Abstract
Triple-negative breast cancer (TNBC) is an aggressive malignancy in which the tumors lack expression of estrogen receptor, progesterone receptor and HER2. Hence, TNBC patients cannot benefit from clinically available targeted therapies and rely on chemotherapy and surgery for treatment. While initially responding to chemotherapy, TNBC patients are at increased risk of developing distant metastasis and have decreased overall survival compared to non-TNBC patients. A majority of TNBC tumors carry p53 mutations, enabling them to bypass the G1 checkpoint and complete the cell cycle even in the presence of DNA damage. Therefore, we hypothesized that TNBC cells are sensitive to cell cycle targeted combination therapy, which leaves non-transformed cells unharmed. Our findings demonstrate that sequential administration of the pan-CDK inhibitor roscovitine prior to doxorubicin treatment is synthetically lethal explicitly in TNBC cells. Roscovitine treatment arrests TNBC cells in the G2/M cell cycle phase, priming them for DNA damage. Combination treatment increased frequency of DNA double strand breaks, while simultaneously reducing recruitment of homologous recombination proteins compared to doxorubicin treatment alone. Furthermore, this combination therapy significantly reduced tumor volume and increased overall survival compared to single drug or concomitant treatment in xenograft studies. Examination of isogenic immortalized human mammary epithelial cells and isogenic tumor cell lines found that abolishment of the p53 pathway is required for combination-induced cytotoxicity; making p53 a putative predictor of response to therapy. By exploiting the specific biological and molecular characteristics of TNBC tumors, this innovative therapy can greatly impact the treatment and care of TNBC patients.
Keywords: triple-negative breast cancer, combination therapy, CDK inhibition, doxorubicin, p53 mutated, DNA damage
Introduction
Triple-negative breast cancer (TNBC) is a clinical diagnosis that afflicts 10–20% of breast cancer patients (1). TNBC tumors are characterized by lacking expression of estrogen receptor (ER), progesterone receptor (PR) and the growth factor receptor HER2. TNBC patients are more likely to be premenopausal, of African descent, have a poor prognosis and have greater risk of recurrence within the first 5 years of diagnosis (2). Less than one-third of metastatic TNBC patients survive 5 years (3). About 70% of TNBC are classified as basal-like breast cancer (BLBC), and share many of the same characteristics, including more likely occurring in patients with BRCA1/BRCA2 gene mutations (4, 5). Dysfunction in the DNA repair pathway, resulting from BRCA-1 mutations, may contribute to TNBC patients initially responding well to chemotherapy; however, many patients’ tumors recur (6). While there are several targeted therapies being developed in clinical trials, including PARP and EGFR inhibitors, there are currently no clinically available and effective targeted therapies for TNBC patients. (6–8).
The majority (54–82%) of TNBC tumors harbor p53 mutations, enabling them to bypass the G1 checkpoint and complete the cell cycle even with unrepaired DNA damage (6, 9, 10). In comparison, only 13% of hormone-receptor positive luminal A tumors have p53 mutations (11). Moreover, 50% of these breast cancers overexpress cyclin D1, inhibiting retinoblastoma (Rb) regulation of E2F (12). Notably, overexpression of cyclin E serves as a poor prognostic marker in breast cancer and correlates to negative ER and PR status (13, 14). Owing to deregulation of the cell cycle in cancer cells, cyclin-dependent kinase (CDK) inhibitors were developed to prohibit tumor cell proliferation and induce apoptosis (15). However, CDK inhibitors have not been effective clinically, despite having promising results both in vitro and in vivo (16, 17). Roscovitine, a pan CDK inhibitor with activity against CDK1, 2, 5, 7 and 9 (18, 19) became the first orally bioavailable drug from this class to go into clinical trials based on the preclinical data showing induction of apoptosis in tumor cells. However, of the 77 solid tumor patients treated with single agent roscovitine, one partial response was seen in hepatocellular carcinoma, 2 prolonged stable disease observed in non small cell lung cancer (14 and >18 months) while stable disease was the best response seen in the remaining solid tumors (20–22). One of the reasons that these CDK inhibitors have not been more effective clinically is that they are either being used as single agents or when they are used in combination therapy, both agents were delivered concomitantly to the patient (23). Additionally, there was no attempt to identify those patients most likely to respond to these agents based on their biology. In fact, very few patients with breast cancer of any subtype were accrued to these trials.
CDK1 participates in the DNA double strand break (DSB) repair pathway homologous recombination (HR). HR faithfully repairs DNA DSBs that occur in late S, G2 and M (24). CDK activity is required for the recruitment of the endonucleases Sae2 or CtIP that excise the DNA DSB to generate single strands during HR in both yeast and mammalian cells, respectively (25, 26). Moreover, CDK activity is required for the recruitment and association of BRCA1 to the MRN (Mre11-Rad50-Nbs1) complex during HR (27). Concordantly, CDK inhibition with roscovitine reduced the recruitment of HR downstream protein RPA34 in irradiated sarcoma cells due to an inability to generate single strands (28). Thus, compromising HR via CDK inhibition may provide a strategy to augment TNBC cell sensitivity to chemotherapy.
No clinically available treatment strategies target the TNBC-deregulated cell cycle to exploit TNBC-cell sensitivity to DNA-damaging agents (e.g. chemotherapeutics). Because TNBC cells have a deregulated G1 checkpoint, enabling them to re-enter the cell cycle while harboring DNA damage, we hypothesized that TNBC cells are sensitive to cell cycle-targeted combination therapy, which leaves non-transformed cells unharmed. Ideally, this therapeutic strategy will be synthetically lethal against TNBC cells by inhibiting pathways that can compensate for one another during tumorigenesis. We therefore examined the effect of combining the pan-CDK inhibitor roscovitine and the chemotherapeutic doxorubicin both sequentially and concomitantly. Our data suggests that sequential treatment of roscovitine prior to doxorubicin is synthetically lethal explicitly in TNBC cells due to p53 pathway ablation.
Materials and Methods
Cell lines and culture conditions
The source of cell lines and culture conditions of the immortalized human mammary epithelial (HMEC) cell lines MCF-10A, 76NE6 and 76NF2V, the breast cancer cell lines MDA-MB-157, MDA-MB-231, HCC1806, MCF-7, ZR75-1, T47D, MDA-MB-468 (Supplemental Table 1A) and the isogenic colorectal cancer cells HCT116 p53 wildtype (p53+/+) and p53 knockout (p53−/−) were previously described (29–31). HEK-293T cells for lentiviral packaging were maintained in DMEM supplemented with 10% FCS. All cells were free of mycoplasma contamination, and their identities were authenticated by karyotype and short tandem repeat analysis using the MD Anderson’s Characterized Cell Line Core Facility (http://www.mdanderson.org/characterized-cell-line-core-facility/index.html) on a routine basis (every 6 months).
Immunofluorescence to detect DNA repair foci
Following treatment of cells with roscovitine (IC50) for 24 hours, doxorubicin (IC50) for 48 hours or both drugs sequentially as indicated, they were fixed in 4% paraformaldehyde for 20 minutes followed by permeabilization with a 0.3% triton solution (20mM Hepes, 50mM NaCl, 3mM MgCl2, 300mM sucrose and TritonX-100) for 20 minutes. Cells were then blocked with 10% BSA and 2% horse serum PBS for one hour. Antibodies were diluted 1:500 and 1:1000 for anti-γ-H2AX (EMD Millipore) and anti-Rad51 (32), respectively, and incubated at 4°C overnight. Secondary goat anti-mouse or goat anti-rabbit antibodies (Alexa Fluor 594 and 488, respectively, EMD Millipore) were diluted at 1:750 and incubated at room temperature at 1 hour. Nuclei were stained with DAPI (Life Technology, Grand Island, NY) at 1μg/ml for 5 minutes room temperature. Cells were mounted with Dako fluorescent mounting medium (Carpentaria, CA). Images were captured using the Olympus FV1000 Laser Confocal Microscope (Tokyo, Japan). Cells with ≥5 foci were considered γ-H2AX positive.
Neutral comet assay
A neutral comet assay was performed according to Trevigen (Gaithersburg, MD) protocols to measure DNA double-strand breaks (DSBs) in HMEC and TNBC cells in response to single and combination drug treatment. Briefly, following treatment, cells were harvested and combined with low-melting agarose, and spread onto CometSlides (Trevigen). Following cell adherence to the slides, cells were lysed with Trevigen lysis solution. The slides were then placed in an electrophoresis chamber with neutral electrophoresis buffer. A current of 21 V was used for 21 minutes. After drying, samples were stained with SYBR Green I and allowed to dry at room temperature in the dark. Images of nuclei were captured using an Eclipse 90i microscope equipped with the NIS-Elements Br 3.10 software program (Nikon, Tokyo, Japan). The tail moment was measured using the CometScore software program (TriTek, Sumerduck, VA).
Xenograft studies
4.5x106 MDA-MB-468 cells in a 1:2 ratio with matrigel (BD Bioscience) were injected into the mammary fat pad of 4- to 6-week-old female Balb/c Nu/nu mice (Taconic, Germantown, NY). Mice received injections on both the right and left flanks. Once the tumors reached a volume (L x W2/2) of 100–150 mm3, mice were treated with vehicle + vehicle, roscovitine (50 mg/kg) 4 days on/3 days off, doxorubicin (2mg/kg) once a week, sequentially with 4 days of roscovitine followed by 1 day of doxorubicin, or concomitantly with 4 days of roscovitine and 1 day of doxorubicin administered on day 4 for four cycles. All drugs were administered i.p. Roscovitine was diluted at 100 mg/ml in DMSO and then further diluted to 10 mg/ml in a carrier solution consisting of 10% Tween 80 (Sigma Aldrich), 20% N-N-dimethylacetamide (Acros Organics, Geel, Belgium) and 70% polyethylene glycol 400 (Sigma-Aldrich) (33). The tumor volume and weight of the mice were measured twice a week. Mice were euthanized when the total tumor burden (sum of right and left tumors) was equal to 1500 mm3. Mice were housed five per cage in sterilized micro-isolator cages (Lab Products, Seaford, DE) furnished with corncob bedding. Mice received care in accordance with the Animal Welfare Act, the National Institutes of Health “Guide for the Care and Use of Laboratory Animals,” and the institutional guidelines of MD Anderson Cancer Center.
Statistical analysis
Each experiment was carried out at least three times. Continuous outcomes were summarized with mean and standard deviations. Comparisons among groups were conducted using Graph Pad Prism software (La Jolla, CA) using either unpaired or paired Student t test with a 95% confidence interval. Overall survival curves were generated with the Kaplan-Meir method and compared using the Mantel-Cox test (Graph Pad Prism Software).
Results
Sequential combination treatment with roscovitine followed by doxorubicin synergistically inhibits TNBC cells
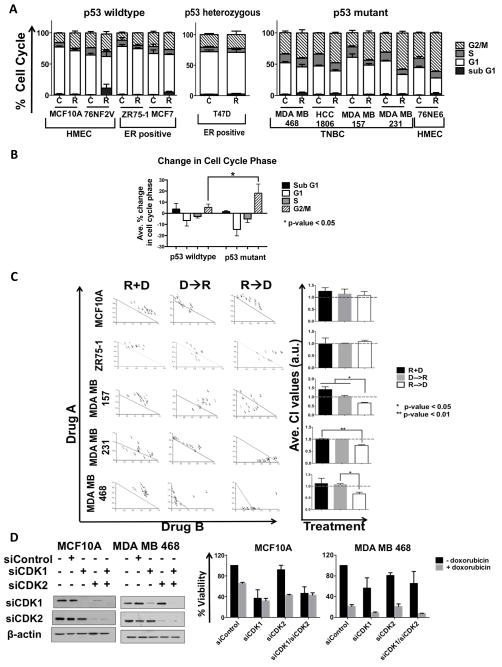
To interrogate if treatment of breast cancer cells with roscovitine would alter their cell cycle profiles based on their different receptor and p53 status, we treated 10 different cell lines, including 3 human mammary epithelial cell lines (HMEC), 3 estrogen and progesterone (ER/PR) positive cell lines and 4 triple negative cell lines, (Supplemental Table 1A) with 20 μM roscovitine for 24 hours followed by flow cytometric analysis (Figure 1A). Roscovitine induced only a 10 percent or less increase in G2/M phase in the p53 wild type HMEC cells (MCF-10A and 76NF2V), p53 wild type ER/PR positive cells (ZR75-1 and MCF-7), and p53 heterozygous ER/PR positive cells (T47D) (34). In contrast, treatment of the p53 mutated TNBC cell lines (MDA-MB-468, MDA-MB-157, HCC 1806) and p53 inactivated HMECs (76NE6) resulted in a 30% and 20% increase of G2/M phase, respectively, demonstrating a significantly greater change (p-value < 0.05) in the G2/M phase with compared to p53 wildtype cells (Figure 1A, 1B, Supplemental Figure 1A).
Figure 1. Sequential administration of roscovitine and doxorubicin is synergistic only in TNBC cells.
(A) A panel of p53 wild type, p53 heterozygous and p53 mutant breast cells, including immortalized human mammary epithelial cells (HMEC cells; MCF-10A, 76NF2V and 76NE6), estrogen receptor positive (ER positive cells; T47D, MCF-7, ZR-75-1) and triple-negative breast cancer (TNBC cells; MDA-MB-468, HCC-1806, MDA-MB-157, and MDA-MB-231) cells were treated with roscovitine (R) at 20 μM for 24 hours followed by flow cytometry for cell cycle analysis. Untreated cells harvested at the same time served as a control (C). (B) Average percent change in cell cycle phase following treatment of roscovitine as compared to untreated cells according to p53 status. The p53 wildtype cell liens included were MCF10A, 76NF2V, MCF7 and ZR75-1; p53 mutant/non-functional cell lines included were 76NE6, MDA MB 468, HCC1806, MDA MB 157 and MDA MB 231 (C), HMEC (MCF-10A), ER positive (ZR75-1) and TNBC (MDA-MB-157, MDA-MB-231, and MDA-MB-468) cells were subjected roscovitine and doxorubicin (D) combination treatment in 96-well plates as described in the Materials and Methods. Drugs were administered both simultaneously (R+D) for 72 hours and sequentially in both directions (D→R or R→D). During sequential treatment the first drug, or drug A, was administered at IC10, IC25 and IC50 concentrations for 24 hours. Following removal of drug A, drug B was administered at IC10-IC50 concentrations for 48 hours. The IC values for all treatment conditions are listed in Supplemental Figure 1B. Following 72 hours of drug treatment, cells were cultured in drug-free medium for an additional 9 days where drug-medium was replaced every 48 hours. Cells were subjected to MTT assay on day 12. Isobolograms and combination index values (CI) were generated using CalcuSyn, in which each point refers to a specific combination of drug A to drug B. CI values <1.0 are synergistic, CI values =1.0 are additive and CI values >1.0 are antagonistic. Average CI values per combination treatment are shown in the bar graphs. (D), MCF10A and MDA MB 468 cells were transiently transfected with siRNA against CDK1, CDK2 or both. Non-targeting siRNA (siControl) served as a control. Western blot analysis with antibodies against CDK1 and CDK2 was used to confirm efficiency of downregulation. Next, transfected cells were subjected to HTSA either in the presence of absence of doxorubicin at IC50 values (30nM for MCF-10A and 25nM for MDA-MB-468 cells), Supplemental figure 1B) for 48 hours. After 48 hours, doxorubicin-containing medium was replaced with drug-free medium every 48 hours until day 12 when cell viability was assessed with MTT. Percent viability for each condition was calculated as compared to siControl is plotted in the bar graphs. For all panels, experiments were repeated three times, and error bars are 95% confidence intervals. The student t-test (two-tailed, equal variance) was employed to derive the p-values.
These results raised the hypothesis that sequential treatment of TNBC cells by roscovitine would accumulate them in the G2/M phase, and when followed by a DNA damaging agent, such as doxorubicin, would lead to synergistic cell killing. We tested this hypothesis by examining the schedule and duration of combination therapy with roscovitine in a panel of 5 cell lines, including HMEC MCF-10A cells, ER positive ZR75-1 and TNBC MDA-MB-157, MDA-MB-231 and MDA-B-468 cell lines (Figure 1C). A high throughput survival assay (HTSA) was used as a means for determining cell survival. This assay evaluates in vitro survival of cells in response to different treatment strategies, either as single agents or in combination (35, 36). As depicted in the diagram (Supplemental Figures 1B and Supplemental 1C), each cell line was treated with three different combination strategies: a) concomitant treatment with roscovitine and doxorubicin; b) sequential treatment with doxorubicin followed by roscovitine and c) sequential treatment with roscovitine followed by doxorubicin. At the conclusion of the experiment (12 days), cell viability was assessed based upon a combinational index (CI) algorithmic that the statistical program CalcuSyn provides. CI values of 0–0.9 are indicative of synergistic response while CI values of 0.9–1.1 or >1.1 indicate an additive or antagonistic response, respectively (37). The CI values demonstrated that concomitant treatment induced an antagonistic or additive response in all cell lines (Figure 1C, R + D). Sequential administration of doxorubicin treatment prior to roscovitine also induced antagonism or additivity in all cell lines (Figure 1C, D → R). However, administration of roscovitine prior to doxorubicin induced a synergistic response only in TNBC cell lines, but an antagonistic response in the p53 wild type HMEC and ER positive cell lines (Figure 1C, R → D) with a significantly lower (p-value <0.05) CI value in all TNBC cell lines (Figure 1C). Furthermore, when TNBC cells were treated with roscovitine first for 24 hours followed by another 48 hours concomitantly with doxorubicin, we measured synergism and additivity (Supplemental Figure 2A). To examine the specificity of the synergistic response to anthracyclines, we also performed sequential treatment of TNBC cells with roscovitine followed by the antimicrotubule chemotherapeutic taxol, which induced an additive and antagonistic response in MDA-MB 157 and MDA-MB 231 cells, respectively (Supplemental Figure 2B). Therefore, only sequential combination treatment of roscovitine followed by doxorubicin can specifically inhibit TNBC cells, and not HMEC or ER positive cells.
Next, we examined whether inhibition of CDK1, CDK2 or both CDKs simultaneously was required for synergism with doxorubicin treatment. MCF-10A, ZR75-1 and MDA-MB-468 cells were transiently transfected with siRNA against either CDK or both, with non-targeting siRNA used as a control (Figure 1D and Supplemental Figure 2C–2D). Cell viability was assessed by HTSA using siCDK1or siCDK2 as single agents and in combination with doxorubicin. While siCDK1 or siCDK1/siCDK2 reduced cell viability in MCF-10A by 50–60%, there was no further reduction in viability in these cells with the addition of doxorubicin. Knockdown of CDK2 did not decrease percent viability, however the addition of doxorubicin treatment again reduced viability by 50–60% in MCF-10A cells (Figure 1E ). Similar results were seen with ZR75-1 cells (Supplemental Figure 2D). Knockdown of CDK1 or both CDKs simultaneously reduced viability by about 50% in MDA-MB-468 cells, whereas siCDK2 caused only 20% cell inhibition. However, the addition of doxorubicin to either siCDK1 or siCDK1/siCDK2 knockdown cells caused 90% cell inhibition only in the TNBC MDA-MB-468 cells but not in the MCF-10A (Figure 1E) or ZR75-1 cells (Supplemental Figure 2D). These results suggest that inhibition of CDK1 combined with doxorubicin treatment is sufficient to induce synergistic cell inhibition in TNBC but not in non-TNBC cells.
Combination treatment increases apoptosis specifically in TNBC cells
To examine cell death in TNBC and HMEC cells in response to single and combination treatments both during and following drug treatment, we used both dye-exclusion assay with propidium iodide (PI) and PARP-1 cleavage as readouts for apoptosis. PI positivity is detected when the cell membrane is compromised, a characteristic of apoptosis (38) as depicted by the use of staurosporine as a positive control in our assays (Figure 2A). Roscovitine-induced PI positivity peaked at 24 hours post drug exposure with no further increase up to 9 days post treatment (Figure 2A, left panel). Doxorubicin treatment steadily reduced cell viability over time; with MDA-MB-157 cells showing over 30% PI positivity at 9 days post drug removal (Figure 2A, middle panel). Combination-sequential treatment of cells with roscovitine followed by doxorubicin induced only a 15% PI positivity in MCF-10A cells, while the combination treatment induced 45% and 30% PI positivity in MDA-MB-157 and MDA-MB-468 cells, respectively. Moreover, only TNBC cells continued to exhibit decreased cell viability, or PI positivity, following release from treatment with maximum PI positivity at 72 hours post treatment in MDA-MB-157 and 120 hours post treatment in MDA-MB-468 cells (Figure 2A, right panel).
Figure 2. Sequential combination treatment of roscovitine and doxorubicin induces apoptosis in TNBC cells.
(A) HMEC (MCF-10A) and TNBC (MDA-MB-157 and MDA-MB-436) cells were treated with roscovitine (R) at IC50 concentrations (13 μM MCF-10A and 32μM and 24μM for MDA-MB-157 and MDA-MB-468, respectively), for 24 hours, doxorubicin (D) (20nM for MCF-10A, 15 nM for MDA-MB-157 and 21 nM for MDA-MB-468) for 48 hours or roscovitine followed doxorubicin (R→D), each at the aforementioned IC50 values for each cell lines. Cell death was assessed directly following drug treatment and after (24 hours,72 hours, 120 hours or 9 days) release into drug-free medium using a dye-exclusion assay with propidium iodide (PI) on unfixed cells using flow cytometry. Staurosporine (S) served as a positive control for each cell line. (B), HMEC and TNBC cells were treated with R, D and R→D (at IC50 values, Supplemental Figure 1B) and harvested immediately following drug treatment or at indicated times post release. Western blot analysis was used to detect full length and cleaved PARP-1. Staurosporine (S) was used as a positive control. Densitometry analysis was performed using Image J. The ratio of cleaved to full length PARP was determined and plotted as bar graphs. (C and D), MCF10A and MDA MB 468 cells were transfected with siControl, siCDK1, siCDK2 or both siCDK1/siCDK2 with or without the sequential addition of doxorubicin (at IC50 concentrations-20nM for MCF-10A and 21nM for MDA-MB-468 cells) treatment for 48 hours. Cells were harvested following drug treatment (top) or 48 hours post release from drug treatment (bottom). Western blot analysis was used to detect full length and cleaved PARP1 in each condition. Densitometry analysis was performed using Image J. The ratio of cleaved to full length PARP was determined and plotted as bar graphs.
PARP-1 cleavage was not observed in MCF-10A cells following any of the treatment arms, or following drug removal (Figure 2B and Supplemental Figure 2E). However, both TNBC cell lines showed sustained levels of PARP-1 cleavage both during and following release from combination treatment (Figure 2B). For example, MDA-MB-468 cells had the highest ratio of PARP-1 cleavage at 24 hours during the combination treatment, which was sustained up to 72 hours post release (Figure 2B) suggesting a persistent apoptotic signal only in TNBC cells and not in MCF-10A cells (Supplemental Figure 2E).
We also examined if the synergism observed between CDK1 siRNA + doxorubicin (Fig 1C–1D) also resulted in higher PARP-1 cleavage. Western blot analysis revealed that MCF-10A cells, showed no change (compared to siControl) in their ability to cleave PARP-1 following either CDK1/2 siRNA or treatment with doxorubicin (Figure 2C top). In contrast, knockdown of CDK1, CDK2 or both increased PARP-1 cleavage in MDA-MB-468 cells compared to siControl transfected cells. Moreover, the addition of doxorubicin to CDK knockdown cells increased cleaved PARP-1 expression (Figure 2D, top). Forty-eight hours post treatment, MCF-10A knockdown and combination treated cells showed complete recovery, expressing only full-length PARP-1 (Figure 2C, bottom). However, MDA-MB-468 cells had persistent cleaved PARP-1 expression in both CDK transiently knockdown cells in the presence or absence of doxorubicin 48 hours post treatment (Figure 2D, bottom). These findings suggest that TNBC cells do not readily recover from pharmacological inhibition or molecular knockdown of CDK combined with doxorubicin, leading to an enduring apoptotic signal.
TNBC bypass the G1 checkpoint in response to combination treatment
The sequential combination treatment of roscovitine followed by doxorubicin resulted in an accumulation of cells in G2/M and generated a polyploid population only in TNBC cell, with 55% of MDA MB 231 cells accumulating in G2/M (Figure 3A and Supplemental Figure 3A). The TNBC polyploid population increased by 20% following the sequential combination therapy, compared to untreated cells, while HMEC cells did not accumulate in G2/M or increase their polyploid population following any of the treatments (Figure 3A). The G2/M and polyploidy population induced by concomitant treatment (45%) was less than sequential (55%) treatment for TNBC cells (Supplemental Figure 3B).
Figure 3. Sequential combination treatment of roscovitine and doxorubicin induces a G2/M arrest and accumulation of polyploidy population.
(A), MCF10A and MDA MB 231 cells were treated with roscovitine (24 hours), doxorubicin (48 hours) or sequential combination treatment followed by cell cycle analysis with flow cytometry. Drugs were administered at IC50 concentrations (see Supplementary Figure 1B). (B), HMEC (MCF-10A) and TNBC (MDA-MB-231 and MDA-MB-468) cells were transfected with siControl, siCDK1, siCDK1 or both siCDK1/siCDK2. Western blot analysis was used to confirm knockdown of CDK1 and CDK2. (C), Cell cycle analysis was performed on HMEC (MCF-10A) and TNBC (MDA-MB-231 and MDA-MB-468) siRNA transfected cells in the absence or presence of doxorubicin (48 hours at IC50 concentration-See Supplementary Figure 1B). (D), MCF10A and MDA MB 157 cells were treated with roscovitine (R), doxorubicin (D) or combination treatment (R→D) at IC50 concentrations, harvested (using RIPA buffer) and subjected to Western blot analysis, with the indicated antibodies. Actin was used for equal loading determination. (E), Image J was used to measure relative protein expression of each antigen. Protein expression was normalized to actin.
Transient knock down of CDK1, CDK2 of both CDKs (Figure 3B) revealed that HMEC cells only had about a 10% increase in G2/M upon CDK knockdown, with the addition of doxorubicin either reverting cells to a G1 accumulation or causing no change in their cell cycle profiles (Figure 3C). Conversely, in TNBC cells, knockdown of CDK1 or CDK1/CDK2 lead to a G2/M accumulation that was augmented with the addition of doxorubicin. In MDA-MB-468 cells, 80% of the cells accumulated in the G2/M when the knockdown of CDK1 or CDK1/CDK2 was combined with doxorubicin treatment as compared to single drug treatments. There was no additional gain in the G2/M phase when both CDKs were knocked down in the presence of doxorubicin compared to CDK1 knockdown plus doxorubicin in TNBC cells (Figure 3C).
In MCF-10A cells the expression of both total and phospho-Rb was reduced, while the expression of p21 and p27 were both increased following single and combination treatment (Figure 3D, 3E). In contrast, treatment of the TNBC MDA-MB-157 cells with single or combination treatment increased both total and phospho Rb with minimal changes in p21 or p27 expression (Figure 3D, 3E). These results suggest that the G1 checkpoint activation is intact only in the HMEC cells. Additionally, p21 was also transcriptionally activated upon combination treatment only in MCF-10A cells (data not shown), suggesting that wild-type p53-induced a G1 arrest that may protect MCF-10A cells against the toxic effects of combination therapy.
Loss of wild-type p53 activity is required for roscovitine/doxorubicin synergistic response
The results from the cell line panel study (Figure 1) suggested that absence of a wild-type p53 could predict for a synergistic response of roscovitine followed by doxorubicin treatment. Since the majority of TNBC cell lines identified to date harbor p53 mutations (39), we utilized two different isogenic cell systems to test this hypothesis: breast epithelial cells (HMECS) 76NF2V and 76NE6 and colorectal cancer cells HCT116 p53+/+ and HCT116 p53−/− cell lines. 76NE6 cells were immortalized from 76N primary HMEC cells via transfection with the viral oncoprotein HPV16-E6, which binds to and degrades p53; while 76NF2V cells were immortalized with the F2V mutant HPV-16E6 gene, which renders cells immortalized but with a functional and will-type p53 (40, 41). Sequential administration of roscovitine followed by doxorubicin resulted in an additive response in p53 wild type cells 76NF2V, while it induced synergism in the p53 inactive 76NE6 cells (Figure 4A), with twice as many cells with polyploid nuclei in 76NE6 compared to 76NF2V cells (Figure 4B). Levels of phospho-and total Rb were reduced following single and combination therapy in 76NF2V cells compared to 76NE6 cells. Additionally, p21 levels were induced only in 76NF2V cells, in response to combination treatments (Figure 4C).
Figure 4. Absence of p53 is required for synergism between roscovitine and doxorubicin.
(A), Isogenic HMEC cells 76NF2V and 76NE6 cells were subjected to sequential roscovitine-doxorubicin treatment via HTSA followed by CalcuSyn analysis. The concentrations used for each agent is listed in Supplemental Figure 1B. Average combination index (CI) are shown for each cell line. (B) 76NF2V and 76NE6 cells were treated with roscovitine (R) for 24 hours, doxorubicin (D) for 48 hours or sequential combination treatment (R→D) at IC50 values (Supplemental Figure 1B). Following fixation, samples were subjected to cell cycle analysis via flow cytometry. Untreated (C) samples served as a control. (C) Cells were treated with R, D or R→D at IC50 values (Supplemental Figure 1B) followed by Western blot analysis of G1 checkpoint proteins. (D), Lentivirus was used to generate stable Rb knockdown 76NF2V cells. Western blot analysis of total Rb was used to confirm knockdown compared to parental and stably expression non-targeting shScamble cells. Rb knockdown and shScramble cells were treated with sequential roscovitine-doxorubicin treatment via HTSA followed by CalcuSyn analysis. Average combination index (CI) are shown for the shSramble the two shRB variants of 76NF2V. (E) Western blot analysis of p53 expression in HCT116 p53−/− cells compared to wildtype HCT116 p53+/+ and MCF10A cells. (F), HCT116 wildtype and knockout cells were subjected to roscovitine-doxorubicin combination treatment via HTSA followed by CalcuSyn analysis. The concentrations used for each agent is listed in Supplemental Figure 1B. Average combination index (CI) are shown for each cell line. (G), Western blot analysis was performed to examine the effect of single and sequential combination drug treatment on G1 checkpoint proteins of HCT116 p53 wildtype and knockout cells. Actin was used as a loading control. (H) HCT116 wildtype and knockout cells treated with R→D at IC50 concentration of each drug and cells were removed either immediately post treatment or 24 hours after drug treatment (R+D+24) were subjected to cell cycle analysis.
Since drug treatment reduced phospho-Rb expression in both MCF-10A (Figure 3D) and 76NF2V (Figure 4C) cells, while remaining elevated in MDA-MB-157 cells, we interrogated if loss of Rb would render 76NF2V cells more synergistic to the combination treatment. Rb was stably knocked down in 76NF2V cells (Figure 4D) and subjected to HTSA followed by CalcuSyn analysis (Figure 4D). Roscovitine-doxorubicin combination treatment induced only additivity or antagonism in the Rb knocked down cells (Figure 4D). Moreover, ablation of the Rb pathway did not cause 76NF2V cells to accumulate more in the G2/M phase compared to shScramble cells (Supplemental Figure 3B). Therefore, Rb inactivation is not sufficient to cause roscovitine-doxorubicin-induced synthetic lethality in HMEC cells.
When HCT116 p53+/+ and HCT116 p53−/− cells were subjected to HTSA and CalcuSyn analysis, p53 wildtype cells responded antagonistically to combination treatment, while deletion of p53 induced synergism in HCT116 cells (Figure 4E, 4F). Sequential roscovitine-doxorubicin treatment reduced phospho-Rb expression in p53 wildtype cells. Moreover, both single and combination drug treatment caused increased expression of p27 and p21 only in the in p53 wildtype cells (Figure 4G). Lastly, combination treatment increased G2/M accumulation and polyploidy in both p53 wildtype and knockout cells. However, combination-treated p53 knockout cells had twice the amount of Sub-G1 cells compared to p53 wildtype cells both during and after treatment (Figure 4H) suggesting that the loss of p53 is required for synergism in tumors cells.
Combination-based treatment increased DNA-double-strand breaks and decreased DNA repair
Since CDK1 activity is required for homologous recombination (HR), we next hypothesized that this combination treatment strategy mediates its activity through the alteration of DNA damage response. To this end, we initially examined a neutral comet assay to quantitate DNA double strand breaks (DSB) in our panel of HMEC and TNBC cells following single and combination treatments (Figure 5A). The read-out of the neutral comet assay is the tail moment, or the amount of DNA in the distance traveled, which measures the extent of DNA DSBs. As expected, doxorubicin treatment induced DNA DSBs, indicated by an increased tail moment, in all cell lines examined (Figure 5A). However, administering roscovitine prior to doxorubicin caused a significant (p value < 0.05) increase in the amount of DNA DSBs only in TNBC cells. Furthermore, doxorubicin treatment induced γ-H2AX foci, a marker of DNA DSBs, in MCF-10A cells and both TNBC cell lines compared to untreated control cells (Figure 5B, C, D). However, treatment with roscovitine prior to doxorubicin significantly increased (p-value < 0.05) the percentage of γ-H2AX positive cells by 20% explicitly in TNBC cells with no increase in MCF-10A cells (Figure 5B, C, D). Moreover, western blot analysis revealed that only combination treatment induced an increase in phospho-H2AX (γ-H2AX) in TNBC MDA MB 157 cells, but not in HMEC MCF-10A cells (Figure 5B).
Figure 5. Sequential combination treatment of roscovitine and doxorubicin causes more DNA double strand breaks while simultaneously reducing downstream homologous recombination proteins.
(A), Neutral comet assay was performed on HMEC (MCF-10A) and TNBC (MDA-MB-157 and MDA-MB-468) cells that were treated with roscovitine (R) for 24 hours, doxorubicin (D) for 48 hours or sequential combination treatment (R→D) at IC50 concentrations (Supplemental Figure 2B). Samples were subjected to a current of 21V for 21 minutes and subsequently stained with SYBR green for visualization (right panels). Images were captured at 10x magnification with an Eclipse 90i microscope equipped with the NIS-Elements Br 3.10 software program. The tail moment (the amount of DNA in the distance traveled) was measured using Comet Score software and plotted as bar graphs (left panel). (B) (A) MDA MB 157aand MCF-10A cells were treated with roscovitine (R), doxorubicin (D) or sequential combination treatment (R→D). Untreated cells were used as a control (C). Following treatment, cells were harvested and Western blot analysis was performed to detect expression of phospho-H2AX and Rad51. (C and D), MCF10A and MDA MB 231 cells were treated with single and combination drug treatment as in panel A and B. DNA repair foci γ-H2AX and Rad51 were detected with immunofluorescence. Dapi was used to detect nuclei. Images were captured at 100X with an Eclipse 90i microscope. (E) Percent of γ-H2AX positive were quantified in HMEC and TNBC cells in response to single and combination drug treatment. Only cells with ≥ 5 foci were considered as positive. One hundred cells per sample, per replicate were counted. (F), Percent of γ-H2AX positive cells with Rad51 foci recruitment were quantified. Cells with ≥ 1 Rad51 foci were considered positive. Images for quantification were captured at 60X with a confocal Olympus FV100 microscope. Experiments were repeated three times, and error bars are 95% confidence intervals. The student t-test (two-tailed, equal variance) was employed to derive the p-values.
Quantification of γ-H2AX positive cells with Rad51 foci was used next to examine the recruitment of downstream HR proteins. Untreated control HMEC and TNBC cells had limited DNA damage, but had over 60% or over 80% cells with Rad51 positivity when γ-H2AX was present, respectively (Figure 5C, D, E). Roscovitine treatment decreased the recruitment of Rad51 in both MCF-10A and MDA-MB-157 cells. Additionally, roscovitine treatment reduced Rad51 protein expression in both MCF-10A and MDA-MB-157 cells (Figure 5B). However, combination treatment significantly reduced (p-value < 0.05) the formation of Rad51 foci compared to doxorubicin treatment only in TNBC cells even though these cells had increased γ-H2AX positivity (Figure 5C–5F). Combination and doxorubicin only treated MCF-10A cells were able to recruit Rad51 foci at a similar percentage (Figure 5 C, 5F). In parallel, while Rad51 expression decreased in combination treated MDA-MB-157 cells, it remained elevated in MCF-10A combination treated cells (Figure 5B). These results suggest that treating TNBC cells with roscovitine prior to doxorubicin can enhance DNA damage while impairing their ability to repair DNA DSBs, regardless of increased DNA damage.
Deletion of p53 allows combination treatment to increase DNA DSBs
HCT116 p53+/+ and HCT116 p53−/− were used to determine the effect of p53 on DNA damage and repair. Presence or absence of p53 had little effect on doxorubicin-induced DNA damage, with 41% and 48% of p53 wildtype and p53 knockout cells having γ-H2AX positivity, respectively (Supplemental Figure 4 A, B, C). However, combination treatment significantly increased (p-value <0 .01) γ-H2AX positive cells only in p53 knockout cells compared to doxorubicin treated cells. Combination treatment did not augment γ-H2AX positivity in p53 wildtype cells. Moreover, combination treated p53 knockout cells had significantly more (p-value <0 .01) γ-H2AX positive cells (by 20%) compared to combination treated p53 wild type cells (Supplemental Figure 4C).
Furthermore, upon doxorubicin treatment, p53 wild type and p53 knockout cells recruited Rad51 to γ-H2AX sites in 63% and 59% of cells, respectively (Supplemental Figure 4A, B, D). Combination treatment had no effect on Rad51 recruitment in p53 wild type cells. However, p53 knockout cells had a significant (p-value <0 .05) decrease in the percent of γ-H2AX positive cells with Rad51 foci compared to doxorubicin treated cells (Supplemental Figure 4A, B, D). Despite having more G2/M cells, p53 knockout cells had less recruitment of Rad51 foci that p53 wild type cells during combination treatment (Figure 4G, Supplemental Figure 4A, B, D). Collectively, these results suggest that deletion of p53 causes tumor cells to have increased DNA damage and reduces the ability of tumor cells to recruit downstream HR proteins in response to roscovitine-doxorubicin combination treatment.
Combination treatment increases overall survival and decreases proliferation in human breast cancer xenograft
MDA-MB-468 cells were used to establish human xenograft tumors in the right and left mammary fat pads of nude mice (Figure 6A). There were five treatment arms: vehicle, roscovitine for 4 days on/3 days off, doxorubicin once a week, concomitant treatment of roscovitine with doxorubicin administered on day 4, or sequential treatment of roscovitine followed by doxorubicin for 4 cycles. Sequential combination treated tumors were the only arm of the study that did not increase in volume while on treatment. Averaging at 125 mm3, sequential combination treated tumors were significantly smaller (p-value <0.01), than vehicle treated tumors that averaged to be 330mm3 on day 26 (Figure 6B, Supplemental figure 5A, C). Indeed, concomitantly treated mice had a 3-fold increase in tumor size on day 26 (Supplemental Figure 5C). Moreover, sequential combination treated tumors were significantly smaller (p-value <0.05) than roscovitine, doxorubicin and concomitant treated tumors during and following treatment (Figure 6B, Supplemental Figure 5A, C). Notably, no measurable difference was observed between vehicle and roscovitine treated mice; supporting clinical findings that roscovitine is inefficient as a single agent (Figure 6A).
Figure 6. Sequential roscovitine-doxorubicin combination treatment is effective and well tolerated in vivo.
(A), A schematic illustrating that 4.5x106 MDA-MB-468 cells in a 1:2 ratio with matrigel were injected into the right and left mammary fat pads of the 4 groups of of 4- to 6-week-old female Balb/c Nu/nu mice. (B), Once tumors reached 100–150 mm3, they were treated with vehicle, roscovitine (50mg/kg) 4 days on/3days off, doxorubicin (2mg/kg) once a week, or combination treatment for four cycles. Weekly tumor volume measurements shown (C), Overall survival graphed as Kaplan Meier curve. Mice treated with concomitant combination drug treatment (4 days of roscovitine with doxorubicin administered on day 4) included in survival analysis. Mice were euthanized if total tumor burden was 1500 mm3 or if mice lost ≥ 20% of initial body weight. Statistical analysis was performed using Mantel-Cox method. (D), Percent BRDU positive cells were averaged from three tumors resected on day 26 per each treatment arm. (E) Western blot analysis performed on tumors resected on day 26. Densitometry analysis performed using Image J.
Additionally, none of the sequential combination treated mice suffered from increased toxicity or tumor burden during the 60-day experiment (Figure 6D and Supplemental Table 2). As such, sequential combination therapy significantly increased overall survival (p-value < 0.05) compared to vehicle, single agent and concomitant treated mice (Figure 6C). Overall toxicity was assessed by weight loss during and following treatment. Neither the roscovitine alone arm nor the combination arm animals suffered any weight loss, while 80% and 50% of doxorubicin and concomitant treated mice had to be sacrificed due to >20% weight loss, respectively, again revealing the limitations of doxorubicin as monotherapy and the importance of drug delivery scheduling (Figure 6C, Supplemental figure 5E, Supplemental Table 2).
These results suggested that treatment with roscovitine could limit doxorubicin-induced toxicities. To test this, a dose escalation study examining weight loss and blood count was performed on non-tumor bearing nude mice that were treated with vehicle, roscovitine (50mg/kg), doxorubicin (5mg/kg or 10 mg/kg) or sequential therapy. At 1 week into treatment, sequential treated mice maintained normal weights compared to doxorubicin treatment at 5mg/kg. However, as the amount of doxorubicin accumulated, the protective of roscovitine against weight loss diminished (Supplemental Figure 6A). Following the completion of treatment, blood was collected from all mice. Treatment of roscovitine prior to doxorubicin was able to rescue the reduction in the white blood cell (WBC) count caused by doxorubicin alone (Supplemental Figure 6B). Additionally, doxorubicin significantly (p- value < .05) reduced the platelet count in mice. However, sequentially treated mice had twice as many platelets (Supplemental Figure 6C). None of the treatments affected the red blood cell count (RBC) (Supplemental Figure 6D). Therefore, sequential combination therapy rescues doxorubicin-induced neutropenia and platelet-loss.
To assess proliferation of tumors in each treatment arm, BRDU incorporation was measured at the end of treatment (day 26) and revealed that both doxorubicin and combination treated tumors had a significant (p-value < 0.05) decrease in proliferation compared to vehicle treated tumors (Figure 6D). Western blot analysis of PARP-1 on tumors resected on day 26 revealed that combination therapy significantly increased cleaved PARP-1 expression (p-value <0.05) compared to doxorubicin treated tumors, indicative of apoptosis (Figure 6E). Based on hemotaoxilyn-eosin staining, tumors from all four treatment arms had similar histology, with combination-treated tumors having marked fibrous (Supplemental Figure 5D). Overall, this data suggests that roscovitine-doxorubicin combination therapy is both well tolerated and efficacious against TNBC.
Discussion
Here we describe that sequential administration of roscovitine followed by doxorubicin is a well-tolerated, synthetic lethal combination treatment strategy that explicitly targets TNBC cells, while leaving non-transformed cells unharmed. Hallmarks of cancer include limitless proliferation and genome instability (42, 43). By targeting CDK1, which has a role in both cell cycle promotion and DNA DSB repair, followed by treatment with a chemotherapeutic, we have developed a novel synthetic lethal combination. Simultaneous drug administration or treating cells with doxorubicin prior to roscovitine was antagonistic in TNBC cells and toxic in vivo. Further, roscovitine followed by doxorubicin treatment inhibited tumor growth in vivo and significantly increased overall survival compared to mice receiving concomitant treatment of the two drugs or either of the drugs as single agents.
Since TNBC cells have lost their G1 to S regulation, treatment of these cells with roscovitine resulted in their accumulation in G2/M phase, while non-TNBC or HMEC cells with a regulated G1 to S transition, accumulated in the G1 phase of he cell cycle. Additionally, combination treatment maintained a G2/M arrest and/or induced polyploid nuclei specifically in TNBC cells. Moreover, knockdown of CDK1, a G2/M specific CDK, was sufficient to augment the percent of G2/M accumulation in the presence of doxorubicin only in TNBC cells. Collectively these results suggest that a sequential treatment strategy that differentially targets the cell cycle in TNBC versus HMEC cells, is more likely to produce a synergistic response only in those cells with a deregulated cell cycle (i.e. TNBC cells).
TNBC cells with p53 mutations had a diminished capacity to induce p21 transcription compared to p53 wildtype HMEC cells. Knockdown of Rb was insufficient to cause a synergistic response in HMEC cells. However, combination treatment was synthetically lethal only in p53-compromised HEMC and tumors cells, whereas the paired isogenic p53 wildtype cells were additive or antagonistic to the combination treatment. Moreover, the cell cycle profile of HMEC and tumor cells lacking p53 activity closely mimicked the cell cycle profile of TNBC cells. Thus, p53 inactivation could serve as a predictor of synergistic response to sequential roscovitine-doxorubicin combination treatment.
Mechanistically, administration of roscovitine prior to doxorubicin caused increased DNA double strand breaks (DSB) while simultaneously inhibiting recruitment of downstream homologous recombination (HR) proteins in TNBC cells. Combination treatment did not subject HMEC cells to increased DNA damage. HR is the preferred method of DNA DSB repair during S and G2/M phase, and CDK1 activity is required for the excision of DNA DSBs during homologous recombination (25). Therefore, inhibiting CDK1 activity (with roscovitine) primes and sensitizes TNBC cells to doxorubicin. Non-transformed cells and tumor cells with an intact p53 pathway were less sensitive to combination treatment because they did not accumulate at the G2/M phase prior to doxorubicin administration.
The requirement of p53 inactivity for the sequential-combination-induced synergism provides a putative predictor of response. TNBC tumors are heterogeneous, with genome analysis to identifying 6 or 4 subgroups within TNBC tumors (44, 45). Thus, identifying patients that would benefit the most from combination treatment, while sparring non-responsive patients to treatment, is crucial to successfully implementing this treatment strategy. Moreover, developing a drug treatment strategy that incorporates the clinically available agent doxorubicin may hasten the adoption of this combination in the clinic.
While the sequential roscovitine followed by doxorubicin treatment was well tolerated in vivo (this study), a phase I clinical trial examining the maximum tolerated dose and efficacy of dinaciclib (the next generation analogue of roscovitine) and the anthracycline epirubicin found that this treatment was very toxic, ending the trial before efficacy could be determined (46). Dose-limiting toxicities included grade 3 neutropenia, syncope and vomiting (46). One solution to increase the efficacy of combination treatment while reducing toxicity is to couple the drugs with a nanocarrier delivery system. Coupling CDK inhibitors or doxorubicin with nanotechnology drug delivery system will limit dispersal of the drug to only the site of action, protecting other organs and tissues from cytotoxicity (47). There is precedence for such a strategy as anti-HER2 immunoliposomes containing doxorubicin were effectively targeted to HER2 overexpressing tumors, increasing the therapeutic benefit of doxorubicin while reducing toxicity in a xenograft mouse model (48). Alternatively, CDK inhibitors could be directly delivered to the tumor site if it was bound to a ligand-mediated active binding nanoparticle. EGFR, which is overexpressed in the majority of TNBC tumors, is a cell surface receptor that provides a putative target to deliver roscovitine to the tumor site. Indeed, EGFR-targeted polymer nanocarriers delivered paclitaxel and ionidamine to multi-drug resistant EGFR-overexpressing tumor cells, increasing drug cytotoxicity (49). Developing EGFR-targeted nanocarriers to deliver to roscovitine directly to the tumor site could increase the therapeutic benefit of CDK inhibition while reducing toxicities. Moreover, liposomal-doxorubicin, which accumulates at tumor sites due to leaky vasculature, is clinically available to treat breast cancer (50). Thus, it would be clinically beneficial to consider incorporating CDK inhibitors or doxorubicin combination treatment with nanoparticle delivery system.
Although doxorubicin and combination treated tumors had the same amount of proliferation, combination treated tumors underwent more apoptosis, indicating increased cyto-toxicity. Furthermore, doxorubicin only treated mice suffered from more weight loss compared to combination treated mice. Thus, the combination therapy of roscovitine followed by doxorubicin can decrease tumor volume while limiting toxicity. Combining chemotherapies with a CDK inhibitor could potentially lead to a reduction in the chemotherapy dose administered to patients. Overall, roscovitine-doxorubicin sequential therapy increased the therapeutic benefit of doxorubicin while reducing toxicity, supporting future clinical studies.
Supplementary Material
Acknowledgments
Financial Support: This research was supported by NIH grants CA87458 and CA1522228 to K. Keyomarsi, by the Center for Clinical and Translational Sciences at NIH, TL1 RR024147 to NA Jabbour-Leung and by NCI CCSG grant CA16672 to M.D. Anderson Cancer Center.
Footnotes
Conflicts of Interest: No conflicts to disclose
References
- 1.Morris GJ, Naidu S, Topham AK, Guiles F, Xu Y, McCue P, et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and End Results database. Cancer. 2007;110:876–84. doi: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]
- 2.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–8. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 3.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 4.Carey L, Winer E, Viale G, Cameron D, Gianni L. Triple-negative breast cancer: disease entity or title of convenience? Nat Rev Clin Oncol. 2010;7:683–92. doi: 10.1038/nrclinonc.2010.154. [DOI] [PubMed] [Google Scholar]
- 5.Palacios J, Honrado E, Osorio A, Cazorla A, Sarrio D, Barroso A, et al. Phenotypic characterization of BRCA1 and BRCA2 tumors based in a tissue microarray study with 37 immunohistochemical markers. Breast Cancer Res Treat. 2005;90:5–14. doi: 10.1007/s10549-004-1536-0. [DOI] [PubMed] [Google Scholar]
- 6.Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. The lancet oncology. 2007;8:235–44. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 7.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 8.Sonnenblick A, de Azambuja E, Azim HA, Jr, Piccart M. An update on PARP inhibitors-moving to the adjuvant setting. Nat Rev Clin Oncol. 2014 doi: 10.1038/nrclinonc.2014.163. [DOI] [PubMed] [Google Scholar]
- 9.Turner N, Moretti E, Siclari O, Migliaccio I, Santarpia L, D’Incalci M, et al. Targeting triple negative breast cancer: Is p53 the answer? Cancer Treat Rev. 2013;39:541–50. doi: 10.1016/j.ctrv.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Engebraaten O, Vollan HK, Borresen-Dale AL. Triple-negative breast cancer and the need for new therapeutic targets. Am J Pathol. 2013;183:1064–74. doi: 10.1016/j.ajpath.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 11.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillett C, Fantl V, Smith R, Fisher C, Bartek J, Dickson C, et al. Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res. 1994;54:1812–7. [PubMed] [Google Scholar]
- 13.Keyomarsi K, Tucker SL, Buchholz TA, Callister M, Ding Y, Hortobagyi GN, et al. Cyclin E and survival in patients with breast cancer. The New England journal of medicine. 2002;347:1566–75. doi: 10.1056/NEJMoa021153. [DOI] [PubMed] [Google Scholar]
- 14.Span PN, Tjan-Heijnen VC, Manders P, Beex LV, Sweep CG. Cyclin-E is a strong predictor of endocrine therapy failure in human breast cancer. Oncogene. 2003;22:4898–904. doi: 10.1038/sj.onc.1206818. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz GK, Shah MA. Targeting the cell cycle: a new approach to cancer therapy. J Clin Oncol. 2005;23:9408–21. doi: 10.1200/JCO.2005.01.5594. [DOI] [PubMed] [Google Scholar]
- 16.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–66. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 17.Malumbres M, Pevarello P, Barbacid M, Bischoff JR. CDK inhibitors in cancer therapy: what is next? Trends Pharmacol Sci. 2008;29:16–21. doi: 10.1016/j.tips.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 18.De Azevedo WF, Leclerc S, Meijer L, Havlicek L, Strnad M, Kim SH. Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human cdk2 complexed with roscovitine. European journal of biochemistry/FEBS. 1997;243:518–26. doi: 10.1111/j.1432-1033.1997.0518a.x. [DOI] [PubMed] [Google Scholar]
- 19.Rizzolio F, Tuccinardi T, Caligiuri I, Lucchetti C, Giordano A. CDK inhibitors: from the bench to clinical trials. Curr Drug Targets. 2010;11:279–90. doi: 10.2174/138945010790711978. [DOI] [PubMed] [Google Scholar]
- 20.Cyclacel. Investigator’s Brochure Seliciclib (CYC202, R-Roscovitine) 2007:1–88. [Google Scholar]
- 21.Galons H, Oumata N, Meijer L. Cyclin-dependent kinase inhibitors: a survey of recent patent literature. Expert Opin Ther Pat. 2010;20:377–404. doi: 10.1517/13543770903524284. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:1770–83. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- 23.Fischer PM, Gianella-Borradori A. Recent progress in the discovery and development of cyclin-dependent kinase inhibitors. Expert Opin Investig Drugs. 2005;14:457–77. doi: 10.1517/13543784.14.4.457. [DOI] [PubMed] [Google Scholar]
- 24.Lieber MR, Ma Y, Pannicke U, Schwarz K. Mechanism and regulation of human non-homologous DNA end-joining. Nat Rev Mol Cell Biol. 2003;4:712–20. doi: 10.1038/nrm1202. [DOI] [PubMed] [Google Scholar]
- 25.Ira G, Pellicioli A, Balijja A, Wang X, Fiorani S, Carotenuto W, et al. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature. 2004;431:1011–7. doi: 10.1038/nature02964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huertas P, Jackson SP. Human CtIP mediates cell cycle control of DNA end resection and double strand break repair. J Biol Chem. 2009;284:9558–65. doi: 10.1074/jbc.M808906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L, Nievera CJ, Lee AY, Wu X. Cell cycle-dependent complex formation of BRCA1.CtIP.MRN is important for DNA double-strand break repair. J Biol Chem. 2008;283:7713–20. doi: 10.1074/jbc.M710245200. [DOI] [PubMed] [Google Scholar]
- 28.Jazayeri A, Falck J, Lukas C, Bartek J, Smith GC, Lukas J, et al. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol. 2006;8:37–45. doi: 10.1038/ncb1337. [DOI] [PubMed] [Google Scholar]
- 29.Band V, Zajchowski D, Kulesa V, Sager R. Human papilloma virus DNAs immortalize normal human mammary epithelial cells and reduce their growth factor requirements. Proc Natl Acad Sci U S A. 1990;87:463–7. doi: 10.1073/pnas.87.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caruso JA, Campana R, Wei C, Su CH, Hanks AM, Bornmann WG, et al. Indole-3-carbinol and its N-alkoxy derivatives preferentially target ERalpha-positive breast cancer cells. Cell Cycle. 2014;13:2587–99. doi: 10.4161/15384101.2015.942210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 32.Yuan J, Chen J. FIGNL1-containing protein complex is required for efficient homologous recombination repair. Proc Natl Acad Sci U S A. 2013;110:10640–5. doi: 10.1073/pnas.1220662110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tirado OM, Mateo-Lozano S, Notario V. Roscovitine is an effective inducer of apoptosis of Ewing’s sarcoma family tumor cells in vitro and in vivo. Cancer Res. 2005;65:9320–7. doi: 10.1158/0008-5472.CAN-05-1276. [DOI] [PubMed] [Google Scholar]
- 34.Vinyals A, Peinado MA, Gonzalez-Garrigues M, Monzo M, Bonfil RD, Fabra A. Failure of wild-type p53 gene therapy in human cancer cells expressing a mutant p53 protein. Gene Ther. 1999;6:22–33. doi: 10.1038/sj.gt.3300786. [DOI] [PubMed] [Google Scholar]
- 35.Lambert LA, Qiao N, Hunt KK, Lambert DH, Mills GB, Meijer L, et al. Autophagy: a novel mechanism of synergistic cytotoxicity between doxorubicin and roscovitine in a sarcoma model. Cancer research. 2008;68:7966–74. doi: 10.1158/0008-5472.CAN-08-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nanos-Webb A, Jabbour NA, Multani AS, Wingate H, Oumata N, Galons H, et al. Targeting low molecular weight cyclin E (LMW-E) in breast cancer. Breast Cancer Res Treat. 2012;132:575–88. doi: 10.1007/s10549-011-1638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–6. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 38.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 39.Chavez KJ, Garimella SV, Lipkowitz S. Triple negative breast cancer cell lines: one tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010;32:35–48. doi: 10.3233/BD-2010-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Band V, De Caprio JA, Delmolino L, Kulesa V, Sager R. Loss of p53 protein in human papillomavirus type 16 E6-immortalized human mammary epithelial cells. J Virol. 1991;65:6671–6. doi: 10.1128/jvi.65.12.6671-6676.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Y, Chen JJ, Gao Q, Dalal S, Hong Y, Mansur CP, et al. Multiple functions of human papillomavirus type 16 E6 contribute to the immortalization of mammary epithelial cells. J Virol. 1999;73:7297–307. doi: 10.1128/jvi.73.9.7297-7307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 43.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–67. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burstein MD, Tsimelzon A, Poage GM, Covington KR, Contreras A, Fuqua S, et al. Comprehensive Genomic Analysis Identifies Novel Subtypes and Targets of Triple-negative Breast Cancer. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-14-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitri Z, Karakas C, Wei C, Briones B, Simmons H, Ibrahim N, et al. A phase 1 study with dose expansion of the CDK inhibitor dinaciclib (SCH 727965) in combination with epirubicin in patients with metastatic triple negative breast cancer. Invest New Drugs. 2015 doi: 10.1007/s10637-015-0244-4. [DOI] [PubMed] [Google Scholar]
- 47.Koo OM, Rubinstein I, Onyuksel H. Role of nanotechnology in targeted drug delivery and imaging: a concise review. Nanomedicine. 2005;1:193–212. doi: 10.1016/j.nano.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Park JW, Hong K, Kirpotin DB, Colbern G, Shalaby R, Baselga J, et al. Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin Cancer Res. 2002;8:1172–81. [PubMed] [Google Scholar]
- 49.Milane L, Duan Z, Amiji M. Development of EGFR-targeted polymer blend nanocarriers for combination paclitaxel/lonidamine delivery to treat multi-drug resistance in human breast and ovarian tumor cells. Mol Pharm. 2011;8:185–203. doi: 10.1021/mp1002653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shehata M, Mukherjee A, Sharma R, Chan S. Liposomal doxorubicin in breast cancer. Womens Health (Lond Engl) 2007;3:557–69. doi: 10.2217/17455057.3.5.557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.