Abstract
Background
Many epithelial ovarian cancer (EOC) risk factors relate to hormone exposure, and elevated estrogen levels are associated with obesity in post-menopausal women. Therefore, we hypothesized that gene-environment interactions related to hormone-related risk factors could differ between obese and non-obese women.
Methods
We considered interactions between 11,441 single nucleotide polymorphisms (SNPs) within 80 candidate genes related to hormone biosynthesis & metabolism and insulin-like growth factors with six hormone-related factors: oral contraceptive use; parity; endometriosis; tubal ligation; hormone replacement therapy; and estrogen use; and assessed whether these interactions differed between obese and non-obese women. Interactions were assessed using logistic regression models and data from 14 case-control studies (6,247 cases; 10,379 controls). Histotype specific analyses were also completed.
Results
SNPs in the following candidate genes showed notable interaction: IGF1R (rs41497346, estrogen plus progesterone hormone therapy, histology = all, p = 4.9×10−6) and ESR1 (rs12661437, endometriosis, histology = all, p = 1.5×10−5). The most notable obesity - gene - hormone risk factor interaction was within INSR (rs113759408, parity, histology = endometrioid, p = 8.8×10−6).
Conclusions
We have demonstrated the feasibility of assessing multi-factor interactions in large genetic epidemiology studies. Follow-up studies are necessary to assess the robustness of our findings for ESR1, CYP11A1, IGF1R, CYP11B1, INSR, and IGFBP2. Future work is needed to develop powerful statistical methods able to detect these complex interactions.
Impact
Assessment of multifactor interaction is feasible, and, here, suggest that the relationship between genetic variants within candidate genes and hormone-related risk factors may vary EOC susceptibility.
Keywords: body mass index, obesity, hormone related factors, SNP, gene-environment interaction, ovarian cancer
INTRODUCTION
Little research has been conducted to determine multifactor gene-environment interaction at the candidate gene or genome-wide level despite the emerging evidence to show that these types of complex relationships do exist (1–3). In addition to the lack of studies assessing complex interactions in cancer risk, only a limited number of studies have assessed gene-environment (GE) interactions by histological subtype, as genetic and environmental risk factors have been found to differ by the histology. Recently, consortia have been established to give the large sample size needed to detect SNPs with small effects, providing the ability to study GE interactions. In April 2005, the Ovarian Cancer Association Consortium (OCAC) was formed; the largest international consortium conducting genetic epidemiology studies for epithelial ovarian cancer (EOC) (4). This international effort comprises more than 40 different genetic epidemiological studies, with the focus on assessing single SNP associations with EOC.
To date, OCAC has identified 18 confirmed novel susceptibility loci that are associated with EOC risk (5–12). In addition to finding new risk loci, GWAS also confirm the biological distinction of the various EOC histologies. For example, risk alleles in 8q24 and 19p13 associate almost exclusively with serous EOC (8, 13), yet those in 2q31 and 17q12 are also associated with other subtypes (8, 14). However, it is hypothesized that the known risk loci are likely to represent only a fraction of the common risk alleles for EOC and that numerous undetected common variant loci still remain to be discovered (15).
In addition to genetic susceptibility loci, there are several confirmed EOC environmental risk factors. Similar to other hormone-related cancers in women, many of these risk factors related to hormone exposure, including: obesity (risk) (16–19); history of endometriosis (risk) (20); estrogen use menopausal hormonal therapy (MHT) (risk) (21); estrogen plus progesterone MHT (risk) (21); oral contraceptive use (protective effect that increases with time of use) (22); parity (protective effect increases with number of live births) (23, 24); tubal ligation (protective) (25); and breast feeding (protective) (26, 27). Similar to genetic risk factors, environmental risk factors also differ by histology (28); for example, endometriosis is associated with risk of only clear cell, low-grade serous, and endometrioid EOC (20, 29). The vast majority of epidemiological studies of EOC risk have focused on marginal effects of genetic and environmental factors. A recent study by OCAC investigators assessed GE interactions across six known genetic risk loci (30). While this study looked at GE by histotype, this study did not investigate a three-way interaction involving obesity.
Obesity is associated with an increase in insulin levels, resulting in an increase in insulin-like growth factor 1 (IGF1) activity (31, 32). Increased levels of adiposity also lead to increased aromatase activity, and thus to an increase in estrogen levels (31, 33–35). After menopause, adipose tissue is the major source of estrogen in women. In breast cancer, evidence suggests that increased estrogen levels might underlie the association between BMI, breast cancer risk and MHT (31). It has been found that in post-menopausal women, the association between breast cancer and BMI is stronger in women who have never received MHT, compared with women who have used MHT (36). Similarly, a recent meta-analysis (2012) found that use of MHT attenuated the effect of BMI on EOC risk (17). A recent OCAC study found that high BMI was associated with increased risk of EOC in 15 case-control studies (16). In addition to finding an association between BMI and EOC risk, they found that this association was more pronounced in borderline serous, invasive endometrioid, and invasive mucinous histotypes. However, they found that MHT did not attenuate the effect of BMI on EOC risk when the analyses were restricted to post-menopausal women. Additionally, they also found no association of BMI with risk of ovarian cancer in the most common serous histotype (16). Based on these data, we hypothesize that GE effects could differ between obese and non-obese women.
Based on the complex relationship between hormone exposure, obesity, growth factors / insulin levels, and genetic factors we hypothesize that GE effects could be histology dependent and differ between obese and non-obese women. This hypothesis is illustrated in Supplemental Figure 1. In this candidate gene study, we sought to detect both two-way and multifactor obesity-gene-environment interactions for EOC risk. Overall, we assessed 11,441 SNPs located within 80 candidate genes related to hormone biosynthesis and metabolism in addition to those in insulin-like growth factors. The case-control analyses were run separately for case groups that involve: (1) all EOC invasive cases; (2) high-grade serous (HGS) invasive cases; and (3) endometrioid (ENDO) invasive cases. Candidate gene analyses specific to the less common histotypes were excluded due to the difficulty of assessing three-way interactions.
MATERIALS AND METHODS
Study Participants
Supplemental Tables 1 and 2 summarize the characteristics of the 14 OCAC studies used to assess GE interactions (37–49). The 14 studies included in this analysis were part of the Collaborative Oncological Gene Environment Consortium (COGS) study in which approximately 200,000 SNPs were genotyped in breast, ovarian and prostate cancers. Each OCAC study included in the analyses had to contribute at least 50 ovarian cancer cases and 50 controls, with controls further required to be sampled from the same population as the cases. Thus, 6,247 invasive cases and 10,379 controls of European descent were included in this analysis. GE interactions have been explored in these studies previously (28) and are described in further detail therein. Each study provided information on age at diagnosis or enrollment, BMI and other reproductive and lifestyle factors as well as information regarding tumor histology (serous, endometrioid, clear cell, mixed, other), tumor behavior (invasive or borderline), and tumor grade (well differentiated, moderately differentiated, poorly differentiated, undifferentiated). All patients provided informed consent, including for passive and active follow-up, using protocols approved by the appropriate Institutional Review Board. Table 1 describes the clinical features of EOC cases (6247 all EOC, 3019 HGS, 961 ENDO) and controls (N = 10379).
Table 1.
Clinical features in EOC cases & controls included in the GE and BMI-GE analyses. Sample sizes vary as not all studies collected data on each lifestyle and reproductive factor.
| Characteristics | Controls: N (%) | Cases: N (%) | P |
|---|---|---|---|
| Age (years) | <.0001 | ||
| Mean ± SD | 57.5 ± 11.6 | 58.3 (11.0) | |
| Age (categorical) | <.0001 | ||
| < 50 years | 2604 (25.1) | 1366 (21.9) | |
| 50 to 55 years | 1424 (13.7) | 946 (15.1) | |
| 55 to 60 years | 1691 (16.3) | 1071 (17.1) | |
| 60 to 65 years | 1629 (15.7) | 1015 (16.2) | |
| > 65 years | 3031 (29.2) | 1849 (29.6) | |
| Young Adult BMI (kg/m2) | <.0001 | ||
| Underweight/Normal (< 25) | 7607 (91.8) | 4427 (89.7) | |
| Overweight/Obese (> 25) | 679 (8.2) | 508 (10.3) | |
| Parity | <.0001 | ||
| (0 full births) | 1415 (14.7) | 1453 (25.1) | |
| (> 0 full births) | 8234 (85.3) | 4328 (74.9) | |
| Breast Feed | <.0001 | ||
| No | 2312 (30.3) | 1641 (39.9) | |
| Yes | 5320 (69.7) | 2467 (60.1) | |
| Oral contraceptive use | <.0001 | ||
| (<= 2 years) | 4895 (47.4) | 3487 (57.1) | |
| (> 2 years) | 5428 (52.6) | 2616 (42.9) | |
| Estrogen use | .44 | ||
| No | 3986 (78.9) | 2250 (78.1) | |
| Yes | 1068 (21.1) | 631 (21.9) | |
| EPP MHT Use | <.0001 | ||
| No | 3420 (67.7) | 2105 (73.3) | |
| Yes | 1631 (32.3) | 765 (26.7) | |
| Endometriosis | <.0001 | ||
| No | 8738 (93.9) | 4802 (90.0) | |
| Yes | 568 (6.1) | 533 (10.0) | |
| Tubal Ligation | <.0001 | ||
| No | 6924 (77.8) | 4692 (83.5) | |
| Yes | 1976 (22.2) | 926 (16.5) | |
| Tumor Grade | |||
| Well-Differentiated | 739 (12.1) | ||
| Moderately Differentiated | 1358 (22.2) | ||
| Poorly Differentiated | 2911 (47.6) | ||
| Undifferentiated | 459 (7.5) | ||
| Other | 647 (10.6) | ||
| Histotypes | |||
| Serous | 3589 (57.4) | ||
| Mucinous | 403 (6.5) | ||
| Endometrioid | 961 (15.4) | ||
| Clear Cell | 468 (7.5) | ||
| Others | 827 (13.2) | ||
Environmental and Genetic Risk Factors
Young Adult BMI
To quantify obesity we used BMI calculated in early adulthood (18–29 years of age) as opposed to BMI at diagnosis as early adulthood BMI would better approximate subjects obesity levels integrated over a lifetime (18, 50), and thus exposure to estrogen derived from adipose tissue. Measurement of weight in early adulthood was conducted in 9 of the 14 studies used for the GE analyses (16); and therefore the three-way BMI-GE interaction analyses were limited to these 9 studies. Five studies reported weight at age 18 (DOV, HAW, HOP, POL, UCI), two studies reported weight ‘in your 20s’ (MAL, USC), and two studies reported weight at age 20 (AUS, GER). The calculated BMIs were classified according World Health Organization (WHO) standards: (<18.5 ‘underweight’; 18.5–24.9 ‘normal weight’; 25–29.9 ‘overweight’; 30–34.9 ‘class I obesity’; 35–39.9 ‘class II obesity’; and ≥40 ‘class III obesity’) (51). From these WHO standards the subjects BMI were further categorized into two groups for GE analyses: (1) underweight or normal weight individuals with BMI less than 25 and (2) overweight or obese individuals BMI greater than 25.
Hormone-Related Environmental Factors
The GE analyses included seven hormone-related environmental factors: oral contraceptive use, parity, breast feeding, tubal ligation status, endometriosis, estrogen MHT, and estrogen plus progesterone MHT. To facilitate testing for multifactor interactions each environmental factor was dichotomized to ensure reasonable sample sizes in the various groups. Oral contraceptive use (years) was divided into (< 1 year; >= 1 year), parity (0 full births; >= 1 full birth), breast feeding was separated into (ever/never), estrogen MHT and estrogen plus progesterone MHT were categorized as (never/ever), while endometriosis and tubal ligation were included in terms of yes/no status.
Genetic Markers
We searched the literature to determine a set of candidate genes related to steroid biosynthesis, estrogen signaling and insulin-like growth factors (IGFs), as we hypothesize that genetic variants within these candidate genes modify EOC risk and that these effects are modified by hormone-related risk factors and obesity (52–54), and identified a list of 80 candidate genes (Supplemental Table 3). Using the National Center for Biotechnology Information (NCBI) website, SNPs were selected within 20 Kb of the first or last exon, as this was expected to sufficiently cover the promoter regions of most genes, as well as SNPs in LD with variation in the gene region (55). Due to power limitations for testing multifactor gene-environment interactions, SNPs were excluded from the analysis if the minor allele frequency (MAF) was less than 10%. This approach extracted 11,441 candidate gene SNPs. The candidate gene SNPs were imputed using the 1000 Genomes project (56), from an original set of > 200,000 genotyped SNPs from the COGS custom Illumina SNP array (57, 58). Details on the number of imputed SNPs for each candidate gene are included in (Supplemental Table 3).
Statistical Analysis
The study population was restricted to individuals of European descent based on LAMP analyses (59) with complete covariate information; and only invasive EOC cases were considered. For analyses involving the MHTs, either estrogen use or estrogen plus progesterone (EPP) use, the cases and controls were further restricted to post-menopausal women. For both the GE and BMI-GE (or GEE) analyses, the presence or absence of the environmental factors were coded as either 0 or 1. Separate analyses were conducted for case groups that included: (1) all invasive EOC cases, (2) HGS cases, and (3) ENDO cases. Analyses were adjusted for age of diagnosis (enrollment), study site and the first 5 principal component scores from a principal component analysis to adjust for population substructure. With the goal to determine gene-environmental effects and not general genetic association, assessment of significance was restricted to the higher level interaction effects (as opposed to “omnibus” tests for both genetic main and interaction effects (60)).
The following logistic regression model was used to assess gene-environment interaction for each SNP. For i = 1, …, n let
where Di represents that disease status (case =1, control = 0) for subject i, Gij represents the number of minor alleles observed for subject i for SNP j, Eik represents the absence or presence of environmental factor k for subject i, and Zi represents a vector of covariates for subject i to account for potential confounding, and each βGE represents a corresponding interaction regression coefficient. For each SNP j and environmental factor k we tested the null hypothesis of no GE interaction versus an alternative hypothesis that a GE interaction is present (i.e., null hypothsis: βGE = 0 vs. alternative hypothesis: βGE ≠ 0). The hypothesis was tested with the likelihood ratio test statistic .
Similarly, to test whether GE interactions could be modified by BMI we considered the following logistic regression model. For i = 1, … n let
where E1i represents the BMI status (low/high) at young adulthood of subject i, E2i represents the presence of absence of the second environmental factor for subject i, and Zi represents a vector of covariates for subject i that account for potential confounding, and each β represents a corresponding regression coefficient. To test whether GE interactions differ between non-obese and obese individuals we test the null hypothesis of no GEE interaction versus an alternative hypothesis of GEE interaction is present (i.e., null hypothesis βGEE = 0 versus alternative hypothesis: βGEE ≠ 0). This hypothesis was tested using a likelihood ratio test statistic .
RESULTS
Gene-Environment Interaction
In total, the GE analyses were run across 11,441 candidate gene SNPs, and included 91,528 GE combinations (11,441 SNPs × (7 Environmental Factors + BMI)), and these analyses were run across 3 separate case groups (All, HGS, ENDO). However, the imputed SNPs were in high linkage disequilibrium, and the analyses across case groups were also highly correlated. The SimpleM method was used to estimate the effective number of independent SNPs tested within each gene (61) (Supplemental Table 3); and in total the analyses were estimated to involve independent 2336 SNPs. Using the estimated effective number of independent tests, the Bonferroni corrections for the number of total candidate gene SNPs was 0.05/2,336 = 2.1 × 10−5, while adjusting for the total number of independent GE combinations gives 0.05/(2,336 × 8) = 2.7 × 10−6 respectively. Several SNP-environment interactions were significant using the former threshold, however using the latter strict threshold, no significant GE was detected. SNPs with GE interaction p < 10−4 are presented in Table 2.
Table 2.
Association with p < 10−4 for GE and BMI-GE analyses. Results highlighted in green or red in Figures 1 and 3. More detailed summaries of these top hits are shown in Supplemental Tables 4 and 5.
| Analysis | Histology | Environment | Gene | SNP | MAF | N cases | N controls | Interaction | P-value |
|---|---|---|---|---|---|---|---|---|---|
| GE | All | EPP MHT | IGF1R | rs41497346 | 0.28 | 2870 | 5051 | −0.577 | 4.92 × 10−6 |
| GE | All | Endometriosis | ESR1 | rs12661437 | 0.34 | 5335 | 9306 | 0.534 | 1.47 × 10−5 |
| GE | ENDO | Estrogen MHT | HSD17B2 | rs2955162 | 0.23 | 405 | 5054 | −1.12 | 3.44 × 10−5 |
| GE | HGS | Endometriosis | CYP11A1 | rs9944175 | 0.24 | 2578 | 9306 | −0.872 | 4.13 × 10−5 |
| GE | ENDO | Estrogen MHT | AKR1C3 | rs61856140 | 0.10 | 405 | 5054 | −1.47 | 5.30 × 10−5 |
| GE | ENDO | Endometriosis | CYP11B2 | rs28526467 | 0.43 | 815 | 9306 | −1.09 | 6.83 × 10−5 |
| GE | ENDO | OC Use | PRL | rs72836169 | 0.10 | 945 | 10323 | −0.827 | 7.09 × 10−5 |
| BMI-GE | ENDO | Parity | INSR | rs8102954 | 0.37 | 778 | 8284 | −2.56 | 8.35 × 10−6 |
| BMI-GE | All | Parity | IGFBP2 | rs869564 | 0.11 | 4934 | 8284 | −2.34 | 1.43 × 10−5 |
| BMI-GE | HGS | OC Use | CYP11B1 | rs113759408 | 0.17 | 2296 | 8250 | 1.49 | 2.18 × 10−5 |
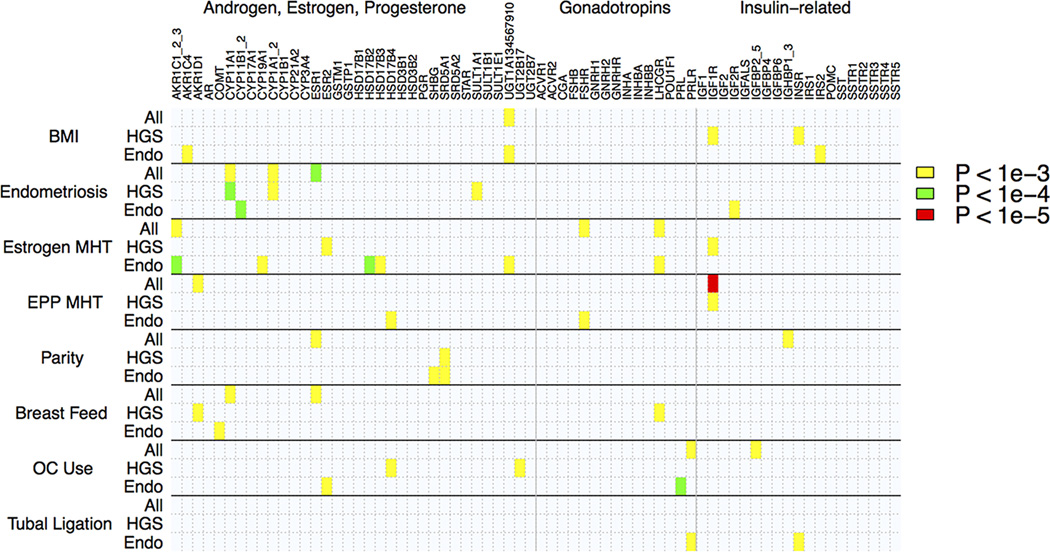
Figure 1 provides an image map that highlights interaction tests of environmental factors and candidate genes with at least one SNP p-value less than pre-defined significance thresholds: p = 10−3, p = 10−4, and p = 10−5. Within this plot, the candidate genes are grouped alphabetically according to their involvement in the production of hormones hypothesized to influence EOC risk (62) (Androgen, Estrogen, Progesterone, Gonadotropins, Insulin-related). A full list of SNPs with minimum p-values (p < 10−3) in candidate genes for the GE interaction analyses are presented in Supplemental Table 4.
Figure 1.
Image map of top p-values for GE interactions results for 80 candidate gene SNPs and 7 hormone related environmental factors as well as BMI.
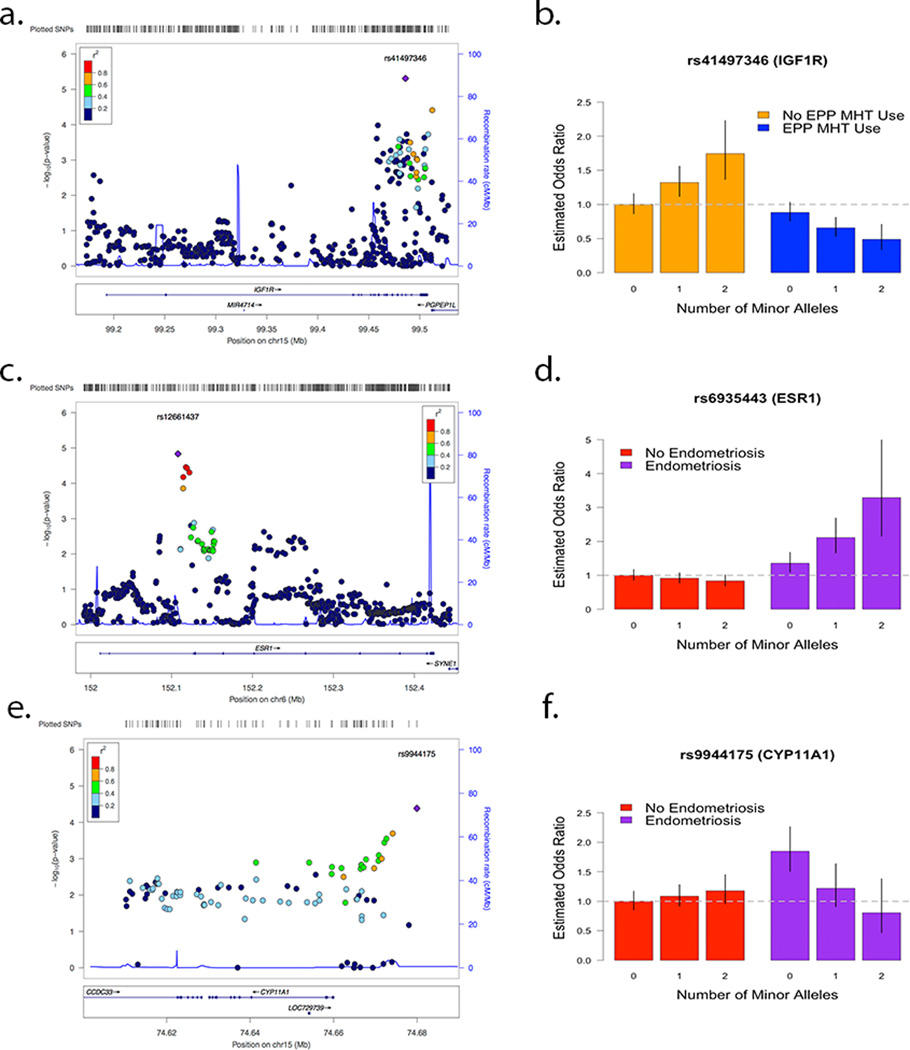
The most statistically significant GE-interaction was IGF1R (rs41497346, estrogen plus progesterone (EPP) MHT, histology = all, OR = .56, p = 4.9×10−6) (Figures 2a, 2b). The marginal odds ratio estimate of rs41497346 was .96 (p = .12). However, within non - EPP MHT users the presence of a minor allele increased risk for EOC (OR = 1.29); while within EPP MHT users rs41497346 provided a protective effect (OR = 0.72). The rs41497346 – EPP MHT interaction estimates were qualitatively similar across each histology included in our candidate gene analyses: HGS (OR = .55, p = 1.7×10−4), and ENDO (OR = .77, p = .38). The next most significant GE interaction result included ESR1 (rs12661437, endometriosis, histology = all, OR = 1.71, p = 1.5 × 10−5) (Figures 2c and 2d), where the minor allele decreased EOC risk in patients with no endometriosis and increased risk in patients with endometriosis. The marginal odds ratio estimate of rs12661437 was .95 (p = .17). However, within women with no endometriosis history, the presence of a rs12661437 minor allele decreased risk for EOC (OR = .92); while within women with a history of endometriosis, the rs12661437 minor allele provided increased risk (OR = 1.59). Subtype specific analyses for rs12661437 also found qualitatively similar effect sizes across all histologies (Supplemental Table 4). Rs12661437 lies in an intron near the 5’ end of ESR1.
Figure 2.
Locus zoom plots and estimated GE interaction effects of top results for IGF1R-Combination use (a,b), ESR1-Endometriosis (c,d), and CYP11A1-Endometriosis (e,f). The vertical black lines represent 95% confidence intervals for estimated odds ratios.
When restricting the cases to HGS, the most notable interaction was for CYP11A1 (rs9944175, endometriosis, histology = HGS, OR = .42, p = 4.1 × 10−5) (Figures 2e and 2f). The marginal odds ratio estimate for HGS EOC risk of rs9944175 was 1.06 (p = .26). However, for women with no history of endometriosis, the estimated effect of one rs9944175 minor allele increased HGS EOC risk (OR = 1.1) but decreased HGS EOC risk in women with a history of endometriosis (OR = .47). This SNP showed no statistically significant interaction for the ENDO histology (OR = .69, p = .18). rs9944175 lies within 20Kb of the 3’ end of CYP11A1.
Multifactor or BMI-Gene-Environment Interactions
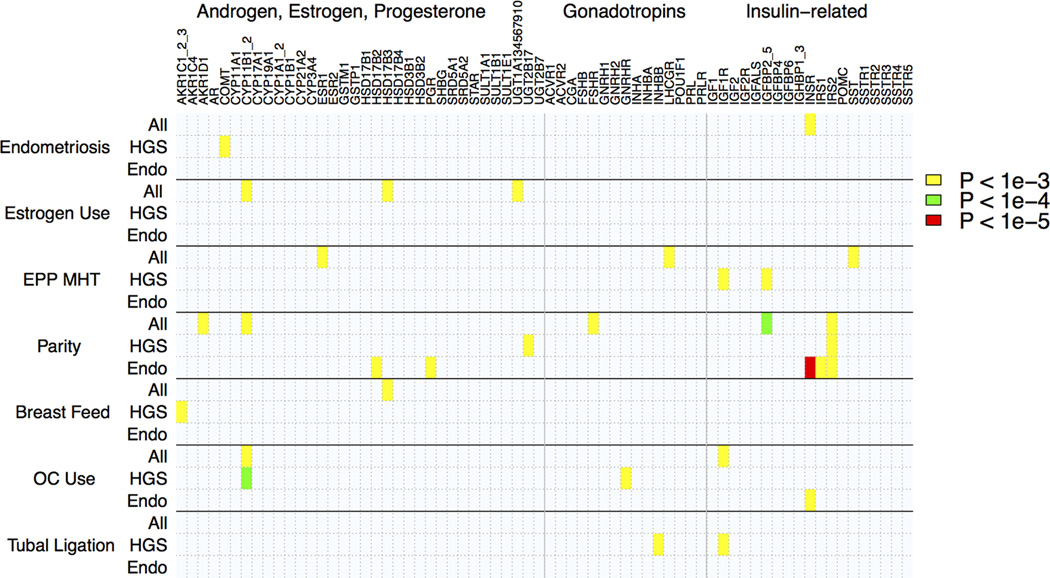
For each gene, SNPs with notable BMI-GE interaction results (p < 10−3) and their estimated interaction effects are presented (Supplemental Table 5). Figure 3 provides an image map that highlights 3-way interaction tests of obesity, lifestyle and reproductive factors, and candidate genes with at least on SNP p-value less than: p = 10−3, p = 10−4, and p = 10−5. This image map groups the candidate genes alphabetically and according to their involvement in the production of hormones hypothesized to influence EOC risk (62) (Androgen, Estrogen, Progesterone, Gonadotropins, Insulin-related). No statistically significant SNPs were detected after Bonferroni correction for the effective number of candidate gene SNPs (p < 2.1 × 10−5). A stricter threshold that adjusts for effective number of candidate gene SNPs by 7 environmental factors in the BMI-GE analyses was p < 3.1 × 10−6.
Figure 3.
Image map of smallest p-values for multi-factor BMI-GE interactions results for Candidate Gene SNPs and 7 non-obesity related environmental factors.
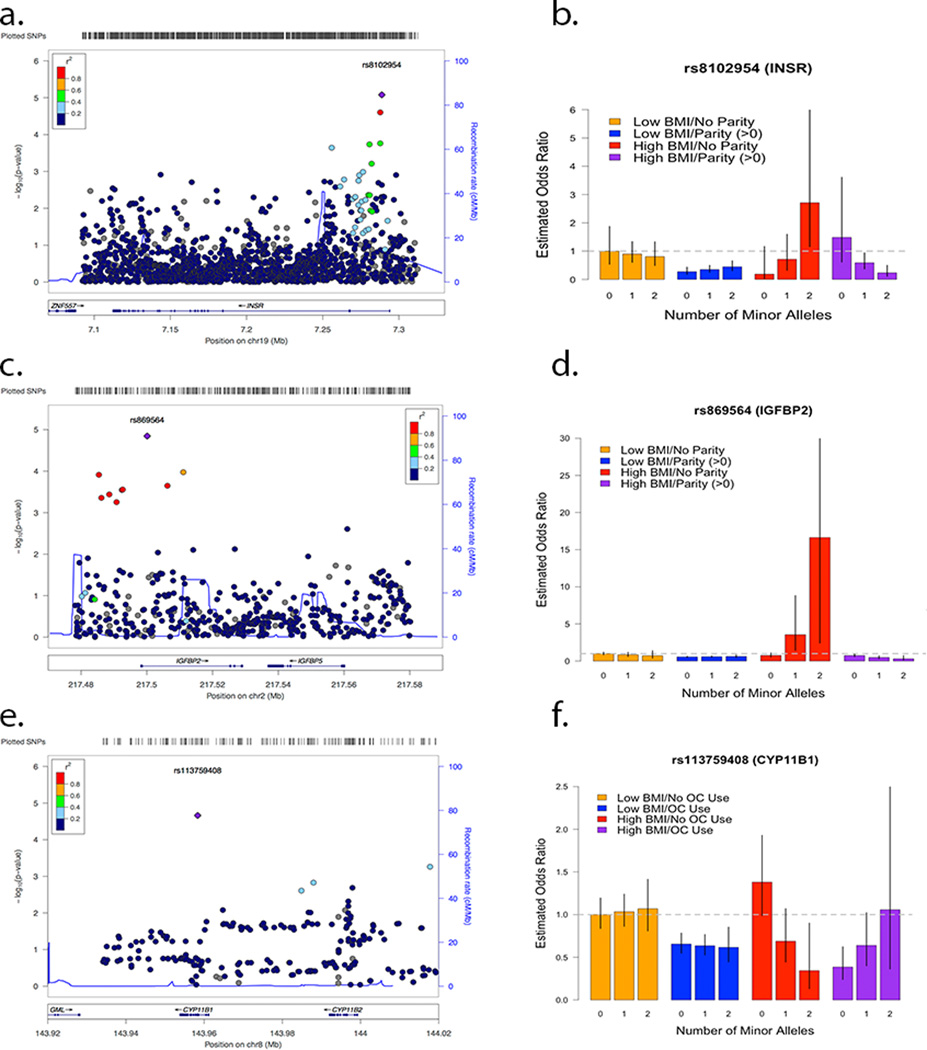
The most statistically significant SNP for the BMI-GE analyses lies in INSR (rs8102954, parity, histology = ENDO, BMI-GE OR = .074, p = 8.83 × 10−6) (Figures 4a and 4b). Within the low BMI women group the estimated SNP – Parity interaction of one rs8102954 minor allele for the ENDO cases was negligible (OR GElow BMI = 1.4, p = .15); while within high BMI women the estimated GE effect is (OR GEhigh BMI = .10, p = .0021). The BMI – GE interaction effect was not significant for analyses with case groups that included all histology and high-grade serous cases. rs8102954 lies in a exonic region near the 3’ end on INSR.
Figure 4.
Locus zoom plots and estimated BMI-GE interaction effects of top results for INSR-Parity-BMI (Histology ENDO) (a,b), IGFBP2-Parity-BMI (Histology All) (c,d), and CYP11B1-OC Use-BMI (Histology HGS) (e,f). The vertical black lines represent 95% confidence intervals for estimated odds ratios.
For case-controls analyses including all histologies, the most notable BMI-GE interaction was IGFBP2 (rs869564, parity, histology = All, BMI – GE OR = .096, p = 1.43 × 10−5) (Figures 4c and 4d). For low BMI women the estimated SNP – parity interaction effect of one rs869564 minor allele was negligible (OR GElow BMI, p = .48); however within high-BMI women the estimated GE interaction effect was (OR GEhigh BMI = .11, p = 4.14 × 10−5). The three-way BMI-GE interaction effect was significant for the HGS cases (BMI – GE OR = .077, p = 1.23 × 10−3), but not the analyses involving the ENDO cases (BMI – GE OR = p = .18). rs869564 resides in an exonic region on the 3’ end of IGFBP2.
For HGS cases, the most statistically significant SNP for the BMI-GE analyses lies in CYP11B1 (rs113759408, oral contraceptive use, histology = HGS, BMI-GE OR = .072, p = 2.2 × 10−5) (Figures 4e and 4f). Within the low BMI women group the estimated SNP-OC use interaction effect of one rs113759408 minor allele for HGS cases was negligible (OR GElow BMI= −.90, p = .41); while within high BMI women the estimated GE effect is large (OR GEhigh BMI= 4.52, p = .0028). The BMI-GE interaction effect was not statistically significant for the ENDO histology (BMI-GE OR= 2.11, p=.24). rs113759408 lies in an intronic region in the middle of CYP11B1.
DISCUSSION
In this paper, we investigated both gene-environment and multifactor obesity-gene environment interactions in epithelial ovarian cancer (EOC) risk. We used 14 case-control studies within the Collaborative Oncological Gene Environment Consortium (COGS) and Ovarian Cancer Association Consortium (OCAC) that provided more than 6,000 cases and 10,000 controls. Our main hypothesis was that some EOC risk due to SNPs could be explained by interactions with environmental factors. Similar to breast and endometrial cancers, many EOC risk factors relate to hormone exposure, and increased levels of estrogen has been associated with obesity in post-menopausal women. Therefore, we hypothesized that gene-environment interactions dealing with hormone-related risk factors could differ between obese and non-obese women. None of the tests of gene-environment interaction and multi-factor obesity-gene-environment interaction were significant at genome-wide level (p = 5×10−8).
The most statistically significant gene-environment interaction result was IGF1R (rs41497346, estrogen plus progesterone MHT, Histology = All, OR = 0.56, p = 4.9 × 10−6). Rs41497346 lies in an intronic region near the 3’ end of IGF1R, and is in the same linkage disequilibrium block as several SNPs hypothesized to have marginal risk in breast cancer (63). High expression levels of IGF1R were reported by Tang et al (64) in tumor tissue samples from 25 of 36 patients with epithelial ovarian cancer. Estrogen use is associated with increased IGF1R expression, while progesterone was associated with decreased IGF1R expression in breast cancer cells (65). Variation within the gene ESR1 was also found to be involved in an interaction involving endometriosis in analyses of all histologies (rs12661437, intronic SNP near 5’ end of gene, p = 1.5 × 10−5), where the minor allele decreased EOC risk in patients with no endometriosis and increased risk in patients with endometriosis. Subtype specific analyses for rs12661437 also found qualitatively similar effect sizes across all histologies. Variation near ESR1 (rs2295190) has been reported to be associated with EOC risk (66); however the SNPs are in low LD (r2 = 0.001).
For the BMI-GE interaction analyses, the most statistically significant results were INSR (rs8102954, parity, histology = ENDO, BMI-GE OR = 0.074, p = 8.83 × 10−6) (Figures 4a and 4b) and IGFBP2 (rs869564, parity, histology = All, BMI – GE OR = 0.096, p = 1.43 × 10−5) (Figures 4c and 4d). No genetic polymorphisms within INSR and IGFBP2 have been associated previously with ovarian cancer risk. Nevertheless, considerable research exists on the role of insulin receptors and cancer as studies have shown that insulin receptors may be involved in the regulation of ovarian cancer cell growth (67) and that increased levels of insulin have been associated with breast and endometrium cancers for which these tumorigenic properties can be modified by insulin receptors (31). Similarly, the role of insulin-like growth factors (IGFs) have been extensive studied for their role in carcinogenesis (68). Specifically, IGFBP2 has been linked ovarian cancer by promoting cancer cell invasion (69), while common variants in IGF1, IGFBP1 and IGFBP3 have been associated with ovarian (70) and endometrial cancers (71). IGFBP2 has also been linked to other hormone-related cancers (72–74).
For the high-grade serous cases, the most statistically significant SNP for the BMI-GE analyses lies in CYP11B1 (rs113759408, oral contraceptive use, Histology = HGS, BMI-GE estimate = 1.49, p = 2.2 × 10−5) (Figures 4e and 4f). Polymorphism rs113759408 lies in an intronic region in the middle of CYP11B1 (between exons 3 and 4), the gene that encodes for steroid 11beta-hydroxylase. Mutations in this gene cause congenital adrenal hyperplasia (OMIM #202010). No research has been published showing a link between EOC risk and variants within this gene. However, genetic variation in CYP11B1 has been reported to be associated with breast cancer risk from a prediction model involving SNP rs4541 in exon 7 of CYP11B1 (75) and the association with serum hormone levels in breast cancer patients (76).
We chose to restrict our analyses to SNPs located within 80 candidate gene and 8 established ovarian cancer reproductive or lifestyle factors. An earlier study investigated 2-way interactions between 6 established SNP risk loci and 5 established environmental risk factors (30). Similar to our study results, their 2-way interaction analyses were not strong enough to rule out the role of chance. While these initial findings suggest that gene-environment interactions play a modest role in EOC risk, genome-wide studies are necessary to fully examine the potential interplay between SNPs and environmental factors.
For the obesity-gene-environment analyses, a strength of this study was the use of young adult BMI (low, high) as opposed to BMI at diagnosis, since young adult BMI may serve as an indicator of obesity integrated over a life-time and adipose-based estrogen exposure (18, 50). While a biological rationale exists for higher-order interactions, very little literature has focused on multi-factor interactions, perhaps due to the challenge of necessary power to detect these higher order interactions. Therefore, a limitation of the multi-factor gene-environment interaction analyses were modest sample sizes: especially for less well documented environmental factors and histology specific analyses (Supplemental Table 7).
In conclusion, we have demonstrated the feasibility of assessing multi-factor interactions in large genetic epidemiology studies. Future work is needed to develop powerful statistical methods able to detect these complex interactions, as they may provide additional information regarding the genetic etiology of ovarian and other hormone – related cancers. Follow-up studies are necessary to assess the robustness of our notable findings in ESR1, CYP11A1, IGF1R, CYP11B1, INSR, and IGFBP2. To further follow-up our investigation of multi-factor gene-environment interactions, we will explore other potential modifiers of gene-environment risk, such as BRCA mutation status, and assess BMI-GE in other hormone-related cancers, such as breast, prostate and endometrial.
Supplementary Material
Acknowledgments
Financial Support:
The Ovarian Cancer Association Consortium is supported by a grant from the Ovarian Cancer Research Fund (PPD/RPCI.07). The COGS project was funded through a European Commission's Seventh Framework Programme grant (agreement number 223175 - HEALTH-F2-2009-223175). This work was supported in part by the National Institutes of Health [P30 CA168524 (B. Fridley), R01 CA112523 (M. Rossing), R01 CA87538 (M. Rossing), R01 CA58598 (M. Goodman), R01 CA61107 (A. Jensen, S. Kjaer), N01 CN55424 (M. Goodman), N01 PC 67001 (M. Goodman), R01 CA122443 (E. Goode), P30 CA15083 (E. Goode), P50 CA136393 (E. Goode), R01 CA76016 (J. Schildkraut), U01 CA71966 (A. Whittemore), R01 CA16056 (K. Moysich), K07 CA143047 (W. Sieh), U01 CA69417 (W. Sieh), R01 CA058860 (H. Anton-Culver), P30 CA14089 (C. Pearce, S. Ramus), R01 CA61132 (M. Pike), N01 PC67010 (C. Pearce), R03 CA113148 (C. Pearce), R03 CA115195 (C. Pearce), R13 CA110770 (F. Modugno)]; Danish Cancer Society (94 222 52); Mermaid 1; U.S. Army Medical Research and Materiel Command (DAMD17-01-1- 0729), National Health & Medical Research Council of Australia (199600 and 400281); Cancer Councils of New South Wales, Victoria, Queensland, South Australia and Tasmania; Cancer Foundation of Western Australia; German Federal Ministry of Education and Research, Program of Clinical Biomedical Research (01GB9401); German Cancer Research Center; US Army Medical Research and Material Command (DAMD17-02-1-0669, DAMD17-02-1-0666); Mayo Foundation; Minnesota Ovarian Cancer Alliance; Fred C. and Katherine B. Andersen Foundation; Radboud University Nijmegen Medical Centre; Intramural Research Program of the National Cancer Institute (N. Wentzensen); Cancer Research UK (C490/A10119 and C490/A10124 (P. Pharoah, H. Song)); Lon V Smith Foundation (LVS-39420); Eve Appeal; OAK Foundation; California Cancer Research Program (00-01389V-20170, 2II0200).
Footnotes
Conflict of Interest: Dr. Goodman, Advisory Board for Johnson and Johnson
References
- 1.Le Marchand L, Hankin JH, Wilkens LR, Pierce LM, Franke A, Kolonel LN, et al. Combined effects of well-done red meat, smoking, and rapid N-acetyltransferase 2 and CYP1A2 phenotypes in increasing colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2001;10:1259–1266. [PubMed] [Google Scholar]
- 2.Shaw GM, Iovannisci DM, Yang W, Finnell RH, Carmichael SL, Cheng S, et al. Endothelial nitric oxide synthase (NOS3) genetic variants, maternal smoking, vitamin use, and risk of human orofacial clefts. Am J Epidemiol. 2005;162:1207–1214. doi: 10.1093/aje/kwi336. [DOI] [PubMed] [Google Scholar]
- 3.Wei S, Wang LE, McHugh MK, Han Y, Xiong M, Amos CI, et al. Genome-wide gene-environment interaction analysis for asbestos exposure in lung cancer susceptibility. Carcinogenesis. 2012;33:1531–1537. doi: 10.1093/carcin/bgs188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolton KL, Ganda C, Berchuck A, Pharaoh PD, Gayther SA. Role of common genetic variants in ovarian cancer susceptibility and outcome: progress to date from the Ovarian Cancer Association Consortium (OCAC) Journal of internal medicine. 2012;271:366–378. doi: 10.1111/j.1365-2796.2011.02509.x. [DOI] [PubMed] [Google Scholar]
- 5.Kuchenbaecker KB, Ramus SJ. Identification of six new susceptibility loci for invasive epithelial ovarian cancer. 2015;47:164–171. doi: 10.1038/ng.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bojesen SE, Pooley KA, Johnatty SE, Beesley J, Michailidou K, Tyrer JP, et al. Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer. Nature genetics. 2013;45:371–384. doi: 10.1038/ng.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pharoah PD, Tsai YY, Ramus SJ, Phelan CM, Goode EL, Lawrenson K, et al. GWAS meta-analysis and replication identifies three new susceptibility loci for ovarian cancer. Nature genetics. 2013;45:362–370. 70e1–70e2. doi: 10.1038/ng.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goode EL, Chenevix-Trench G, Song H, Ramus SJ, Notaridou M, Lawrenson K, et al. A genome-wide association study identifies susceptibility loci for ovarian cancer at 2q31 and 8q24. Nature genetics. 2010;42:874–879. doi: 10.1038/ng.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song H, Ramus SJ, Tyrer J, Bolton KL, Gentry-Maharaj A, Wozniak E, et al. A genome-wide association study identifies a new ovarian cancer susceptibility locus on 9p22.2. Nature genetics. 2009;41:996–1000. doi: 10.1038/ng.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen H, Fridley BL, Song H, Lawrenson K, Cunningham JM, Ramus SJ, et al. Epigenetic analysis leads to identification of HNF1B as a subtype-specific susceptibility gene for ovarian cancer. Nature communications. 2013;4:1628. doi: 10.1038/ncomms2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Permuth-Wey J, Lawrenson K, Shen HC, Velkova A, Tyrer JP, Chen Z, et al. Identification and molecular characterization of a new ovarian cancer susceptibility locus at 17q21. 31. Nature communications. 2013;4:1627. doi: 10.1038/ncomms2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolton KL, Ganda C, Berchuck A, Pharaoh PD, Gayther SA. Role of common genetic variants in ovarian cancer susceptibility and outcome: progress to date from the Ovarian Cancer Association Consortium (OCAC) Journal of internal medicine. 2012;271:366–378. doi: 10.1111/j.1365-2796.2011.02509.x. [DOI] [PubMed] [Google Scholar]
- 13.Bolton KL, Tyrer J, Song H, Ramus SJ, Notaridou M, Jones C, et al. Common variants at 19p13 are associated with susceptibility to ovarian cancer. Nature genetics. 2010;42:880–884. doi: 10.1038/ng.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen H, Fridley BL, Song H, Lawrenson K, Cunningham JM, Ramus SJ, et al. Epigenetic analysis leads to identification of HNF1B as a subtype-specific susceptibility gene for ovarian cancer. Nature communications. 2013;4:1628. doi: 10.1038/ncomms2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolton KL, Ganda C, Berchuck A, Pharaoh PD, Gayther SA. Role of common genetic variants in ovarian cancer susceptibility and outcome: progress to date from the ovarian cancer association consortium (OCAC) J Intern Med. 2012 doi: 10.1111/j.1365-2796.2011.02509.x. [DOI] [PubMed] [Google Scholar]
- 16.Olsen CM, Nagle CM, Whiteman DC, Ness R, Pearce CL, Pike MC, et al. Obesity and risk of ovarian cancer subtypes: evidence from the Ovarian Cancer Association Consortium. Endocrine-related cancer. 2013;20:251–262. doi: 10.1530/ERC-12-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cancer CGoESoO. Ovarian cancer and body size: individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLoS Med. 2012;9:e1001200. doi: 10.1371/journal.pmed.1001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leitzmann MF, Koebnick C, Danforth KN, Brinton LA, Moore SC, Hollenbeck AR, et al. Body mass index and risk of ovarian cancer. Cancer. 2009;115:812–822. doi: 10.1002/cncr.24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feigelson HS, Jonas CR, Teras LR, Thun MJ, Calle EE. Weight gain, body mass index, hormone replacement therapy, and postmenopausal breast cancer in a large prospective study. Cancer Epidemiology Biomarkers & Prevention. 2004;13:220–224. doi: 10.1158/1055-9965.epi-03-0301. [DOI] [PubMed] [Google Scholar]
- 20.Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case–control studies. The lancet oncology. 2012;13:385–394. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cancer CGoESoO. Menopausal hormone use and ovarian cancer risk: individual participant meta-analysis of 52 epidemiological studies. The Lancet. 2015 doi: 10.1016/S0140-6736(14)61687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cancer CGoESoO. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23 257 women with ovarian cancer and 87 303 controls. The Lancet. 2008;371:303–314. doi: 10.1016/S0140-6736(08)60167-1. [DOI] [PubMed] [Google Scholar]
- 23.Whittmore AS, Harris R, Itnyre J. Characteristics relating to ovarian cancer risk: collaborative analysis of 12 US case-control studies II. Invasive epithelial ovarian cancers in White women. American journal of epidemiology. 1992;136:1184–1203. doi: 10.1093/oxfordjournals.aje.a116427. [DOI] [PubMed] [Google Scholar]
- 24.Adami H, Lambe M, Persson I, Ekbom A, Hsieh C, Trichopoulos D, et al. Parity, age at first childbirth, and risk of ovarian cancer. The Lancet. 1994;344:1250–1254. doi: 10.1016/s0140-6736(94)90749-8. [DOI] [PubMed] [Google Scholar]
- 25.Sieh W, Salvador S, McGuire V, Weber RP, Terry KL, Rossing MA, et al. Tubal ligation and risk of ovarian cancer subtypes: a pooled analysis of case-control studies. International journal of epidemiology. 2013;42:579–589. doi: 10.1093/ije/dyt042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li DP, Du C, Zhang ZM, Li GX, Yu ZF, Wang X, et al. Breastfeeding and ovarian cancer risk: a systematic review and meta-analysis of 40 epidemiological studies. Asian Pacific journal of cancer prevention : APJCP. 2014;15:4829–4837. doi: 10.7314/apjcp.2014.15.12.4829. [DOI] [PubMed] [Google Scholar]
- 27.Luan NN, Wu QJ, Gong TT, Vogtmann E, Wang YL, Lin B. Breastfeeding and ovarian cancer risk: a meta-analysis of epidemiologic studies. The American journal of clinical nutrition. 2013;98:1020–1031. doi: 10.3945/ajcn.113.062794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pearce CL, Rossing MA, Lee AW, Ness RB, Webb PM, Chenevix-Trench G, et al. Combined and interactive effects of environmental and GWAS-identified risk factors in ovarian cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22:880–890. doi: 10.1158/1055-9965.EPI-12-1030-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case? control studies. The lancet oncology. 2012;13:385–394. doi: 10.1016/S1470-2045(11)70404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearce CL, Rossing MA, Lee AW, Ness RB, Webb PM, for Australian Cancer S et al. Combined and interactive effects of environmental and GWAS-identified risk factors in ovarian cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2013;22:880–890. doi: 10.1158/1055-9965.EPI-12-1030-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 32.Pugeat M, Crave JC, Elmidani M, Nicolas MH, Garoscio-Cholet M, Lejeune H, et al. Pathophysiology of sex hormone binding globulin (SHBG): relation to insulin. The Journal of steroid biochemistry and molecular biology. 1991;40:841–849. doi: 10.1016/0960-0760(91)90310-2. [DOI] [PubMed] [Google Scholar]
- 33.Tchernof A, Despres J. Sex steroid hormones, sex hormone-binding globulin, and obesity in men and women. Hormone and metabolic research= Hormon-und Stoffwechselforschung= Hormones et metabolisme. 1999;32:526–536. doi: 10.1055/s-2007-978681. [DOI] [PubMed] [Google Scholar]
- 34.Key TJ, Allen NE, Verkasalo PK, Banks E. Energy balance and cancer: the role of sex hormones. Proceedings of the Nutrition Society. 2001;60:81–89. doi: 10.1079/pns200068. [DOI] [PubMed] [Google Scholar]
- 35.Group EHBCC. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. Journal of the National Cancer Institute. 2003;95:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 36.Armer JM, Radina ME, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported symptoms. Nursing research. 2003;52:370–379. doi: 10.1097/00006199-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Merritt MA, Green AC, Nagle CM, Webb PM. Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. International Journal of Cancer. 2008;122:170–176. doi: 10.1002/ijc.23017. [DOI] [PubMed] [Google Scholar]
- 38.Ness RB, Dodge RC, Edwards RP, Baker JA, Moysich KB. Contraception methods, beyond oral contraceptives and tubal ligation, and risk of ovarian cancer. Annals of epidemiology. 2011;21:188–196. doi: 10.1016/j.annepidem.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Royar J, Becher H, Chang-Claude J. Low-dose oral contraceptives: Protective effect on ovarian cancer risk. International journal of cancer. 2001;95:370–374. doi: 10.1002/1097-0215(20011120)95:6<370::aid-ijc1065>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 40.Lurie G, Wilkens LR, Thompson PJ, McDuffie KE, Carney ME, Terada KY, et al. Combined oral contraceptive use and epithelial ovarian cancer risk: time-related effects. Epidemiology. 2008;19:237–243. doi: 10.1097/EDE.0b013e31816334c5. [DOI] [PubMed] [Google Scholar]
- 41.Glud E, Kjaer SK, Thomsen BL, Høgdall C, Christensen L, Høgdall E, et al. Hormone therapy and the impact of estrogen intake on the risk of ovarian cancer. Archives of internal medicine. 2004;164:2253–2259. doi: 10.1001/archinte.164.20.2253. [DOI] [PubMed] [Google Scholar]
- 42.Kelemen LE, Sellers TA, Schildkraut JM, Cunningham JM, Vierkant RA, Pankratz VS, et al. Genetic variation in the one-carbon transfer pathway and ovarian cancer risk. Cancer Res. 2008;68:2498–2506. doi: 10.1158/0008-5472.CAN-07-5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia-Closas M, Brinton LA, Lissowska J, Richesson D, Sherman ME, Szeszenia-Dabrowska N, et al. Ovarian cancer risk and common variation in the sex hormone-binding globulin gene: a population-based case-control study. BMC cancer. 2007;7:60. doi: 10.1186/1471-2407-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ziogas A, Gildea M, Cohen P, Bringman D, Taylor TH, Seminara D, et al. Cancer risk estimates for family members of a population-based family registry for breast and ovarian cancer. Cancer Epidemiology Biomarkers & Prevention. 2000;9:103–111. [PubMed] [Google Scholar]
- 45.Balogun N, Gentry-Maharaj A, Wozniak EL, Lim A, Ryan A, Ramus SJ, et al. Recruitment of newly diagnosed ovarian cancer patients proved challenging in a multicentre biobanking study. Journal of Clinical Epidemiology. 2011;64:525–530. doi: 10.1016/j.jclinepi.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Pike MC, Pearce CL, Peters R, Cozen W, Wan P, Wu AH. Hormonal factors and the risk of invasive ovarian cancer: a population-based case-control study. Fertility and sterility. 2004;82:186–195. doi: 10.1016/j.fertnstert.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 47.Hoyo C, Berchuck A, Halabi S, Bentley RC, Moorman P, Calingaert B, et al. Anthropometric measurements and epithelial ovarian cancer risk in African–American and White women. Cancer Causes & Control. 2005;16:955–963. doi: 10.1007/s10552-005-3205-y. [DOI] [PubMed] [Google Scholar]
- 48.Rossing MA, Cushing-Haugen KL, Wicklund KG, Doherty JA, Weiss NS. Menopausal hormone therapy and risk of epithelial ovarian cancer. Cancer Epidemiology Biomarkers & Prevention. 2007;16:2548–2556. doi: 10.1158/1055-9965.EPI-07-0550. [DOI] [PubMed] [Google Scholar]
- 49.McGuire V, Felberg A, Mills M, Ostrow K, DiCioccio R, John E, et al. Relation of contraceptive and reproductive history to ovarian cancer risk in carriers and noncarriers of BRCA1 gene mutations. American journal of epidemiology. 2004;160:613–618. doi: 10.1093/aje/kwh284. [DOI] [PubMed] [Google Scholar]
- 50.Engeland A, Tretli S, Bjørge T. Height, body mass index, and ovarian cancer: a follow-up of 1.1 million Norwegian women. Journal of the National Cancer Institute. 2003;95:1244–1248. doi: 10.1093/jnci/djg010. [DOI] [PubMed] [Google Scholar]
- 51.World Health Organisation W. Physical status: the use and interpretation of anthropometry. Report of a World Health Organisation Expert Committee. 1995 [PubMed]
- 52.Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004;23:6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 53.Canzian F, Cox DG, Setiawan VW, Stram DO, Ziegler RG, Dossus L, et al. Comprehensive analysis of common genetic variation in 61 genes related to steroid hormone and insulin-like growth factor-I metabolism and breast cancer risk in the NCI breast and prostate cancer cohort consortium. Hum Mol Genet. 2010;19:3873–3884. doi: 10.1093/hmg/ddq291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olson SH, Bandera EV, Orlow I. Variants in estrogen biosynthesis genes, sex steroid hormone levels, and endometrial cancer: a HuGE review. Am J Epidemiol. 2007;165:235–245. doi: 10.1093/aje/kwk015. [DOI] [PubMed] [Google Scholar]
- 55.Smith AV, Thomas DJ, Munro HM, Abecasis GR. Sequence features in regions of weak and strong linkage disequilibrium. Genome research. 2005;15:1519–1534. doi: 10.1101/gr.4421405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Consortium GP. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pharoah PD, Tsai Y-Y, Ramus SJ, Phelan CM, Goode EL, Lawrenson K, et al. GWAS meta-analysis and replication identifies three new susceptibility loci for ovarian cancer. Nature genetics. 2013;45:362–370. doi: 10.1038/ng.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Couch FJ, Wang X, McGuffog L, Lee A, Olswold C, Kuchenbaecker KB, et al. Genome-wide association study in BRCA1 mutation carriers identifies novel loci associated with breast and ovarian cancer risk. PLoS genetics. 2013;9:e1003212. doi: 10.1371/journal.pgen.1003212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sankararaman S, Sridhar S, Kimmel G, Halperin E. Estimating local ancestry in admixed populations. Am J Hum Genet. 2008;82:290–303. doi: 10.1016/j.ajhg.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kraft P, Yen YC, Stram DO, Morrison J, Gauderman WJ. Exploiting gene-environment interaction to detect genetic associations. Hum Hered. 2007;63:111–119. doi: 10.1159/000099183. [DOI] [PubMed] [Google Scholar]
- 61.Gao X, Starmer J, Martin ER. A multiple testing correction method for genetic association studies using correlated single nucleotide polymorphisms. Genetic epidemiology. 2008;32:361. doi: 10.1002/gepi.20310. [DOI] [PubMed] [Google Scholar]
- 62.Risch HA. Hormonal etiology of epithelial ovarian cancer, with a hypothesis concerning the role of androgens and progesterone. Journal of the National Cancer Institute. 1998;90:1774–1786. doi: 10.1093/jnci/90.23.1774. [DOI] [PubMed] [Google Scholar]
- 63.Christopoulos PF, Msaouel P, Koutsilieris M. The role of the insulin-like growth factor-1 system in breast cancer. Molecular cancer. 2015;14:43. doi: 10.1186/s12943-015-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang J, Li J, Zeng G, Tang Y, Tian W, He J, et al. Antisense oligonucleotide suppression of human IGF-1R inhibits the growth and survival of in vitro cultured epithelial ovarian cancer cells. J Ovarian Res. 2013;6:71. doi: 10.1186/1757-2215-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clarke RB, Howell A, Anderson E. Type I insulin-like growth factor receptor gene expression in normal human breast tissue treated with oestrogen and progesterone. British journal of cancer. 1997;75:251. doi: 10.1038/bjc.1997.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doherty JA, Rossing MA, Cushing-Haugen KL, Chen C, Van Den Berg DJ, Wu AH, et al. ESR1/SYNE1 polymorphism and invasive epithelial ovarian cancer risk: an Ovarian Cancer Association Consortium study. Cancer Epidemiology Biomarkers & Prevention. 2010;19:245–250. doi: 10.1158/1055-9965.EPI-09-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalli KR, Falowo OI, Bale LK, Zschunke MA, Roche PC, Conover CA. Functional Insulin Receptors on Human Epithelial Ovarian Carcinoma Cells: Implications for IGF-II Mitogenic Signaling. Endocrinology. 2002;143:3259–3267. doi: 10.1210/en.2001-211408. [DOI] [PubMed] [Google Scholar]
- 68.Gallagher EJ, LeRoith D. The proliferating role of insulin and insulin-like growth factors in cancer. Trends in Endocrinology & Metabolism. 2010;21:610–618. doi: 10.1016/j.tem.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee E-J, Mircean C, Shmulevich I, Wang H, Liu J, Niemistö A, et al. Insulin-like growth factor binding protein 2 promotes ovarian cancer cell invasion. Molecular Cancer. 2005;4:7. doi: 10.1186/1476-4598-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Terry KL, Tworoger SS, Gates MA, Cramer DW, Hankinson SE. Common genetic variation in IGF1, IGFBP1, and IGFBP3 and ovarian cancer risk. Carcinogenesis. 2009:bgp257. doi: 10.1093/carcin/bgp257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McGrath M, Lee IM, Buring J, De Vivo I. Common genetic variation within IGFI, IGFII, IGFBP-1, and IGFBP-3 and endometrial cancer risk. Gynecologic Oncology. 2011;120:174–178. doi: 10.1016/j.ygyno.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garner CP, Ding YC, John EM, Ingles SA, Olopade OI, Huo D, et al. Genetic variation in IGFBP2 and IGFBP5 is associated with breast cancer in populations of African descent. Human genetics. 2008;123:247–255. doi: 10.1007/s00439-008-0468-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neuhausen SL, Brummel S, Ding YC, Singer CF, Pfeiler G, Lynch HT, et al. Genetic variation in insulin-like growth factor signaling genes and breast cancer risk among BRCA1 and BRCA2 carriers. Breast cancer research : BCR. 2009;11:R76. doi: 10.1186/bcr2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cust AE, Allen NE, Rinaldi S, Dossus L, Friedenreich C, Olsen A, et al. Serum levels of C-peptide, IGFBP-1 and IGFBP-2 and endometrial cancer risk; Results from the European prospective investigation into cancer and nutrition. International Journal of Cancer. 2007;120:2656–2664. doi: 10.1002/ijc.22578. [DOI] [PubMed] [Google Scholar]
- 75.Listgarten J, Damaraju S, Poulin B, Cook L, Dufour J, Driga A, et al. Predictive models for breast cancer susceptibility from multiple single nucleotide polymorphisms. Clinical Cancer Research. 2004;10:2725–2737. doi: 10.1158/1078-0432.ccr-1115-03. [DOI] [PubMed] [Google Scholar]
- 76.Dudenkov TM, Ingle JN, Buzdar A, Robson ME, Kubo M, Batzler A, et al. Genes associated with serum estrone, estrone conjugates, and androstenedione concentrations in postmenopausal women with estrogen receptor-positive breast cancer; ASCO Annual Meeting Proceedings; 2014. p. 593. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.