current is more pronounced in WT compared with fmr1 KO tissue. Dash lines indicate zero-current level. (B) Histogram shows significant difference of current density for the tonic component between WT (black bar) and fmr1 KO (gray bar) mice.
Tonic GABAA currents are downregulated in the subiculum of fmr1 KO mice
Besides the block of phasic events, application of PTX 100 μM to slices obtained from WT mice revealed a shift of the holding current, likely due to the block of GABAA receptors responsible for the tonic component (Fig. 2A). An appreciable shift in holding current was rarely seen in fmr1 KO tissue (Fig. 2A), suggesting a reduced tonic component as compared with WT. The current density recorded in WT and fmr1 KO tissue was downregulated by ~91% (WT: 5.32 ± 0.88 pA/pF, n = 7; fmr1 KO: 0.48 ± 0.13 pA/pF, n = 7; P = 0.00014, Fig. 2B).
Next, we investigated mRNA expression for the α5 and δ subunits in the subiculum of WT and fmr1 KO tissue. We found that fmr1 KO mice presented with ~26% reduction in α5 mRNA expression compared with WT (R = 0.74 ± 0.03 with GAPDH, P < 0.0001 and R = 0.73 ± 0.02 with TBP, P < 0.0001; n = 4 WT and 4 fmr1 KO mice, in 5 experiments; Fig. 3). The mRNA for the δ subunit in fmr1 KO mouse subiculum also presented with ~35% reduction compared with WT (R = 0.65 ± 0.06 with GAPDH, P < 0.0001 and R = 0.64 ± 0.03 with TBP, P < 0.0001; n = 4 WT and 4 fmr1 KO mice, in 5 experiments; Fig. 3). Data obtained using GAPDH as housekeeping gene were not significantly different than data obtained using TBP (n.s. in Fig. 3).
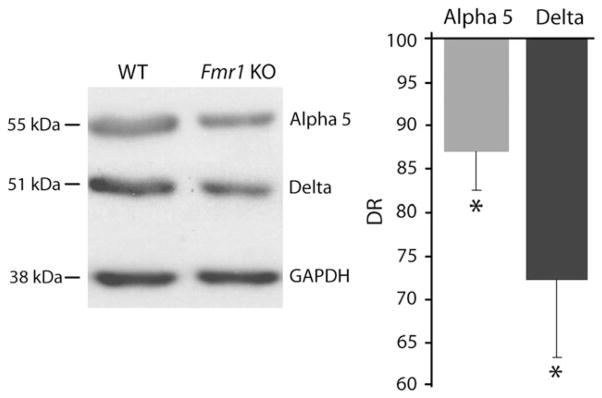
Finally, we carried out western blot analysis to investigate whether the different expression of mRNA levels was paralleled by similar changes at the protein level. Figure 4 shows western blots performed on WT and fmr1 KO tissue for both the subunits. GAPDH was used as loading control. Quantification reveals that expression of the α5 subunit was reduced by ~13% in fmr1 KO versus WT (DR = 87.0 ± 4.6%, P < 0.05; n = 4 WT and 4 fmr1 KO mice, 5 experiments; Fig. 4). The δ subunit was underexpressed by ~28% in fmr1 KO animals compared with WT (DR = 72.0 ± 9.0%, P < 0.05; n = 4 WT and 4 fmr1 KO mice, 4 experiments; Fig. 4).
Figure 4.
The α5 and δ subunits are underexpressed at protein level in subiculum of fmr1 KO mice. Western blots performed in WT (left) and fmr1 KO (right) tissue for both GABAA subunits in subiculum. On the right, DRs obtained from western blot experiments reveal underexpression of α5 (light gray bar) and δ (dark gray bar) GABAA receptor subunits.
Discussion
We have found in this study that the tonic component of the GABAA receptor–mediated inhibition is markedly altered in pyramidal cells of the fmr1 KO mouse subiculum. This conclusion was supported by experiments in which we compared the electrophysiological and molecular characteristics of the GABAA receptor subunits in the subiculum of fmr1 KO and WT mice. In contrast, no changes were detected in current density and kinetic parameters for the phasic inhibitory events analyzed with patch-clamp recordings only.
By using real-time RT-PCR and western blot analysis, we have discovered in the fmr1 KO mouse subiculum a significant decrease of α5 and δ subunits, both at mRNA and protein level. These subunits are considered to be an essential component of the tonic GABAA receptor, at least in the hippocampus (Sperk et al. 1997; Caraiscos et al. 2004). Interestingly, previous molecular studies have found δ subunit underexpression in hippocampus and neocortex (D’Hulst et al. 2006; Gantois et al. 2006), whereas no changes were observed with the α5 subunit in the neocortex of fmr1 KO mice (D’Hulst et al. 2006). This evidence, along with our results, suggests therefore that subunits mediating the tonic GABAA current may be altered in a site-specific manner in this mouse model of FXS. The amplitude of the tonic current recorded in WT subicular neurons is similar to the one reported for pyramidal cell in C57BL6 mice (Glykys et al. 2008). The electrophysiological data obtained in the fmr1 KO’s subiculum, however, identify a marked reduction in tonic GABAA receptor–mediated currents and thus support the hypothesis that a reduction in α5 and δ subunit expression can lead to a decrease in tonic inhibition. However, the reduced expression of these 2 subunits at a molecular level (~60%) and protein level (~50%) cannot fully explain the degree of functional impairment (~91%) seen by comparing the tonic GABAA receptor–mediated currents recorded from subicular neurons in fmr1 KO and WT mice. We cannot exclude that the discrepancy between molecular, protein, and current reductions is due, at least in part, to alterations of the mRNA translation and/or of the trafficking of the receptors to the membrane surface, as suggested for the glutamatergic receptors in fragile X model (Kooy 2003; Bear et al. 2004). In addition, it cannot be excluded also that other subunits responsible for tonic GABAA receptors may be underexpressed in fmr1 KO animals. Scimemi et al. (2005) have indeed proposed that subunits involved in tonic inhibition have still to be identified. In fact, in their study on tonic inhibition in CA1 pyramidal neurons from epileptic rats they observed, after the block of α5 subunit, a residual neurosteroids-insensitive tonic current; therefore, they excluded that this was due to α5 and δ subunits. On the contrary, Glykys et al. (2008) did not observe any residual tonic current in CA1 of α5–δ double-KO mice. The discrepancy between the 2 studies can be due to the different species and models used: C57BL6 KO mice versus Sprague–Dawley pilocarpine-treated rats (where mossy fiber sprouting and reorganization of the network have been reported). In addition, no one has ever investigated this aspect of tonic currents in the subiculum.
Electrophysiological analysis of the phasic GABAA receptor–mediated events failed in revealing any significant difference between fmr1 KO and WT mice. This conclusion is in agreement with data reported by Centonze et al. (2008), who found no difference in peak amplitude and time constants of the IPSCs recorded from striatal spiny neurons in fmr1 KO compared with the WT. However, their data revealed an increased frequency in FXS tissue. This discrepancy may reflect differences in brain structure thus underscoring the region specificity of the changes in neurotransmission that characterize this model of FXS. Moreover, it should be remarked that underexpression of some phasic GABAA receptor subunits has been reported in the hippocampus and cortex of fmr1 KO mice (El Idrissi et al. 2005; D’Hulst et al. 2006). Beyond region specificity, this difference further suggests a compensatory mechanism involving other GABAA receptor subunits. Thus, changes in subunit expression and in function can diverge.
Several evidences suggest that alterations in GABAA receptor subunits can be good candidates for neurodevelopmental and neuropsychiatric anomalies seen in human syndromes of mental retardation. In particular, it has been reported that altered expression of subunit for tonic GABAA receptors is implicated in learning and memory processes (D’Hulst and Kooy 2007). Deletion of the delta subunit of GABAA receptor occurs in patients affected by 1p36 deletion syndrome, characterized by moderate to severe psychomotor retardation and epilepsy (Windpassinger et al. 2002). In addition, altered expression of GABAA receptor subunits has been observed in the brain of other models of mental retardation, such as Angelman (DeLorey et al. 1998) and Prader–Willi syndrome (Lucignani et al. 2004). Because it has been reported reductions in the expression of α1, α3 and α4, β1 and β2, γ1 and γ2, and δ subunits at an mRNA level (D’Hulst et al. 2006; Gantois et al. 2006) and of β subunit at protein level (El Idrissi et al. 2005) in fragile X syndrome, we cannot exclude that impairment of GABAergic functional inhibition is due also to alterations in GABAA receptors not containing α5 or δ subunits, may be in structures outside subiculum. Atack et al. (2006) showed that a selective antagonist for α5 subunit enhance LTP in mice. However, alterations in LTP and LTD have been reported in fragile X mice (Huber et al. 2002; Li et al. 2002; Antar et al. 2004; Aschrafi et al. 2005; Zhao et al. 2005), suggesting that the final phenotype derives from a combination of dysfunctions that occur at the same time. This is the first evidence showing a reduced functionality of GABAA receptors in an animal model of mental retardation. However, further investigations of GABAA currents would be necessary.
Tonic inhibition, beyond learning and memory, plays a critical role in the context of epilepsy. It has been reported that reduction of the tonic inhibition in α5 or δ KO mice is sufficient to induce epileptiform hyperexcitability in CA3 (Glykys and Mody 2006) or greater susceptibility to pentylenetetrazol-induced seizures (Spiegelman et al. 2002). In addition, altered expression of GABAA receptor subunits has been observed in the hippocampus of other epileptic animals (Peng et al. 2004; Nishimura et al. 2005; Payne et al. 2006; Zhang et al. 2007).
In line with these observations and as proposed by D’Hulst and Kooy (2007), an altered expression of the GABAA receptor subunits may have functional consequences that relate to the behavioral and neurological phenotype of FXS. In addition, it has been reported that 48-h administration of neurosteroids, such as 3α-OH-5β-pregnan-20-one (3α, 5β-THP) or 17 β-estradiol (E2) + progesterone, to female rats increased expression of the δ subunit in CA1 hippocampus (Shen et al. 2005). Because the ability to express δ subunit in fmr1 KO mice is reduced but not completely compromised, administration of neurosteroids could represent a possible treatment for FXS.
Acknowledgments
Funding
Canadian Institutes of Health Research (MOP 8109, MOP 43964); the Savoy Foundation; Italian Ministry of University (PRIN 2007CX2R77).
Footnotes
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Conflict of Interest: None declared.
Notes
G.C. is a postdoctoral fellow of the Fragile X Research Foundation of Canada in partnership with Canadian Institutes of Health Research. We thank Dr J.M. Fritschy (University of Zurich, Switzerland) for the gift of the α5 GABAA receptor antibody. We thank Mr J. Roy for technical support and Ms T. Papadopoulos for editorial assistance.
References
- Antar LN, Afroz R, Dictenberg JB, Carroll RC, Bassell GJ. Metabotropic glutamate receptor activation regulates fragile X mental retardation protein and Fmr1 mRNA localization differentially in dendrites and at synapses. J Neurosci. 2004;24:2648–2655. doi: 10.1523/JNEUROSCI.0099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antar LN, Bassell GJ. Sunrise at the synapse: the FMRP mRNP shaping the synaptic interface. Neuron. 2003;37:555–558. doi: 10.1016/s0896-6273(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Aschrafi A, Cunningham BA, Edelman GM, Vanderklish PW. The fragile X mental retardation protein and group I metabotropic glutamate receptors regulate levels of mRNA granules in brain. Proc Natl Acad Sci. 2005;102:2180–2185. doi: 10.1073/pnas.0409803102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atack JR, Bayley PJ, Seabrook GR, Wafford KA, McKernan RM, Dawson GR. L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for alpha5-containing GABAA receptors. Neuropharmacology. 2006;51:1023–1029. doi: 10.1016/j.neuropharm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Bakker CE, Oostra BA. Understanding fragile X syndrome: insights from animal models. Cytogenet Genome Res. 2003;100:111–123. doi: 10.1159/000072845. [DOI] [PubMed] [Google Scholar]
- Bakker CE, Verheij C, Willemsen R, van der Helm R, Oerlemans F, Vermey M, Bygrave A, Hoogeveen AT, Oostra BA, Reyniers E, et al. Fmr1 knockout mice: a model to study fragile X mental retardation. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Benini R, Avoli M. Rat subicular networks gate hippocampal output activity in an in vitro model of limbic seizures. J Physiol. 2005;566:885–900. doi: 10.1113/jphysiol.2005.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, et al. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. Proc Natl Acad Sci. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Rossi S, Mercaldo V, Napoli I, Ciotti MT, De Chiara V, Musella A, Prosperetti C, Calabresi P, Bernardi G, et al. Abnormal striatal GABA transmission in the mouse model for the fragile X syndrome. Biol Psychiatry. 2008;63:963–973. doi: 10.1016/j.biopsych.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- D’Antuono M, Merlo D, Avoli M. Involvement of cholinergic and GABAergic systems in the fragile X knockout mice. Neurosci. 2003;119:9–13. doi: 10.1016/s0306-4522(03)00103-9. [DOI] [PubMed] [Google Scholar]
- DeLorey TM, Handforth A, Anagnostaras SG, Homanics GE, Minassian BA, Asatourian A, Fanselow MS, Delgado-Escueta A, Ellison GD, Olsen RW. Mice lacking the β3 subunit of the GABAA receptor have the epilepsy phenotype and many of the behavioral characteristics of Angelman syndrome. J Neurosci. 1998;18:8508–8514. doi: 10.1523/JNEUROSCI.18-20-08505.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hulst C, De Geest N, Reeve SP, Van Dam D, De Deyn PP, Hassan BA, Kooy RF. Decreased expression of the GABAA receptor in fragile X syndrome. Brain Res. 2006;1121:238–245. doi: 10.1016/j.brainres.2006.08.115. [DOI] [PubMed] [Google Scholar]
- D’Hulst C, Kooy RF. The GABAA receptor: a novel target for treatment of fragile X? Trends Neurosci. 2007;30:425–431. doi: 10.1016/j.tins.2007.06.003. [DOI] [PubMed] [Google Scholar]
- El Idrissi A, Ding XH, Scalia J, Trenkner E, Brown WT, Dobkin C. Decreased GABAA receptor expression in the seizure-prone fragile X mouse. Neurosci Lett. 2005;377:141–146. doi: 10.1016/j.neulet.2004.11.087. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Brewer JB, Desmond JE, Glover GH. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;276:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- Gantois I, Vandesompele J, Speleman F, Reyniers E, D’Hooge R, Severijnen LA, Willemsen R, Tassone F, Kooy RF. Expression profiling suggests underexpression of the GABAA receptor subunit delta in the fragile X knockout mouse model. Neurobiol Dis. 2006;21:346–357. doi: 10.1016/j.nbd.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mody I. Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABAA receptor α5 subunit-deficient mice. J Neurophysiol. 2006;95:2796–2807. doi: 10.1152/jn.01122.2005. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooy RF. Of mice and the fragile X syndrome. Trends Genet. 2003;19:148–154. doi: 10.1016/s0168-9525(03)00017-9. [DOI] [PubMed] [Google Scholar]
- Li J, Pelletier MR, Velazquez J-LP, Carlen P. Reduced cortical synaptic plasticity and GluR1 expression associated with Fragile X mental retardation protein deficiency. Mol Cell Neurosci. 2002;19:138–151. doi: 10.1006/mcne.2001.1085. [DOI] [PubMed] [Google Scholar]
- Lucignani G, Panzacchi A, Bosio L, Moresco RM, Ravasi L, Coppa I, Chiumello G, Frey K, Koeppe R, Fazio F. GABAA receptor abnormalities in Prader-Willi syndrome assessed with positron emission tomography and [11C]flumazenil. Neuroimage. 2004;22:22–28. doi: 10.1016/j.neuroimage.2003.10.050. [DOI] [PubMed] [Google Scholar]
- Mertens S, Benke D, Mohler H. GABAA receptor populations with novel subunit combinations and drug binding profiles identified in brain by α5- and δ-subunit-specific immunopurification. J Biol Chem. 1993;268:5965–5973. [PubMed] [Google Scholar]
- Miyashiro KY, Beckel-Mitchener A, Purk TP, Becker KG, Barret T, Liu L, Carbonetto S, Weiler IJ, Greenough WT, Eberwine J. RNA cargoes associating with FMRP reveal deficits in cellular functioning in Fmr1 null mice. Neuron. 2003;37:417–431. doi: 10.1016/s0896-6273(03)00034-5. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Schwarzer C, Gasser E, Kato N, Vezzani A, Sperk G. Altered expression of GABAA and GABAB receptor subunit mRNAs in the hippocampus after kindling and electrically induced status epilepticus. Neurosci. 2005;134:691–704. doi: 10.1016/j.neuroscience.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Oostra BA, Chiurazzi P. The fragile X gene and its function. Clin Genet. 2001;60:399–408. doi: 10.1034/j.1399-0004.2001.600601.x. [DOI] [PubMed] [Google Scholar]
- Payne HL, Donoghue PS, Connelly WMK, Hinterreiter S, Tiwari P, Ives JH, Hann V, Sieghart W, Lees G, Thompson CL. Aberrant GABAA receptor expression in the dentate gyrus of the epileptic mutant mouse Stargazer. J Neurosci. 2006;26:8600–8608. doi: 10.1523/JNEUROSCI.1088-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the δ subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–8639. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radonic A, Thulke S, Mackay IM, Landt O, Siegart W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun. 2004;313:856–862. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- Scimemi A, Semyanov A, Sperk G, Kullmann DM, Walker MC. Multiple and plastic receptors mediate tonic GABAA receptor currents in the hippocampus. J Neurosci. 2005;25:10016–10024. doi: 10.1523/JNEUROSCI.2520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PE, Green C. Spatial correlates of firing patterns of single cells in the subiculum of the freely moving rat. J Neurosci. 1994;14:2339–2356. doi: 10.1523/JNEUROSCI.14-04-02339.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases δ GABAA receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49:573–586. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABAA receptor subunits in the rat hippocampus I: immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- Spiegelman I, Li Z, Banerjee PK, Mihalek RM, Homanics GE, Olsen RW. Behavior and physiology of mice lacking the GABAA-receptor δ subunit. Epilepsia. 2002;43:3–8. doi: 10.1046/j.1528-1157.43.s.5.8.x. [DOI] [PubMed] [Google Scholar]
- Todd PK, Mack KJ, Malter JS. The fragile X mental retardation protein is required for type-I metabotropic glutamate receptor-dependent translation of PSD-95. Proc Natl Acad Sci. 2003;100:14374–14378. doi: 10.1073/pnas.2336265100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windpassinger C, Kroisel PM, Wagner K, Petek E. The human γ-aminobutyric acid A receptor delta (GABRD) gene: molecular characterisation and tissue-specific expression. Gene. 2002;292:25–31. doi: 10.1016/s0378-1119(02)00649-2. [DOI] [PubMed] [Google Scholar]
- Wozny C, Knopp A, Lehmann TN, Heinemann U, Behr J. The subiculum: a potential site of epileptogenesis in human temporal lobe epilepsy. Epilepsia. 2005;46:17–21. doi: 10.1111/j.1528-1167.2005.01066.x. [DOI] [PubMed] [Google Scholar]
- Zhang N, Wei W, Mody I, Houser CR. Altered localization of GABAA receptor subunits on dentate granule cell dendrites influences tonic and phasic inhibition in a mouse model of epilepsy. J Neurosci. 2007;27:7520–7531. doi: 10.1523/JNEUROSCI.1555-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M-G, Toyoda H, Ko SW, Ding H-K, Wu L-J, Zhuo M. Deficits in trace fear memory and long-term potentiation in a mouse model for fragile X syndrome. J Neurosci. 2005;25:7385–7392. doi: 10.1523/JNEUROSCI.1520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]