Summary
Purpose
Cytochrome P450 cholesterol side-chain cleavage enzyme (P450scc) catalyzes the initial step in the biosynthesis of neurosteroids within the brain. We sought to determine which cells express P450cc and whether neurosteroids play a role in the regulation of epileptogenesis following pilocarpine-induced status epilepticus (SE).
Methods
Rats experienced uninterrupted SE or SE terminated with diazepam at 60, 120, and 180 min. P450scc induction in CA3 hippocampus was determined by double immunolabeling with P450scc antiserum and monoclonal antibodies against GFAP (astrocytes), RIP (oligodendrocytes), or heme oxygenase-1 (microglia).
Results
SE was associated with P450scc induction in many astrocytes and a small number of microglia and oligodendrocytes in the hippocampal CA3 strata radiatum and lacunosum-moleculare. The extent of P450scc induction increased with increasing SE duration. Paradoxically, increased P450scc induction in rats experiencing SE for 180 min or more was associated with the delayed onset of spontaneous recurrent seizures. Treatment with the 5α-reductase inhibitor finasteride (100 mg/kg/day for 25 days), which inhibits the synthesis of γ-aminobutyric acid (GABA)A receptor modulating neurosteroids such as allopregnanolone, was associated with a significant reduction in time to the onset of spontaneous seizures in rats exposed to 180-min but not 90-min SE.
Discussion
P450scc is induced by SE in a diverse population of hippocampal glia. Induction of P450scc is associated with the delayed onset of spontaneous seizures. Conversely, inhibition of neurosteroid synthesis accelerated the onset of spontaneous seizures, but only in animals exhibiting significant increases in P450scc. These findings suggest that induction of neurosteroid synthesis in reactive glial cells is associated with delayed onset of spontaneously recurrent seizures.
Keywords: Neurosteroid, Epileptogenesis, Glia, Finasteride, Pilocarpine, Temporal lobe epilepsy
The initial rate-limiting step in the biosynthesis of steroid hormones is the conversion of cholesterol to pregnenolone, which is catalyzed by cytochrome P450 side-chain cleavage enzyme (P450scc) located in the inner mitochondrial membrane. By virtue of its role in regulating the production of precursor steroid hormones, P450scc also influences the biosynthesis of reduced steroid hormone metabolites such as allopregnanolone and allotetrahydr-oxycorticosterone, which act as positive allosteric modulators of γ-aminobutyric acid (GABA)A receptors (Lambert et al., 2003). GABAA-receptor modulating neurosteroids can be synthesized in the periphery and also in the nervous system by neurons and glial cells (Jung-Testas et al., 1989; Zwain & Yen, 1999; Mellon & Griffin, 2002). It is well recognized that these steroids possess anticonvulsant properties (Rogawski & Reddy, 2004), and we recently presented evidence that they may serve as endogenous regulators of epileptogenesis (Biagini et al., 2006).
Temporal lobe epilepsy with hippocampal sclerosis is the most common type of epilepsy in adults and is often characterized by the presence of an initial precipitating injury, which is then followed by recurrent seizures after a latent period of variable duration (Mathern et al., 2002). Rodents experiencing status epilepticus (SE) induced by convulsants such as pilocarpine and kainic acid or, alternatively, by repetitive stimulation of limbic structures, exhibit a disorder with similar clinical and neuropathologic features (Avoli et al., 2002). In these models, neuronal cell loss, in vulnerable brain regions such as the hippocampal CA3 area, is associated with activation of astrocytes and invasion of damaged tissue by microglial cells. In fact, intense activation of neurons as occurs during SE can induce dramatic astrocytic reactivity (Steward et al., 1991). Because neurosteroidogenic enzymes are present in astrocytes, the histopathologic changes that occur with epileptogenesis could influence neurosteroid synthesis and the availability of neurosteroids for modulation of GABAA-receptor function. In fact, in our recent study, we found that P450scc is induced in the hippocampal formation of rats following pilocarpine-induced SE (Biagini et al., 2006). This increase was detected mainly in presumptive glial cells and occurred during the latent period before the appearance of spontaneous recurrent seizures. In the present study, we extend these prior observations by showing that P450scc is induced in a diverse population of glial cells, including astrocytes, oligodendrocytes, and microglia. In addition, we have found that the extent of P450scc induction in these cells is dependent on SE duration. Finally, we confirm our previous observation that the latent period is reduced by neurosteroid synthesis inhibition with the 5α-reductase inhibitor finasteride, but only with SE of sufficient duration to induce P450scc. Taken together, our observations strengthen the view that GABAA-receptor modulating neurosteroids play a role in regulating epileptogenesis following SE.
Methods
Animals and treatments
Male Sprague–Dawley adult (8-week-old) rats (Harlan, S. Pietro al Natisone, Italy) were housed under controlled temperature (23 ± 1ºC), humidity (about 60%), and light–dark cycle (light from 7:00 to 19:00 hours). All experimental procedures were approved by the respective institutional animal care committees. Animals were treated with intraperitoneal (i.p.) pilocarpine (380 mg/kg) as described by Biagini et al. (2006). To prevent discomfort caused by peripheral muscarinic receptor stimulation, rats were pretreated with subcutaneous scopolamine methylnitrate (1 mg/kg) 30 min before the pilocarpine. Animal behavior was monitored after pilocarpine injection and scored according to Racine’s classification (Racine, 1972). Rats that did not reach a stage 5 response to pilocarpine (i.e., continuous tonic–clonic seizures) were discarded; a stage 5 response for 30 min was considered as SE. Seizures were quelled after 180, 120, 90, or 60 min with i.p. diazepam (20 mg/kg). A further group was instead exposed to uninterrupted SE. After recovering from SE, animals were video recorded 8 h/day, 7 days/week to identify the time of appearance and to estimate the frequency of spontaneous seizures. Only stage 5 (generalized tonic– clonic) seizures were scored. Control rats, injected i.p. with saline, did not develop any of these symptoms. Subgroups of animals exposed to 180 or 90 min SE were treated with finasteride [100 mg/kg, subcutaneous (s.c.) for 25 days during days 4–28 following SE] or vehicle (30% hydroxypropyl-β-cyclodextrin) as described previously (Biagini et al., 2006).
Immunohistochemistry
For tissue analysis, nonepileptic control and pilocarpine- treated epileptic rats were sacrificed 3 days after saline or pilocarpine treatment. Rats were anesthetized with chloral hydrate (450 mg/kg, i.p.) and perfused via the ascending aorta with 100 ml saline followed by 100 ml 4% paraformaldehyde and picric acid (0.3%) dissolved in 0.1 M phosphate buffer (pH 6.9). Brains were then postfixed overnight in the same fixative at 4ºC and, after cryoprotection by immersion in 15% and 30% sucrose–phosphate buffer solutions, were frozen and cut horizontally with a freezing microtome in serial 50 μm– thick sections. Sections were, respectively, incubated with several markers of neuronal and glial cells: a polyclonal anti-P450scc antibody (1:200; Chemicon, Temecula, CA, U.S.A.); a monoclonal antiglial fibrillary acidic protein (GFAP, 1:500, Sigma-Aldrich, Milan, Italy) antibody for astrocytes; a monoclonal antiheme oxygenase-1 (HO-1, 1:500, Stressgen, Victoria, BC, Canada) for the activated microglia; and a monoclonal anti- RIP (1:6,000, Chemicon) antibody for oligodendrocytes. Immunohistochemistry was made with the avidin–biotin complex (ABC) technique and diaminobenzidine as chromophore (Biagini et al., 2006). For double immunolabeling experiments, antibodies were incubated in two steps. First, we incubated sections with the respective monoclonal antibody (GFAP, HO-1, and RIP) and developed the reaction with horseradish peroxidase (Amersham, Milan, Italy) and diaminobenzidine (Biagini et al., 2006). Then, we incubated the stained sections with the polyclonal antibody against P450scc and developed the reaction with alkaline phosphatase (Amersham), using nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate as chromophores (Biagini et al., 1994). Stained sections were analyzed with KS300 (Carl Zeiss Vision GmbH, Munich, Germany) image analysis software as detailed previously (Biagini et al., 1994, 2006). Data from two different fields in each hippocampus of three sections were averaged. Background values were obtained from areas that did not contain any stained cells. Stained profiles were discriminated from background, and the field area (FA) corresponding to the region covered by specifically stained cell profiles was measured. Cell profile counts were determined in each field for profiles greater in diameter than a minimum cutoff value, taken to be 4 μm for glial cells.
Data analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences, version 8.0 (SPSS, Chicago, IL, U.S.A.). Results were analyzed with oneway analysis of variance (ANOVA) and the Tukey’s test for multiple comparisons. The Kaplan–Meier method was used to estimate the onset of stage 5 spontaneous seizures after SE. Curves were compared by the log rank test. Data are presented as mean ± standard error of the mean (SEM) and differences were considered statistically significant if p < 0.05.
Results
Colabeling with P450scc and glial markers in the CA3 region following pilocarpine SE
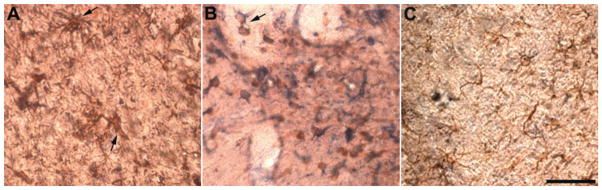
The P450scc antibody identified immunopositive neurons and glial cells in control rats, as shown previously (Biagini et al., 2006). Three days after uninterrupted SE, the number of glial cells immunopositive for P450scc was markedly increased (170.4 ± 19.3/mm2, p < 0.01, five rats) in strata radiatum and lacunosum-moleculare of the CA3 region of pilocarpine-treated animals, compared with control values (50.7 ± 11.4/mm2, five rats). P450scc-positive cells in the CA3 region were predominantly co-labeled by GFAP antibody, indicating that they were astrocytes (Fig. 1A). The astrocytes were hypertrophic with increased cell volume and highly ramified and enlarged cell processes (arrows in Fig. 1A). In addition to astrocytes, the population of P450scc-positive glial cells included a small number of HO-1–positive microglial cells, some of which were clearly located in blood vessel walls (arrow in Fig. 1B). Immunostaining with an antibody anti-RIP also identified the presence of P450sccpositive oligodendrocytes; however, these cells were more faintly stained by the P450scc antibody than astrocytes or microglial cells (Fig. 1C).
Figure 1.
Photomicrographs illustrating the colocalization of cytochrome P450 cholesterol side-chain cleavage enzyme (P450scc) with glial cell-specific markers in the CA3 region 3 days after pilocarpine-induced status epilepticus (SE) (>180 min continuous seizures). Glial cell–specific markers were revealed using horseradish peroxidase and diaminobenzidine as chromophore (appearing brownish). P450scc antibody immunoreactivity was revealed with alkaline phosphatase, using nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate as chromophores (appearing bluish). (A) Double-staining with GFAP monoclonal antibody, which specifically identified P450scc-positive astrocytes (arrows) and P450 polyclonal antibody. (B) A monoclonal antibody raised against heme oxygenase-1 identified P450scc-positive microglial cells (arrow). (C) P450scc-positive oligodendrocytes as visualized with an anti-RIP antibody. Note that astrocytes and microglial cells are more intensely stained with the anti-P450scc antibody than are oligodendrocytes. Scale bar, 50 μm.
Correlation between SE duration, P450scc induction, and latency to the onset of spontaneous recurrent seizures
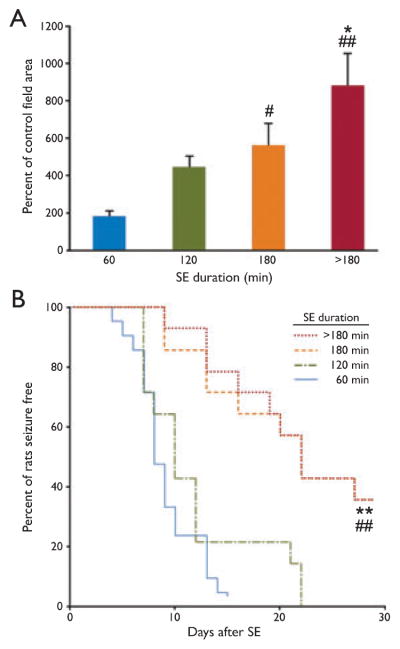
Next, we investigated the possible existence of a correlation between SE duration and P450scc induction. SE was aborted by administering diazepam after 60 (18 rats), 120 (16 rats), or 180 (12 rats) min from SE induction. As shown in Fig. 2A, there was a progressive increase in P450scc immunoreactivity in the strata radiatum and lacunosum-moleculare of the CA3 region with duration of SE. The increase was statistically significant compared with control in the 180 min and >180 min (9 rats) groups. Moreover, the increases in the 180 min and >180 min groups were also significantly different with respect to the 60 min group; the >180 min group was also significantly different from the 120 min group (p < 0.05) but not the 180 min group.
Figure 2.
(A) Relationship between the duration of status epilepticus (SE) and extent of increase in cytochrome P450 cholesterol side-chain cleavage enzyme (P450scc) immunoreactivity, expressed as percentage of control values. P450scc levels were evaluated 3 days after SE as the area of specific immunoreactivity (field area of specific profiles). In the >180 min group, animals did not receive treatment to abort the seizures; in the other groups, seizures were quelled with diazepam (20 mg/kg, i.p., 60, 120, or 180 min after the onset of SE). There is a progressive increase in P450scc induction with duration of SE, but a statistically significant (p < 0.01) difference from control values was obtained only in the 180-min and >180-min groups. #p < 0.05 vs. 60-min group; ##p < 0.01 vs. 60- min group; *p < 0.05 vs. 120-min group. (B) Kaplan- Meier analysis of the latency to the onset of the first spontaneous stage 5 seizure in pilocarpine-treated rats that had experienced SE of various durations. The onset was significantly delayed in animals experiencing SE for 180 and >180 min compared with 60 and 120 min. There were no significant differences between the 60- and 120-min groups or between the 180- and >180-min groups. ##p < 0.01 vs. 60-min group; **p < 0.01 vs. 120-min group. Epilepsia © ILAE
In further experiments, we compared the onset of spontaneous recurrent seizures in groups of rats experiencing different durations of SE as in Fig. 2A. As shown in Fig. 2B, the overall onset was earlier in the 60 min (21 rats) and 120 min (14 rats) groups compared with the 180 min (14 rats) and >180 min (14 rats) groups (p < 0.01). The 50% points in the Kaplan-Meier curves for the shorter duration groups were advanced by approximately 13 days.
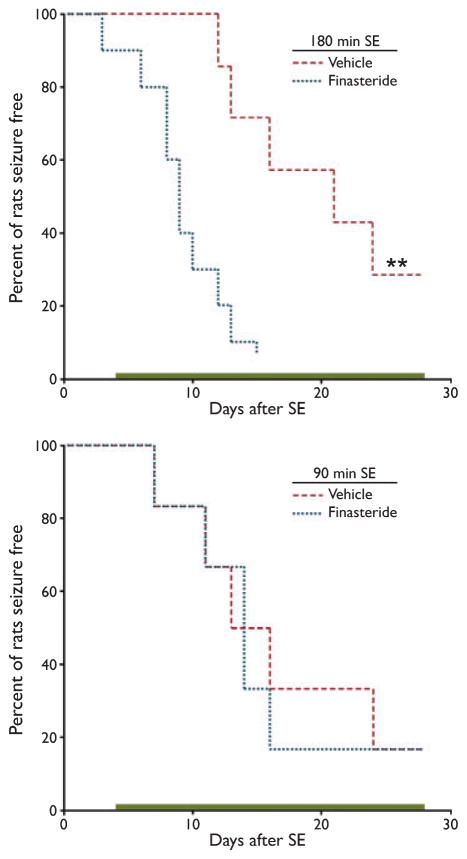
To examine the role of endogenous neurosteroids in regulating the rate of onset of spontaneous recurrent seizures, we determined the effect of finasteride treatment on groups of animals experiencing SE for a duration of 180 or 90 min. The animals were treated with daily s.c. injections of 100 mg/kg finasteride (100 mg/kg in 30% hydroxypropyl-β-cyclodextrin) or vehicle starting 3 days after SE and continuing for 25 days. The finasteride-treated 180- and 90 min groups consisted of 10 and 6 rats, respectively; the corresponding vehicle-treated groups consisted of 7 and 6 animals. In the 180-min SE groups, finasteride treatment accelerated the first occurrence of a stage 5 seizure (Fig. 3A). In contrast, there was no effect of finasteride treatment in the 90-min SE groups (Fig. 3B).
Figure 3.
Effects of treatment with finasteride during the latent period after status epilepticus (SE) on the onset of spontaneous recurrent seizures. Top, the time to the first spontaneous stage 5 seizure following 180-min SE is accelerated in rats treated with finasteride compared with those receiving vehicle (30% hydroxypropyl-β-cyclodextrin) (**p < 0.01, log rank test). Bottom, an experiment similar to that shown in the top panel, except that the duration of SE was 90 min. There is no significant difference between the finasteride and vehicle groups. Bars indicate 25-day period of finasteride administration. Epilepsia © ILAE
Discussion
It is well recognized that neurosteroidogenic enzymes are expressed in glia as well as neurons (Mellon & Griffin, 2002). P450scc, the enzyme responsible for the initial step in de novo neurosteroid synthesis is present in various brain regions, including the hippocampus, and is expressed in greater quantity in astrocytes and oligodendrocytes than in neurons (Mellon & Griffin, 2002). The CA3 hippocampal subfield is particularly vulnerable to damage after pilocarpine-induced SE, and there is associated glial cell reactivity (Liu et al., 1994; Belluardo et al., 1996). Consequently, we have been interested in the possibility that P450scc is induced following pilocarpine SE.
Previously, we found that P450scc immunoreactivity greatly increases in presumptive glial cells in the hippocampus of rats that had experienced SE (Biagini et al., 2006). In the present study we extended these observations by identifying P450scc immunopositivity in reactive astrocytes identified by a GFAP monoclonal antibody as well as in RIP-positive oligodendrocytes, although the extent of P450scc labeling was less than in astrocytes. In addition, we demonstrated strongly P450scc-positive microglial cells, identified by an antibody against HO-1. Therefore, P450scc is induced in a diverse population of glia following SE.
In our previous study, we showed that greater P450scc induction is associated with a more prolonged latency to develop stage-5 spontaneously recurrent seizures in rats exposed to 60-or 180-min SE (Biagini et al., 2006). Here, we have confirmed these results and demonstrate that SE of at least 180 min is required to obtain a significant difference in the length of the latent period. Therefore, rats that experienced 60- or 120-min SE had a similar time course for the development of spontaneously recurrent seizures. In accordance with our previous results, animals exposed to SE lasting 180 min or more had significantly greater P450scc immunoreactivity when compared with groups exposed to 60-min SE. Overall, the onset of spontaneous seizures was progressively delayed with increasing SE duration and P450scc immunoreactivity increased in a corresponding manner. Therefore, our present results confirm the relationship between P450scc induction and duration of the latent period suggested by our previous study.
We have hypothesized that the relationship between P450scc induction and the delay in the onset of stage 5 seizures is caused by the increased synthesis of GABAA receptor modulatory neurosteroids, such as allopregnanolone, which we propose can retard epileptogenesis. This hypothesis is supported by our results with the neurosteroid synthesis inhibitor finasteride. In our prior study and in the new experiments presented herein, treatment with finasteride significantly accelerated the onset of spontaneous seizures, but only in those animals that had experienced 180-min SE. The finding that the duration of the latent period was unaffected by finasteride in the 90-min SE group is intriguing, since it suggests that the effect of finasteride requires substantial induction of P450scc and enhanced neurosteroid synthesis. Alternatively, it may be that the short time window precludes observing an effect of neurosteroid synthesis inhibition. In any case, the results demonstrate that the acceleration in seizure onset observed with finasteride is not due to its nonspecific proconvulsant actions, and, indeed, we have failed to observe seizure induction in control animals (Biagini et al., 2006).
In conclusion, we have found that P450scc is induced in diverse glial cell types following pilocarpine-induced SE, and that the extent of this induction is related to the duration of SE. Moreover, the duration of the latent period is prolonged in association with increased cellular P450scc content. We have been able to link the alterations in P450scc to neurosteroid synthesis through the use of the synthesis-inhibitor finasteride. Our results provide additional support for the concept that neurosteroid synthesis is increased in reactive glial cells and that these neurosteroids modulate the epileptogenic process.
Acknowledgments
This work was supported by the Pierfranco and Maria Luisa Mariani Foundation (R-06-50), the Emilia-Romagna Region (PRIER 2007/09), the Canadian Institutes of Health Research (Grant MT-8109), and the Savoy Foundation.
Footnotes
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosure: The authors declare no conflicts of interest.
References
- Avoli M, D’Antuono M, Louvel J, Kçhling R, Biagini G, Pumain R, D’Arcangelo G, Tancredi V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system. Prog Neurobiol. 2002;68:167–207. doi: 10.1016/s0301-0082(02)00077-1. [DOI] [PubMed] [Google Scholar]
- Belluardo N, Mudo G, Jiang XH, Condorelli DF. Induction of astroglial gene expression by experimental seizures in the rat: spatio-temporal patterns of the early stages. Glia. 1996;16:174–186. doi: 10.1002/(SICI)1098-1136(199602)16:2<174::AID-GLIA9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Biagini G, Frasoldati A, Fuxe K, Agnati LF. The concept of astrocyte- kinetic drug in the treatment of neurodegenerative diseases: evidence for L-deprenyl-induced activation of reactive astrocytes. Neurochem Int. 1994;25:17–22. doi: 10.1016/0197-0186(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Biagini G, Baldelli E, Longo D, Pradelli L, Zini I, Rogawski MA, Avoli M. Endogenous neurosteroids modulate epileptogenesis in a model of temporal lobe epilepsy. Exp Neurol. 2006;201:519–524. doi: 10.1016/j.expneurol.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Jung-Testas I, Hu ZY, Baulieu EE, Robel P. Neurosteroids: biosynthesis of pregnenolone and progesterone in primary cultures of rat glial cells. Endocrinology. 1989;125:2083–2091. doi: 10.1210/endo-125-4-2083. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Prog Neurobiol. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Liu Z, Nagao T, Desjardins GC, Gloor P, Avoli M. Quantitative evaluation of neuronal loss in the dorsal hippocampus in rats with long-term pilocarpine seizures. Epilepsy Res. 1994;17:237–247. doi: 10.1016/0920-1211(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Adelson PD, Cahan LD, Leite JP. Hippocampal neuron damage in human epilepsy: Meyer’s hypothesis revisited. Prog Brain Res. 2002;135:237–251. doi: 10.1016/s0079-6123(02)35023-4. [DOI] [PubMed] [Google Scholar]
- Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–394. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Reddy DS. Neurosteroids: endogenous modulators of seizure susceptibility. In: Rho JM, Sankar R, Cavazos J, editors. Epilepsy: scientific foundations of clinical treatment. Marcel Dekker; New York: 2004. pp. 319–355. [Google Scholar]
- Steward O, Torre ER, Tomasulo R, Lothman E. Neuronal activity up-regulates astroglial gene expression. Proc Natl Acad Sci U S A. 1991;88:6819–6823. doi: 10.1073/pnas.88.15.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwain IH, Yen SS. Neurosteroidogenesis in astrocytes, oligoden-drocytes, and neurons of cerebral cortex of rat brain. Endocrinology. 1999;140:3843–3852. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]