Abstract
While calpain-1 activation is required for LTP induction by theta burst stimulation (TBS), calpain-2 activation limits its magnitude during the consolidation period. A selective calpain-2 inhibitor applied either before or shortly after TBS enhanced the degree of potentiation. In the present study, we tested whether the selective calpain-2 inhibitor, Z-Leu-Abu-CONH-CH2-C6H3 (3, 5-(OMe)2 (C2I), could enhance learning and memory in wild-type (WT) and calpain-1 knockout (C1KO) mice. We first showed that C2I could reestablish TBS-LTP in hippocampal slices from C1KO mice, and this effect was blocked by PD98059, an inhibitor of ERK. TBS resulted in PTEN degradation in hippocampal slices from both WT and C1KO mice, and C2I treatment blocked this effect in both mouse genotypes. Systemic injection of C2I 30 min before training in the fear-conditioning paradigm resulted in a biphasic dose-response curve, with low doses enhancing and high doses inhibiting freezing behavior. The difference between the doses needed to enhance and inhibit learning matches the difference in concentrations producing inhibition of calpain-2 and calpain-1. A low dose of C2I also restored normal learning in a novel object recognition task in C1KO mice. Levels of SCOP, a ERK phosphatase known to be cleaved by calpain-1, were decreased in dorsal hippocampus early but not late following training in WT mice; C2I treatment did not affect the early decrease in SCOP levels but prevented its recovery at the later time-point and prolonged ERK activation. The results indicate that calpain-2 activation limits the extent of learning, an effect possibly due to temporal limitation of ERK activation, as a result of SCOP synthesis induced by calpain-2-mediated PTEN degradation.
Keywords: calpain-1, calpain-2, learning, SCOP, ERK, hippocampus
Graphical abstract
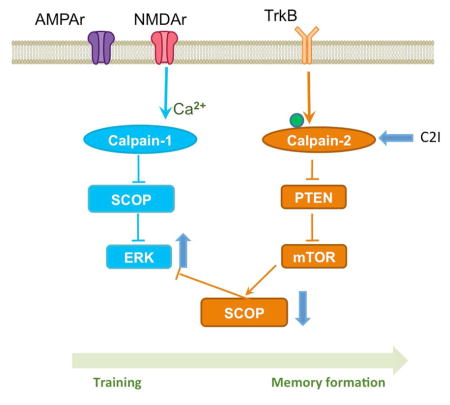
Calpains are calcium-dependent proteases, which play critical roles in both physiological and pathological conditions in the central nervous system. Two major calpain isoforms are present in mammalian brain: calpain-1 (aka, μ-calpain) and calpain-2 (aka, m-calpain)(Croall and Ersfeld, 2007; Goll et al., 2003; Ono and Sorimachi, 2012; Wu and Lynch, 2006). While calpain-1 activation is necessary for the formation of long-term potentiation (LTP) elicited by theta burst stimulation (TBS) in field CA1 of hippocampus, calpain-2 activation during the 1 h-consolidation period after TBS limits the extent of synaptic potentiation, and its inhibition results in enhanced LTP (Wang et al., 2014). During LTP induction, calpain-1 activation results in the truncation of suprachiasmatic nucleus circadian oscillatory protein (SCOP), a negative regulator of extracellular signal-regulated kinase (ERK), leading to ERK activation. On the other hand, calpain-2 activation during the consolidation period stimulates mTOR-dependent SCOP protein synthesis through calpain-mediated degradation of phosphatase and tensin homologue (PTEN), which limits the duration of ERK activation (Briz et al., 2013; Wang et al., 2014).
Transient ERK activation has been repeatedly shown to be necessary for LTP induction (Jin and Feig, 2010; Kelleher et al., 2004), and as mentioned above, this could be related to calpain-1 mediated SCOP degradation (Wang et al., 2014). ERK activation has also been shown to be involved in learning of various hippocampus-dependent tasks (Menard et al., 2015; Peng et al., 2010; Xia and Storm, 2012). A previous study indicated that training in novel object recognition or in fear conditioning resulted in SCOP degradation and ERK activation in hippocampus 5 min after training (Shimizu et al., 2007). However, SCOP levels returned to normal 30 min after training, suggesting that the mechanism linking TBS to calpain activation, PTEN degradation and re-synthesis of SCOP could also take place in hippocampus following a learning episode. To further evaluate the differential roles of calpain-1 and calpain-2 in learning and memory, we first tested the effects of a selective calpain-2 inhibitor (Z-Leu-Abu-CONH-CH2-C6H3(3,5-(OMe)2) (C2I) (Li et al., 1996), on the impairment in LTP we previously reported in C1KO mice (Zhu et al., 2015). We also verified that calpain-2 was activated following TBS in slices from C1KO mice, as assessed with PTEN truncation, which we previously reported to be degraded by calpain-2 but not calpain-1 (Briz et al., 2013). We then tested the effects of various doses of C2I on learning and memory using the fear-conditioning paradigm in WT mice and the effects of a low dose of C2I on novel object recognition in WT and C1KO mice, which we have also shown to be impaired in the C1KO mice (Zhu et al., 2015). Finally, we tested the effects of C2I treatment on the levels of SCOP and activated/phosphorylated ERK in dorsal and ventral hippocampus at 5 and 50 min after training, to further establish the role of calpain-2 in learning consolidation. Our results demonstrate that calpain-2 activation limits the extent of learning by restricting the temporal activation of ERK. These results further strengthen the links between LTP and learning, as both genetic and pharmacological manipulations affect both processes through the same molecular mechanisms.
Experimental Procedures
Animal use in all experiments followed National Institutes of Health guidelines, and all protocols were approved by the Institution Animal Care and Use Committee of Western University of Health Sciences. Calpain-1 knock-out (C1KO) mice on a C57BL/6 background were obtained from a breeding colony established from breeding pairs generously provided by Dr. Chishti (Tufts University). C57BL/6 mice were purchased from The Jackson Laboratory and were used as the corresponding wild-type (WT).
Acute hippocampal slice preparation
Hippocampal slice preparation and electrophysiological recording were performed as previously reported (Sun et al., 2015). Adult male mice (3- to 4-month-old) were anesthetized with halothane and decapitated. Brains were quickly removed and transferred to oxygenated, ice-cold cutting medium as follows (in mM): NaCl (124), NaHCO3 (26), glucose (10), KCl (3), 1KH2PO4 (1.25), MgSO4 (5), and CaCl2 (3.4). Hippocampal transversal slices (350 μm thick) were prepared using a Mc-Ilwain-type tissue chopper and transferred to an interface recording chamber and exposed to a warm, humidified atmosphere of 95% O2/5% CO2 and continuously perfused with oxygenated and preheated (33 ± 0.5°C) aCSF (in mM): NaCl (110), KCl (5), CaCl2 (2.5), MgSO4 (1.5), KH2PO4 (1.24), D-glucose (10), NaHCO3 (27.4), at a rate of 1.4 ml/min.
Electrophysiology
After 2 h of incubation at 33.0 ± 0.5°C in the recording chamber, a single glass pipette filled with 2 M NaCl was used to record field EPSPs (fEPSPs) elicited by stimulation of the Schaffer collateral pathway with twisted nichrome wires (single bare wire diameter, 50 μm) placed in CA1 stratum radiatum. Responses were recorded through a differential amplifier (DAM 50, World Precision Instruments) with a 10 kHz low-pass and 0.1 Hz high-pass filter. LTP was induced by two different paradigms: high-frequency stimulation (HFS, 100 Hz, 1 s), TBS (10 bursts of 4 pulses at 100 Hz delivered at 5 Hz). For LTP experiments, stimulation intensity was adjusted to elicit 40% of the maximal EPSP slope, except when otherwise indicated. Responses during baseline and after stimulation were recorded every 20 s. Data were collected and digitized by Clampex, and the slope of fEPSP was analyzed. LTP level was normalized to the average slope of responses recorded during the 10 min baseline, and responses were recorded for at least 40 min after LTP induction.
Western blot
To analyze activity-dependent regulation of different proteins, CA1 mini-slices were obtained by dissecting out the CA1 region before transferring them to the recording chamber, as previously reported (Wang et al., 2014). After 2 h incubation in aCSF, slices were subjected to different stimulations. Ten minutes after stimulation, slices were collected for Western blotting assay. Slices were lysed and protein concentrations were measured using the BCA protein assay kit (Thermo Scientific). Equal amounts of proteins were processed for SDS-PAGE and Western blot. The primary antibodies used were suprachiasmatic nucleus circadian oscillatory protein (SCOP) (1:1000, Millipore), actin (1:10,000, Millipore), phospho-ERK (1:3000, Cell Signaling Technology), ERK (1:3000, Cell Signaling Technology), phospho-AKT S473 (1:2000, Cell Signaling Technology), AKT (1:2000, Cell Signaling Technology).
Immunohistochemistry
For immunohistochemistry, vehicle or C2I (200 nM) was added to the slice incubation medium at the time of TBS application; 10 min later, slices were collected and fixed in 4% PFA for 1 h and cryoprotected in 30% sucrose for 1 h at 4°C, and sectioned on a freezing microtome at 20 μm. Sections were blocked in 0.1 M PBS containing 10% goat serum and 0.4% Triton X-100, and then incubated with primary antibodies PTEN (1:200 Cell Signaling Technology) in 0.1 M PBS containing 5% goat serum and 0.4% Triton X-100 overnight at 4°C. Sections were washed 3 times (10 min each) in PBS and incubated in AlexaFluor-594 goat anti-rabbit IgG (A-11037, Invitrogen) for 2 h at room temperature. Localization of the stimulation and recording electrodes was evident in the sections, and we were able to reproducibly analyze mean fluorescence intensity (MFI) in a 500 μm2 area close to the recording electrode. For puncta analysis, a Nikon C1 confocal laser-scanning microscope 100 × objective was used.
Behavioral testing
Novel Object Recognition (NOR) task
The Novel Object Recognition task was conducted in an open field arena with two different kinds of objects. Both objects were consistent in height and volume but were different in shape and appearance. During habituation, animals were allowed to explore an empty arena. Twenty-four hours after habituation, animals were exposed to the familiar arena with two identical objects added. One h or 24 h later, mice were allowed to explore the open field in the presence of one familiar object and a novel object to test short-term or long-term recognition memory. Time spent exploring each object was measured and a discrimination index percentage was calculated as Tnew/(Tnew + Told) × 100%.
Fear Conditioning
Mice were housed individually with normal 12/12 h daylight cycle. They were handled daily for 5 d before training. On training day, mice were first injected intraperitoneally with a calpain-2 selective inhibitor (dosage from 0.03–10mg/kg body weight). Thirty minutes later, they were placed in the fear conditioning chamber (H10–11M-TC, Coulbourn Instruments) located in the center of a sound-attenuating cubicle (Coulbourn Instruments). The conditioning chamber was cleaned with 10% ethanol to provide a background odor. A ventilation fan provided a background noise at -55dB. After a 2 min exploration period, one or three tone-footshock pairings separated by 1 min intervals were delivered. The 85 dB 2 kHz tone lasted for 30 s, and footshock intensity was set at 0.75 mA and it lasted for 2 s. Footshocks coterminated with the tone. Mice remained in the training chamber for another 30 s before being returned to their home cages. Context test was performed 1 d after training. Mice were placed back into the original conditioning chamber, and their behaviors were recorded for 5 min. On day 3, animals were subjected to cue/tone test. The same conditioning chamber was modified by changing its metal grid floor to a plastic sheet, white metal walls to plastic walls gridded with red tapes, and odor from ethanol to acetic acid. The ventilation fan was turned off to reduce background noise, and the ceiling light was changed from yellow to white. Mice were placed in the altered chamber for 5 min to measure freezing level in the altered context; and after this 5 min period, a tone (85 dB, 2 kHz) was delivered for 1 min to measure freezing to tone. Mice behavior was recorded with the Freezeframe software and analyzed with Freezeview software (Coulbourn Instruments). Motionless bouts lasting 1 s were considered as freezing. The percentage of time animal froze was calculated, and the group means with SEM and accumulative distribution of percentage freeze were analyzed. All experiments were performed blind, as the experimenter had no knowledge of the treatment (saline or drugs).
Statistical analyses
Data are presented as means ± S.E.M. For experiments where only two groups were compared, two-tail t-test was used for determining statistical significance. When more than 2 groups were compared, we used one-way ANOVA or two-way ANOVA followed by Bonferroni test to determine statistical significance. P values less than 0.05 were considered statistically significant.
Results
A selective calpain-2 inhibitor rescued TBS-induced LTP impairment in hippocampal slices from C1KO mice through ERK activation and enhanced HFS-induced LTP in WT mice
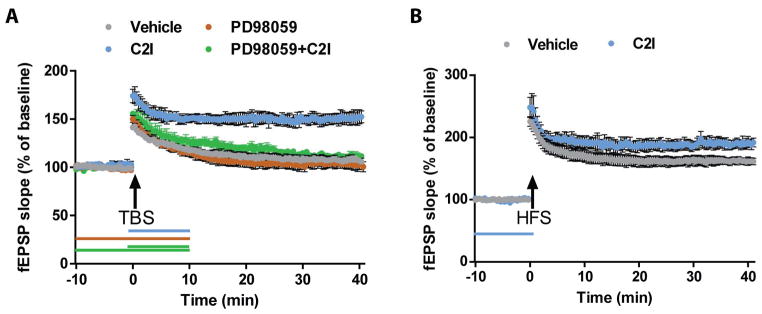
We previously reported that TBS-induced LTP induction was impaired in hippocampal slices from C1KO mice (Zhu et al., 2015), and that a selective calpain-2 inhibitor (C2I) could enhance LTP whether applied before or after TBS in hippocampal slices from WT mice (Wang et al., 2014). To directly test whether C2I could rescue TBS-LTP in slices from C1KO mice, we applied C2I for 10 min immediately before TBS in hippocampal slices from C1KO mice. As previously reported, synaptic responses returned to basal levels 30 min after TBS in untreated slices (EPSP slope values at 40 min: 105 ± 2% of baseline response) (Fig. 1A). However, the same stimulation protocol performed in the presence of C2I (200 nM) elicited stable LTP in hippocampal slices from C1KO mice (EPSP slope values at 40 min: 152 ± 7% of baseline response). Application of the MEK inhibitor PD98059 10 min before TBS prevented TBS-induced LTP in the presence of C2I in slices from C1KO mice (EPSP slope values at 40 min: 110 ± 3% of baseline response), indicating that the enhancing effect of C2I required ERK activation (Fig. 1). Application of PD98059 alone had no effect on baseline responses and on the lack of LTP in slices from C1KO mice.
Figure 1. A calpain-2 selective inhibitor rescued TBS-induced LTP impairment in hippocampal slices from C1KO mice, an effect blocked by PD9805, and increased HFS-induced LTP in WT mice.
A. Vehicle (grey) or calpain-2 inhibitor (200 nM, blue) was applied for 10 min after TBS; PD98059 (50 μM) was applied alone (brown) for 20 min starting 10 min before TBS; PD98059 (50 μM) and calpain-2 inhibitor (200 nM) (green). B. Vehicle (grey) or Calpain-2 inhibitor (200 nM, blue) was applied for 10 min before HFS (*p<0.05, as compared to vehicle). Results are means ± S.E.M. of 4–6 slices from 3 animals.
We also previously reported that HFS and TBS engage different signaling pathways to induce LTP, although the existence of several cross-talks between these pathways could facilitate or impair LTP triggered by different patterns of electrical stimulation (Zhu et al., 2015). It was thus interesting to determine the effects of C2I on HFS-induced LTP. Pretreatment of hippocampal slices from WT mice with C2I (200 nM) for 10 min before HFS enhanced the magnitude of LTP (Fig. 1B; LTP at 40 min: 161 ± 5% in control and 188 ± 8% of baseline value after C2I, p < 0.05; Student’s t-test).
A selective calpain-2 inhibitor inhibited TBS-induced PTEN degradation in hippocampal slices from WT mice and C1KO mice
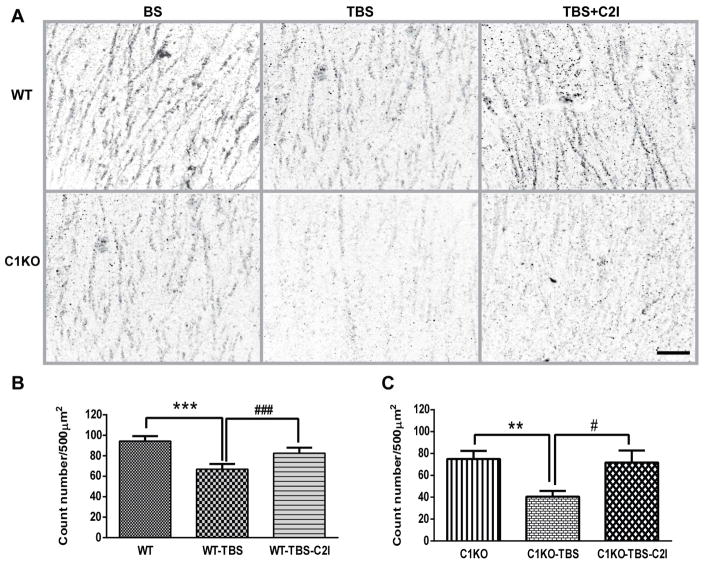
Our previous study indicated that during the LTP consolidation period following TBS, calpain-2 was activated, as evidenced by the decrease in the levels of PTEN around the stimulating electrode (Wang et al., 2014). In order to verify that calpain-2 was also activated by TBS in hippocampal slices from C1KO mice as in slices from WT mice, we compared PTEN immunostaining after TBS in slices from WT and C1KO mice. Ten minutes following TBS, PTEN immunostaining in apical dendrites of field CA1 close to the recording electrode was clearly decreased, as compared to that observed under basal conditions (Fig. 2). Treatment of slices with C2I (200 nM) blocked TBS-induced PTEN decrease in slices from both WT and C1KO mice (Fig. 2). Quantitative analysis revealed a significant decrease in PTEN levels in apical dendrites in both WT and C1KO mice, and this effect was completely blocked by treatment of slices with C2I (Fig. 2). These results confirm that calpain-2 is activated by TBS in hippocampal slices from both WT and C1KO mice.
Figure 2. Blockade of TBS-induced PTEN degradation by calpain-2 inhibitor treatment in hippocampal slices from WT and C1KO mice.
A. PTEN immunoreactivity 10 min after TBS delivered in slices treated with vehicle or calpain-2 inhibitor (200 nM) for 10 min after TBS. Scale bar: 20 μm. B. Quantitative analysis of PTEN immunostaining in slices from WT mice; results are means ± S.E.M. of 4–6 slices from 3 animals (***p< 0.001, as compared to WT group; ###p< 0.001, as compared to WT-TBS group). C. Quantitative analysis of PTEN immunostaining in slices from C1KO mice; results are means ± S.E.M. of 4–6 slices from 3 animals (**p< 0.01, as compared to C1KO group; #p< 0.05, as compared to C1KO-TBS group).
Biphasic effects of a selective calpain-2 inhibitor on learning and memory in WT mice
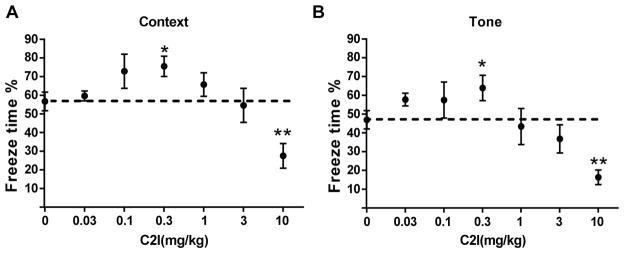
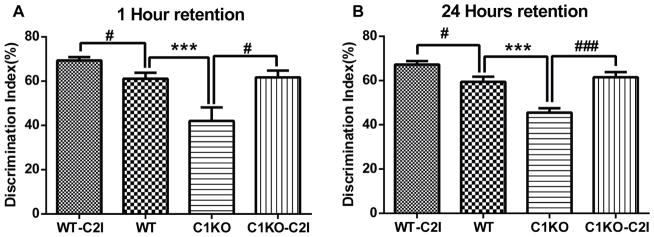
While still debated, there is a general consensus that LTP represents a cellular mechanism of learning and memory. As C2I enhanced LTP in hippocampal slices from WT mice applied before TBS, we expected that C2I treatment immediately before training would enhance learning in WT mice. To test the effects of C2I in learning and memory, WT mice were injected i.p. with various doses of C2I 30 min before training in the fear-conditioning paradigm (Fig. 3). They were tested 24 h in the context conditioning stimulus and 48 h later with the tone conditioning stimulus. The dose response relationshipe for both context and tone learning exhibited an inverted U-shape, with low doses enhancing learning and high doses inhibiting learning. Significant increase in freezing time in both context -and tone-elicited fear-conditioning was observed at a dose of 0.3 mg/kg, as compared to vehicle-treated mice. On the other hand, a dose of 10 mg/kg significantly inhibited learning for both context and tone fear conditioning. We previously reported that the Ki of C2I for calpain-2 was 25 nM versus a Ki of 940 nM for calpain-1 (Wang et al., 2014). The relative difference between the inhibitory doses (3–10 mg/kg) and the stimulatory doses (0.1–0.3 mg/kg) represents a factor of 30, which is close to the ratio between the Kis for calpain-1 and calpain-2 (a factor of 40). As the optimal dose to enhance learning in WT mice was 0.3 mg/kg, we used this dose for further experiments using the Novel Object Recognition test in WT and C1KO mice. As previously reported (Zhu et al., 2015), C1KO mice exhibited impairment in this task as compared to WT mice at both 1 h and 24 h after training (Fig. 4). Treatment with C2I (0.3 mg/kg) enhanced learning in both WT and C1KO mice at both 1 h and 24 h retention time. Moreover, following C2I treatment there was no significant difference in novel object exploration time between WT and C1KO mice.
Figure 3.
Biphasic effects of a selective calpain-2 inhibitor on learning and memory in WT mice WT mice were treated with C2I (A&B 0.03–10 mg/kg;) 30 min before training. Results are expressed as percent time mice exhibited freezing behavior. A. Quantitative analysis of percent freezing for each group in context memory. B. Quantitative analysis of percent freezing for each group in tone memory. Results represent means ± S.E.M. of 6–18 mice. *p< 0.05, **p<0.01, as compared to vehicle controls.
Figure 4. A calpain-2 selective inhibitor enhanced learning and memory in WT mice and rescued learning and memory impairment in C1KO mice.
WT and C1KO mice were treated with vehicle or calpain-2 inhibitor (0.3mg/kg) 30 min before training. Discrimination index for novel-object recognition was determined one hour (A) and 24 hour (B), after training. Results are means ± S.E.M. of 6 mice for each group (***p< 0.001 as compared to WT group; #p< 0.05, ##p< 0.01, ###p< 0.001, as compared to vehicle group).
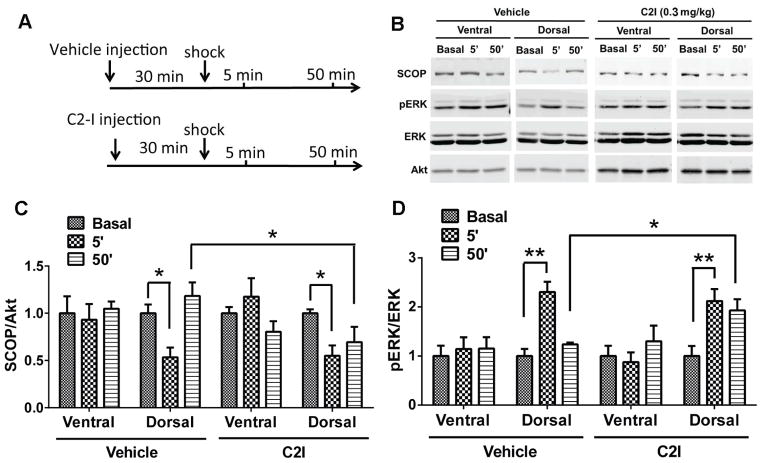
A calpain-2 selective inhibitor prolonged ERK activation in dorsal hippocampus
Fear conditioning training was shown to be associated with decreased levels of SCOP and increased levels of p-ERK in hippocampus 5 min after training, although levels of SCOP and pERK returned to basal levels 50 min after training (Shimizu et al., 2007). This pattern of changes for SCOP and p-ERK is very similar to what we observed in field CA1 following TBS-induced LTP (Wang et al., 2014). As we showed that this pattern was due to calpain-2-mediated truncation of PTEN and the stimulation of mTOR signaling leading to SCOP resynthesis, we tested the effect of C2I on the changes in SCOP and p-ERK in dorsal and ventral hippocampus at 5 and 50 min after training in the fear conditioning task (Fig. 5).
Figure 5. A calpain-2 selective inhibitor prolonged ERK activation in dorsal hippocampus.
A. Experimental design: Calpain-2 inhibitor (0.3 mg/kg) or Vehicle were injected (i.p.) in WT mice 30 min before training; dorsal and ventral hippocampi were collected 5 min and 50 min after training. B. Representative western blots for SCOP, Akt, p-ERK and ERK under various experimental conditions. C&D. Quantitative analysis of the levels of SCOP (normalized to Akt values) and p-ERK/ERK ratios. * p < 0.05 **p<0.01.
As previously reported (Shimizu et al., 2007), SCOP levels were decreased and p-ERK levels increased in dorsal but not ventral hippocampus 5 min after training. C2I injection (0.3 mg/kg) 30 min before training had no effect on these changes in SCOP or p-ERK. Likewise, SCOP and p-ERK levels returned to basal levels 50 min after training in vehicle-treated mice. However, following C2I treatment, SCOP levels remained decreased and p-ERK levels increased, as compared to vehicle-treated mice. No significant changes were observed in ventral hippocampus under any conditions.
Discussion
Our results provide further information regarding the respective roles of calpain-1 and calpain-2 in LTP and in learning and memory. We previously reported that calpain-1 activation was necessary for TBS-induced LTP in field CA1 of hippocampal slices, as it was impaired in C1KO mice. In contrast, calpain-2 activation during the consolidation period that follows TBS limited the extent of potentiation. The current results indicate that calpain-2 activation following TBS is independent of calpain-1 activation, as it was also observed in slices from C1KO mice, as assessed with PTEN truncation. Furthermore, in the absence of calpain-1, selective calpain-2 inhibition immediately before and after TBS resulted in LTP, an effect blocked by a MEK inhibitor, suggesting that the previously described signaling cascade involving calpain-2 ---→ PTEN degradation --→ mTOR stimulation --→ SCOP synthesis and ERK inactivation is as active in slices prepared from C1KO mice as in slices from WT mice. This is probably due to the fact that BDNF is likely to be released in field CA1 of C1KO mice, resulting in ERK activation and calpain-2 phosphorylation/activation, as we previously described (Wang et al., 2014; Zadran et al., 2010). These results indicate that calpain-1 activation is sufficient but not necessary to trigger LTP by TBS, as TBS can still elicit LTP in the absence of calpain-1, as long as calpain-2 is inhibited. Alternatively, it is conceivable that another signaling mechanism might be responsible for triggering LTP following TBS in the presence of a selective calpain-2 inhibitor. In particular, we showed that LTP elicited by high frequency stimulation (HFS) was normal in field CA1 of hippocampal slices from C1KO mice and this effect required cAMP and PKA activation (Zhu et al., 2015), as reported by other laboratories (Kim et al., 2010). Considering the numerous crosstalks between various signaling pathways involved in LTP (Baudry et al., 2015), we cannot exclude the possibility that selective blockade of calpain-2 can alter the signaling pathway(s) triggered by TBS, bypassing the requirement for calpain-1 activation. Note however, that this type of LTP relies on ERK activation, as blocking ERK activation prevented the rescue of LTP provided by calpain-2 inhibition. Several studies have shown that adenylate cyclase activity can trigger ERK activation (Goto et al., 2015; Hasegawa et al., 2014; Shimizu et al., 2007), suggesting that in the absence of calpain-1, TBS could trigger LTP by a pathway involving adenylate cyclase and ERK activation. On the other hand, calpain-2 activation also modifies the pathway triggered by HFS, as calpain-2 inhibition enhanced HFS-LTP. The mechanism underlying this effect is not clear, but could also be related to the cross-talk between the pathways engaged by HFS and TBS.
Our results further confirm the opposite roles of calpain-1 and calpain-2 in learning and memory. While dose-response curves of a number of manipulations or drugs affecting learning and memory exhibit an inverted U-shape (Baldi et al., 2005; Monte-Silva et al., 2009; Salehi et al., 2010), which is not always easy to account for, the results with the selective calpain-2 inhibitor we used are well explained with the differential affinities of the compound for calpain-2 and calpain-1. Thus, at low doses, C2I is expected to inhibit calpain-2 and therefore to enhance hippocampal LTP and learning. On the other hand, high doses of C2I are expected to inhibit calpain-1, resulting in LTP and learning impairment. In support of this interpretation, the differences in doses producing learning enhancement or inhibition are closely related to the differences in Kis for the compound to inhibit calpain-2 or calpain-1. On the other hand, while C2I was able to rescue TBS-LTP in hippocampal slices from C1KO mice, it still produced learning impairment at high doses in WT mice under conditions where calpain-1 would be blocked. In addition, low doses of C2I also enhanced learning in C1KO mice. These results confirm that calpain-1 activation is required for learning of certain types of information, while calpain-2 activation limits the extent of learning. C1KO mice were impaired both in fear conditioning (Zhu et al., 2015) and in novel object recognition, and C2I was able to enhance learning in both types of learning in both WT and C1KO mice.
We previously proposed that the opposite functions of calpain-1 and calpain-2 in LTP were due to their links to selective signaling pathways. We presented evidence that calpain-1, by cleaving the negative ERK regulator, SCOP, was playing a critical role in activating ERK and triggering sequences of events leading to LTP. In contrast, calpain-2 activation in the consolidation period would selectively cleave PTEN, resulting in mTOR activation, local protein synthesis stimulation, leading to recovery of SCOP levels and the termination of ERK activation. The present results provide strong supportive evidence for a similar sequence taking place in learning and memory formation. Thus we confirmed previously reported results indicating that SCOP levels are rapidly and transiently decreased after training in hippocampus, while levels of p-ERK are correspondingly rapidly and transiently increased (Shimizu et al., 2007). However, when mice were pretreated with a selective calpain-2 inhibitor, the initial decrease in SCOP levels and corresponding increase in p-ERK were not affected, supporting the idea that calpain-1 but not calpain-2 activation is involved in SCOP degradation. However, pretreatment with the inhibitor at a dose that stimulated learning prevented the recovery of SCOP levels, and maintained p-ERK levels elevated. These results further support the idea that calpain-2 activation is delayed, as compared to the rapid calpain-1 activation, and is involved in limiting the extent of ERK activation and learning. Thus the same biochemical machinery providing a molecular brake on LTP is involved in limiting the extent of learning of hippocampus-dependent tasks. We previously argued that such a mechanism could account for the beneficial effects of space training as opposed to massed training (Baudry et al., 2014; Shimizu et al., 2007; Wang et al., 2013).
Conclusions
The present results clearly indicate that calpain-1 and calpain-2 play opposite roles in learning and memory. Thus calpain-1 activity is required for certain types of learning and memory, while calpain-2 limits the extent of learning. These opposite functions of calpain-1&2 are related to their opposite functions in hippocampal LTP, further strengthening the links between LTP and learning and memory. Finally, the present results indicate that a selective calpain-2 inhibitor could provide a potential treatment for a number of disorders associated with learning and memory impairment. As shown here, not only did the compound enhance learning under normal conditions, but it was also effective in a genetic model where learning was impaired.
Highlights.
A selective calpain-2 inhibitor rescues the impairment in TBS-induced LTP in C1KO mice.
Low doses of the calpain-2 inhibitor enhances learning & memory in WT and C1 KO mice.
Calpain-2 activation during training limits the extent of ERK activation and learning.
Acknowledgments
This work was supported by grant P01NS045260-01 from NINDS (PI: Dr. C.M. Gall). XB is also supported by funds from the Daljit and Elaine Sarkaria Chair. The authors are very grateful to Dr. A. Chishti (Tufts University) for providing us breeding pairs of calpain-1 KO to generate the mice used in this study.
Footnotes
Author Contributions: YL performed the electrophysiological and the behavioral experiments analyzed the data and wrote the manuscript; JD performed the immunohistochemical experiments, YW performed Western blots and analyzed the data; GZ performed parts of the Western blots experiments. XB designed some of the experiments, and wrote the manuscript; MB designed the experiments, directed the studies and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldi E, Efoudebe M, Lorenzini CA, Bucherelli C. Spatial navigation in the Morris water maze: working and long lasting reference memories. Neurosci Lett. 2005;378:176–180. doi: 10.1016/j.neulet.2004.12.029. [DOI] [PubMed] [Google Scholar]
- Baudry M, Zhu G, Liu Y, Wang Y, Briz V, Bi X. Multiple cellular cascades participate in long-term potentiation and in hippocampus-dependent learning. Brain Res. 2014 doi: 10.1016/j.brainres.2014.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briz V, Hsu YT, Li Y, Lee E, Bi X, Baudry M. Calpain-2-mediated PTEN degradation contributes to BDNF-induced stimulation of dendritic protein synthesis. J Neurosci. 2013;33:4317–4328. doi: 10.1523/JNEUROSCI.4907-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croall DE, Ersfeld K. The calpains: modular designs and functional diversity. Genome Biology. 2007;8 doi: 10.1186/gb-2007-8-6-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll DE, Thompson VF, Li HQ, Wei W, Cong JY. The calpain system. Physiological Reviews. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- Goto A, Nakahara I, Yamaguchi T, Kamioka Y, Sumiyama K, Matsuda M, Nakanishi S, Funabiki K. Circuit-dependent striatal PKA and ERK signaling underlies rapid behavioral shift in mating reaction of male mice. Proc Natl Acad Sci U S A. 2015;112:6718–6723. doi: 10.1073/pnas.1507121112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa Y, Mukai H, Asashima M, Hojo Y, Ikeda M, Komatsuzaki Y, Ooishi Y, Kawato S. Acute modulation of synaptic plasticity of pyramidal neurons by activin in adult hippocampus. Front Neural Circuits. 2014;8:56. doi: 10.3389/fncir.2014.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SX, Feig LA. Long-term potentiation in the CA1 hippocampus induced by NR2A subunit-containing NMDA glutamate receptors is mediated by Ras-GRF2/Erk map kinase signaling. PLoS One. 2010;5:e11732. doi: 10.1371/journal.pone.0011732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Kim M, Huang T, Abel T, Blackwell KT. Temporal sensitivity of protein kinase a activation in late-phase long term potentiation. PLoS Comput Biol. 2010;6:e1000691. doi: 10.1371/journal.pcbi.1000691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Ortega-Vilain AC, Patil GS, Chu DL, Foreman JE, Eveleth DD, Powers JC. Novel peptidyl alpha-keto amide inhibitors of calpains and other cysteine proteases. J Med Chem. 1996;39:4089–4098. doi: 10.1021/jm950541c. [DOI] [PubMed] [Google Scholar]
- Menard C, Gaudreau P, Quirion R. Signaling pathways relevant to cognition-enhancing drug targets. Handb Exp Pharmacol. 2015;228:59–98. doi: 10.1007/978-3-319-16522-6_3. [DOI] [PubMed] [Google Scholar]
- Monte-Silva K, Kuo MF, Thirugnanasambandam N, Liebetanz D, Paulus W, Nitsche MA. Dose-dependent inverted U-shaped effect of dopamine (D2-like) receptor activation on focal and nonfocal plasticity in humans. J Neurosci. 2009;29:6124–6131. doi: 10.1523/JNEUROSCI.0728-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y, Sorimachi H. Calpains - An elaborate proteolytic system. Biochimica Et Biophysica Acta-Proteins and Proteomics. 2012;1824:224–236. doi: 10.1016/j.bbapap.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Peng DJ, Zhou JY, Wu GS. Post-translational regulation of mitogen-activated protein kinase phosphatase-2 (MKP-2) by ERK. Cell Cycle. 2010;9:4650–4655. doi: 10.4161/cc.9.23.13957. [DOI] [PubMed] [Google Scholar]
- Salehi B, Cordero MI, Sandi C. Learning under stress: the inverted-U-shape function revisited. Learn Mem. 2010;17:522–530. doi: 10.1101/lm.1914110. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Phan T, Mansuy IM, Storm DR. Proteolytic degradation of SCOP in the hippocampus contributes to activation of MAP kinase and memory. Cell. 2007;128:1219–1229. doi: 10.1016/j.cell.2006.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Zhu G, Liu Y, Standley S, Ji A, Tunuguntla R, Wang Y, Claus C, Luo Y, Baudry M, Bi X. UBE3A Regulates Synaptic Plasticity and Learning and Memory by Controlling SK2 Channel Endocytosis. Cell Rep. 2015;12:449–461. doi: 10.1016/j.celrep.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Briz V, Chishti A, Bi X, Baudry M. Distinct roles for mu-calpain and m-calpain in synaptic NMDAR-mediated neuroprotection and extrasynaptic NMDAR-mediated neurodegeneration. J Neurosci. 2013;33:18880–18892. doi: 10.1523/JNEUROSCI.3293-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhu G, Briz V, Hsu YT, Bi X, Baudry M. A molecular brake controls the magnitude of long-term potentiation. Nat Commun. 2014;5:3051. doi: 10.1038/ncomms4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HV, Lynch DR. Calpain and synaptic function. Molecular Neurobiology. 2006;33:215–236. doi: 10.1385/MN:33:3:215. [DOI] [PubMed] [Google Scholar]
- Xia Z, Storm DR. Role of signal transduction crosstalk between adenylyl cyclase and MAP kinase in hippocampus-dependent memory. Learn Mem. 2012;19:369–374. doi: 10.1101/lm.027128.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadran S, Jourdi H, Rostamiani K, Qin Q, Bi X, Baudry M. Brain-derived neurotrophic factor and epidermal growth factor activate neuronal m-calpain via mitogen-activated protein kinase-dependent phosphorylation. J Neurosci. 2010;30:1086–1095. doi: 10.1523/JNEUROSCI.5120-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Liu Y, Wang Y, Bi X, Baudry M. Different patterns of electrical activity lead to long-term potentiation by activating different intracellular pathways. J Neurosci. 2015;35:621–633. doi: 10.1523/JNEUROSCI.2193-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]