Abstract
Rationale
The lowest nicotine threshold "dose" in cigarettes discriminated from a cigarette containing virtually no nicotine may help inform the minimum dose maintaining dependence.
Objectives
Spectrum research cigarettes (from NIDA) differing in nicotine content were used to evaluate a procedure to determine discrimination thresholds.
Methods
Dependent smokers (n=18; 13 M, 5 F) were tested on ability to discriminate cigarettes with nicotine contents of 11, 5, 2.4, and 1.3 mg/g, one per session, from the “ultra-low” cigarette with 0.4 mg/g, after having discriminated 16 mg/g from 0.4 mg/g (all had 9–10 mg “tar”). Exposure to each was limited to 4 puffs/trial. All subjects were abstinent from smoking overnight prior to each session, and the number of sessions was determined by the participant’s success in discrimination behavior on >80% of trials. Subjective perceptions and behavioral choice between cigarettes were also assessed and related to discrimination behavior.
Results
The median threshold was 11 mg/g, but the range was 2.4 mg/g to 16 mg/g, suggesting wide variability in discrimination threshold. Compared to the ultra-low, puff choice was greater for the subject’s threshold dose but only marginal for the subthreshold (next lowest nicotine) cigarette. Threshold and subthreshold also differed on subjective perceptions but not withdrawal relief.
Conclusions
Under these testing conditions, threshold content for discriminating nicotine via cigarettes may be 11 mg/g or greater for most smokers, but some can discriminate nicotine contents one-half or one-quarter this amount. Further study with other procedures and cigarette exposure amounts may identify systematic differences in nicotine discrimination thresholds.
Keywords: Nicotine, Discrimination, Cigarette smoking, Threshold, Choice, Subjective effects
Introduction
Identification of the lowest “dose” of nicotine in cigarettes that reinforces tobacco use in humans likely has major implications for understanding dependence on cigarette smoking, as well as for public policy on nicotine regulation (Hatsukami et al. 2010; Sofuoglu and LeSage 2012). For example, establishing the maximum nicotine content in cigarettes at a very low level, far below that of current brands, could result in nicotine exposure from smoking below that necessary for the onset or maintenance of dependence (Benowitz and Henningfield 1994). Although a broad array of research is needed to identify such a threshold dose for reinforcement (e.g. Goodwin et al. 2015; Grebenstein et al. 2015; Hatsukami et al. 2012; Hatsukami et al. 2013a; Hecht 2012), this dose likely is not lower than the threshold dose for discriminating nicotine’s interoceptive stimulus effects. In other words, it is unlikely that a dose that could not be discriminated by smokers would continue to support nicotine reinforcement. Thus, the minimum dose of nicotine reliably discriminable from tobacco cigarettes containing no nicotine (i.e. a “placebo”) may help inform the likely threshold dose for dependence.
Nicotine discrimination has been extensively studied in non-human animals (e.g. Smith and Stolerman 2009) and, to a much lesser extent, in humans (see Perkins 2011). However, no research has directly assessed the threshold dose for nicotine discrimination via cigarette smoking, even though studies of discrimination threshold with alcohol, caffeine, opioids, and other drugs have been conducted in humans (e.g., Jackson et al. 2001; Mumford et al. 1994; Rush et al. 1995; Preston and Bigelow 1998). Recent research has explored the important and interesting question of discrimination and reinforcement of nicotine administered in novel fashion via oral capsules in never-smokers (Duke et al. 2015), but threshold dose per se was not specifically examined. To our knowledge, the only directly relevant research in humans on nicotine discrimination threshold dose is our study of nicotine administered by nasal spray (Perkins et al. 2001). We found in 18 smokers that the median threshold dose for discriminating nicotine from placebo spray was 3 µg/kg (about 0.2 mg for a typical 70 kg human), fairly consistent with a small study on choice of IV nicotine dose in smokers (Sofuoglu et al. 2008).
However, the generalizability of these results to discrimination of nicotine via cigarette smoking is uncertain. Nicotine administration by spray (or IV) may underestimate the threshold dose in cigarettes, since sensory stimuli unique to smoke inhalation may make discrimination of nicotine per se from cigarettes more difficult than nicotine via other methods of administration lacking those sensory stimuli (e.g. Donny et al. 2007; Rose 2006). On the other hand, very rapid drug administration methods, such as smoke inhalation (versus spray or IV, which do not involve inhalation), often result in stronger acute responses (e.g., Henningfield and Keenan 1993), and so even smaller doses of smoked nicotine may be readily discriminable by smokers. The answer to this question, the minimum nicotine content that can be reliably discriminated from a completely nicotine-free cigarette, could establish a threshold “dose” for smoked nicotine’s interoceptive stimulus effects. Because doses below that minimum would not be discriminable, by definition, results of this research could ultimately help to inform identification of the amount of nicotine content in cigarettes below which tobacco dependence likely could not be maintained (Benowitz and Henningfield 1994; Hatsukami et al. 2013a; see also Goodwin et al. 2015).
Yet, practical factors may require that this research to assess nicotine discrimination threshold dose via cigarettes be done in a manner different from that in prior studies using non-tobacco forms of nicotine administration. According to U.S. law (i.e. the Tobacco Control Act; see U.S. Govt 2009), elimination of all nicotine from a tobacco cigarette (i.e. to produce a true “placebo”) cannot be mandated. Thus, to relate findings to U.S. tobacco regulation policy (and perhaps even to obtain tobacco products for testing purposes), any cigarette nicotine “threshold” doses would have to be in comparison with a cigarette that still has some nicotine content. This procedure clearly differentiates research on nicotine discrimination via cigarette smoking from that using administration via spray, IV, or capsule placebos, which are devoid of any nicotine. Furthermore, as required in any drug discrimination research (e.g. Glennon and Young 2011; Holtzman 1990), that comparison cigarette also should be as similar as possible to the higher nicotine content cigarettes on the non-nicotine constituents to ensure discrimination is based on interoceptive effects of nicotine per se, rather than these other constituents, which may be psychoactive (Hoffman and Evans 2013).
Until very recently, no such cigarettes existed. However, research cigarettes that differ across a range of specific nicotine contents, including some with very low contents, have become available through the National Institute on Drug Abuse (NIDA). Critically, these “Spectrum” research cigarettes (see Methods) manipulate the actual nicotine content of the tobacco used, rather than by engineering nicotine intake via the filter ventilation, etc., as in commercial brands labeled low in “yield” (e.g. “lights”; Hatsukami et al. 2013b). This nicotine “yield” in commercial brands, as determined by the Federal Trade Commission (FTC method, via machines), is based on a specific amount of smoke for chemical analysis (St. Charles et al. 2010). Yet, most brands allow ventilation of smoke directly into ambient air through the holes engineered in the paper, bypassing the cigarette’s tip and lowering machine-based yield. Human smokers can overcome effects of this ventilation, such as by covering over the holes to inhale a greater amount of the smoke than in the machine testing (Benowitz et al. 1983; Marian et al. 2009; Strasser et al. 2005). This flexibility in smoke intake renders these yield values of limited utility in assessing delivery of a cigarette’s nicotine dose (Henningfield et al. 1994). Thus, in contrast with commercial brands, smokers cannot easily obtain greater nicotine intake from Spectrum research cigarettes with lower contents, potentially allowing study of nicotine discrimination in humans via smoking. This study evaluated an initial procedure by which to identify the lowest nicotine content cigarette that dependent smokers can distinguish from those containing almost no nicotine (labeled here “ultra-low” in nicotine content, since a “placebo” does not exist), based on 4 puffs exposure to each of these Spectrum research cigarettes. We also explored whether this nicotine threshold dose for behavioral discrimination of cigarettes was associated with the concomitant subjective perceptions and subsequent choice behavior of participants in response to these cigarettes, as often found in other drug discrimination research (e.g. Johanson 1991) but never before evaluated with nicotine via smoke inhalation. Some human research suggests that behavioral drug discrimination may be more sensitive to dose than self-reported subjective effects (e.g., Perkins et al. 1994; Preston and Bigelow 1998). Our main purpose was to examine whether this procedure could serve as a starting point to guide future research in assessing discrimination threshold doses of nicotine via cigarette smoke inhalation.
Methods
Participants
Eligible subjects were those 18 (out of 29) able to discriminate between the two Spectrum cigarettes most widely differing in nicotine content (see next section) and thus could provide a discrimination threshold dose (see Perkins et al. in press). All were dependent smokers who preferred non-menthol cigarettes, to eliminate variability in one key non-nicotine constituent, menthol, that could alter discrimination behavior (a topic warranting focused research; e.g. Strasser et al. 2013). Nicotine dependence was confirmed by assessing presence of DSM-V criteria (APA 2013), using a structured interview updated from Breslau et al. (1994). All also completed the Fagerstrom Test of Nicotine Dependence (FTND; Heatherton et al. 1991). Subjects in the present study of discrimination threshold dose determination (n=18; 13 M, 5 F) had mean (SD) characteristics of 33.4 (11.1) years old, 16.3 (5.4) cigs/day, and 4.8 (1.6) FTND score.
Investigational cigarettes
Spectrum investigational research cigarettes, manufactured by 22nd Century Group (Clarence NY; http://www.xxiicentury.com/), were obtained from NIDA’s Drug Supply Program after we submitted an application for an Investigational Tobacco Product to the Center for Tobacco Products at the Food and Drug Administration (FDA). Recently, Spectrum cigarettes were investigated in a 6-week trial to determine the impact of nicotine reduction in smokers not currently interested in quitting (Donny et al. 2015). Selected for the current study were all those non-menthol Spectrum cigarettes that differed in nicotine contents but were similar on “tar” yield. Including the cigarette all were able to discriminate in order to be eligible for this assessment of the threshold dose for nicotine discrimination, Spectrum nicotine contents were approximately 16, 11, 5, 2.4, 1.3, and 0.4 mg of nicotine per gram of tobacco (i.e. mg/g), and all had about 9–10 mg “tar.” (To compare them with commercial brands, these research cigarettes correspond to 0.8, 0.7, 0.26, 0.12, 0.07, and 0.03 mg nicotine “yields” by FTC method, as reported in http://grants.nih.gov/grants/guide/notice-files/NOT-DA-14-004.html. Typical commercial brands yield about 0.9 mg nicotine, with roughly 10 mg “tar”; Jarvis et al. 2001; USDHHS 2010.) As described below in Procedures, all discrimination testing involved comparing the very lowest content cigarette, 0.4 mg/g, with each of the higher nicotine content cigarettes. Thus, the 0.4 mg/g is sometimes called the “ultra-low” nicotine cigarette, to characterize it in a manner that clearly differentiates it from the others.
Control of exposure from each cigarette
Intake from all cigarettes was standardized at 4 puffs per trial via portable Clinical Research Support System (CReSS; Borgwaldt KC, Inc., Richmond VA), with one puff every 30 sec. A new cigarette was used on each trial. The precise timing and 2-sec duration of each puff were determined by computer-presented instructions to the participant, standardizing smoke intake at approximately 60 ml per puff, a pattern consistent with most ad lib puffing (Blank et al. 2009; June et al. 2012; Perkins et al., 2012). This exposure rate was intended to allow intervals between trials of 15 min while minimizing smoking satiation or toxicity in these initially overnight-deprived smokers. (Otherwise, providing a full cigarette per trial would increase chances of satiation and toxicity well before completion of testing, and it would require much longer intervals between trials and/or multiple sessions with each pair of cigarettes to be discriminated; see below). This amount of exposure over each 3-hr session was no more than that expected from ad lib smoking in the morning after overnight abstinence, which typically results in multiple cigarettes per hour and 10–12 puffs per cigarette (e.g. Hatsukami et al. 1988; Mooney et al. 2006). Also, smoking 4 puffs would be expected to capture the amount of exposure at the onset of one’s expectations about a cigarette, which could substantially influence the amount of subsequent self-administration of that cigarette (i.e. reinforcement), as well as other responses (e.g., Gu et al. 2015; Hasenfratz et al. 1993). In the only prior tests on discriminating drug via inhaled smoke, to our knowledge, marijuana similarly was provided in just 2 or 4 puffs per administration (Chait et al. 1988). In summary, then, based on our prior research, this exposure per trial would be expected to deliver sufficient nicotine by which to perceive the cigarette’s interoceptive or subjective effects but prevent onset of satiation or toxicity (e.g. Perkins et al. 1996; 2001).
Procedures
General
Given a lack of prior research with nicotine discrimination via cigarettes, most procedures were adapted from our prior study in humans of nicotine discrimination threshold dose via nasal spray (Perkins 2011; Perkins et al. 2001). Those procedures themselves were based on standard methods of research testing discrimination of nicotine in animal models (see Smith and Stolerman 2009; Stolerman 1989) and of other drugs in humans (e.g. Holtzman 1990; Preston 1991; Rush et al. 1995). The initial development and evaluation of these procedures for testing nicotine discrimination by tobacco smoking, once the Spectrum research cigarettes became available, are detailed elsewhere (Perkins et al. in press). All study sessions involved testing for discrimination between the ultra-low 0.4 mg/g nicotine content cigarette versus one of the higher nicotine content Spectrum cigarettes (>1 mg/g). As noted, all participants in this study were those able to discriminate the highest content Spectrum cigarette of 16 mg/g versus 0.4 mg/g cigarette at initial testing, so that a threshold dose for nicotine discrimination could be assessed. (In other words, anyone unable to learn to discriminate the two cigarettes most different in nicotine content could not be tested for a discrimination threshold dose, since even higher nicotine content Spectrum cigarettes were not available.)
Spectrum cigarettes with nicotine contents of 16, 11, 5, 2.4, and 1.3 mg/g were separately tested, one per session, on discriminability from the 0.4 mg/g cigarette. After the first session, in which subjects learned the discrimination procedure and showed ability to discriminate 16 mg/g vs 0.4 mg/g, the order of the other nicotine content cigarettes individually compared with 0.4 mg/g across sessions was arranged systematically, either in descending or ascending fashion. Thus, the order was progressively lower for half the subjects (“descending” subgroup), to 11 mg/g, then 5 mg/g, etc., and progressively higher for the other subjects (“ascending” subgroup), starting with 1.3 mg/g, then 2.4 mg/g, and 5 mg/g, and so on. Subjects were randomly assigned to these descending and ascending subgroups, which allowed us to verify that discrimination threshold dose did not differ as a function of the order in which the other cigarettes were administered, consistent with our prior threshold dose study with nicotine spray (Perkins et al. 2001). Each subject’s total number of sessions was determined by the success of discrimination behavior, as subject participation ended when the lowest nicotine content cigarette he or she reliably discriminated from the ultra-low 0.4 mg/g cigarette was identified.
Specific session procedures
Subjects were abstinent overnight prior to each session, confirmed by CO≤10 ppm (SRNT 2002) assessed by BreathCO CO monitor (Vitalograph, Lenexa, KS). Withdrawal also was assessed upon arrival with the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes and Hatsukami, 1986), as each item was rated on a 0–100 visual analog scale (VAS) and averaged for a total withdrawal score. The MNWS was not used to confirm abstinence but to evaluate any potential differences between cigarettes in the decline in withdrawal from pre-smoking baseline.
Specific procedures were virtually identical on each session, as subjects were initially “trained” to discriminate which cigarette was which, and then “tested” on their ability to discriminate them. The two cigarettes compared in each session were presented in random order, once per 15 min trial, and identified by letter code during the initial training trials (e.g. “A” or “B” in session 1, “C” vs “D” in session 2, “E” vs “F” in session 3, and so on). (Training conditions for these participants, all of whom learned to discriminate the two cigarettes most different in nicotine content, 16 mg/g vs 0.4 mg/g, as noted, varied slightly between the first 10 and last 8 subjects. Training involved 2 trials, one for each cigarette, for the first 10 subjects, and 4 trials, two for each cigarette, for the last 8 subjects, as explained elsewhere in the development of these procedures; Perkins et al. in press. Threshold doses for those with 2 vs. 4 training trials were not different in this study, as indicated below in the Results.) For training, subjects were instructed to “evaluate these cigarettes based on your overall subjective feelings” since it was “important that you learn how to tell the difference between the two cigarettes.” Immediately following the 4 puffs in each trial, subjects completed a brief 8-item measure assessing subjective perceptions to examine whether they may relate to discrimination behavior. These 8 items asked subjects to rate the cigarette on how “satisfying”, “strong”, “harsh”, “smooth”, and “similar to own brand” it was, and how much “nicotine”, “flavor”, and “liking” they experienced, each via 0–100 VAS, anchored by “not at all” to “very much” (Perkins et al. 2012). To standardize motivation to learn this discrimination, subjects were told each correct cigarette identification during the subsequent testing trials would be reinforced by adding $1 to their total participant payment (as in Perkins et al. 2001).
These training trials were then followed by 6 testing trials, in which subjects were uninformed of the cigarette identification (i.e. kept blind), to assess acquisition of discrimination. The two types of cigarettes were presented in random order across testing trials, once per 15 min (but the same type never more than twice in succession). After completing the self-report VAS measure of subjective perceptions, they then circled which letter code (e.g. “A” or “B”, “C” or “D”) they believed identified the cigarette based on how they perceived it during the training trials. Based on the rate of drug-appropriate responding criterion used in most prior studies of drug discrimination with humans (see Takada 1996; see also Duke et al. 2015), “successful” discrimination between the cigarettes was a priori defined here by at least 80% correct identifications (i.e. ≥5 out of 6 trials). Subjects completed the MNWS withdrawal measure after the last testing trial.
Finally, subjects then completed two additional trials, again 15 min apart, involving “choice” of puffs from the two cigarettes made available concurrently. These trials were intended to gauge the relative reinforcing effects of the cigarettes differing in nicotine contents, as in prior studies of nicotine reinforcement (e.g. Perkins et al. 1996). In each of these two trials, they were given both of the cigarettes, the ultra-low 0.4 mg/g and the higher nicotine content cigarette for discrimination during the testing trials, and informed of their letter codes (as during training trials). They were then instructed to take a total of 4 puffs from any combination of the two cigarettes, solely according to their own preference (e.g. all 4 from one or from the other, or from a mix of the two). As in the prior trials, the timing and duration of each chosen puff were controlled by computer-presented instructions. The number of times the higher nicotine Spectrum cigarette (all greater than the 0.4 mg/g) was chosen, out of 8 total puff opportunities from the two choice trials, was the measure of nicotine’s relative reinforcement. This study protocol was approved by the University of Pittsburgh Institutional Review Board.
Descending versus ascending order of cigarettes across sessions
In the descending order subgroup, subjects correctly identifying the two cigarettes with >80% accuracy progressed to the next lowest nicotine content cigarette for comparison versus the ultra-low 0.4 mg/g cigarette at the subsequent session, and so on. If they failed to identify the cigarettes with at least 80% accuracy, the subsequent session involved a repeat of the training and testing trials with the same pair of cigarettes to verify inability to reliably discriminate them (i.e. their “subthreshold” dose). If subjects were able to reliably discriminate them during that session, they continued on to the next lowest nicotine content cigarette at the subsequent session. Yet, if they still failed to discriminate the cigarettes this second time, the subsequent session involved repeat training and testing at the next highest nicotine content cigarette (i.e. the lowest content cigarette that they previously successfully discriminated) to verify reliable discrimination of that cigarette (identified as their “threshold” dose). Correspondingly, in the ascending order subgroup, subjects were presented with progressively higher nicotine content cigarettes across sessions to discriminate versus the ultra-low 0.4 mg/g, beginning with 1.3 mg/g, if they earlier failed to reliably discriminate the lower nicotine content cigarettes. Once successful, the subsequent session involved a repeat training and testing of the next lowest nicotine content cigarette to verify inability to reliably discriminate it (i.e. their “subthreshold” dose) from the ultra-low 0.4 mg/g. The last session involved a repeat of training and testing of the lowest nicotine content cigarettes they were able to discriminate from the ultra-low, to verify reliability of that discrimination (again, their “threshold” dose). These procedures are as in Perkins et al. (2001).
Data Analyses
Discrimination threshold and subthreshold doses were identified for each individual subject according to the a priori criteria noted previously (i.e. the threshold dose was the lowest nicotine content cigarette accurately discriminated from the 0.4 mg/g ultra-low on at least 80% of trials, and the subthreshold dose was the next lowest nicotine content cigarette). The primary result of interest was the median threshold dose for the entire sample, after confirming no difference in thresholds for the ascending versus descending subgroups (or for those with 2 vs 4 training trials). Subjective perceptions of the threshold and subthreshold cigarettes were calculated as the difference between effects of the higher nicotine content vs. ultra-low cigarette during all trials. These cigarette perceptions were compared using multivariate analysis of variance (MANOVA), with follow-up univariate ANOVAs for each individual VAS response. Withdrawal at baseline vs. post-testing trials for the threshold vs. subthreshold cigarettes was assessed with ANOVA. The non-parametric Wilcoxon signed rank test (z) was used to analyze choice between the higher nicotine content and ultra-low nicotine cigarettes, within each session. The absolute number of puff choices (out of 8 total) for each cigarette was compared to gauge relative reinforcement between the cigarettes differing in nicotine content. Finally, given our limited power for between-groups comparisons, subject characteristics were related to number of puff choices for exploratory purposes only.
Results
Threshold doses
In preliminary comparisons of threshold doses between subgroups, no differences were found between the ascending and descending subgroups, with medians of 11 mg/g and the range of thresholds from 2.4–16 mg/g for each subgroup. Similarly, median threshold dose was 11 mg/g for those receiving 2 vs. 4 training trials each session. Thus, results were combined between these subgroups to provide data for the entire sample. As indicated by these subgroup comparisons, the median threshold dose was 11 mg/g for the entire sample of 18, with the range of 2.4–16 mg/g. Only the 1.3 mg/g cigarette, with nicotine content just above the ultra-low, could not be discriminated by any subject from the 0.4 mg/g ultra-low cigarette.
Individual variability in discrimination ability, under the conditions of this research, is suggested by the distribution of cigarette nicotine content thresholds, which was 16 mg/g for 3 subjects, 11 mg/g for 9, 5 mg/g for 4, and 2.4 mg/g for 2. To explore potential factors associated with these threshold differences, we examined demographic characteristics between the 12 whose threshold was higher, 11 mg/g or greater, versus the 6 whose threshold was lower, 5 mg/g or less. No significant differences were found between those with higher vs. lower thresholds, respectively (mean±SD), for age (32.9±11.9 vs. 34.5±10.0) or cigarettes/day (15.5±4.9 vs. 17.8±6.5), both t(16)<1, or for FTND (4.3±1.7 vs. 5.8±0.8), t(16)=2.07, p=.06. (Too few women were included to examine potential sex differences, but just 1 of 5 women, compared to 5 of 13 men, comprised those with the lower thresholds.)
Subjective perception, withdrawal, and choice responses
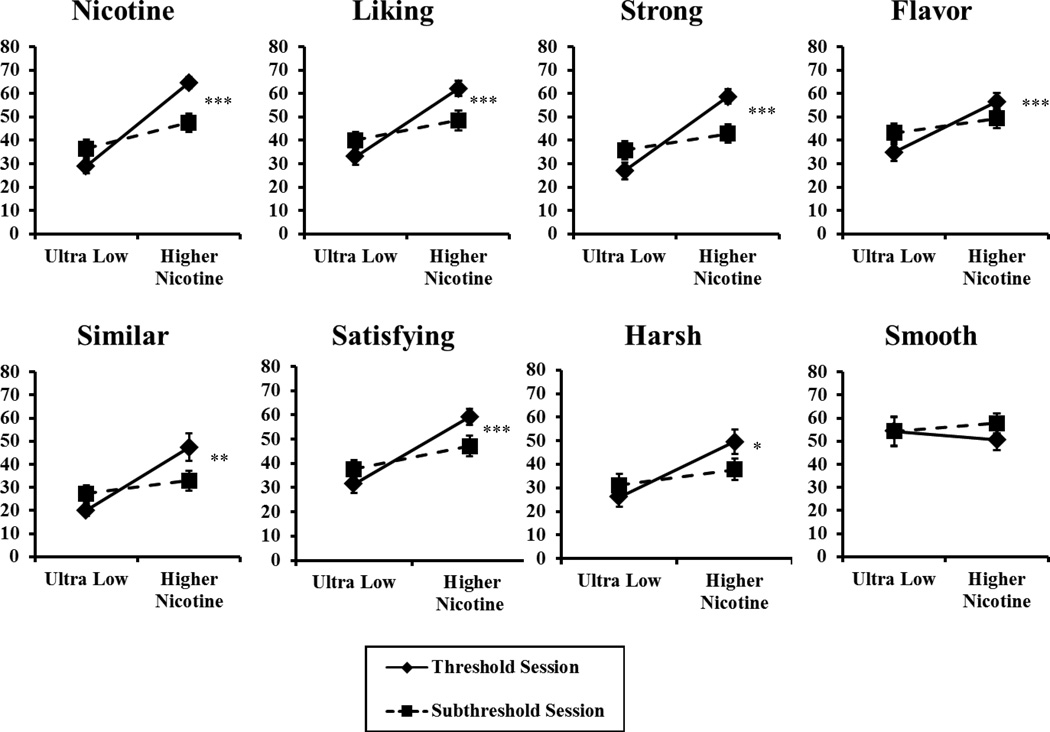
As expected, we found greater subjective perceptions of the threshold vs. subthreshold nicotine cigarettes, F (8,10)=11.40, p<.001 in MANOVA. In the follow-up univariate ANOVAs, significant differences were found for all the individual items, except “smooth” (Figure 1). Yet, MNWS withdrawal decreased substantially from pre-smoking baseline to the last smoking discrimination testing trial, F(1, 17)=26.89, p<.001 (i.e. main effect of pre- vs post-smoking). This withdrawal relief occurred regardless of which cigarette was being compared with the 0.4 mg/g ultra-low (i.e. no main effect of threshold vs subthreshold cigarette, F(1,17)<1, or interaction of cigarette by trial, F(1,17)=1.28, p>.20). Means (±SEM) at baseline vs. post-testing trials, respectively, were 47.0±5.3 vs. 26.4±4.2 for the threshold cigarette, and 46.1±4.9 vs. 30.9±5.3 for the sub-threshold cigarette.
Figure 1.
Mean (SEM) subjective perception on each 0–100 VAS item for all subjects (n=18) in response to smoking their threshold or their subthreshold nicotine Spectrum cigarettes on separate sessions, each vs. the ultra-low (0.4 mg/g) comparison cigarette. (* p<.05, **p<.01, and ***p<.001 for difference between the higher nicotine content cigarettes in univariate ANOVAs).
Somewhat similar to the subjective effects differences between cigarettes, when available concurrently with the ultra-low nicotine cigarette, the choice of puffs was significantly greater for the threshold dose, 5.3±0.4 vs. 2.7±0.4 (mean±SEM), z = 2.51, p<.05, but only marginally greater for the subthreshold dose, 4.8±0.4 vs. 3.2±0.4, z = 1.66, p<.10. However, these puff choices for the threshold and subthreshold cigarettes were not different when compared with each other (i.e. between-sessions), z = 1.57, p>.10.
Discussion
Our findings generally confirm feasibility and efficacy of this procedure for assessing discrimination threshold dose for nicotine from cigarette smoke inhalation, although refinement of this approach warrants further study. Within the limitations of this study (e.g. exposure of just 4 puffs per trial), our results suggest that the nicotine threshold “dose", the lowest cigarette nicotine content that is discriminable from a cigarette nearly devoid of nicotine (the ultra-low, 0.4 mg/g), may be 11 mg/g for many smokers, based on the median threshold in this sample of 18 nicotine dependent adults. However, this threshold appears to vary widely across the nicotine contents available from these Spectrum research cigarettes comparable on tar, as the 11 mg/g nicotine content cigarette was the specific threshold for just half the participants. The threshold was higher, 16 mg/g, for 3 subjects, while it was much lower, at 5 or 2.4 mg/g (i.e. one-half or one-quarter of the median threshold content), for 6 subjects. It is possible that some of those whose threshold was 11 mg/g may have been able to discriminate cigarettes with nicotine contents intermediate between 5 and 11 mg/g, if such Spectrum cigarettes had been available for testing. Yet, no one was able to discriminate the 1.3 mg/g content cigarette from the ultra-low 0.4 mg/g, suggesting smoking 1.3 mg/g content cigarettes may be comparable to smoking a nearly non-nicotine cigarette, which should not be able to maintain dependence (Benowitz and Henningfield 1994). Nevertheless, substantially more research is required to fully evaluate the degree to which these very low nicotine content cigarettes may attenuate the onset and persistence of dependence (e.g. Hatsukami et al. 2013a; 2013b), especially whether their long-term use aids ability to quit tobacco smoking (Donny et al. 2015).
We examined possible associations of other responses to smoking these cigarettes with each subject’s nicotine threshold dose versus subthreshold dose. In comparison with the ultra-low cigarette, puff choice was significantly greater for the threshold dose but only marginally greater for the subthreshold cigarette, perhaps consistent with the notion that nicotine’s discriminability is related to its reinforcing efficacy (e.g. Harvey et al. 2004; Perkins 2009; Sofuoglu et al. 2008). The threshold and subthreshold cigarettes also differed in subjective perceptions reported by participants. On the other hand, this difference in perception included most of the items assessed, some of which might not be expected to differ due to interoceptive stimulus effects of nicotine per se. In particular, VAS items of “harsh” and “flavor” differed between the two cigarettes, although another such item, “smooth”, did not differ (Figure 1). By their self-report nature, the basis for responding on these items is not known, but the differential nicotine contents of these Spectrum cigarettes may influence some of their exteroceptive effects, aside from their interoceptive stimulus effects, despite being comparable on tar contents. Yet, these cigarettes did not differ in the significant degree to which they relieved withdrawal from baseline following overnight abstinence. This observation is fully consistent with prior laboratory-based research showing substantial acute withdrawal relief from smoking regardless of the cigarette’s nicotine content (e.g. Butschky et al. 1995). However, we hasten to add that this study was not specifically designed to compare the cigarettes on withdrawal relief, as each cigarette was compared with the ultra-low cigarette within the same session, minimizing differences in total nicotine exposure from smoking between these cigarettes.
Moreover, for reasons explained, each session of discrimination testing here necessarily compared the varying nicotine content cigarettes with a cigarette still containing minimal nicotine, the 0.4 mg ultra-low. Even minimal nicotine content cigarettes may produce significant brain receptor occupancy (Brody et al. 2009), which could lead to interoceptive stimulus effects that are difficult to discriminate from modestly higher nicotine content cigarettes. A true threshold dose for discriminating the presence versus complete absence of nicotine in cigarettes (i.e. a placebo matched with the others on tar), if it could be provided in testing, may be lower for all of these smokers. It is also conceivable that the potential individual differences in sensitivity to the discriminative stimulus effects of nicotine in cigarettes may be even broader than the 7-fold range (2.4 mg/g vs 16 mg/g) indicated by our findings (see also Grebenstein et al. 2015). This possibility is further suggested by the range of nicotine threshold doses compared with placebo via nasal spray in our earlier study, varying by over 100-fold (Perkins et al. 2001). In the current study, we did not observe any systematic differences as a function of threshold dose, but only a few were examined (age, cigarettes/day, FTND) in this relatively homogeneous and small sample of dependent smokers. Controlled research to identify individual differences that may affect threshold discrimination of nicotine via cigarettes likely would require large study samples comprising subgroups widely differing in specific characteristics of interest.
Finally, we should reiterate that the threshold for nicotine discrimination via cigarette smoking assessed here may be specific to our testing procedures (see Perkins 2011; Smith and Stolerman 2009). For practical reasons, owing to the avoidance of smoke toxicity across the number of trials required, subjects took only 4 controlled puffs on each cigarette administered in a trial. More subjects may have discriminated cigarettes lower in nicotine contents than their threshold identified here if they had taken more puffs. Future studies should examine how different testing procedures and amounts of exposure per trial can alter the discrimination threshold of nicotine via cigarettes, and perhaps via other methods of administration (e.g. Duke et al. 2015). Such research should also determine the influence of certain individual differences in nicotine discrimination threshold to gauge what factors may relate to variable sensitivity to nicotine’s interoceptive stimulus effects (Perkins 2009; Perkins et al. 1994; see also Grebenstein et al. 2015). If possible, methods from preclinical research on nicotine discrimination may inform studies in humans using cigarettes varying in nicotine contents (e.g. Pittenger and Bevins 2013; Shoaib and Stolerman 1996; Smith and Stolerman 2009; Stolerman 1989).
Acknowledgments
Research reported in this publication was supported by the National Institute on Drug Abuse and Food and Drug Administration Center for Tobacco Products (CTP) (U54 DA031659). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Footnotes
No authors have any potential conflicts of interest to report.
References
- American Psychiatric Association (APA) Diagnostic and Statistical Manual-V. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Benowitz NL, Hall SM, Herning RI, Jacob P, Jones RT, Osman A-L. Smokers of low-yield cigarettes do not consume less nicotine. New Engl J Med. 1983;309:139–142. doi: 10.1056/NEJM198307213090303. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE. Establishing a nicotine threshold for addiction. New Engl J Med. 1994;331:123–125. doi: 10.1056/NEJM199407143310212. [DOI] [PubMed] [Google Scholar]
- Blank MD, Disharoon S, Eissenberg T. Comparison of methods for measurement of smoking behavior: mouthpiece-based computerized devices versus direct observation. Nicotine Tob Res. 2009;11:896–903. doi: 10.1093/ntr/ntp083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. DSM-IIIR nicotine dependence in young adults: prevalence, correlates and associated psychiatric disorders. Addiction. 1994;89:743–754. doi: 10.1111/j.1360-0443.1994.tb00960.x. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Costello MR, Abrams AL, Scheibal D, Farahi J, et al. Brain nicotinic acetylcholine receptor occupancy: effect of smoking a denicotinized cigarette. Int J Neuropsychopharmacol. 2009;12:305–312. doi: 10.1017/S146114570800922X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butschky MF, Bailey D, Henningfield JE, Pickworth WB. Smoking with nicotine delivery decreases withdrawal in 12-hour abstinent smokers. Pharmacol Biochem Behav. 1995;50:91–96. doi: 10.1016/0091-3057(94)00269-o. [DOI] [PubMed] [Google Scholar]
- Chait LE, Evans SM, Grant KA, Kamien JB, Johanson CE, Schuster CR. The discriminative stimulus and subjective effects of smoked marijuana in humans. Psychopharmacology. 1988;94:206–212. doi: 10.1007/BF00176846. [DOI] [PubMed] [Google Scholar]
- Donny EC, Denlinger RL, Tidey JW, et al. Randomized trial of reduced-nicotine standards for cigarettes. New Engl J Med. 2015;373:1340–1349. doi: 10.1056/NEJMsa1502403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction. 2007;102:324–334. doi: 10.1111/j.1360-0443.2006.01670.x. [DOI] [PubMed] [Google Scholar]
- Duke AN, Johson MW, Reissig CJ, Griffiths RR. Nicotine reinforcement in never-smokers. Psychopharmacology. 2015;232:4243–4252. doi: 10.1007/s00213-015-4053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Young R. Drug Discrimination: Application to Medicinal Chemistry and Drug Studies. New York: John Wiley & Sons; 2011. [Google Scholar]
- Goodwin AK, Hiranita T, Paule MG. The reinforcing effects of nicotine in humans and nonhuman primates: a review of intravenous self-administration evidence and future directions for research. Nicotine Tob Res. 2015;17:1297–1310. doi: 10.1093/ntr/ntv002. [DOI] [PubMed] [Google Scholar]
- Grebenstein PE, Burroughs D, Roiko SA, Pentel PR, LeSage MG. Predictors of the nicotine reinforcement threshold, compensation, and elasticity of demand in a rodent model of nicotine reduction policy. Drug Alc Depend. 2015;151:181–193. doi: 10.1016/j.drugalcdep.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Lohrenz T, Salas R, et al. Belief about nicotine selectively modulates value and reward prediction error signals in smokers. Proc Nat Acad Sci. 2015;112:2539–2544. doi: 10.1073/pnas.1416639112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey DM, Yasar S, Heishman SJ, Panlilio LV, Henningfield JE, Goldberg SR. Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology. 2004;175:134–142. doi: 10.1007/s00213-004-1818-6. [DOI] [PubMed] [Google Scholar]
- Hasenfratz M, Jacober A, Battig K. Smoking-related subjective and physiological changes: pre- to postpuff and pre- to postcigarette. Pharmacol Biochem Behav. 1993;46:527–534. doi: 10.1016/0091-3057(93)90540-a. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Benowitz NL, Donny E, Henningfield J, Zeller MR. Nicotine reduction: Strategic research plan. Nicotine Tob Res. 2013a;15:1003–1013. doi: 10.1093/ntr/nts214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Biener L, Leischow SJ, Zeller MR. Tobacco and nicotine product testing. Nicotine Tob Res. 2012;14:7–17. doi: 10.1093/ntr/ntr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Heishman SJ, Vogel RI, Denlinger RL, et al. Dose-response effects of Spectrum Research Cigarettes. Nicotine Tob Res. 2013b;15:1113–1121. doi: 10.1093/ntr/nts247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Perkins KA, LeSage MG, Ashley DL, Henningfield JE, Benowitz NL, Backinger C, Zeller M. Nicotine reduction revisited: science and future directions. Tob Control. 2010;19:436–445. doi: 10.1136/tc.2009.035584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Pickens RW, Svikis DS, Hughes JR. Smoking topography and nicotine blood levels. Addict Behav. 1988;13:91–95. doi: 10.1016/0306-4603(88)90031-7. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Research opportunities related to establishing standards for tobacco products under the Family Smoking Prevention and Tobacco Control Act. Nicotine Tob Res. 2012;14:18–28. doi: 10.1093/ntr/ntq216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K-O. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol. 1993;61:743–750. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Kozlowski LT, Benowitz NL. A proposal to develop meaningful labeling for cigarettes. JAMA. 1994;272:312–314. [PubMed] [Google Scholar]
- Holtzman SG. Modern Methods in Pharmacology. Vol. 6. New York: Wiley-Liss, Inc.; 1990. Discriminative stimulus effects of drugs: Relationship to potential for abuse; pp. 193–210. [Google Scholar]
- Hoffmann AC, Evans SE. Abuse potential of non-nicotine tobacco smoke constituents: acetaldehyde, nornicotine, cotinine, and anabasine. Nicotine Tob Res. 2013;15:622–632. doi: 10.1093/ntr/nts192. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Jackson A, Stephens DN, Duka T. A low dose alcohol drug discrimination in social drinkers: relationship with subjective effects. Psychopharmacology. 2001;157:411–420. doi: 10.1007/s002130100817. [DOI] [PubMed] [Google Scholar]
- Jarvis MJ, Boreham R, Primatesta P, Feyerabend C, Bryant A. Nicotine yield from machine-smoked cigarettes and nicotine intakes in smokers: evidence from a representative population. J of NCI. 2001;93:134–138. doi: 10.1093/jnci/93.2.134. [DOI] [PubMed] [Google Scholar]
- Johanson C-E. Discriminative stimulus effects of psychomotor stimulants and benzodiazepines in humans. In: Glennon RA, Jarbe TUC, Frankenheim J, editors. Drug Discrimination: Applications to Drug Abuse Research. NIDA Research Monograph 116. Washington, DC: U.S. Government Printing Office; 1991. pp. 181–196. [PubMed] [Google Scholar]
- June KM, Norton KJ, Rees VW, O’Connor RJ. Influence of measurement setting and home smoking policy on smoking topography. Addict Behav. 2012;37:42–46. doi: 10.1016/j.addbeh.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marian C, O’Connor RJ, Djordjevic MV, Rees VW, Hatsukami DK, Shields PG. Reconciling human smoking behavior and machine smoking patterns: implications for understanding smoking behavior and the impact on laboratory studies. Cancer Epid Biomarkers Prev. 2009;18:3305–3320. doi: 10.1158/1055-9965.EPI-09-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney M, Green C, Hatsukami D. Nicotine self-administration: cigarettes versus nicotine gum diurnal topography. Hum Psychopharmacol. 2006;21:539–548. doi: 10.1002/hup.808. [DOI] [PubMed] [Google Scholar]
- Mumford GK, Evans SM, Kaminski BJ, Preston KL, Sannerud CA, Silverman K, Griffiths RR. Discriminative stimulus and subjective effects of theobromine and caffeine in humans. Psychopharmacology. 1994;115:1–8. doi: 10.1007/BF02244744. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Discriminative stimulus effects of nicotine in humans. In: Henningfield JE, London E, Pogun S, editors. Nicotine Psychopharmacology. New York: Springer-Verlag; 2009. pp. 369–400. [Google Scholar]
- Perkins KA. Nicotine discrimination in humans. In: Glennon RA, Young R, editors. Drug Discrimination: Application to Medicinal Chemistry and Drug Studies. New York: John Wiley & Sons; 2011. pp. 463–481. Chapter 15 in. [Google Scholar]
- Perkins KA, DiMarco A, Grobe JE, Scierka A, Stiller RL. Nicotine discrimination in male and female smokers. Psychopharmacology. 1994;116:407–413. doi: 10.1007/BF02247470. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Sanders M, Meeker J, Wilson A. Threshold doses for nicotine discrimination in smokers and nonsmokers. Psychopharmacology. 2001;155:163–170. doi: 10.1007/s002130000660. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Weiss D, Fonte C, Caggiula A. Nicotine preference in smokers as a function of smoking abstinence. Pharmacol Biochem Behav. 1996;55:257–263. doi: 10.1016/s0091-3057(96)00079-2. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. The reliability of puff topography and subjective responses during ad lib smoking of a single cigarette. Nicotine Tob Res. 2012;14:490–494. doi: 10.1093/ntr/ntr150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Kunkle N, Michael VC, Karelitz JL, Donny EC. Assessing discrimination of nicotine in humans via cigarette smoking. Nicotine Tob Res. doi: 10.1093/ntr/ntw082. (in press) in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger ST, Bevins RA. Interoceptive conditioning in rats: effects of using a single training dose or a set of 5 different doses of nicotine. Pharmacol Biochem Behav. 2013;114:82–89. doi: 10.1016/j.pbb.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL. Drug discrimination methods in human drug abuse liability evaluation. Br J Addiction. 1991;86:1587–1594. doi: 10.1111/j.1360-0443.1991.tb01752.x. [DOI] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE. Opioid discrimination in humans: discriminative and subjective effects of progressively lower training dose. Behav Pharmacol. 1998;9:533–543. doi: 10.1097/00008877-199811000-00009. [DOI] [PubMed] [Google Scholar]
- Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology. 2006;184:274–285. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- Rush CR, Critchfield TS, Troisi JR, Griffiths RR. Discriminative stimulus effects of diazepam and buspirone in normal volunteers. J Exper Anal Behav. 1995;63:277–294. doi: 10.1901/jeab.1995.63-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M, Stolerman IP. Brain sites mediating the discriminative stimulus effects of nicotine in rats. Behav Brain Res. 1996;78:183–188. doi: 10.1016/0166-4328(95)00245-6. [DOI] [PubMed] [Google Scholar]
- Smith JW, Stolerman IP. Recognising nicotine: the neurobiological basis of nicotine discrimination. In: Henningfield JE, London E, Pogun S, editors. Nicotine Psychopharmacology. New York: Springer-Verlag; 2009. pp. 295–333. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, LeSage MG. The reinforcement threshold for nicotine as a target for tobacco control. Drug Alc Depend. 2012;125:1–7. doi: 10.1016/j.drugalcdep.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Yoo S, Hill KP, Mooney M. Self-administration of intravenous nicotine in male and female cigarette smokers. Neuropsychopharmacol. 2008;33:715–720. doi: 10.1038/sj.npp.1301460. [DOI] [PubMed] [Google Scholar]
- SRNT subcommittee. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- St. Charles FK, Kabbani AA, Borderding MF. Estimating tar and nicotine exposure: human smoking versus machine generated smoke yields. Reg Tox Pharmacol. 2010;56:100–110. doi: 10.1016/j.yrtph.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Stolerman IP. Discriminative stimulus effects of nicotine in rats trained under different schedules of reinforcement. Psychopharmacology. 1989;97:131–138. doi: 10.1007/BF00443427. [DOI] [PubMed] [Google Scholar]
- Strasser AA, Ashare RL, Kaufman M, Tang KZ, Mesaros AC, Blair IA. The effect of menthol on cigarette smoking behaviors, biomarkers and subjective responses. Cancer Epid Biomarkers Prev. 2013;22:382–389. doi: 10.1158/1055-9965.EPI-12-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser AA, Ashare RL, Kozl.owski LT, Pickworth WB. The effect of filter vent blocking and smoking topography on carbon monoxide levels in smokers. Pharmacol Biochem Behav. 2005;82:320–329. doi: 10.1016/j.pbb.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Takada K. Drug discrimination studies in humans: a review of methodologies. Meth Find Exp Clin Pharmacol. 1996;18(suppl 1):187–196. [Google Scholar]
- U.S. Department of Health and Human Services (USDHHS) A Report of the Surgeon General. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease. [PubMed] [Google Scholar]
- U.S. Govt. Family Smoking Prevention and Tobacco Control Act, Pub. L. No. 111–31. 2009 http://www.gpo.gov/fdsys/pkg/PLAW-111publ31/pdf/PLAW-111publ31.pdf.