Summary
Taste memories allow animals to modulate feeding behavior in accordance with past experience and avoid the consumption of potentially harmful food [1]. We have developed a single-fly taste memory assay to functionally interrogate the neural circuitry encoding taste memories [2]. Here, we screen a collection of Split-GAL4 lines that label small populations of neurons associated with the fly memory center - the mushroom bodies (MB) [3]. Genetic silencing of PPL1 dopamine neurons disrupts conditioned, but not naïve feeding behavior, suggesting these neurons are selectively involved in the conditioned taste response. We identify two PPL1 subpopulations that innervate the MB α lobe and are essential for aversive taste memory. Thermogenetic activation of these dopamine neurons during training induces memory, indicating these neurons are sufficient for the reinforcing properties of bitter tastant to the MBs. Silencing of either the intrinsic MB neurons, or the output neurons from the α lobe, disrupts taste conditioning. Thermogenetic manipulation of these output neurons alters naïve feeding response, suggesting that dopamine neurons modulate the threshold of response to appetitive tastants. Taken together, these findings detail a neural mechanism underlying the formation of taste memory and provide a functional model for dopamine-dependent plasticity in Drosophila.
Etoc Blub
Masek et al investigate the central brain circuitry that regulates taste memory by selectively manipulating small classes of dopamine neurons in the fruit fly. These experiments yield a neural circuit that sets the threshold of food acceptance, conferring taste memory.
Results and Discussion
Animals form robust taste memories in order to modify food choice in accordance with prior experience [1, 4]. In both flies and mammals, bitter-tasting quinine is a potent inducer of aversive taste memory [2, 5, 6]. Applying fructose solution to the tarsi (feet) of a starved, tethered fly induces the Proboscis Extension Reflex (PER), a robust feeding behavior [2, 7]. PER can be modified based on previous experience [8]. The repeated paired application of fructose to the tarsi and quinine to the proboscis results in a robust reduction of the PER response to the subsequent application of fructose alone [2]. Here, we investigate the neural circuitry regulating aversive taste memory in Drosophila.
While taste neurons localize to many areas of the body including the wings, ovipositor and internal mouthparts, the majority of taste sensillae reside on the tarsi and labellum [9, 10]. These sensillae contain the dendrites of gustatory neurons that express defined subsets of 68 gustatory receptors (Gr) [11, 12]. The gustatory system contains distinct functional classes of neurons that confer attraction or repulsion to tastants [13]. Sweet-sensing neurons express an array of sugar receptors, including Gr5a, Gr61a and a number of Gr64 receptors and are required for the response to all tested sugars, glycerol and fatty acids while bitter-sensing neurons are marked by expression of the bitter receptor Gr66a and respond to bitter substances [14–18]. Artificial activation of sugar-sensing neurons in the proboscis or tarsi triggers feeding response, while activation of bitter-sensing neurons in the proboscis triggers avoidance [2, 19, 20]. Primary taste neurons project to distinct regions of the subesophageal zone (SEZ) depending on the peripheral locations of the sensory dendrites as well as on the particular Gr they express [17, 21]. The SEZ then transmits taste signals to higher order brain structures that regulate feeding choice and conditioned taste memory [2, 22]. The mushroom body (MB) comprises a central brain neuropil consisting of ~2200 intrinsic and extrinsic neurons that are required for many types of fly memories, including gustatory memory [23–25]. The MBs are required for aversive taste memory, but the neural circuitry through which the MBs receive and transmit information during taste conditioning has not been identified [8, 22].
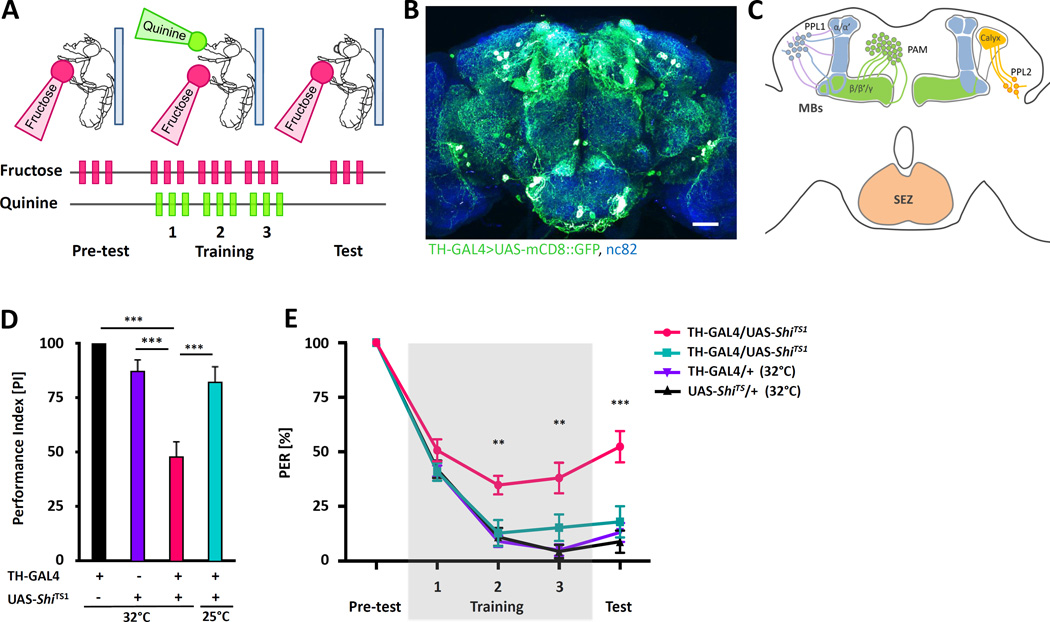
The neurotransmitter dopamine is implicated in learning and memory in insects and the fly brain contains ~300 dopamine neurons (DANs) that project to diverse brain regions, including the MBs. Dopamine regulates memory formation in a number of different modalities including visual memory and multiple forms of olfactory memory [3, 26–28]. We employed a single-fly aversive taste memory assay where flies form conditioned PER suppression following the simultaneous pairing of quinine and fructose (Figure 1A; [2, 8]). To examine the role of dopamine in aversive taste memory, we silenced the majority of DANs by expressing the temperature sensitive dominant-negative dynamin GTPase Shibire (ShiTS1) under control of tyrosine hydroxylase-GAL4 (TH-GAL4) and tested flies for memory ([29, 30]; Figure 1B and C). Silencing of TH-GAL4 labeled DANs including the PPL1, PPL2 and portion of PAM clusters of neurons that project to the MB for the entire period prior to training through testing impairs aversive taste memory, though memory is not completely abolished suggesting a dopamine-independent component or involvement of DANs that are not labeled by TH-GAL4 (Figure 1D). The reduction in PER suppression was significant after two training trials and persisted during testing in the absence of bitter quinine (Figure 1E). These findings suggest synaptic release from DANs is required for aversive taste memory.
Figure 1. Aversive taste conditioning is dependent on neurotransmission from dopamine neurons.
A) Schematic of aversive taste memory assay. A pretest of stimulation with 100mM fructose (pink) alone is followed by three training trial triplets, where fructose is immediately followed by the application of 10mM quinine (green) to the extended proboscis. Only fructose is provided during the test and PER is measured. B) A fly brain expressing CD8::GFP under control of TH-GAL4 (green) reveals broad ramifications of DANs throughout the central brain. Background staining is nc82 antibody (blue). Scale bar represents 20 µm. C) Dopamine clusters that are labeled by TH-GAL4 are innervating vertical lobes (PPL1) and horizontal lobes (PAM) of the MBs as well as to other brain neuropiles (modified from [36]). D) Average of performance index during testing reveals TH-GAL4>UAS-ShiTS1 (pink) flies do not suppress PER after pairing fructose with quinine to the levels of control flies (n=10–22). E) Flies expressing ShiTS1 in TH-GAL4 neurons (TH-GAL4>UAS-ShiTS1) show a significant reduction in PER suppression as early as the second training trial when tested at the non-permissive temperature of 32°C, but not at the permissive temperature of 25°C when TH-GAL4 neurons are active (n=10–22). All data represent the mean ± SEM, **p<0.01, *** p<0.001.
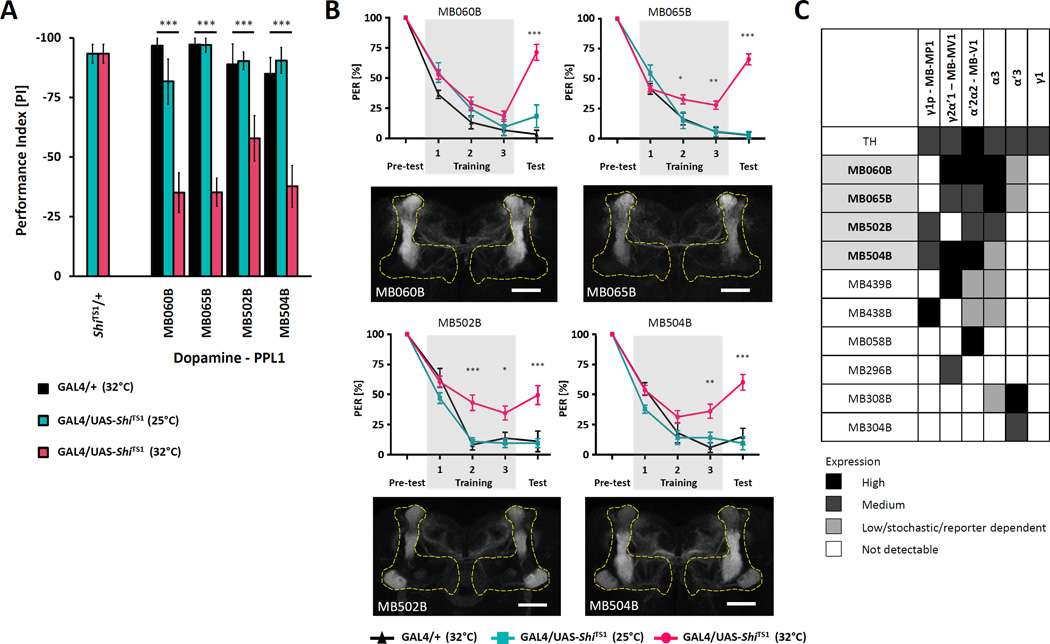
We screened a collection of 29 split-GAL4 lines that selectively and sparsely label MB-innervating DANs for aversive taste memory [3, 31]. Small populations of DANs were silenced by expressing ShiTS1 in neurons labeled by dopamine-specific split-GAL4 lines. Experimental flies harboring both split-GAL4 and UAS-ShiTS1 transgenes were compared to controls harboring UAS-ShiTS1 alone (Figure S1A, B). We found impaired memory in four lines that drive expression in subpopulations of PPL1 neurons and in two lines that label PAM neurons indicating that these two dopamine populations are required for aversive taste memory (Figure 2A and Figure S1B, C)
Figure 2. PPL1 neurons are required for aversive taste memory.
A) Mean PER suppression for PPL1 expressing lines during testing shows impaired PER suppression in flies expressing TRPA1 and tested at 32°C (pink) compared to controls harboring split-GAL4 constructs alone (black) or tested at 25°C (cyan). (n=10–56). B) Aversive taste memory was disrupted when four lines that express in the PPL1 cluster of DANs were silenced. MB060B (n=10–29), MB065B (n=12–56), MB502B (n=10–27), MB504B (n=10–36)(p<0.05). Lower panels: Innervation patterns of PPL1 neurons in MB lobes visualized with pJFRC225-5xUAS-IVS-myr:: smGFP-FLAG reporter in VK00005.. Scale bar represents 20µm. C) Specific expression of identified PPL1 DANs. Only the MB-V1 and MB-α3 clusters are present in all the identified lines. (The gray scale represents subjectively determined intensity of terminals in the MB). All data represent the mean ± SEM, * p<0.05, ** p<0.01, *** p<0.001
Monitoring of PER during the three training trials revealed that silencing of PPL1 subpopulations impairs PER suppression as early as the second training trial (MB065B and MB502B) or during the third training trial (MB060B and MB504B) and this phenotype persists during testing in the absence of quinine (Figure 2B). Twelve cells in the PPL1 cluster include six cell types of dopaminergic neurons that innervate the vertical and lateral horizontal portion of the MB lobes [3, 27, 32–34] named after the sites of innervation in the MB lobes: PPL1-α′2α2 (MB-V1), PPL1-α3, PPL1-α′3, PPL1-γ2α′1 (MB-MV1), PPL1-γ1pedc (MB-MP1) and PPL1-γ1. The lines MB060B, MB065B, MB502B and MB504B that were identified as hits in the screen label overlapping subset of these PPL1 neurons (Figure 2C). All identified lines show strong expression in the combination of PPL1-α′2α2 (MB-V1) and PPL1-α3, suggesting dopamine signaling to the vertical lobes of the MBs is critical for aversive taste memory (Figure 2B, C). Silencing only one of these dopaminergic neuron populations (MB058B) did not lead to disruption of the taste memory (Figure 2C and Figure S1A) suggesting that the collective action of these dopaminergic neurons may be required. Previous studies showed that PPL1-γ1pedc (MB-MP1) mediates the reinforcing property of electric shock and bitter taste for olfactory and visual learning [28, 35–37]. Interestingly, we found a strong impairment of taste memory by MB060B and MB065B, lines that broadly label PPL1 dopaminergic neurons but not the PPL1-γ1pedc (MB-MP1). PPL1-γ1pedc (MB-MP1) and PPL1-γ2 α′1 (MB-MV1) regions were shown to be involved in aversive olfactory memory [35] and these regions are labeled by lines MB438B and MB439B, which conversely did not show a taste memory impairment (Figure 2C and Figure S1A). Therefore, these findings indicate different PPL1 neurons regulate aversive taste memory and aversive olfactory memory. Whether the different subsets represents the distinction between two learned modalities (taste vs olfaction), the difference between the reinforcers (quinine vs electric shock) or between the behaviors (PER vs avoidance walking response) needs to be further investigated by varying the conditioning properties and testing protocol within each behavioral assay.
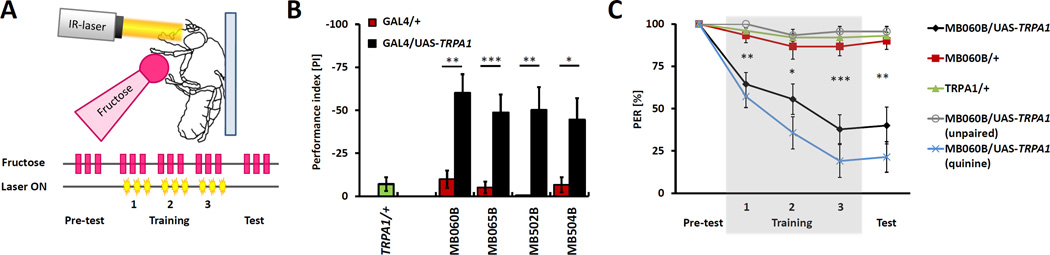
Activating PPL1 or PAM clusters of dopaminergic neurons can substitute for the unconditioned stimulus in the induction of associative olfactory or visual memory [28, 36–39]. PPL1 were shown to function in aversive memory, whereas PAM cluster primarily in reward memory [27, 33, 40]. We therefore asked if activation of the PPL1 neurons we identified is sufficient to provide the aversive conditioning cue in the absence of aversive quinine. Infrared-based activation of neurons expressing Drosophila Transient receptor potential 1 (TRPA1) has been shown to have precise temporal and spatial specificity [2, 41–43]. Tethered flies expressing the thermo-receptor TRPA1 under control of split-GAL4 lines labeling PPL1 neurons were trained by providing fructose to their tarsi while the PPL1 neurons were activated with an infrared laser. We targeted the laser beam to the head of the fly to specifically activate only neurons in the brain but not in the ventral nerve cord (Figure 3A). Significant PER suppression was observed in all four lines MB065B, MB060B, MB502B and MB504B (Figure 3B, Figure S2). The time course of PER suppression by direct activation of these PPL1 DANs during training was comparable to that obtained when using quinine paired with fructose (Figure 3C). There was no effect of infrared light targeting in flies harboring either the UAS-TRPA1 transgene or the split-GAL4 driver alone, indicating that the PER suppression observed following PPL1 activation is specific to TRPA1 activation in target neurons. Thus, activation of PPL1 dopaminergic neurons labeled by these lines is both necessary and sufficient for the formation of aversive taste memory (Figure 3B, C and Figure S2A–C). We also tested MB058B line expressing in subpopulation of identified DANs (Figure 2C) but did not achieved significant memory, suggesting that perhaps a larger subset of DANs is necessary for functional reinforcement in taste memory (Figure S2D). To confirm that formation of aversive taste memory is specific to these lines, we tested line MB438B that expresses in a dopamine cluster previously shown to be involved in formation of aversive olfactory memory [36]. Activation of these neurons together with fructose presentation did not elicit any PER suppression (Figure S2E).
Figure 3. Thermogenetic activation of PPL1 DANs substitutes for a bitter punishment.
A) Schematic of inducible activation of DANs. Infrared-laser stimulation (yellow) is substituted for quinine stimulation to activate DANs following the application of fructose (pink). B) Activation of the four lines labeling the PPL1 cluster (black) that are required for memory formation result in significant PER suppression. Flies harboring GAL4 transgenes (red) or UAS-TRPA1 (green) alone showed no reduction (MB502B) or not significant reduction (all other lines) compared to naïve response (n=10–15). C) Pairing laser stimulation with the presentation of fructose in flies expressing UAS-TRPA1 under the control of MB060B-GAL4 results in PER suppression during training and test (black). Pairing fructose with quinine (without laser stimulation) also leads to PER suppression to a similar level (blue). Unpaired presentation of laser and fructose (grey), where laser onset precedes fructose application, does not lead to PER suppression. Control flies harboring GAL4 transgenes (red) or UAS-TRPA1 (green) alone showed no significant suppression of PER (n=10–15). All data represent the mean ± SEM * p<0.05, ** p<0.01, *** p<0.001
The formation of memory requires simultaneous presentation of the conditional and unconditional stimuli, and the unpaired presentation of fructose and the activation of bitter-sensing neurons fails to induce memory [2]. Unpairing dopamine neuron activation and fructose by presenting fructose following infrared stimulation did not induce PER suppression, supporting the notion that the activation of PPL1 neurons serves as a predictive unconditional stimulus (Figure 3C). Therefore, the formation of aversive taste memory requires coincident thermogenetic activation of the PPL1-α′2α2 (MB-V1) and PPL1-α3 with fructose presentation.
To verify previous reports suggesting the MBs are required for aversive taste memory [8] we assayed lines broadly labeling the MBs as well as lines that selectively label the γ neurons, the α/β, or α′/β′ neurons [3]. Silencing of MB-specific lines reveals a significant defect in tested lines widely expressing in MB intrinsic neurons (MB010B and MB152B) as well as in more specific lines including MB009B and MB131B that exclusively label γ and MB418B that labels α′/β′ neurons suggesting multiple subtypes of neurons are required for memory formation (Figure S3).
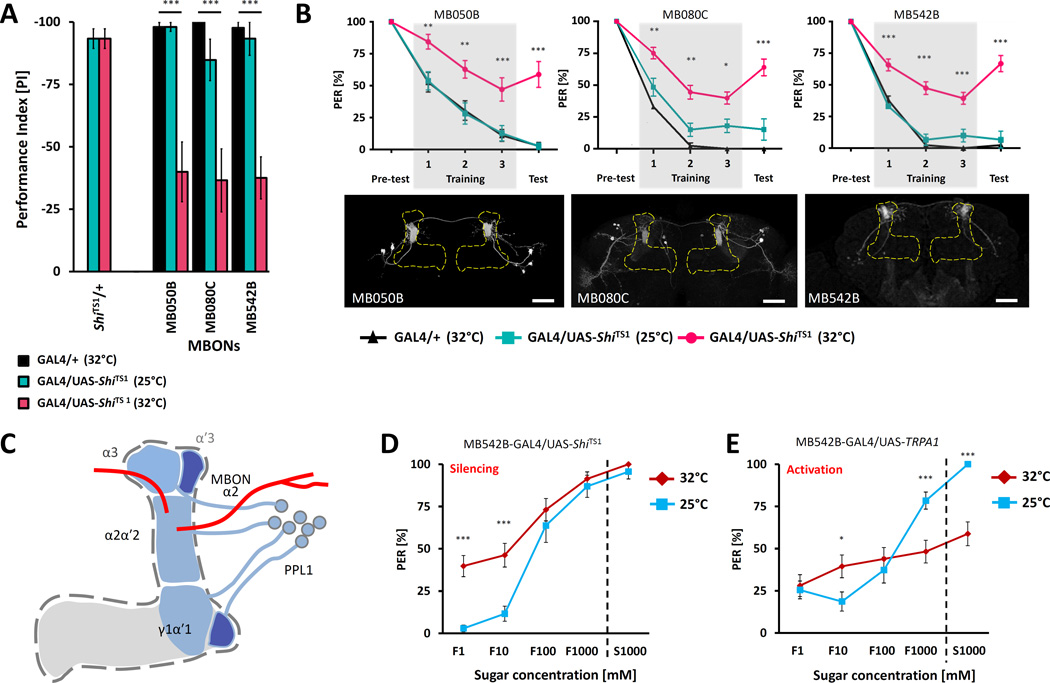
While the MBs are known to be required for taste memory, the neuronal targets and downstream output neurons regulating aversive taste memory have not been previously identified [8]. We tested 26 lines that selectively label mushroom body output neurons (MBONs) and found that memory was impaired when synaptic output was blocked for the duration of the memory assay in three lines expressing in MB outputs from vertical lobes (Figure 4A, B). The line MB080C labels MBON-α2sc (MB-V2α) that has dendrites located exclusively in the α2 compartment of the MB vertical lobes that is innervated by the identified PPL1-α′2α2 (MB-V1) neurons [44]. The other line, MB050B, labels MBON-α2sc (MB-V2α) and additional MBONs in the same cluster. Common expression of MBON-α2sc suggests an essential role for this MBON cell type in aversive taste memory. The third line (MB542B) labels MBON-α2p3p projecting from the α2 compartment but not in the MBON-α2sc subset. These findings support a model where DANs signal to the vertical MB lobes, resulting in altered MB output that is critical for the formation of aversive taste memory (Figure 4C).
Figure 4. The α2 MB output neurons modulate naïve sugar response.
A) Average PER suppression reveals impaired memory in flies expressing ShiTS1 in α2 MBONs at 32°C (pink) compared to controls harboring Split-GAL4 constructs alone (black) or tested at 25°C (cyan) (n=10–36). B) Three independent lines that label the α2 MBONs disrupt taste memory when used to express ShiTS1 and tested at non-permissive temperature of 32°C (red). MB050B (n=12–17), MB080C (n=11–36), MB542B (n=10–33). Lower panels: Innervation patterns of the identified MBONs lines visualized with pJFRC225-5xUAS-IVS-myr::smGFP-FLAG reporter in VK00005. Scale bar represents 20µm. C) Possible pathway regulating aversive taste conditioning. Identified DANs of PPL1 cluster projects regions of vertical lobes of MBs including α2 and α3. MBONs projecting from the α2 region of MBs to dorsal region of the lateral horn (MB080C and MB050B) or to superior medial protocerebrum (MB542B). D) Flies expressing ShiTS1 in the identified output neurons (MB542B) blocks activity of these neurons at a restrictive temperature of 32°C (red) and leads to increase preference to low sugar concentrations compared to measurement at permissive temperature of 25°C (blue)(n=23–31). E) Flies expressing TRPA1 in the same output neurons (MB542B) reduce their PER response to high concentrations of sugars upon activation of TRPA1 (red) at an elevated temperature of 32°C compared to control measured at a low temperature of 25°C (blue)(n=34–38). All data represent the mean ± SEM * p<0.05, ** p<0.01, *** p<0.001
Taste memory involves experience-dependent changes in the naïve response to tastants. The MBs are essential for conditioned changes in PER but dispensable for innate feeding behavior [8, 45, 46]. Our findings raise the possibility that the induction of taste memories involves modulation of synaptic signaling in the MB by the MB-V1/α3 population of PPL1 DANs that in turn alters the output of a subset of MBONs adjusting the naïve response to fructose. To test this hypothesis, we silenced or activated the identified MBONs with ShiTS1 or TRPA1 respectively, and measured the naïve response to a range of fructose concentrations and a high concentration of sucrose. The silencing of α2α3/α′3 MBONs labeled by the MB542B line through targeted expression of ShiTS1 increased PER in response to low concentration of fructose when tested at 32°C, but did not alter the response to high concentrations of sugars, suggesting these neurons suppress PER under innate conditions (Figure 4D). Conversely, flies expressing TRPA1 in the same populations of MBONs, responded normally to low concentrations of fructose but suppressed PER response to high concentrations of fructose and sucrose when tested at 32°C (Figure 4E). We observed similar changes in the lines MB050B and MB080C expressing ShiTS1 (Figure S4C, D). At the non-permissive temperature of 32°C, the response to 1mM–100mM fructose increased, but did not differ at 1M fructose. Conversely, expression of TRPA1 under control of MB080C reduced response to high concentrations of both sugars but not to low concentration of fructose (Figure S4C). Expressing TRPA1 in MB050B led to a significant reduction in PER to high and low concentration of sugars (Figure S4D) suggesting that activation of these neurons modulates the threshold for sugar response independent of their concentration. We did not observe any changes in PER when only UAS-TRPA1 or UAS- ShiTS1 was present (Figure S4B). Also, no effect on PER was observed after silencing or activating MB-V1/α3 dopamine lines that are required for aversive taste memory, suggesting that the modulation of naïve feeding response is downstream of MBs but not upstream (Figure S4E–G) or by modulation of identified DANs (Figure S4H–K). These findings provide evidence that the MBON-α2sc (MB-V2α) and MBON-α2p3p neurons modulate food acceptance in accordance with previous feeding experience. Therefore, we have identified dopaminergic inputs to the MBs and MBONs that appear to change the response threshold for taste neurons gating PER to mediate aversive taste conditioning responses.
Conclusions
Diverse dopaminergic neuronal populations are involved in distinct types of learning and memory including different behaviors and different modalities [27, 28, 32, 33, 35, 36, 40, 45, 47]. Therefore, it is possible that these DANs are activated by distinct sensory modalities, are specific for particular behaviors, and modulate distinct regions of the MBs. Thermogenetic activation of the PPL1 DANs to the MB vertical lobes is sufficient to induce memory formation in the absence of quinine reinforcement, supporting the notion that these DANs are activated by bitter-sensing neurons, and signal the aversive cue to the MBs. Activation of PPL1 neurons was previously shown to be sufficient to elicit aversive olfactory memory when activated during presentation of the conditioned stimulus [2, 35]. Further, a different subpopulation of PPL1 neurons, MB-MP1, is required for olfactory memories conditioned with aversive DEET provided as a tastant [37]. These findings raise the possibility that distinct subsets of PPL1 neurons are not only differentially involved in reinforcing different modalities, but also different aversive taste memories within the MBs and highlight the need for interrogating memory circuitry at the sub-cluster or single-neuron levels.
Taken together, we identify a neural circuit where the α2/α3 population of PPL1 DANs signal through the α neurons in the MBs, and likely confer conditioned changes in the α2 populations of MBONs that alter the naïve taste response resulting in an acquired, conditioned aversive response to appetitive tastants. Therefore, these findings establish the outlines of a central brain dopamine-modulated circuit that modulates conditioned taste aversion.
Experimental Procedures
Fly stocks and maintenance
Drosophila stocks were maintained on standard cornmeal/agar/molasses medium (Jazz mix, Fisher Scientific) at 25°C and 60% humidity in a LD incubator with 12:12 light/dark cycle. The split-GAL4 lines used in this study are described in [3]. Further information and line generation is available at http://splitgal4.janelia.org. For more detail see Supplemental Methods.
Gustatory taste memory
PER induction was performed in one-week old mated females as described previously [2, 17]. For more detail see Supplemental Methods.
Thermogenetic manipulation of neural function
Flies were prepared as described above for behavioral experiments [2]. For neural activation experiments each GAL4 line was crossed to UAS-TRPA1 or WT control (Canton-S). Flies were water-satiated and the laser beam was focused on head. The laser setup is described in [2] and the Supplemental Methods).
For experiments silencing neural subsets, split-GAL4 males were crossed to virgin females harboring a UAS-ShiTS1 transgene [30]. To inactivate neurons, the glass slide was placed on a heat block (AccuBlock™, Labnet International,INC.) at 32°C. Neurons were inactivated throughout the pretest, training and testing process.
Taste preference
Proboscis extension reflex response was used to measure feeding response to fructose (1–1000 mM), sucrose (1000 mM). The assay was performed as described before [16]. See Supplemental Methods for details.
Statistics
Each genotype represents >10 flies assayed. Statistical analyses were performed using InStat software (GraphPad Software Inc.). During single pretest, three training sessions and test, each fly was sampled three times with the same tastant in each session (total 15 stimulations per experiment) and their responses were pooled for values ranging from 0 to 3 per session. Group analysis using Multiple t-test and Sidak-Bonferroni Multiple Comparison Method for statistical significance was performed on the raw data from single flies for comparison of different genotypes and/or different experimental conditions (32°C vs 25°C). Wilcoxon signed rank test (non-parametric) with two-tailed P value was used to test memory score significance on single groups. In figures, graphs bars are mean values and error bars are standard error of the mean. The significance level of statistical tests was set to 0.05. P-values in figures are shown as * p<0.05, ** p<0.01, *** p<0.001.
Supplementary Material
Highlights.
-
-
A specific subset of PPL1 dopamine neurons is required for aversive taste memory.
-
-
Thermogenetic activation of these neurons during acquisition induces taste memory.
-
-
Taste memory is dependent on the V2 cluster of mushroom body output neurons.
-
-
The V2 mushroom body output neurons alter the threshold of feeding response.
Acknowledgments
We are grateful to H. Tanimoto (Tohoku University Graduate School of Life Sciences, Japan) and the mushroom body consortium at the Janelia Research Campus for feedback and guidance, and Flylight team at the Janelia Research Campus for anatomical data. This work was supported by grants from the National Institute of General Medical Sciences P20GM103440 and P20 GM103650 to A.C.K. and P.M., National Institute of Neurological Disorders and Stroke award R01NS08252 to A.C.K., an NSF EPSCoR award to K.W., and HHMI funding to Y.A. and G.M.R. Support also came from a Systems X Award to ACK from the Swiss NSF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gamiz F, Gallo M. Taste learning and memory: a window on the study of brain aging. Front Syst Neurosci. 2011;5:91. doi: 10.3389/fnsys.2011.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keene AC, Masek P. Optogenetic induction of aversive taste memory. Neuroscience. 2012;222:173–180. doi: 10.1016/j.neuroscience.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aso Y, H D, Yu Y, Johnston RM, Iyer N, Ngo TB, Dionne H, Abbott LF, Axel R, Tanimoto H, Rubin GM. The neuronal architecture of the mushroom body provides a logic for associative learning. eLIFE. 2014 doi: 10.7554/eLife.04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Law E, Nuttley WM, van der Kooy D. Contextual taste cues modulate olfactory learning in C. elegans by an occasion-setting mechanism. Curr Biol. 2004;14:1303–1308. doi: 10.1016/j.cub.2004.06.066. [DOI] [PubMed] [Google Scholar]
- 5.Perisse E, Portelli G, Le Goas S, Teste E, Le Bourg E. Further characterization of an aversive learning task in Drosophila melanogaster: intensity of the stimulus, relearning, and use of rutabaga mutants. Journal of comparative physiology. A, Neuroethology, sensory, neural, and behavioral physiology. 2007;193:1139–1149. doi: 10.1007/s00359-007-0266-2. [DOI] [PubMed] [Google Scholar]
- 6.Darling FM, Slotnick BM. Odor-cued taste avoidance: a simple and efficient method for assessing olfactory detection, discrimination and memory in the rat. Physiol Behav. 1994;55:817–822. doi: 10.1016/0031-9384(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 7.Dethier VG. The Hungry Fly. Cambridgee, MA: Harvard University Press; 1976. [Google Scholar]
- 8.Masek P, Scott K. Limited taste discrimination in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14833–14838. doi: 10.1073/pnas.1009318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vosshall LB, Stocker RF. Molecular architecture of smell and taste in Drosophila. Annu Rev Neurosci. 2007;30:505–533. doi: 10.1146/annurev.neuro.30.051606.094306. [DOI] [PubMed] [Google Scholar]
- 10.Isono K, Morita H. Molecular and cellular designs of insect taste receptor system. Front Cell Neurosci. 2010;4:20. doi: 10.3389/fncel.2010.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clyne PJ, Warr CG, Carlson JR. Candidate taste receptors in Drosophila. Science. 2000;287:1830–1834. doi: 10.1126/science.287.5459.1830. [DOI] [PubMed] [Google Scholar]
- 12.Scott K, Brady R, Jr, Cravchik A, Morozov P, Rzhetsky A, Zuker C, Axel R. A chemosensory gene family encoding candidate gustatory and olfactory receptors in Drosophila. Cell. 2001;104:661–673. doi: 10.1016/s0092-8674(01)00263-x. [DOI] [PubMed] [Google Scholar]
- 13.Montell C. A taste of the Drosophila gustatory receptors. Current opinion in neurobiology. 2009;19:345–353. doi: 10.1016/j.conb.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wisotsky Z, Medina A, Freeman E, Dahanukar A. Evolutionary differences in food preference rely on Gr64e, a receptor for glycerol. Nat Neurosci. 2011;14:1534–1541. doi: 10.1038/nn.2944. [DOI] [PubMed] [Google Scholar]
- 15.Jiao Y, Moon SJ, Wang X, Ren Q, Montell C. Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila. Curr Biol. 2008;18:1797–1801. doi: 10.1016/j.cub.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masek P, Keene AC. Drosophila fatty acid taste signals through the PLC pathway in sugar-sensing neurons. PLoS Genet. 2013;9:e1003710. doi: 10.1371/journal.pgen.1003710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Moon SJ, Köttgen M, Jiao Y, Xu H, Montell C. A taste receptor required for the caffeine response in vivo. Current biology : CB. 2006;16:1812–1817. doi: 10.1016/j.cub.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 19.Marella S, Fischler W, Kong P, Asgarian S, Rueckert E, Scott K. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki T, Ito K. Neural architecture of the primary gustatory center of Drosophila melanogaster visualized with GAL4 and LexA enhancer-trap systems. The Journal of comparative neurology. 2010;518:4147–4181. doi: 10.1002/cne.22433. [DOI] [PubMed] [Google Scholar]
- 21.Thorne N, Chromey C, Bray S, Amrein H, Building C, Carolina N. Taste Perception and Coding in Drosophila. Current. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Pool AH, Scott K. Feeding regulation in Drosophila. Curr Opin Neurobiol. 2014;29C:57–63. doi: 10.1016/j.conb.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heisenberg M. MUSHROOM BODY MEMOIR. Group. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 24.Davis RL. Traces of Drosophila memory. Neuron. 2011;70:8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waddell S. Dopamine reveals neural circuit mechanisms of fly memory. Trends Neurosci. 2010;33:457–464. doi: 10.1016/j.tins.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masek P, Keene AC. Dopamine: on the threshold of sleep. Curr Biol. 2012;22:R949–R951. doi: 10.1016/j.cub.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Claridge-Chang A, Roorda RD, Vrontou E, Sjulson L, Li H, Hirsh J, Miesenbock G. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogt K, Schnaitmann C, Dylla KV, Knapek S, Aso Y, Rubin GM, Tanimoto H. Shared mushroom body circuits underlie visual and olfactory memories in Drosophila. Elife. 2014;3:e02395. doi: 10.7554/eLife.02395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J Neurobiol. 2003;54:618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- 30.Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 31.Pfeiffer BD, Ngo TT, Hibbard KL, Murphy C, Jenett A, Truman JW, Rubin GM. Refinement of tools for targeted gene expression in Drosophila. Genetics. 2010;186:735–755. doi: 10.1534/genetics.110.119917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mao Z, Davis RL. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Frontiers in neural circuits. 2009;3:5. doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu C, Placais PY, Yamagata N, Pfeiffer BD, Aso Y, Friedrich AB, Siwanowicz I, Rubin GM, Preat T, Tanimoto H. A subset of dopamine neurons signals reward for odour memory in Drosophila. Nature. 2012;488:512–516. doi: 10.1038/nature11304. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka NK, Tanimoto H, Ito K. Neuronal assemblies of the Drosophila mushroom body. The Journal of comparative neurology. 2008;508:711–755. doi: 10.1002/cne.21692. [DOI] [PubMed] [Google Scholar]
- 35.Aso Y, Siwanowicz I, Bracker L, Ito K, Kitamoto T, Tanimoto H. Specific dopaminergic neurons for the formation of labile aversive memory. Curr Biol. 2010;20:1445–1451. doi: 10.1016/j.cub.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aso Y, Herb A, Ogueta M, Siwanowicz I, Templier T, Friedrich AB, Ito K, Scholz H, Tanimoto H. Three dopamine pathways induce aversive odor memories with different stability. PLoS Genet. 2012;8:e1002768. doi: 10.1371/journal.pgen.1002768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das G, Klappenbach M, Vrontou E, Perisse E, Clark CM, Burke CJ, Waddell S. Drosophila learn opposing components of a compound food stimulus. Curr Biol. 2014;24:1723–1730. doi: 10.1016/j.cub.2014.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Placais PY, Trannoy S, Friedrich AB, Tanimoto H, Preat T. Two pairs of mushroom body efferent neurons are required for appetitive long-term memory retrieval in Drosophila. Cell Rep. 2013;5:769–780. doi: 10.1016/j.celrep.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 39.Galili DS, Dylla KV, Ludke A, Friedrich AB, Yamagata N, Wong JY, Ho CH, Szyszka P, Tanimoto H. Converging circuits mediate temperature and shock aversive olfactory conditioning in Drosophila. Curr Biol. 2014;24:1712–1722. doi: 10.1016/j.cub.2014.06.062. [DOI] [PubMed] [Google Scholar]
- 40.Burke CJ, Huetteroth W, Owald D, Perisse E, Krashes MJ, Das G, Gohl D, Silies M, Certel S, Waddell S. Layered reward signalling through octopamine and dopamine in Drosophila. Nature. 2012;492:433–437. doi: 10.1038/nature11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flood TF, Iguchi S, Gorczyca M, White B, Ito K, Yoshihara M. A single pair of interneurons commands the Drosophila feeding motor program. Nature. 2013;499:83–87. doi: 10.1038/nature12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pulver SR, Pashkovski SL, Hornstein NJ, Garrity PA, Griffith LC. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. Journal of neurophysiology. 2009;101:3075–3088. doi: 10.1152/jn.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aso Y, Hattori D, Yu Y, Johnston RM, Iyer NA, Ngo TT, Dionne H, Abbott L, Axel R, Tanimoto H, et al. The neuronal architecture of the mushroom body provides a logic for associative learning. Elife. 2014;3 doi: 10.7554/eLife.04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwaerzel M, Monastirioti M, Scholz H, Friggi-Grelin F, Birman S, Heisenberg M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zars T, Fischer M, Schulz R, Heisenberg M. Localization of a short-term memory in Drosophila. Science. 2000;288:672–675. doi: 10.1126/science.288.5466.672. [DOI] [PubMed] [Google Scholar]
- 47.Riemensperger T, Völler T, Stock P, Buchner E, Fiala A. Punishment Prediction by Dopaminergic Neurons in Drosophila. Current. 2005;15:1953–1960. doi: 10.1016/j.cub.2005.09.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.