Abstract
The neuropeptide PDF promotes the normal sequencing of circadian behavioral rhythms in Drosophila, but its signaling mechanisms are not well understood. We report daily rhythmicity in responsiveness to PDF in critical pacemakers called small LNvs. There is a daily change in potency, as great as 10-fold higher, around dawn. The rhythm persists in constant darkness, does not require endogenous ligand (PDF) signaling, or rhythmic receptor gene transcription. Furthermore, rhythmic responsiveness reflects the properties of the pacemaker cell type, not the receptor. Dopamine responsiveness also cycles, in phase with that of PDF, in the same pacemakers, but does not cycle in large LNv. The activity of RalA GTPase in s-LNv regulates PDF responsiveness and behavioral locomotor rhythms. Additional, cell autonomous PDF signaling reversed the circadian behavioral effects of lowered RalA activity. Thus RalA activity confers high PDF responsiveness, providing a daily gate around the dawn hours to promote functional PDF signaling.
Keywords: Drosophila, Circadian Rhythms, GPCR, PDF, RalA, Dopamine
INTRODUCTION
The Drosophila brain contains ~150 neurons that function as circadian pacemakers controlling daily physiological and behavioral rhythms. The neurons interact extensively via conventional neurotransmission and via neuropeptidergic signals to organize temporal information across the network and to mediate entrainment to environmental signals (Collins et al., 2014; Chung et al., 2009; Guo et al., 2014, Hermann-Luibl et al., 2014; Kunst et al., 2014; Parisky et al., 2008; Renn et al., 1999; Shafer et al., 2008).
Cellular properties among the Drosophila pacemaker cells differ greatly (e.g., Helfrich-Forster et al., 2007; Nitabach and Taghert, 2008). Notably, the neuropeptide Pigment Dispersing Factor (PDF) is produced by about 10% of the pacemakers (called ventrolateral neurons, LNv, Helfrich-Forster, 1997). PDF mediates pacemaking functions by the LNv neurons (Renn et al., 1999; Beckwith and Ceriani, 2015; Dissel et al., 2014; Yao and Shafer, 2014), but how it does so remains a mystery. PDF signaling promotes molecular cycles among clock cells (Cusumano et al., 2009; Im et al., 2011; Lear et al., 2009; Lin et al., 2004; Peng et al., 2003; Yoshii et al., 2011); it also supports the stability of TIMELESS (Seluzicki et al., 2014) and PER (Li et al., 2014). Recent in vivo whole brain scanning of pacemaker calcium activities across the full 24 hr day revealed PDF is responsible (in part) for the non-synchronous activity patterns normally exhibited by the different pacemaker groups (Liang et al., 2016).
Here we investigate the extent to which PDF responsiveness by identified pacemakers may change over the course of a day, and whether such changes may constrain PDF modulation to specific time domains. In mammals, cellular properties within circadian pacemakers undergo striking diurnal changes (e.g., An et al., 2012; Itri et al., 2010). Likewise, in the Drosophila circadian system, many cellular properties reflect the strong influence of clock outputs. The small LNv (s-LNv) undergo daily morphological changes (e.g., Gorostiza et al., 2014), and the large LNv (l-LNv) display biochemical (Kula-Eversole et al., 2010) and physiological rhythms (e.g., Cao and Nitabach, 2008).
PDF receptor (Hyun et al., 2005; Lear et al., 2005; Mertens et al., 2005) is broadly expressed across the pacemaker network by about half the total cell number (Im and Taghert, 2010). PDF receptor is coupled to Gsα (Hyun et al., 2005; Lear et al., 2006; Mertens et al., 2005) and to the IP3 receptor (Agrawal et al., 2013). Pharmacologically, PDF sensitivity is displayed across the circadian network (Im and Taghert, 2010; Pírez et al., 2013; Shafer et al., 2008; Yao and Shafer, 2014). Here we report that sensitivity does indeed follow a circadian cycle (a daily change in potency as great as 10-fold), in a transcription- and ligand-independent manner, and in a manner that reflects its cellular context. Moreover, the change gates functional PDF signaling to a narrow period around dawn and it involves regulation by the small GTPase, RalA.
RESULTS
PDF sensitivity shows circadian variation in s-LNv cells
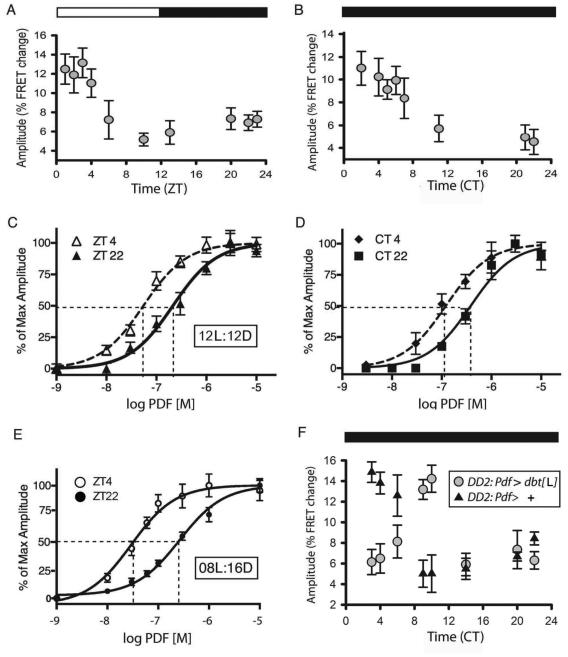
To ask whether PDF sensitivity changes over the course of the day in vivo, we tested a single concentration of the peptide (10−07 M - within the linear range of the PDF concentration-effect curve - Shafer et al., 2008) on the s-LNv subgroup of clock cells, using the transgenic EPAC-FRET sensor for cyclic nucleotide levels (Figure 1). Under a 12:12 light/dark (LD) regimen, the amplitudes of FRET responses to PDF were highest during the four-hour period ZT1-4 (immediately after lights-on). They exhibited ~50% of that peak value at all other times of the day tested (Figure 1A). The diurnal change in PDF sensitivity persisted on the second day of constant darkness (DD2 - Figure 1B) with peak values early in the subjective day. To evaluate the daily change, we chose two times representing periods of either “high” (ZT4) or “low” (ZT22) sensitivity states and determined full concentration-effect curves (Figure 1C, D): s-LNv were ~5-fold more sensitive to PDF at ZT4 (EC50: 5.5 × 10−08) versus ZT22 (EC50: 2.4 × 10−07). Sensitivity to PDF increased from an EC50 of 3.7 × 10−07 at CT22 (CT = Circadian Time) to 1.2 × 10−07 at CT4 (these times were 36 vs. 42 hr respectively after release into constant darkness). Values and statistical analyses of EC50s and maximum amplitudes for all experiments are reported in Table 1.
Figure 1. Daily changes in response to PDF by M neurons.
A. The size of maximal responses by s-LNv to 10−07 M PDF varies over the course of the day. A one-way analysis of variance revealed a statistically significant difference (P<0.001). A post-hoc pairwise comparison (Tukey Test) revealed differences in amplitude (with P<0.01) between the following pairs: ZT1:ZT10, and ZT1:ZT13; ZT2:ZT10 and ZT2:ZT13; ZT3:ZT10, ZT3:ZT13, and ZT3:ZT22. We only compared amplitude measures with normal distributions. N=58 brains with 214 ROIs analyzed; each ROI represents 0ne to four s-LNvs. B. The daily variation persists on the 2nd day of constant darkness (DD2). A one-way analysis of variance revealed a statistically significant difference across time of day in small LNv (P<0.001). A post-hoc pairwise comparison (Tukey Test) revealed differences in amplitude (with P<0.001) between CT2:CT22 for DD and ZT2:ZT7, ZT11, ZT22, &ZT23 for LD, suggesting peak broadening and a possible phase shift. N= 26 brains with 96 ROIs analyzed. C. Concentration-effect curves for PDF at two different times of day in brains from flies experiencing a 12:12 LD cycle. D. Concentration-effect curves for PDF at two different times of day in brains from flies at DD2. E. Concentration-effect curves for PDF at two different times of day in brains from flies raised under short day conditions - 08:16 light:dark cycle. F. The phase of maximal response amplitude in DD2 in M pacemakers the mis-expressing DBT[L] variant (pdf > dbtL, n = 24 brains and 85 ROIs) versus control (pdf > +, n = 20 brains and 64 ROIs). A two-way analysis of variance revealed differences (P<0.001). A post-hoc pairwise comparison (Tukey Test) within genotypes indicated significant differences at CT 3, 4 and at CT 9, 10 (P<0.001).
Table 1.
EC50 and Maximal Amplitude of Dose Response Curves.
| Genotype | Cell Type |
Daylength
LD:DD |
PDF EC50
ZT4 |
PDFEC50
ZT22 |
Max Ampl PDF
ZT4 |
Max Ampl PDF
ZT22 |
|---|---|---|---|---|---|---|
| Pdf > Epac50A | s-LNV | 12:12 | 5.4 × e-8 | 2.1 × e-7** | 17.4 ± 1.7 | 17.5 ± 1.2 |
| Pdf > Epac50A | s-LNV | 08:16 | 2.9 × e-8 | 2.6 × e-7** | 20.1 ± 1.8 | 17.1 ± 0.9 |
| Pdf > Epac50A ; Pdfr | s-LNV | 12:12 | 8.8 × e-8 | 4.1 × e-7** | 22.8 ± 1.6 | 23.2 ± 1.6 |
| pdfr[han], Pdf > Epac50A ; Pdfr | s-LNV | 12:12 | 1.0 × e-7 | 5.1 × e-7* | 23.9 ± 1.6 | 22.6 ± 1.3 |
| Pdf > Epac50A; pdf[01] | s-LNV | 12:12 | 9.9 × e-8 | 3.6 × e-7* | 17.3 ± 1.7 | 16.9 ± 1.4 |
| c929 > Epac50A | l-LNV | 12:12 | 2.0 × e-8 | 3.2 × e-8 | 18.2 ± 1.7 | 21.9 ± 1.9 |
| c929 > Epac50A | SE2 | 12:12 | 2.6 × e-8 | 2.4 × e-8 | 33.9 ± 4.3 | 35.7 ± 5.5 |
| Pdf > Epac50A ; ralaDN | s-LNV | 12:12 | 3.2 × e-7 | 4.4 × e-7 | 16.0 ± 1.1 | 16.9 ± 1.6 |
| Pdf > Epac50A ; ralaCA | s-LNv | 12:12 | 8.2 × e-8 | 9.1 × e-8 | 18.7 ± 1.8 | 16.8 ± 1.1 |
|
PDF EC50
CT4 |
PDF EC50
CT22 |
Max Ampl PDF
CT4 |
Max Ampl PDF
CT22 |
|||
| Pdf > Epac50A | s-LNV | 12:12 | 1.2 × e-7 | 3.7 × e-7** | 17.8 ± 1.2 | 19.0 ± 1.3 |
|
DA EC50
ZT4 |
DA EC50
ZT22 |
Max Ampl DA
ZT4 |
Max Ampl DA
ZT22 |
|||
| Pdf > Epac50A | s-LNV | 12:12 | 4.7 × e-6 | 2.2 × e-5** | 15.3 ± 1.2 | 14.7 ± 2.8 |
| Pdf > Epac50A | l-LNV | 12:12 | 5.1 × e-6 | 4.7 × e-6 | 15.3 ± 1.3 | 16.0 ± 1.3 |
| Pdf > Epac50A ; ralaDN | s-LNV | 12:12 | 2.9 × e-5 | 4.8 × e-5 | 13.6 ± 1.5 | 14.4 ± 1.9 |
| Pdf > Epac50A ; ralaCA | s-LNv | 12:12 | 5.7 × e-6 | 6.4 × e-6 | 14.0 ± 1.4 | 13.6 ± 2.0 |
Significant differences in EC50 values (p>0.001), between ZT4 vs ZT22 and between CT4 vs. CT22 were only observed in s-LNv, with the exception of those expressing ralaDN or ralaCA. No Maximal Amplitude daily differences were observed between ZT4 and ZT22 in any cells.
Significant differences in EC50 values (p>0.0001), between ZT4 vs ZT22 and between CT4 vs. CT22 were only observed in s-LNv, with the exception of those expressing ralaDN or ralaCA. No Maximal Amplitude daily differences were observed between ZT4 and ZT22 in any cells.
We next asked whether the diurnal change in PDF sensitivity was modified according to day length. Drosophila reared under short day (SD) conditions (08hr:16hr) displayed greatly increased average responses to 10−07 M PDF compared to those reared under 12hr:12hr, and to long day (16hr:08hr) conditions (Suppl. Figure 1A). A more complete analysis revealed that PDF sensitivity cycled across a full ~10 fold difference under SD (Figure 1E): the EC50 was 2.6 × 10−07 at ZT22, and 2.9 × 10−08 at ZT4 (Table 1). Finally, we tested the direct involvement of the molecular circadian clockwork in setting the phase of this rhythm by mis-expressing the ‘long’ variant of the DOUBLETIME (DBT) kinase in LNv (Pdf > dbt[L]). The L variant produces long circadian locomotor periods in this genotype (Muskus et al., 2007) and when expressed exclusively in PDF neurons, it produced a ~7-8 hr delayed phase in the peak of PDF sensitivity over the course of two days in constant darkness, compared to the control genotype (Figure 1F). This finding is consistent with cell autonomous regulation by the circadian clock. In summary, there is a daily change in PDF sensitivity in s-LNv pacemakers, which, is enhanced under SD conditions, and which reflects cell autonomous control by the circadian clock.
To derive an estimate for how quickly PDF sensitivity can be normally regenerated in vivo, we applied neuraminidase (NM) to isolated brains; NM disrupts glycan modifications present on membrane proteins. We anticipated that PDF responses in s- LNv would be disrupted due to modification of diverse molecules, but in particular to adenylate cyclase 3 (AC3), which is coupled to PDFR in s-LNv (Duvall and Taghert, 2012), and which in mammals displays glycosylation that is functionally-important (Henion et al., 2011). PDF responses were indeed degraded by short-term NM treatment (Suppl. Figure 1B & C). This was true for both “morning-type” (M: s-LNv) and “evening-type” (E: LNd) pacemakers (see Stoleru et al., 2004; Duvall and Taghert, 2013). Notably, M and E pacemakers displayed full recovery of PDF sensitivity in cultured brains within ~ 2hr (albeit with cell-type specific rates - (Suppl. Figure 1D-F & H). The degree of recovery varied according to time of day: after a single 60 min recovery period, recovery was highest in the morning (Suppl. Figure 1G & H). Thus critical aspects of PDF signaling complexes (their composition, number and/or location) are dynamic within pacemaker cell groups in vivo, and are most quickly regenerated around dawn.
Changes in PDF sensitivity persist despite excess receptor expression and also in the absence of the PDF ligand
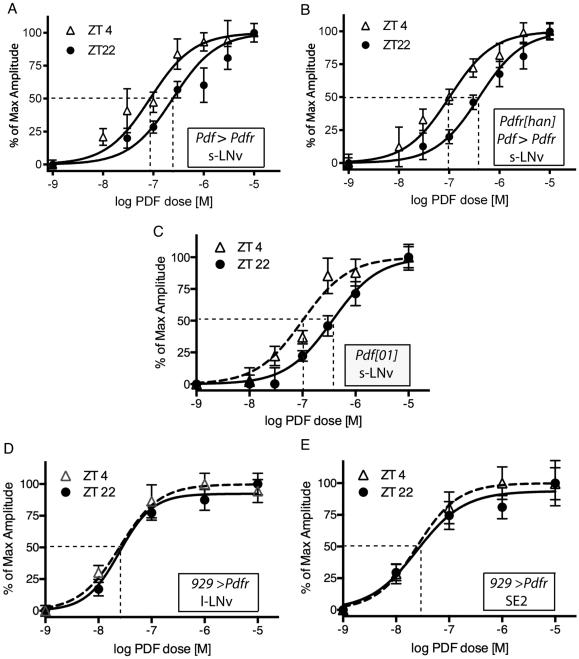
We asked whether the changes in PDF sensitivity in s-LNv were the product of daily changes in Pdfr transcription. Many transcripts display daily rhythms of abundance within pacemaker neurons of the fly brain as outputs of the circadian clock (Kula-Eversole et al., 2010; Abruzzi et al., 2011). Pdfr message levels in s-LNv are higher at ZT12 than ZT0, according to microarray determinations from purified neurons (Kula-Eversole et al., 2010). That pattern of enrichment is approximately 12 hours out of phase to that which we describe here for PDF sensitivity. We previously reported that over-expressed PDFR confers ectopic sensitivity to PDF onto large LNv (l-LNv) and, for s-LNv, over-expression adds ~10% to the maximal amplitude of their normal response to PDF (Shafer et al., 2008). We over-expressed UAS-Pdfr in a wild-type background using the same non-rhythmic Pdf-GAL4 driver. In that over-expression background, PDF sensitivity in s-LNv revealed a persistent daily change in sensitivity, with EC50 values of 6.7 × 10−08 levels at ZT4 and 2.5 × 10−07 at ZT22 (Figure 2A; Table 1). We also tested the effects of PDFR over-expression in a Pdfr −/− background (han5304 a strongly hypomorphic mutant allele – Hyun et al., 2005). In that mutant background, there is no PDF sensitivity in s-LNv (Shafer et al., 2008). In the present experiments, the main source of receptor gene transcription was the UAS-Pdfr transgene driven by the non-rhythmic Pdf-Gal4 driver. In this combined han mutant/Pdfr over-expression background, PDF sensitivity in s-LNv displayed a significant daily change (Figure 2B; Table 1). We next asked whether the daily change in PDF sensitivity reflected ligand-induced receptor endocytosis, by measuring responses in pdf01 flies (these chronically lack PDF neuropeptide). We recorded a similar, significant change in the EC50 values for PDF sensitivity (Figure 2C; Table 1). Together these observations suggest the mechanisms underlying daily changes in PDF sensitivity in s-LNv are largely post-transcriptional and ligand-independent.
Figure 2. Daily changes in response to PDF following genetic manipulation of PDF or PDFR.
A. Concentration-effect curves of PDF responses at two different times of day in s-LNv over-expressing PDFR. B. Concentration-effect curves of PDF responses at two different times of day in s-LNv over-expressing PDFR in a Pdfr null mutant (han5534) background. C. Concentration-effect curves of PDF responses at two different times of day in s-LNv in a Pdf mutant (Pdf01) background. D. Concentration-effect curves of PDF responses at two different times of day in l-LNv over-expressing PDFR. E. Concentration-effect curves of PDF responses at two different times of day in SE2 neurons over-expressing PDFR.
Ectopic PDF receptor expression confers PDF sensitivity that is time-of-day invariant
The daily rhythm of PDF sensitivity in s-LNv could reflect a property of the PDF receptor, or it could reflect the cellular context - the specific properties of s-LNv pacemakers. To discriminate between these possibilities we targeted ectopic expression of PDF-R using c929(dimm)-GAL4 to confer sensitivity to neurons that do not otherwise respond to PDF (cf. Shafer et al., 2008). Specifically, we studied (i) l-LNv pacemakers as well as (ii) two large non-pacemaker neurons of the sub-esophageal neuromeres (SE2, they express dFMRFa peptides (Schneider et al., 1993); SE2 do not express the molecular clockwork and are not traditionally considered part of the canonical circadian pacemaker network. For both ectopic sites, we measured concentration-effect curves to PDF at the peak and trough times (ZT4 and ZT22). Under the conditions tested, ectopic PDF sensitivity was again conferred onto the l-LNv pacemakers, but it showed no time-of-day variation: EC50 values were 2.0 × 10−08 at ZT4 versus 3.2 × 10−08 at ZT22 (Figure 2D; Table 1). Likewise, in the non-pacemaker SE2 neurons, ectopic PDF sensitivity appeared very similar at both time points: EC50 values were 2.6 ×10−08 at ZT4 versus 2.4 × 10−08 at ZT22 (Figure 2E; Table 1). These observations suggest that the cycling mechanism(s), which produces normal daily changes in PDF sensitivity, likely involves properties reflecting the cellular context, and not properties specific to the PDF receptor molecule.
In s-LNv, daily changes also occur in sensitivity to other Gs alpha-coupled receptors
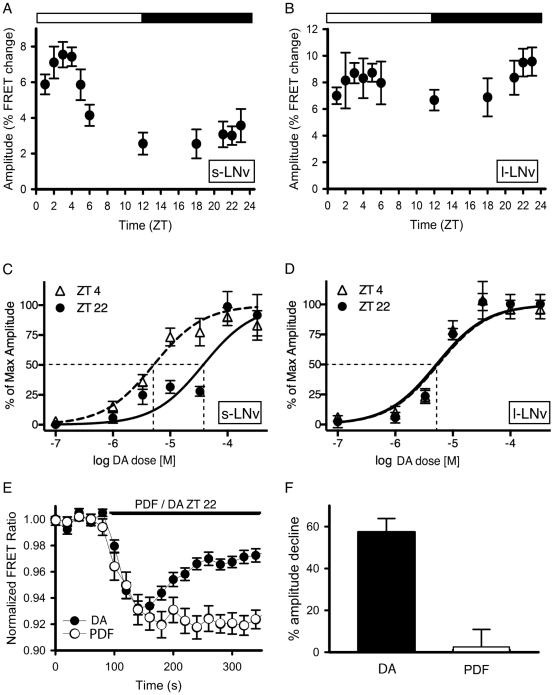
Because PDF-R-based responses varied according to cellular context, we next asked whether similar daily changes might also be observed for other Gs-coupled GPCRs in the s-LNvs. Dopamine (DA) generates cAMP increases in vivo, in both small and l-LNv, the latter via one or more D1-like dopamine receptors (Shang et al. 2011; Duvall and Taghert, 2012). We first measured responses to a single (sub-maximal) concentration of DA throughout the day. The s-LNv displayed a rhythm of responsiveness, with a peak phase in synchrony with the peak phase of PDF responsiveness, just after lights-on (Figure 3A). In contrast, FRET responses to DA in l-LNv did not significantly vary over the 24-hr time period tested (Figure 3B). With full concentration-effect curve measures, we observed a ~10-fold change in the EC50 measured for DA in s-LNv at ZT4 (4.8 × 10−06) versus at ZT22 (4.8 × 10−05) (Figure 3C; Table 1). In contrast, DA sensitivity in l-LNv was invariant at the two times tested: EC50 = 5.8 × 10−06 (ZT4) versus 5.6 × 10−06 (ZT22) (Figure 3D; Table 1). PDF and DA sensitivities co-vary in daily sensitivity measures, and the daily variation in PDF signaling is context-dependent (i.e., it is seen in s-LNv, but not in l-LNv). These facts suggest potential coordinate rhythmic regulation of the Gs-alpha signaling system in s-LNv. PDF-R in s-LNv is coupled to the AC3 isoform of adenylate cyclase (Duvall and Taghert, 2012); we asked whether this adenylate cyclase isoform might also be coupled to DA-R(s). RNAi targeting of AC13E and of ACX-A both partially reduced DA responsiveness in s-LNv, whereas targeting of AC3 did not (Suppl. Figure 2-top). Likewise, over-expression of AC3 completely eliminated PDF autoreceptor sensitivity in s-LNv, but it had no effect on DA responsiveness in these same pacemaker neurons (Duvall and Taghert, 2012). We conclude that the daily rhythms of PDF and DA responsiveness in s-LNv are not due to regulation of a common AC.
Figure 3. Daily changes in response to DA by M neurons.
A. The sizes of response amplitudes by s-LNv to 3 × 10−05 M DA measured over the course of the day. N = 36 brains with 124 ROIs analyzed. B. The sizes of the response amplitudes by l-LNv to 3 × 10−05 M DA do not vary over the course of the day. A one-way analysis of variance revealing a statistically significant difference across time of day for s-LNv neurons (P<0.001), but not l-LNv neurons (P=0.574). A post-hoc pairwise comparison (Tukey Test) of small LNv data revealed differences in amplitude (P<0.01) between ZT1- ZT22; ZT2- ZT12, ZT21, ZT22, and ZT23; ZT3- ZT12, ZT21, ZT22, and ZT23; ZT4- ZT12, and ZT22. We only compared amplitude measures with normal distributions. N = 33 brains with 178 ROIs analyzed. Eacg ROI represents one to two l-LNv’s. C. Concentration-effect curves of DA responses at two different times of day in s-LNv. D. Concentration-effect curves of DA responses at two different times of day in l-LNv. E. A single example of Epac-camps FRET signal generation in an s-LNv in response to 3×10−05 M dopamine versus 10−06 M PDF. F. Response attenuation, 180 sec after initial agonist exposure in a static bath, was 57.5 +/− 6.4% for DA (n = 53 cells) and 1.2 +/− 9.7% for PDF (n = 35 cells).
We noted that FRET response waveforms in the face of sustained exposure to DA were different from those characteristically seen in response to sustained exposure to PDF. Specifically, DA responses peaked within 1-2 minutes (as did PDF responses) but then rapidly attenuated, whereas PDF responses remained at or near their maximal amplitudes for >5 minutes and for as long as free peptide remained available (Figure 3E and F; see also Shafer et al., 2008). In parallel, we also tested the ability of the PDF neuropeptide and of dopamine to provoke recruitment of β-arrestin (β-ARR) to the plasma membrane in cells expressing PDF-R or either of two Drosophila D1-like receptors. β-ARR recruitment can activate membrane trafficking resulting in rapid GPCR internalization, thereby truncating initial signaling events. Exposure to PDF consistently failed to recruit β–ARR1-GFP or β-ARR2-GFP translocation in hEK-293 cells that expressed functional PDF-R (Suppl. Figure 2 - bottom). Addition of GRK2 or of a dominant negative Dynamin (Johnson et al., 2003) did not affect this result (see Suppl. data). Most other Drosophila neuropeptides potently recruit β-ARR2 translocation following activation of their cognate receptors in hEK cells (Johnson et al., 2003), including DH31 (Johnson et al., 2004). The DH31–R and PDF-R share considerable molecular similarity as members of a group derived from ancestors of the calcitonin/CGRP receptors. (Hewes and Taghert, 2000). DA potently recruited β-ARR2-GFP translocation (Suppl. Figure 2). Thus the display of transient short term, G protein-dependent signaling by the D1-type receptor in vivo is correlated with its ability to recruit β-ARR translocation in hEK-293. Neither property is displayed by PDFR.
Manipulation of the RalA GTPase specifically in PDF neurons mimics daily changes in PDF sensitivity in s-LNv
The combined evidence suggested that daily changes in the membrane of the s-LNv (perhaps involving trafficking) leads to daily rhythms of PDF sensitivity. We used RNAi transgene screening to evaluate potential mediators of vesicle trafficking in PDF neurons (W. Li and P.H. Taghert, unpublished) and in so doing, turned attention to the gene encoding the rho-related G protein, RalA (Wang et al., 2004). RalA controls diverse cellular activities through discrete effectors, including the promotion of vesicle exocytosis via interactions with the exocyst (Chen et al., 2011). The exocyst is a multi-subunit protein complex associated with post-Golgi, cargo-filled vesicles and it controls multiple membrane trafficking processes (Gentry et al., 2014). Knockdown of several exocyst components often, though not uniformly, produced a circadian locomotor syndrome that included increased arrhythmicity in DD, accompanied by longer circadian behavioral periods (Suppl. Table 1).
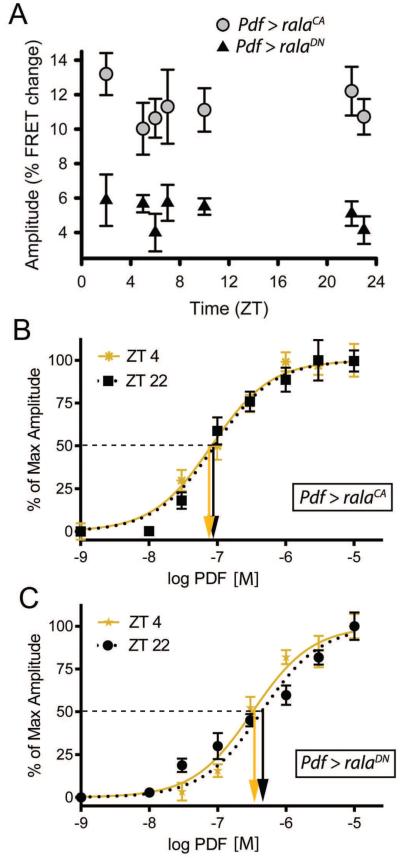
We first tested for the possible influence of RalA on sensitivity to PDF by expressing transgenes encoding the N19V constitutively active (CA) or the S25N dominant negative (DN) forms of RalA (Sawamoto et al., 1999; Mirey et al., 2003) in PDF neurons exclusively (Pdf> UAS rala-CA and Pdf> UAS rala-DN). We measured the phase of daily changes in PDF sensitivity to a single sub-maximal concentration (Figure 4A) and also obtained full PDF concentration-effect curves at ZT4 and ZT22 using the EPAC FRET reporter (Figure 4B, C and Table 1). Brains mis-expressing either of the two RalA variants were typically tested simultaneously in the same imaging chamber to reduce the possibility that any differential effects observed were due to technical differences in presentation (see Methods). Both transgenes eliminated the rhythmic change we had observed in sensitivity to PDF, but did so in opposite ways. CA-expressing s-LNv displayed high levels of sensitivity throughout the day, while DN-expressing s-LNv correspondingly displayed consistently lowered levels (Figure 4A). The EC50 values for RalA (CA)-expressing s-LNv were not significantly different from each other (Figure 4B): at ZT4 it was 8.2 ×10−08 and at ZT22 it was 9.1 ×10−08. Likewise for RalA (DN)-expressing s-LNv, the ZT4 and ZT22 values were not different from each other (Figure 4C): 3.2 ×10−07 and 4.4 ×10−07 respectively. Furthermore, the values for both timepoints in (CA)- expressing s-LNv were not significantly different from that of control brains at ZT4 (5.4 × 10−08). In contrast, the EC50 value at ZT22 for both for (CA)-expressing and (DN)-expressing s-LNv were significantly different from that of the control genotype, 2.1 × 10−07. Thus the DN isoform of RalA biased s-LNv’s to a “low” sensitivity state, at both ZT4 and ZT22, whereas the CA isoform of RalA biased the same neurons to a high sensitivity state at both time points.
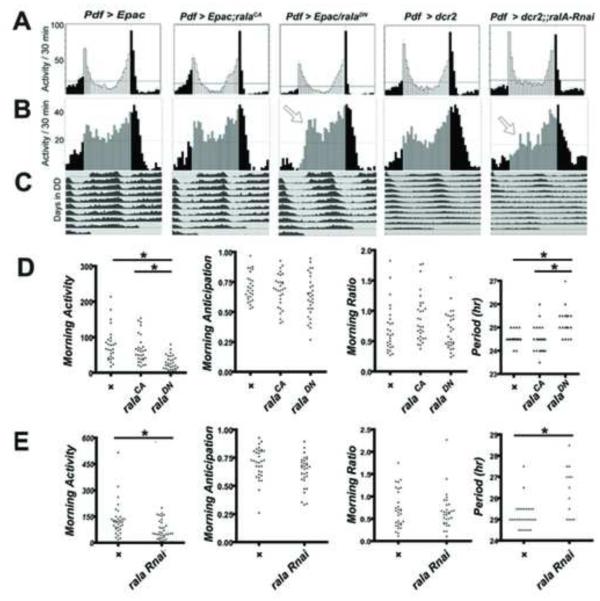
Figure 4. Manipulation of RalA activity in M pacemaker neurons affects their daily cycle of PDF sensitivity.
A. FRET responses to a single dose of PDF (10−07 M) at different times of day in two different genotypes: PDF > ralaCA, (grey circles; n = 21 brains with 62 ROIs analyzed) and Pdf > ralaDN (n = 24 brains with 84 ROIs analyzed). B. Concentration-effect curves for PDF sensitivity following expression of ralaCA in PDF neurons at ZT4 (gold) and ZT22 (black). C: Concentration-effect curves for PDF sensitivity following expression of ralaDN in PDF neurons at ZT4 (gold) and ZT22 (black). All genotypes contain UAS-Epac-camps. Downward arrows indicate values of EC50’s. At ZT4, the EC50 for ralaCA is not significantly different from control EC50 values (p =0.139), while the value for ralaDN is significantly different (p < 0.0001). At ZT22, the EC50 for ralaCA is significantly different from control EC50 values (p <0.0001), while the value for ralaDN is not significantly different (p = 0.133).
To extend these observations, we also asked whether these same genetic manipulations in these same neurons similarly altered responses to DA (Suppl. Figure 3). Indeed, the DN isoform of RalA biased s-LNv’s to a “low” DA sensitivity state at both time points with EC50 values that were not significantly different from each other: the ZT4 value was 2.9 × 10−05 and the ZT22 value was 4.8 × 10−05. Furthermore, the CA isoform of RalA biased the same neurons to a high DA sensitivity state again with EC50 values that were not significantly different from each other: the ZT4 value was 5.7 ×10−06 and the ZT22 EC50 value was 6.4 ×10−06. Thus RalA isoforms had similar effects on s-LNv sensitivities to PDF and to DA. Based on these genetic interactions, we tested the hypothesis that RalA and PDF-R co-associate in living cells by studying their localization when co-transfected in hEK-293 cells (Suppl. Figure 4 - top). Most cells displayed numerous puncta indicative of highly co-localized RalA and PDFR; in some instances, RalA-enriched vesicles surrounded PDFR-containing cargo. We observed these discrete points of co-localization near (or perhaps at) the plasma membrane. As indicated by the time series shown, such puncta typically did not maintain fixed positions for more than a few seconds. These observations support the hypothesis that RalA can affect the distributions of PDFR and or its signaling complexes within s-LNv. We asked whether RalA manipulation in vivo produced deleterious effects on s-LNv viability or on its neuropeptide expression. We found no consistent differences between these experimental genotypes and their control in apparent morphology or in peptide immunoreactivity (Suppl. Figure 4 - bottom).
RalA manipulations produce circadian locomotor phenotypes that correlate with effects on PDF autoreceptor signaling
We reasoned that if RalA normally affected PDF receptor signaling in M cells, then genetic manipulations of rala should produce changes in behavior comparable to that observed when PDF autoreceptor signaling is altered. PDF signaling via autoreceptors in s-LNv (M pacemakers) (i) promotes morning activity (total activity 3 hr before lights-ON), (ii) advances the phase of the morning peak, (iii) increases the ratio of the morning to evening peak and (iv) shortens the circadian period (Choi et al. 2014). We therefore recorded daily locomotor rhythms in Pdf > ralaCA and Pdf > ralaDN variants (Figure 5), both in combination with the UAS-EPAC FRET transgene (to make the observations directly comparable with imaging results).
Figure 5. Manipulation of RalA activity in M neurons affects circadian locomotor patterns.
(A): LD group eductions for progeny of Pdf-Gal4 flies crossed to UAS-Epac (control) (n=14), UAS-ralaCA (n=16), UAS-ralaDN (n=16), UAS-dcr2 (control) (n=16) or UAS-dcr2, rala RNAi (n=15). (B): DD group eductions on DD1-2. Results shown are from a single experiment and representative of two independent experiments. Arrows highlight delayed phase of activity peak in the subjective morning. (C). Average group actograms displayed in double-plotted format over DD1-9. Total morning activity (total activity 3 hr before lights-ON) for the three genotypes. (D). Behavioral indices for the three genotypes involving RalA isoforms, including Morning Activity, Morning anticipation, Morning Ratio. and circadian period (τ) in DD3-9. (E) The same four behavioral indices for the two genotypes involving RalA RNAi. * indicates p < 0.01; ** indicates P < 0.001. See also Suppl. Table 1.
Under LD conditions, morning activity, anticipation and the morning ratio (morning vs. evening activity peaks) were very comparable in scale between control (Pdf > w1118) and the RalA-CA-expressing flies. However morning activity and anticipation were both reduced in flies expressing the RalA-DN variant (Figure 5D). By examining activity in the first day of DD (the first subjective morning, cf., Lear et al., 2009), we found the morning activity peak was delayed but not reduced by RalA-DN expression (Figure 5B). Finally, the RalA-DN variant increased the circadian period significantly, whereas circadian period following expression of the RalA-CA variant was largely similar to control (in one of three experiments we observed a slight though significant decrease; when combined the three experiments together revealed no significant difference). To confirm the authenticity of these rala genetic behavioral effects, we used targeted RNAi expression as an independent method of manipulating rala levels (Figure 5A-C). Indeed, reducing rala RNA levels specifically in LNv (using Pdf-Gal4) reduced morning activity, delayed the morning peak phase and increased circadian period (Figure 5E). Using a conditional gal80ts design, we confirmed that the behavioral effects of these rala manipulations were due to changes in the physiology of adult neurons and not due to the altered development of PDF neurons (Suppl. Figure 5). The rala [DN]-dependent effects on Morning activity and on the lengthening of circadian period were limited to flies that experienced the restrictive temperature (Suppl. Figure 6). These and all other behavioral results are compiled in Suppl. Tables 1 & 2. We also measured the behavioral effects of the CA and DN rala variants when expressed throughout the entire circadian pacemaker network by combining them with tim(UAS)-Gal4 and found largely congruent results (Suppl. Figure 7). Both isoforms produced increased morning activity. Under constant conditions, RalA-CA produced no effect on τ, whereas RalA-DN lengthened it, comparable to when RalA-DN expression was limited to PDF neurons. In summary, RalA manipulations that bias s-LNv to a steady low PDF sensitivity state also alter morning activity and increase circadian period. These results support the conclusion that functionally-effective PDF signaling in M neurons (signaling above the threshold necessary for specific behavioral consequence) is confined to a period just after lights-on by a RalA-dependent “morning gate”.
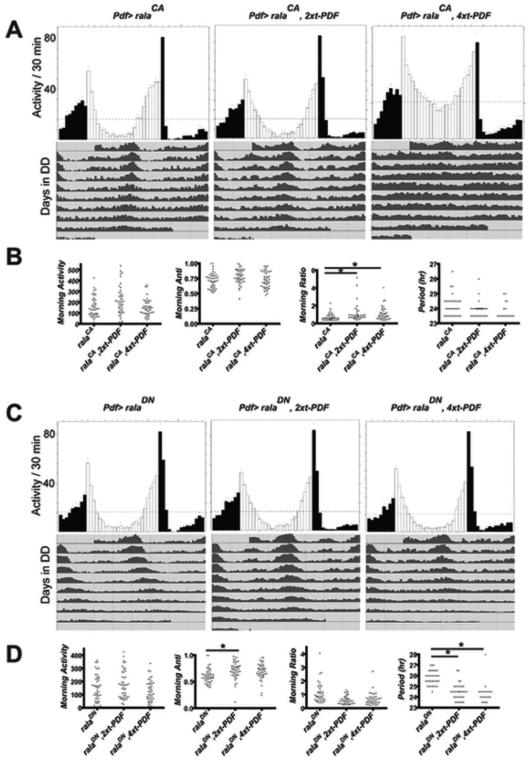
Expression of a tethered-PDF reverses RalA-DN effects in M neurons
To test the hypothesis of a morning gate for behaviorally-effective PDF receptor signaling, we asked whether increased availability of PDF ligand for PDF receptors in s-LNv could affect behavioral results produced by manipulating RalA. We reasoned that if high PDF sensitivity conferred by RalA-CA represented a true ceiling to effective PDF signaling, additional PDF ligand would not substantially change behavioral measures found with RalA-CA expression. However, if the DN isoform of RalA confers a low sensitivity state by decreasing PDF receptor number or quality (or otherwise limits its spatial distribution), additional PDF ligand could potentially reverse such behavioral effects. Indeed, these expectations were largely met. We used a tethered PDF transgenic strategy (t-PDF) to limit additional PDF ligand to the LNv’s. Choi et al. (2014) reported that 4 copies of t-Pdf driven by Pdf-Gal4 (but not 1 or 2 copies) significantly lowered circadian period. We found that t-Pdf combined with ralaCA produced a minor increase in the Morning Ratio (Figure 6B) and that t-Pdf combined with ralaDN produced a minor increase in the Morning Anticipation (Figure 6D). More significantly, we found that, in each of three separate experiments, combining t-Pdf with ralaDN lowered circadian period by more than one hr (Figure 6C and D, and Suppl. Table 1). In contrast, combining t-Pdf with ralaCA had no such effect on period (Figure 6A and B, and Suppl. Table 1). Concerning control genotypes, two and four copies of t-PDF alone did not change circadian period, neither did the coupling of two or four copies of a scrambled tethered PDF with RalA-DN, nor coupling of 4 copies of a tethered DH31 peptide with Ral-DN (Suppl. Table 2). Most of these genotypes exhibited increased percentages of arrhythmic flies, but not to a greater extent than four copies of t-PDF alone. Likewise, none of these “control” tethered peptides significantly changed circadian period when coupled to RalA-CA. Thus, under constant conditions, neurons biased to an invariant low-PDF sensitivity state can nevertheless trigger additional PDF receptor signaling with circadian behavioral consequence, if provided additional PDF ligand. However, additional PDF ligand does not provoke consequential PDF receptor signaling in neurons biased to an invariant high sensitivity state. We also noted that the morning peak of activity in Pdf > ralaDN flies enigmatically displayed a normal phase (Figure 6C), which is different from the result obtained when this genotype included the UAS-Epac transgene (Figure 5) and when we assayed the behavior using a gal80ts transgene (Suppl. Figures 5 and 6).
Figure 6. Additional PDF signaling in M cells from t-PDF transgenes reverses the RalA-DN but does not influence the Ral-CA behavioral phenotype.
(A) and (C) LD group eductions (top) and DD group actograms (bottom). Results in (A) and (C) are from a single representative experiment, from a total of three independent experiments. (A) Pdf > ralaCA (left; n = 16 for LD and DD conditions), Pdf > ralaCA , 2x-tPdf (center, n = 15, LD and = 14 DD), and Pdf > ralaCA , 4x-tPdf (right, n = 16 LD and = 12 DD). (B) Scatterplots of Morning Activity, Morning Anticipation, Morning Ratio and τ (DD3-9) for the three different genotypes: data compiled from three independent experiments. (C) Pdf > ralaDN (left; n = 15 LD and = 12 DD), Pdf > ralaDN , 2x-tPdf (center, n = 16 LD and = 15 DD), and Pdf > ralaDN , 4x-tPdf (right, n = 16 LD and= 14 DD). (D) Scatterplots of Morning Activity, Morning Anticipation, Morning Ratio and τ (DD3-9) for the three different genotypes: data compiled from three independent experiments. * for Morning Ratio and for Morning Anticipation, values are significantly different (p < 0.05) by one-way ANOVA followed by Tukey’s post hoc test; for Period (p < 0.01).
DISCUSSION
The neuropeptide PDF promotes proper behavioral sequencing dictated by the circadian timing system. This work has revealed a fundamental aspect underlying PDF physiological mechanisms. While s-LNv pacemakers respond to pharmacological doses of PDF at all times of day, functional PDF signaling (signaling that is behaviorally-consequential) is constrained to a narrow time window around dawn. The constraining mechanism is cell type-specific, receptor-selective, ligand-independent, and RalA-biased. Prior studies of PDF signaling have primarily determined the spatial aspects of receptor expression (Lear et al., 2009; Im and Taghert, 2010, Duvall and Taghert, 2012; 2013; Yao and Shafer, 2014). However, many cellular properties of pacemaker neurons vary over time. Pírez et al. (2013) found pharmacological evidence for a rhythm of PDF sensitivity in certain target neurons: supermaximal concentrations (10−05 M) of PDF produced larger amplitude responses in Ellipsoid Body (non-circadian pacemaker) target neurons in the morning versus evening. Here we show that critical pacemaker neurons use the 24-hr clock to change responsiveness (as much as 10-fold) to key neurotransmitter substances, including to the PDF neuropeptide. These results argue strongly that the temporal/pharmacological details of neuropeptide signaling are equally critical to properly understand neuropeptide modulation of neuronal circuits. In particular, they demonstrate that the responsiveness of the target cell, not just the level of signaling ligand, can be rhythmic. A corollary of this observation is that the times of maximal responsiveness predict when significant neuropeptide signaling occurs.
Our work builds on a broad consensus that Drosophila PDF signaling proceeds early in the photophase (Park et al., 2000; Cusumano et al., 2009; Zhang et al., 2009; Im et al., 2011; Collins et al., 2014; Choi et al., 2014). The specific thought is that ligand availability (via PDF release) is greatest in the morning. However, this important point remains an inference: there are as yet no direct observations of PDF release in vivo. In fact, declines in PDF immunohistochemical detection happen over many hours (Park et al., 2000) and PDF neurons exhibit electrophysiological activity throughout the day (Cao and Nitabach, 2008). We propose that the inferred peak of PDF availability and the demonstrated peak of PDF responsiveness are co-aligned to occur just after dawn for optimal, rhythmic neuropeptide modulation. Why does the gate for high PDF signaling occur in the early morning hours? Genetic evidence suggests PDF and Cryptochrome signals converge to drive proper PER cycles (Cusumano et al., 2009; Zhang et al., 2009; Im et al., 2011) due to co-expression of CRY and PDFR in specific pacemakers (Im et al., 2011). CRY levels fall quickly after light exposure (Busza et al., 2004), and so the temporal alignment of optimal PDF sensitivity with maximal CRY levels supports a co-activation model in the early morning hours. Together these observations support the hypothesis that PDF signaling in the early morning hours, when responsiveness is highest, has the greatest behavioral consequence.
What mechanisms underlie daily changes in PDF sensitivity of s-LNv pacemakers?
We observed a daily rhythm in the dependence of the cyclic-nucleotide response on the concentration of PDF (potency), rather than the maximal response (efficacy). In a multi-step process, this could be produced by changes in receptor number, distribution and/or quality, or could result from the rhythmic effects of a competitive antagonist. In addition, if one or more steps in the process are saturated under normal conditions, the change could occur after the initial receptor-ligand interaction. Zhang and Emery (2012) reported that GW182 positively modulates PDFR signaling via miRNA-dependent gene silencing and subsequent inhibition of dunce (phosphodiesterase) RNA. GW182 is unlikely to cause the daily change in PDF sensitivity in s-LNv because GW182 actions were attributed completely to its functions within E pacemakers (Zhang and Emery, 2012). Nevertheless, this possibility requires future investigation. In the mammalian liver, CRY inhibits glucagon-mediated cAMP accumulation by interaction with Gs α activity (Zhang et al., 2010). However a CRY-based mechanism to negatively regulate PDF sensitivity after the morning hours appears unlikely in s-LNv because CRY protein is degraded rapidly in the early morning light, when PDF-R and DA-R signaling appear greatest.
Several previous studies have shown that signaling via 7-transmembrane (7TM) proteins varies based on circadian physiology. Most notably, odorant sensory neurons in Drosophila display daily rhythms in olfactory responses caused by circadian accumulation of olfactory receptors in their dendrites that is driven by changes in the GPR kinase 2 (Tanoue et al., 2008). However, a GPRK2-based mechanism appears unlikely to explain PDFR daily changes in s-LNv: while Drosophila olfactory receptors are 7TM proteins, their polarity in the membrane is inverted relative to GPCRs and they function as ligand-gated ion channels (Benton et al., 2006). Likewise in the mouse suprachiasmatic nucleus, where the neuropeptide VIP signaling appears orthologous to PDF signaling in Drosophila (Vosko et al., 2007), levels of the VPAC2 receptor display diurnal and/ or circadian rhythmicity (Cagampang et al., 1998 Shinohara et al., 1999; An et al., 2012). However, whether such changes in accumulation translate to daily changes in VIP potency have not been investigated. The daily cycling of dopamine (D1-like) receptor responsiveness in Drosophila s-LNv parallels that of PDF receptor responsiveness, and recalls the work of Andretic and Hirsh (2000) who reported circadian variation in D2-like receptor responsiveness based on behavioral assays. Our current results suggest that rhythmic DA sensitivity in s-LNv may also have functional importance for s-LNv pacemaker outputs. Finally, Pdfr RNA levels also cycle in PDF neurons (K. Abruzzi and M Rosbash, pers. commun.) but with a peak phase around dusk, whereas PDF sensitivity in these neurons peaks 12 hr later. Likewise, we observed a normal daily rhythm in PDF responses when PDFR expression was driven only by the non-rhythmic Pdf GAL4 promoter.
We strongly implicate the small GTPase RalA in the mechanism underlying regulation of PDF sensitivity: we found that CA and DN isoforms of RalA in vivo respectively force PDF sensitivity to opposite endpoints within a dynamic range that the s-LNv normally traverses over the course of each day. Rala RNA levels do not cycle in vivo in LNv, according to information derived from single cell RNA profiling (K. Abruzzi and M. Rosbash, personal communication): instead we suspect RalA activity must cycle to produce the changes in PDFR sensitivity. Active RalA binds diverse downstream effectors and modulates several cellular activities including secretory vesicle trafficking, growth of the neuromuscular junction, and regulation of the exocyst complex (Gentry et al., 2014). We speculate there is a daily generation of distinctive PDF receptors (distinguished by their location, post-translational modification and/or by other signaling components) and that RalA is needed to promote their timely fusion with the plasma membrane. However, other RalA-based mechanisms are also possible (Nakashima et al., 1999; Jullien-Flores et al., 2000; Matsuzaki et al., 2002; Godin et al., 2010). Regardless of the precise interactions between the GPCR and RalA, finding a close apposition of RalA with PDFR in hEK cells (Suppl. Figure 4) (as previously demonstrated for metabotropic glutamate receptors (Bhattacharya et al., 2004)) supports the hypothesis that RalA efficiently biases PDFR receptor activity.
Lack of short-term attenuation of PDF receptor signaling
While PDF sensitivity displays striking temporal variation over a long (24 hr) time course (Figure 1), we observe that in the short term, PDFR signaling properties are remarkably stable. PDFR activation failed to recruit cytoplasmic β-ARR2-GFP translocation in vitro (Suppl. Figure 2), unlike the D1-Rs and unlike many other Drosophila neuropeptide GPCRs (Johnson et al., 2003, 2004). The stability of PDFR signaling is reminiscent of another class B GPCR, the parathyroid hormone receptor (Viladarga et al., 2014), and of certain GPCRs, like Type 1 mGluRs and CB1, which display agonist-independent, constitutive rates of endocytosis (Bhattacharya et al., 2004; LeTerrier et al., 2006). Notably, type 1 mGluR and CB1 are regulated by different control systems, including RalA (Dhami and Ferguson, 2006).
The role of rhythmic PDF signaling in the regulation of behavior
To determine if changes in PDF sensitivity regulate diurnal locomotor behavior, we expressed RalA isoforms with additional (cell-autonomous) PDF signaling in M neurons and found that the expression of tethered PDF on the surface of M cells effectively suppressed the behavioral effects of RalA DN, but that there were no interactions with the high RalA activity state (RalA CA). Our interpretation of these results is two-fold. First, although changing RalA activity levels is bound to alter many aspects of M cell physiology, RalA-DN effects within M cells on circadian behavior are largely explained by its effects on PDF signaling. Secondly, because the RalA CA genotype confers constant high levels of PDF sensitivity, with little change in behavior, the circadian rhythm in PDF responsiveness is not critically required to support behavioral rhythms. This combination of cell-biological and behavioral observations suggests that a high level of PDF response is important early in the photophase, but that the assumption of a low level at other times is not critical. Therefore we propose the hypothesis that the daily change in PDF sensitivity in M neurons allows PDFR signaling to reach this necessary, high-sensitivity level in the morning. PDF signaling below this level has little influence on circadian behaviors and above it, does not appreciably add to rhythmic behavior. Other cellular processes may require that the level of PDF signaling declines during other phases of the circadian cycle. At present, the role of this daily change in responsiveness remains unclear, it may be energetically or physiologically unfavorable to maintain high PDFR (and other GPCR) signaling throughout the day.
Further research will now ask which molecular mechanisms elevate PDF responsiveness in pacemaker neurons. We favor the possibility that a specific population of PDF receptors is promoted during the neuron’s period of high sensitivity, as a result of an action of RalA and that a similar mechanism exists for DA receptors. Together these observations may provide an entry for biochemical experiments to address this emerging theme in the neurobiology of circadian rhythms.
METHODS
Fly Rearing and Stocks
We reared Drosophila on yeast suppl emented with cornmeal/agar. Newly-eclosed males were collected each morning and entrained 4-7 days in a 12h:12h Light:Dark (LD) cycle. The 12:12 L:D cycle was maintained throughout rearing, except when flies were instead maintained in constant darkness for 1-2 days following the initial 3-4 days of entrainment in L:D. RNAi stocks were obtained from VDRC and TRiP stock centers; rala RNAi was tested along with UAS-dcr2. All other UAS lines and Gal4 lines have been described previously: w; UAS-Epac1camps50A (Shafer et al., 2008), w, Pdf-Gal4(M) (Park et al., 2000), w1118; UAS—dbtL (Muskus et al., 2007), UAS-ralaCA (G20V) and w;UAS-ralaDN (Mirey et al., 2003; Sawamoto et al., 1999), w;UAS-Pdfr (16) (Mertens et al., 2005), w;;pdf01 (Renn et al., 1999); Pdfr-han5534 (Hyun et al., 2005); 2x- and 4x-tethered PDF fly stocks (w; UAS-2xtPDF and w; UAS -4xtPDF) (Choi et al., 2014). To test the effects of t-PDF transgenes in combination with rala transgene variants, we first made stable stocks with genotypes Pdf-GAL4 (M); UAS-ralaCA and Pdf-GAL4 (M);; UAS-ralaDN. Test combinations were created by crossing them to:
58F1aF5a (III) to add 2x-tPDF
58M2aM3a/CyO; 58F1aF5a/TM6c to add 4x-tPDF
P1-a2b1/CyO; +/Tm6c (II) to add 2x-Scrpdf
P1-a2b4/ CyO; P1-b3b5/Tm6c to add 4x-Scrpdf
P2-2c/ CyO; P2-B2B3/Tm3 to add 4x-tDH31
For conditional Gal4 activity, we using the following genotypes: Pdf-GAL4 (M)/ tubGal80ts; UAS-ralaCA and Pdf-GAL4 (M)/ tubGal80ts ; +/ UAS-ralaDN. Flies were raised at 18°C until eclosion, then maintained at 18°C or at 29°C, for at least 3 days prior to testing.
FRET Imaging
Methods generally followed those of Shafer et al., (2008) and Duvall and Taghert (2012). One to two days prior to testing, the rearing temperature was raised to 29°C to enhance UAS transgene expression. Flies were chilled on ice for 11-13 minutes, dissected in chilled calcium-free HL3 (Stewart et al., 1994). 2-4 brains were studied on poly-lysine-treated plastic dishes (35×10mm Falcon polystyrene) containing 2.9 mLs standard HL3: typically, a mutant line and its genetic control were tested simultaneously (in blind fashion) for a direct comparison. Image capture and X,Y,Z stage movements were set via SLIDEBOOK 4.1 (Intelligent Imaging Innovations) which controlled a Prior H101Plan Power Stage. Regions of interest were defined for one to four neurons in each of 1-2 optical planes of each hemi-segment of each brain. Following 3 minutes (9 ratio recordings) of baseline YFP/CFP measurements, 0.9mLs of bath saline was removed, combined with 100μL of HL3 containing either PDF or dopamine, of various concentrations, and then restored to the bath (dilution factor of 1/3). We collected only a single round of data for any isolated brain (no repeated dosing). We used synthetic PDF at >90% purity (Neo-MPS, San Diego, CA), and dopamine (Sigma-Aldrich, St. Louis, MO). We tested normality in the data using the Shapiro-Wilk test in SigmaPlot (SYSTAT, San Jose, CA), and calculated EC50 and Maximum Amplitude values, and performed ANOVA analyses followed by post hoc Tukey tests, using Prism 5.0 (GraphPad Software, La Jolla, CA). Calculations involved a 3-parameter nonlinear fit of normalized data, constrained to zero at the bottom and 100 at the top of the concentration-effect curve, and used a standard Hill slope.
Analysis of concentration-effect curves
Some of the concentration-effect curves showed an indication that there may be more than one population of cells in terms of responses to treatment (for example, Figures 3C). To examine this question more fully, we tested the ability of a single Gaussian function to describe such distributions in each of two ways. The first was a parametric test of skewness and curtosis combined into a single probability, performed using STATA (v12.1; StataCorp, College Station TX). The second was a non-parametric test to compare the cumulative distribution of the data to that predicted by the mean and standard deviation of the data (SigmaPlot v11, Systat Software San Jose, CA). These tests indicated that several of the data sets indeed were unlikely to be described by a single Gaussian curve. However the two tests did not agree; in only 2 cases did both tests indicate significant deviation out of the 10 in which one or both did (a total of 28 data sets were examined using both tests, or 56 tests). This observation suggested that the impression of non-normal distributions was not consistent or robust. Accordingly, we analyzed all the data in terms of a single population mean and standard deviation. Further description of these analyses is described in Suppl. Information.
Immunocytochemistry
Methods followed those of Im and Taghert (2010). We performed confocal imaging with a Nikon A1R microscope with settings consistent for all specimens, and adjusted contrast for Figures in Photoshop. See also Suppl. Information.
Locomotor Activity
We monitored locomotor activity in 4–6 d old males for 6 days under 12:12 LD and then for 9 days under in DD, using Trikinetics Activity Monitors. We assessed rhythmicity by normalizing activity from DD Days 3–9 and used a Χ2 periodogram with a 95% confidence cutoff, and also SNR analysis (Levine et al., 2002). We defined arrhythmic flies by displaying a power value less than 10 and width value less than 2, or a τ estimate < 18 hr or > 30 hr. See also Suppl. Information.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Tom Schwartz, Michael Nitabach, the Bloomington Stock Center and the VDRC for Drosophila stocks, Steve Ferguson for plasmids, Katherine Abruzzi and Michael Rosbash for sharing unpublished information, and Dongkook Park, Erik Herzog and Stephan Dissel for critically reading a draft of this manuscript. We thank Jennifer Trigg for excellent technical assistance, Dennis Oakley of the Bakewell Neuroimaging Laboratory and the Washington University Center for Cellular Imaging (WUCCI) for assistance, and members of the laboratory for advice and helpful comments. LBD was supported by NIH 5-T32-GM08151-27 and by 5-T32-EY013360-10. The work was supported by a grant from the NIH (R01 MH067122) to PHT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
MK, LBD, WL, XL and CR performed experiments; MK, LBD, WL, XL and PHT conceived of the experiments; MK, LBD, WL, XL CR JHS and PHT analyzed the data; MK, LBD, JHS and PHT wrote the manuscript.
LITERATURE CITED
- Abruzzi KC, Rodriguez J, Menet JS, Desrochers J, Zadina A, Luo W, Tkachev S, Rosbash M. Drosophila CLOCK target gene characterization: implications for circadian tissue-specific gene expression. Genes Dev. 2011;5:2374–86. doi: 10.1101/gad.178079.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal T, Sadaf S, Hasan G. A genetic RNAi screen for IP3/Ca²? coupled GPCRs in Drosophila identifies the PdfR as a regulator of insect flight. PLoS Genet. 2013;9:e1003849. doi: 10.1371/journal.pgen.1003849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, Tsai C, Ronecker J, Bayly A, Herzog ED. Spatiotemporal distribution of vasoactive intestinal polypeptide receptor 2 in mouse suprachiasmatic nucleus. J Comp Neurol. 2012;520:2730–41. doi: 10.1002/cne.23078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andretic R, Hirsh J. Circadian modulation of dopamine receptor responsiveness in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2000;97:1873–78. doi: 10.1073/pnas.97.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith EJ, Ceriani MF. Experimental assessment of the network properties of the Drosophila circadian clock. J Comp Neurol. 2015;523:982–96. doi: 10.1002/cne.23728. [DOI] [PubMed] [Google Scholar]
- Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M, Babwah AV, Godin C, Anborgh PH, Dale LB, Poulter MO, Ferguson SS. Ral and phospholipase D2-dependent pathway for constitutive metabotropic glutamate receptor endocytosis. J Neurosci. 2004;24:8752–61. doi: 10.1523/JNEUROSCI.3155-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busza A, Emery-Le M, Rosbash M, Emery P. Roles of the two Drosophila CRYPTOCHROME structural domains in circadian photoreception. Science. 2004;304:1503–06. doi: 10.1126/science.1096973. [DOI] [PubMed] [Google Scholar]
- Cagampang FR, Sheward WJ, Harmar AJ, Piggins HD, Coen CW. Circadian changes in the expression of vasoactive intestinal peptide 2 receptor mRNA in the rat suprachiasmatic nuclei. Brain Res Mol Brain Res. 1998;54:108–12. doi: 10.1016/s0169-328x(97)00327-6. [DOI] [PubMed] [Google Scholar]
- Cao G, Nitabach MN. Circadian control of membrane excitability in Drosophila melanogaster lateral ventral clock neurons. J Neurosci. 2008;28:6493–501. doi: 10.1523/JNEUROSCI.1503-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XW, Leto D, Xiao J, Goss J, Wang Q, Shavit JA, Xiong T, Yu G, Ginsburg D, Toomre D, Xu Z, Saltiel AR. Exocyst function is regulated by effector phosphorylation. Nat Cell Biol. 2011;13(5):580–588. doi: 10.1038/ncb2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Cao G, Tanenhaus AK, McCarthy EV, Jung M, Schleyer W, Shang Y, Rosbash M, Yin JC, Nitabach MN. Autoreceptor control of peptide/neurotransmitter corelease from PDF neurons determines allocation of circadian activity in Drosophila. Cell Rep. 2012;2:332–44. doi: 10.1016/j.celrep.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung BY, Kilman VL, Keath JR, Pitman JL, Allada R. The GABA(A) receptor RDL acts in peptidergic PDF neurons to promote sleep in Drosophila. Curr Biol. 2009;19:386–90. doi: 10.1016/j.cub.2009.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B, Kaplan HS, Cavey M, Lelito KR, Bahle AH, Zhu Z, Macara AM, Roman G, Shafer OT, Blau J. Differentially timed extracellular signals synchronize pacemaker neuron clocks. PLoS Biol. 2014;12:e1001959. doi: 10.1371/journal.pbio.1001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusumano P, Klarsfeld A, Chélot E, Picot M, Richier B, Rouyer F. PDF-modulated visual inputs and cryptochrome define diurnal behavior in Drosophila. Nat Neurosci. 2009;12:1431–37. doi: 10.1038/nn.2429. [DOI] [PubMed] [Google Scholar]
- Dahdal D, Reeves DC, Ruben M, Akabas MH, Blau J. Drosophila pacemaker neurons require g protein signaling and GABAergic inputs to generate twenty-four hour behavioral rhythms. Neuron. 2010;68:964–77. doi: 10.1016/j.neuron.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhami GK, Ferguson SS. Regulation of metabotropic glutamate receptor signaling, desensitization and endocytosis. Pharmacol Ther. 2006;111:260–71. doi: 10.1016/j.pharmthera.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Dissel S, Hansen CN, Özkaya Ö , Hemsley M, Kyriacou CP, Rosato E. The logic of circadian organization in Drosophila. Curr Biol. 2014;24:2257–66. doi: 10.1016/j.cub.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall LB, Taghert PH. The circadian neuropeptide PDF signals preferentially through a specific adenylate cyclase isoform AC3 in M pacemakers of Drosophila. PLoS Biol. 2012;10:e1001337. doi: 10.1371/journal.pbio.1001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvall LB, Taghert PH. E and M circadian pacemaker neurons use different PDF receptor signalosome components in Drosophila. J Biol Rhythms. 2013;28:239–48. doi: 10.1177/0748730413497179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández MP, Berni J, Ceriani MF. Circadian remodeling of neuronal circuits involved in rhythmic behavior. PLoS Biol. 2008;6:e69. doi: 10.1371/journal.pbio.0060069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry LR, Martin TD, Reiner DJ, Der CJ. Ral small GTPase signaling and oncogenesis: More than just 15 minutes of fame. Biochim Biophys Acta. 2014;1843:2976–88. doi: 10.1016/j.bbamcr.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin CM, Ferreira LT, Dale LB, Gros R, Cregan SP, Ferguson SS. The small GTPase Ral couples the angiotensin II type 1 receptor to the activation of phospholipase C-delta 1. Mol Pharmacol. 2010;77:388–95. doi: 10.1124/mol.109.061069. [DOI] [PubMed] [Google Scholar]
- Gorostiza EA, Depetris-Chauvin A, Frenkel L, Pírez N, Ceriani MF. Circadian pacemaker neurons change synaptic contacts across the day. Curr Biol. 2014;24(18):2161–67. doi: 10.1016/j.cub.2014.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Cerullo I, Chen X, Rosbash M. PDF neuron firing phase-shifts key circadian activity neurons in Drosophila. Elife. 2014:3. doi: 10.7554/eLife.02780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfrich-Förster C, Shafer OT, Wülbeck C, Grieshaber E, Rieger D, Taghert P. Development and morphology of the clock-gene-expressing lateral neurons of Drosophila melanogaster. J Comp Neurol. 2007;500:47–70. doi: 10.1002/cne.21146. [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C. Development of pigment-dispersing hormone-immunoreactive neurons in the nervous system of Drosophila melanogaster. J Comp Neurol. 1997;380:335–54. doi: 10.1002/(sici)1096-9861(19970414)380:3<335::aid-cne4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Henion TR, Faden AA, Knott TK, Schwarting GA. β3GnT2 maintains adenylyl cyclase-3 signaling and axon guidance molecule expression in the olfactory epithelium. J Neurosci. 2011;31:6578–86. doi: 10.1523/JNEUROSCI.0224-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann-Luibl C, Yoshii T, Senthilan PR, Dircksen H, Helfrich-Förster C. The ion transport peptide is a new functional clock neuropeptide in the fruit fly Drosophila melanogaster. J Neurosci. 2014;34:9522–36. doi: 10.1523/JNEUROSCI.0111-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewes RS, Taghert PH. Neuropeptides and neuropeptide receptors in the Drosophila melanogaster genome. Genome Res. 2001;11:1126–42. doi: 10.1101/gr.169901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun S, Lee Y, Hong ST, Bang S, Paik D, Kang J, Shin J, Lee J, Jeon K, Hwang S, Bae E, Kim J. Drosophila GPCR Han is a receptor for the circadian clock neuropeptide PDF. Neuron. 2005;48:267–78. doi: 10.1016/j.neuron.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Im SH, Taghert PH. PDF receptor expression reveals direct interactions between circadian oscillators in Drosophila. J Comp Neurol. 2010;518:1925–45. doi: 10.1002/cne.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SH, Li W, Taghert PH. PDFR and CRY signaling converge in a subset of clock neurons to modulate the amplitude and phase of circadian behavior in Drosophila. PLoS One. 2011;6:e18974. doi: 10.1371/journal.pone.0018974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itri JN, Vosko AM, Schroeder A, Dragich JM, Michel S, Colwell CS. Circadian regulation of a-type potassium currents in the suprachiasmatic nucleus. J Neurophysiol. 2010;103:632–40. doi: 10.1152/jn.00670.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EC, Bohn LM, Barak LS, Birse RT, Nässel DR, Caron MG, Taghert PH. Identification of Drosophila neuropeptide receptors by G protein-coupled receptors-beta-arrestin2 interactions. J Biol Chem. 2003;278:52172–78. doi: 10.1074/jbc.M306756200. [DOI] [PubMed] [Google Scholar]
- Johnson EC, Bohn LM, Taghert PH. Drosophila CG8422 encodes a functional diuretic hormone receptor. J Exp Biol. 2004;207:743–48. doi: 10.1242/jeb.00818. Pt 5. [DOI] [PubMed] [Google Scholar]
- Jullien-Flores V, Mahé Y, Mirey G, Leprince C, Meunier-Bisceuil B, Sorkin A, Camonis JH. RLIP76, an effector of the GTPase Ral, interacts with the AP2 complex: involvement of the Ral pathway in receptor endocytosis. J Cell Sci. 2000;113:2837–44. doi: 10.1242/jcs.113.16.2837. [DOI] [PubMed] [Google Scholar]
- Kula-Eversole E, Nagoshi E, Shang Y, Rodriguez J, Allada R, Rosbash M. Surprising gene expression patterns within and between PDF-containing circadian neurons in Drosophila. Proc Natl Acad Sci U S A. 2010;107:13497–502. doi: 10.1073/pnas.1002081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst M, Hughes ME, Raccuglia D, Felix M, Li M, Barnett G, Duah J, Nitabach MN. Calcitonin gene-related peptide neurons mediate sleep-specific circadian output in Drosophila. Curr Biol. 2014;24:2652–64. doi: 10.1016/j.cub.2014.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lear BC, Merrill CE, Lin JM, Schroeder A, Zhang L, Allada R. A G protein-coupled receptor, groom-of-PDF, is required for PDF neuron action in circadian behavior. Neuron. 2005;48:221–27. doi: 10.1016/j.neuron.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Lear BC, Zhang L, Allada R. The neuropeptide PDF acts directly on evening pacemaker neurons to regulate multiple features of circadian behavior. PLoS Biol. 2009;207:e1000154. doi: 10.1371/journal.pbio.1000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier C, Lainé J, Darmon M, Boudin H, Rossier J, Lenkei Z. Constitutive activation drives compartment-selective endocytosis and axonal targeting of type 1 cannabinoid receptors. J Neurosci. 2006;26:3141–53. doi: 10.1523/JNEUROSCI.5437-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Guo F, Shen J, Rosbash M. PDF and cAMP enhance PER stability in Drosophila clock neurons. Proc Natl Acad Sci U S A. 2014;111:E1284–90. doi: 10.1073/pnas.1402562111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Holy TE, Taghert PH. Synchronous Drosophila circadian pacemakers display non-synchronous Ca2+ rhythms in vivo. Science. 2016;351:976–81. doi: 10.1126/science.aad3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Stormo GD, Taghert PH. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J Neurosci. 2004;24:7951–57. doi: 10.1523/JNEUROSCI.2370-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki T, Hanai S, Kishi H, Liu Z, Bao Y, Kikuchi A, Tsuchida K, Sugino H. Regulation of endocytosis of activin type II receptors by a novel PDZ protein through Ral/Ral-binding protein 1-dependent pathway. J Biol Chem. 2002;277:19008–18. doi: 10.1074/jbc.M112472200. [DOI] [PubMed] [Google Scholar]
- Mertens I, Vandingenen A, Johnson EC, Shafer OT, Li W, Trigg JS, De Loof A, Schoofs L, Taghert PH. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron. 2005;48:213–19. doi: 10.1016/j.neuron.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Mirey G, Balakireva M, L'Hoste S, Rosse C, Voegeling S, Camonis J. A Ral guanine exchange factor-Ral pathway is conserved in Drosophila melanogaster and sheds new light on the connectivity of the Ral, Ras, and Rap pathways. Mol Cell Biol. 2003;23:1112–24. doi: 10.1128/MCB.23.3.1112-1124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muskus MJ, Preuss F, Fan JY, Bjes ES, Price JL. Drosophila DBT lacking protein kinase activity produces long-period and arrhythmic circadian behavioral and molecular rhythms. Mol Cell Biol. 2007;27:8049–64. doi: 10.1128/MCB.00680-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima S, Morinaka K, Koyama S, Ikeda M, Kishida M, Okawa K, Iwamatsu A, Kishida S, Kikuchi A. Small G protein Ral and its downstream molecules regulate endocytosis of EGF and insulin receptors. EMBO J. 1999;8:3629–42. doi: 10.1093/emboj/18.13.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol. 2008;18:R84–93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, Kang K, Liu X, Garrity PA, Rosbash M, Griffith LC. PDF cells are a GABA-responsive wake promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–82. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Helfrich-Forster C, Lee G, Liu L, Rosbash M, Hall JC. Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proc Natl Acad Sci. USA. 2000;97:3608–13. doi: 10.1073/pnas.070036197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y, Stoleru D, Levine JD, Hall JC, Rosbash M. Drosophila free-running rhythms require intercellular communication. PLoS Biol. 2003;1:E13. doi: 10.1371/journal.pbio.0000013. Epub 2003 Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pírez N, Christmann BL, Griffith LC. Daily rhythms in locomotor circuits in Drosophila involve PDF. J Neurophysiol. 2013;110:700–8. doi: 10.1152/jn.00126.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SCP, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons both cause severe abnormalities of circadian behavioral rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Winge P, Koyama S, Hirota Y, Yamada C, Miyao S, Yoshikawa S, Jin MH, Kikuchi A, Okano H. The Drosophila Ral GTPase regulates developmental cell shape changes through the Jun NH(2)-terminal kinase pathway. J Cell Biol. 1999;146:361–72. doi: 10.1083/jcb.146.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LE, Sun ET, Garland DJ, Taghert PH. An immunocytochemical study of the FMRFamide neuropeptide gene products in Drosophila. J Comp Neurol. 1993;337:446–60. doi: 10.1002/cne.903370308. [DOI] [PubMed] [Google Scholar]
- Seluzicki A, Flourakis M, Kula-Eversole E, Zhang L, Kilman V, Allada R. Dual PDF signaling pathways reset clocks via TIMELESS and acutely excite target neurons to control circadian behavior. PLoS Biol. 2014;18(12):e1001810. doi: 10.1371/journal.pbio.1001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Kim DJ, Dunbar-Yaffe R, Nikolaev VO, Lohse MJ, Taghert PH. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron. 2008;58:223–37. doi: 10.1016/j.neuron.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Haynes P, Pírez N, Harrington KI, Guo F, Pollack J, Hong P, Griffith LC, Rosbash M. Imaging analysis of clock neurons reveals light buffers the wake-promoting effect of dopamine. Nat Neurosci. 2011;14:889–95. doi: 10.1038/nn.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara K, Funabashi T, Kimura F. Temporal profiles of vasoactive intestinal polypeptide precursor mRNA and its receptor mRNA in the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 1999;63:262–67. doi: 10.1016/s0169-328x(98)00289-7. [DOI] [PubMed] [Google Scholar]
- Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol A. 1994;17:179–91. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- Tanoue S, Krishnan P, Chatterjee A, Hardin PE. G protein-coupled receptor kinase 2 is required for rhythmic olfactory responses in Drosophila. Curr Biol. 2008;18:787–94. doi: 10.1016/j.cub.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilardaga JP, Jean-Alphonse FG, Gardella TJ. Endosomal generation of cAMP in GPCR signaling. Nat Chem Biol. 2014;10:700–6. doi: 10.1038/nchembio.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosko AM, Schroeder A, Loh DH, Colwell CS. Vasoactive intestinal peptide and the mammalian circadian system. Gen Comp Endocrinol. 2007;152:165–75. doi: 10.1016/j.ygcen.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li G, Sugita S. RalA-exocyst interaction mediates GTP-dependent exocytosis. J Biol Chem. 2004;279:19875–81. doi: 10.1074/jbc.M400522200. [DOI] [PubMed] [Google Scholar]
- Yao Z, Shafer OT. The Drosophila circadian clock is a variably coupled network of multiple peptidergic units. Science. 2014;343:1516–20. doi: 10.1126/science.1251285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii T, Wülbeck C, Sehadova H, Veleri S, Bichler D, Stanewsky R, Helfrich-Förster C. The neuropeptide pigment-dispersing factor adjusts period and phase of Drosophila's clock. J Neurosci. 2009;29:2597–610. doi: 10.1523/JNEUROSCI.5439-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Lear BC, Seluzicki A, Allada R. The CRYPTOCHROME photoreceptor gates PDF neuropeptide signaling to set circadian network hierarchy in Drosophila. Curr Biol. 2009;19:2050–55. doi: 10.1016/j.cub.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang EE, Liu Y, Dentin R, Pongsawakul PY, Liu AC, Hirota T, Nusinow DA, Sun X, Landais S, Kodama Y, Brenner DA, Montminy M, Kay SA. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16:1152–56. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Emery P. GW182 controls Drosophila circadian behavior and PDF-receptor signaling. Neuron. 2013;78:152–65. doi: 10.1016/j.neuron.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.