Abstract
Kidney cancer is the 6th most common cancer in the US and its incidence is increasing. The treatment of this malignancy took a major step forward with the recent introduction of targeted therapeutics such as the kinase inhibitors. Unfortunately, kinase inhibition is associated with the onset of resistance after 1–2 years of treatment. Regorafenib, like many multi-kinase inhibitors, was designed to block the activities of several key kinase pathways involved in oncogenesis (Ras/Raf/MEK/ERK) and tumor angiogenesis (VEGF-receptors), and we have recently shown that it also possesses soluble epoxide hydrolase (sEH) inhibitory activity which may be contributing to its salutary effects in patients. Since sEH inhibition results in increases in the DHA-derived epoxydocosapentaenoic acids (EDPs) which we have previously described to possess anti-cancer properties, we asked whether the addition of DHA to a therapeutic regimen in the presence of regorafenib would enhance its beneficial effects in vivo. We now show that the combination of regorafenib and DHA results in a synergistic effect upon tumor invasiveness as well as p-VEGFR attenuation. In addition, this combination showed a reduction in tumor weights, greater than each agent alone, in a mouse xenograft model of human RCC, yielding the expected oxylipin profiles; this data was supported in several RCC cell lines which showed similar results in vitro. Since DHA is the predominant component of fish oil, our data suggest that this non-toxic dietary supplement could be administered with regorafenib during therapy for advanced RCC and could be the basis of a clinical trial.
INTRODUCTION
Renal-cell carcinoma (RCC) arises from the renal tubular epithelium (1, 2), is the most common malignancy of the kidney, and is the 6th most common cancer in the US. In contrast to many other cancers, the incidence of RCC is increasing likely due to smoking as well as the increased prevalence of the metabolic syndrome in the Western world (3–7). When localized to the kidney, surgical resection is usually curative, however once the cancer metastasizes the survival statistics, even with currently available novel therapies, are dismal. Among non-surgical treatments of RCC, the immune modulators were historically associated with a very low success rate (8), likely related to immune suppression in the tumor microenvironment possibly through local generation of tryptophan metabolites (9, 10). Enter the era of targeted therapeutics which has resulted in the discovery of drugs possessing antiangiogenic activity via abrogation of vascular endothelial growth factor (VEGF) and other tyrosine kinase receptor signaling pathways involved in tumor growth and angiogenesis (11–14). However, while these approaches represented a major advance in the field, they are unfortunately associated with a high level of resistance after 1–2 years of treatment (15, 16), and furthermore, some are linked with a troublingly high rate of systemic hypertension (17). Therefore, novel approaches are urgently needed to improve the efficacy of these drugs. In the current study, we examined the use of the tyrosine kinase inhibitors in combination with compounds that we hypothesized would attenuate tumor resistance.
Regorafenib is a second generation multi-kinase inhibitor that blocks the activity of kinases involved in the regulation of oncogenesis (Ras/Raf/MEK/ERK) and tumor angiogenesis (VEGF-R1, -R2, and –R3) (13). This drug is a marked improvement over the first generation compounds (e.g. sorafenib) due to its higher specific activity leading to greater pharmacological potency (13). The antitumor activity of regorafenib has been demonstrated in a variety of preclinical models and is associated with its kinase inhibitory effects, which results in suppression of cell proliferation, induction of apoptosis, and inhibition of tumor angiogenesis (13, 18, 19), the latter being a key area of investigation for therapies of highly angiogenic RCC (20). We have recently shown that these multikinase inhibitors block soluble epoxide hydrolase (sEH), a key enzyme that metabolizes bioactive lipids of inflammation (21). Because inhibition of sEH stabilizes these lipids thereby prolonging their beneficial effects on angiogenesis and inflammation, we asked whether it is possible to capitalize on this enzymatic activity to enhance the salutary effects of these specific kinase inhibitors in RCC.
sEH hydrolyzes epoxygenated fatty acids generated by the P450 metabolism of omega-3 and omega-6 polyunsaturated fatty acids (PUFA). Among these PUFAs, sEH metabolizes epoxyeicosatrienoic acids (EETs), which are P450 products of arachidonic acid (ARA), and epoxydocosapentaenoic (EDPs), which are also P450 products but derive from docosahexaenoic acid (DHA), to their less bioactive diols (diols of EETs and EDPs, dihydroxyeicosatrienoic acids, DHETs) and dihydroxydocosapentaenoic acids, DiHDPEs; respectively; Fig. 1) (22). While EETs possess anti-inflammatory (23) and anti-hypertensive (24) properties, they have been shown to be pro-angiogenic (25–27), a property which can clearly be detrimental in the treatment of highly angiogenic tumors such as RCC. In addition, recent studies have suggested that EETs can promote the progression of cancer (28, 29), while other studies have contradicted these findings (30). In contrast, EDPs, which are also stabilized by sEH inhibitors (Fig. 1) have the opposite effect on angiogenesis (31), hence we focus on the DHA metabolites of sEH in our study.
Figure 1.
EET and EDP synthesis occurs through cytochrome P450 family enzymes naturally via oxidation of unsaturated bonds of precursor fatty acids.
The EET and EDP epoxygenated products are degraded by sEH into their respective diols. Inhibition of sEH by regorafenib or other means results in accumulation of epoxygenase metabolites.
We hypothesized that the sEH inhibitory activity of regorafenib will result in marked increases in the anti-angiogenic and anti-hypertensive EDPs which will be enhanced in the presence of exogenously administered DHA, the most abundant component of dietary fish oil supplements. We now show that the combination of DHA and regorafenib causes a decrease in HuVEC cell invasion as a measure of tumor angiogenesis as well as synergistically decreasing cell viability across three human RCC lines. Furthermore, by employing a xenograft model of RCC in athymic nude mice, we demonstrate a decrease in tumor mass in vivo associated with the expected target effects and plasma oxylipin changes. Thus, once validated in human studies, novel therapy based on the addition of the dietary supplement DHA to regorafenib has the potential to result in an enhanced therapeutic efficacy of this kinase inhibitor for treatment of advanced RCC.
MATERIALS AND METHODS
Cell culture
Human Umbilical Vein Endothelial Cells (HuVEC; Lonza, Walkersville, MD, USA) were grown in endothelial basal medium (EBM-2) supplemented with growth factors. The RCC cell-lines, 786-O(VHL−/−), Caki-1(VHL+/+), and Renca (VHL+/+) were obtained from the American Type Culture Collection (Manassas, VA, USA) and the Renal proximal tubule epithelial cells (RPTEC or “normal human kidney, NHK”) were a primary (i.e. non-immortalized) line acquired from Lonza, which were cultured in renal epithelial cell growth medium (REGM; Lonza). All ATCC and Lonza cell lines undergo extensive authentication tests during the accessioning process as described on their website; in addition all cells were frequently tested for mycoplasma in the author’s laboratory. 786-0 and Caki-1 and Renca cells were maintained in RPMI and NHK cells were grown cultured in DMEM, both supplemented with 10% FBS, 100 units/mL streptomycin, and 100 mg/mL penicillin. Cells were maintained at 5% CO2 and at 37°C. All cell lines were used with a passage number of two and confirmed to be free of mycoplasma, per monthly laboratory testing.
Animals and Treatments
All animal studies were approved by the University of California Davis Animal Use and Care Committee and were performed in accordance with the National Institutes of Health Guide for the care and use of laboratory animals. 36 four week old male athymic nude Nu/Nu mice (Harlan Laboratories, Madison, WI) were acclimated to housing conditions for one week and were kept under a 12 h light-dark cycle with free access to water and food for the duration of the experiment.
Subsequently, mice were injected a suspension containing 786-O cells at 0.5×106 mixed in 30% of non-growth factor reduced Matrigel (Corning Inc., Corning, NY, USA) subcutaneously in the flank region as previously described (32). Tumor growth was monitored twice a week for each mouse using a digital caliper. Tumor volume (mm3) was calculated as length*(width2/2). When tumor volume reached approximately 100 mm3 (around 3–4 weeks of inoculation) treatments and diets began.
Mice were randomly divided into two experimental dietary groups: control diet (5% corn oil) or a 1% DHA enriched diet (17.5 g DHA and 52.5 g corn oil/kg). DHA ethyl ester replaced corn oil to retain equal dietary fat between both isocaloric diets. The detailed composition of the diets is described in Supplementary Table S1. Half of the mice in each dietary group were given a daily administration of either 10 mg/kg regorafenib or vehicle (PEG400/125 mM aqueous methanesulfonic acid (80/20)) via oral gavage. Treatments continued for three weeks. Body weights and tumor sizes were measured every two days. At the end of the experiment, plasma and tissues were harvested for immunohistochemistry and oxylipin analysis.
Endothelial Cell Invasion Assay
HuVECs were grown in 24-well plates containing transwell inserts of 8 µm pore polyvinylpyrrolidone-free polycarbonate filters coated with Matrigel on the upper compartment at a density of 1×105 cells in EBM-2 media containing 0.1% BSA (33). EBM-2 media consisting of 10% BSA was added in the bottom compartment of the well as a chemoattractant. Both upper and lower chambers contained one of the following treatments: 1 µM arachidonic acid (ARA), 1 µM DHA, 1 µM DHA plus 1 µM regorafenib, 1 µM regorafenib, 1 µM linoleic acid (LA) or DMSO. Cells were incubated at 37 °C for 20 hr to allow for migration. Afterwards, transwells containing cells were washed in PBS, fixed in 5% glutaraldehyde, and stained with 0.5% Toluidine Blue. Next, the upper wells were gently scraped to allow for imaging and quantification of cells that had migrated towards the lower compartment of the transwell inserts.
MTT Assay
Cell viability was assayed by plating cells in 96-well plates at a density of 3×103 cells. After 24 hr, NHK, 786-0, Caki-1, and Renca cells were treated with 1µM of the fatty acids LA, ARA, eicosapentaenoic acid (EPA), and DHA each with the presence or absence of 1 µM regorafenib and DMSO control. After 24 hr of treatment, cells were quantified via hemocytometer and treated with media containing MTT solution (1mg/ml thiazolyl blue tetrazolium bromide) for 3 h. Afterwards, the MTT solution was removed and the blue crystalline precipitate internalized by the cells were dissolved with DMSO. Finally, plates were placed in a plate reader to measure visible absorbance at 570nm.
Immunoblotting
HuVECs were grown at a density of 2 × 105 cells in six well plates. After serum starvation for 6 hr in EBM-2 media containing 0.1% bovine serum albumin (BSA), cells were treated with 1 µM omega-6 linoleic acid (LA), 1 µM LA + regorafenib, 1 µM DHA, or 1 µM DHA + 1 µM regorafenib for 24 hr. Cells were then lysed and total cell lysates were analyzed for proteins of interest using antibodies against phosphorylated VEGFR-2 and β-actin (Cell Signaling Technology, Inc., Beverly, MA, USA).
Immunoblotting of tumor tissue was performed as previously described (34). Briefly, after the indicated treatments, the tissues were washed with PBS, lysed, and subjected to immunoblotting. For the xenograft tissue tumors, proteins were extracted with T-PER. The membranes were blocked in 5% nonfat dry milk for 1 hr at room temperature, incubated with antibodies (β-actin, pVEGFR-2, VEGFR-2, pERK1/2, and ERK1/2), and then probed with HRP-tagged anti-mouse or anti-rabbit IgG antibodies. The signal was detected using ECL solutions (Thermo Fisher Scientific, Waltham MA, USA). Densitometry was performed using ImageJ software.
Oxylipin Analysis
The method for quantitative profiling of oxylipin was performed as previously described (35). Briefly, plasma samples were extracted using solid-phase extraction cartridges. Samples were eluted through the cartridges, dried and then reconstituted by adding 200 nM 1-cyclohexyl-dodecanoic acid urea (CUDA) methanol solution. Oxylipins were then detected using high performance liquid chromatography electrospray ionization tandem mass spectrometry (HPLC-ESI-MS/MS). The optimized conditions of chromatographic separation have been reported previously (36) as have the instrument parameters including MRM transitions (35) (Applied Biosystems, 4000 QTRAP tandem mass spectrometer, Foster City, CA).
Statistical analysis and synergy calculations
All data were analyzed for significance in SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). Cell numbers from invasion assay, tumor weights, oxylipin quantification, and tumor volumes were analyzed for significance by One-Way ANOVA at P < 0.05. Where significant differences were found, a Tukey's Multiple Comparison Test was performed at a probability of α = 0.05. The data are presented as means ± s.e.m. Different letters appearing above bars in bar graphs designates that significant differences were found while bars sharing the same letter indicate significance was not achieved. Bars having two letters (such as ‘bc’) indicates that significance was not achieved compared to group ‘b’ or group ‘c’.
Synergy was assessed by calculating the combination index (CI) values using CalcuSyn software which provides a quantitative definition for additive effect (CI = 1), synergism (CI < 1), and antagonism (CI > 1) in combination treatments.
RESULTS AND DISCUSSION
Co-administration of regorafenib and DHA suppresses vascular endothelial cell invasion and is associated with attenuated angiogenesis markers
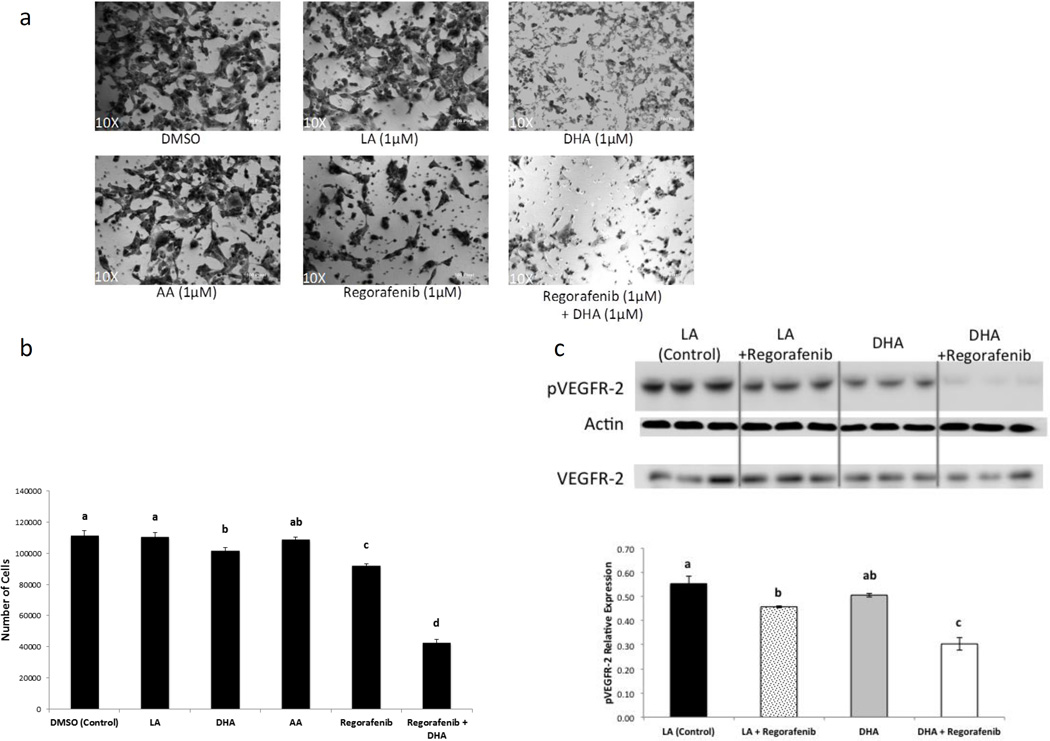
Since the addition of DHA in the presence of sEH inhibition provided by regorafenib would be expected to increase local EDP levels (Fig. 1) and thereby attenuate angiogenesis (31), we first evaluated this property in an in vitro model of angiogenesis (37, 38). Since we previously reported an increase in HuVEC proliferation and infiltration when treated with EETs, specifically 11, 12-EET and 14, 15-EET which are generated from ARA (31), we utilized the omega-6 PUFA linoleic acid (LA), the predominant PUFA found in corn oil, as an additional control. HuVEC were grown on matrigel in transwell plates, in which cells that infiltrated the matrigel were enumerated in order to assay for invasive potential (see Materials and Methods). After treatment with 1 µM ARA, 1 µM DHA, 1 µM DHA + 1 µM regorafenib, 1 µM regorafenib, 1 µM LA or DMSO for 20 h, invading HuVEC were imaged (Fig. 2a) and quantitated (Fig. 2b). The cells treated concurrently with DHA and regorafenib were found to be the least invasive of all conditions tested, with a reduction of ~60% compared to DMSO control. This combination likely resulted in a higher amount of EDPs which comes about with a high availability of DHA in concert with the inhibition of sEH afforded by regorafenib. To confirm target inhibition by regorafenib, we evaluated its kinase activity on the phosphorylated (kinase-active) form of VEGFR-2 (39) and showed that pVEGFR-2 expression was lower in cells treated with regorafenib after both linoleic acid (LA) and DHA treatments alone, but more pronounced with the combination (Fig. 2c), thereby confirming target engagement with regorafenib. Regorafenib alone demonstrated a non-significant decrease in VEGFR-2 expression (data not shown).
Figure 2.
- HuVECs were grown on Matrigel and treated with 1 µM LA, 1 µM ARA, 1 µM DHA, 1 µM DHA + 1 µM regorafenib, 1 µM regorafenib, or DMSO for 20 hr. The cells transiting the Matrigel were photographed (see Materials and Methods).
- Quantification of cells was performed by counting the cells in wells (n=3 for each treatment, repeated in triplicates).
- In a separate parallel experiment, HuVEC cells were grown to confluence and treated with the indicated compounds for 24 hr and immunoblotted for pVEGFR-2 and β-actin as a loading control. VEGFR-2 was also immunoblotted under the same conditions in a separate blot.
These experiments were each repeated at least three times. *Bars in graphs indicate mean ± SEM. Different letters above bars indicate statistical difference among groups (p<0.05).
Linoleic acid, LA; Docosahexaenoic acid, DHA; Arachidonic acid, AA.
These results are consistent with previous data showing that EDPs inhibit VEGF-induced cell migration in HuVEC after being treated with 19,20-EDP (31). Further, these findings demonstrate a synergistic effect of DHA and regorafenib on endothelial cells to suppress angiogenesis, primarily via suppression of endothelial cell migration likely via high levels of EDP.
The Combination of Regorafenib and DHA synergistically decreases survival of kidney cancer cells in vitro
We next assessed cell viability in vivo utilizing two human kidney cancer lines (786-0, Caki-1) and the mouse kidney cancer cell line Renca, as well as primary (non-immortalized) normal human kidney epithelial (NHK) cells as controls. All cells were treated with 1µM of the fatty acids LA, ARA, EPA, and DHA each, in the presence or absence of 1 µM regorafenib and DMSO control. LA, which is the major polyunsaturated fatty acid comprising corn oil, served as the in vitro control treatment that would best mimic the conditions of corn oil administration in vivo such that the two experiments could be compared. EPA was used to discern if the mitigation of cell viability was due to an omega-3 effect or specifically to DHA. After 24 h of treatment, both Regorafenib + DHA and DHA alone decreased cell viability in all three of the cancer lines with no significant effect on NHK cells, however a greater decrease in cell viability was found with the former treatment (Fig. 3). Furthermore, cells were quantified from an experiment performed in parallel to the MTT assay on the four cell types (insert, Fig. 3); these data demonstrate that the combination of regorafenib with DHA resulted in synergistic responses after 24 h of incubation. The therapeutic efficacy was assessed by calculating combination index (CI) values using CalcuSyn software (40). Analysis of combination therapeutic indexes revealed synergistic effects by demonstrating the CI values in the range of 0.61 to 0.85 (synergy defined as CI <1) with the combination of regorafenib and DHA alone among the three RCC lines. Antagonistic interactions (CI>1) were found with linoleic acid and arachidonic acid with CI calculations 1.14 and 1.23, respectively. These findings demonstrate that the combination of regorafenib and DHA produced a synergistic decrease in several RCC, but not normal renal epithelial cell, viability.
Figure 3.
Cell viability was assayed via MTT in NHK, 786-0, Caki-1, and Renca cells
NHK, 786-0, Caki-1, and Renca cells were treated with 1 µM of the fatty acids LA, ARA, EPA, and DHA each with the presence or absence of 1 µM regorafenib and DMSO control. After 24 h of treatment, an MTT assay was performed and cells were counted via hemocytometer. The DHA and regorafenib combination index (CI) was calculated using the CalcuSyn software as discussed in Materials and Methods. These experiments were each repeated at least three times. *Bars in graph indicate mean ± SEM. Asterisks (*) above bars indicate statistical difference compared to DMSO treatment of identical cell line (p<0.05).
The Combination of Regorafenib and DHA decreases tumor growth in vivo
In light of previous data from one of our laboratories demonstrating that treatment with EDP concurrently with sEH inhibition attenuated both tumor growth and angiogenesis (31), we next asked whether concurrent addition of regorafenib and DHA synergizes in an in vivo xenograft model of human RCC employing the 786-0 (VHL−/−) human RCC cell line used in several previous studies (39, 41, 42). Male athymic Nu/Nu mice were started on the diets and pharmacological treatments after the 786-0 xenografts achieved a volume of ~100 mm3. The mice were given free access to either a diet with fat originating from corn oil, which is naturally high in the omega-6 PUFA LA, or a 1% enriched DHA diet. The DHA concentration in the diet was determined by metabolic body size using an average daily food intake of 5g/day/mouse which translates to ~3.1g/day of DHA in a 70 kg human. This amount is achievable through consuming fish oil supplementation and in fact has been recommended to decrease progression in IgA nephropathy, a common renal disease (43).
Mice were given either regorafenib (10 mg/kg/day) or vehicle control administered by oral gavage. Tumors and terminal plasma were collected after 18 days of intervention for immunoblot and oxylipin analysis, respectively. There was no significant difference between treatment groups in body weights after 18 days indicating a lack of general toxicity (Fig. 4a); tumor weights (Fig. 4b) and volume (Supplementary Fig. S1) were found to be the smallest in the mice treated with regorafenib while ingesting the DHA diet (~1.9 fold decrease) and there was a synergistic decrease of the combination as compared to DHA or regorafenib administered alone.
Figure 4.
The combination of regorafenib and DHA reduces tumor weight with the expected on-target effects.
- Body weights were measured at the indicated times;
- Tumor weights were determined;
- Terminal tumors were immunoblotted with pVEGFR-2, pERK1/2, or ERK1/2, and β-actin in was used as a loading control.
*Lines and bars in graphs indicate mean ± SEM. Different letters above bars indicate statistical difference among groups (p<0.05).
To evaluate the target effects of regorafenib in the xenografted animals, we evaluated the MAPK and VEGFR pathways which are known receptor tyrosine kinase targets (13). Immunoblotting of the tumors for pVEGFR-2 demonstrated the most dramatic reduction in the tumors from the DHA+Regorafenib treated mice with minimal effects upon these proteins in the other animals (Fig. 4c), indicating that regorafenib attenuates the active forms of both MAPK and VEGFR species, consistent with the HuVEC data (see Fig. 2c). Since we have previously shown an sEH inhibitory effect of regorafenib similar to sorafenib (44), the influence of regorafenib and DHA in the in vivo model is likely specific to this combination.
The DHA diet resulted in an increase in all CYP450 metabolites of DHA in murine plasma
While the circulating plasma oxylipin profile can suggest the mechanism of the observations, these data do not always correlate with what is occurring at the local (i.e. tissue) level (45). The EDP species are rapidly metabolized to their diol constituents due to the actions of sEH, however the inhibitory actions on this catabolic enzyme from an sEH-inhibitor, as we have shown for sorafenib (46, 47), were evident in the plasma (31). Terminal plasma oxylipin analysis showed the expected higher levels of 7(8)-EDP, 10(11)- EDP, 13(14)- EDP, 16(17)- EDP, and 19(20)- EDP in mice treated with the DHA diet compared to the corn oil diet groups (Fig. 5a). An increase in the corresponding diols was also observed as 10(11)-DiHDPE, 13(14)-DiHDPE, 16(17)-DiHDPE, and 19(20)-DiHDPE in the DHA fed mice (Fig. 5b). The production of these diols was anticipated due to the enriched dietary DHA.
Figure 5.
Plasma oxylipin analysis shows increased levels of EDPs in the mice fed with the DHA diet compared with the other mice.
- EDPs;
- DHA-derived diols;
- The epoxide to diol ratio as a measure of sEH inhibition.
*Bars in graphs indicate mean ± SEM. Different letters above bars indicate statistical difference among groups (p<0.05).
To assess in vivo sEH inhibition we examined the ratio of epoxide to their corresponding diol products in the plasma. The sum epoxide-to-diol ratio was found to be ~2.2 fold in the corn oil diet + regorafenib treatment group compared to the corn oil diet alone, with greatest difference being ~3.6 fold increase found in the 16(17)- EDP -to-16(17)-DiHDPE (fig. 5c). Surprisingly, the epoxide-to-diol ratio in the plasma of the DHA fed mice did not reflect sEH inhibition as the concentrations were found to be about the same for all of the measured species, and even lower in the 10(11)-EDP-to-10(11)-DiHDPE and 13(14)-EDP-to-13(14)-DiHDPE. This was also observed in an earlier experiment performed with sorafenib rather than regorafenib treatments (data not shown). Recently it has been identified that the omega-3 derived EDPs are turned over more rapidly than the corresponding omega-6 derived EETs as sEH has a preference for these DHA-derived epoxygenated metabolites (47). Thus, it is conceivable that due to this preference in substrate and the abundance of EDP in the blood, sEH enzyme levels may be upregulated and higher in the DHA diet fed mice, resulting in a greater amount of epoxide turnover to diols leading to a decrease in the epoxide-to-diol ratio in the plasma, although this may not be representative of tissue. Future investigations may elucidate this observation by measuring circulating EET and EDP concentrations or measuring oxylipins in other tissues.
CONCLUSION
We have shown that combination treatment of DHA with regorafenib results in a synergistic efficacy over regorafenib or DHA alone in inhibiting growth in an in vivo xenograft model of VHL-mut RCC. We further show that there is a decrease in markers of angiogenesis and that this growth inhibition is accompanied by the expected target effects. We provide evidence that tumor growth attenuation likely occurs as a result of increasing levels of EDPs due to the sEH inhibitory property of regorafenib (Fig. 6). Until a clinical trial is accomplished, patient use of the common and readily available dietary supplement, fish oil, can therefore be recommended in those individuals undergoing regorafenib treatment for advanced RCC.
Figure 6.
Proposed mechanism of combining regorafenib treatment with dietary DHA to inhibit RCC growth.
sEH inhibition by regorafenib reduces degradation of DHA-derived epoxides and leads to increased EDP bioavailability resulting in attenuation of angiogenesis and likely an increase in vasodilation (which was not measured in this study). The kinase inhibitor activity of regorafenib impedes VEGF and MAPK/Raf signaling which likely further decreases angiogenesis and concomitant RCC tumor growth.
Supplementary Material
Acknowledgments
Financial Support
This work was supported by NIH grants 1R01CA135401-01A1, 1R03CA181837-01, and 1R01DK082690-01A1, the Medical Service of the US Department of Veterans’ Affairs, and Dialysis Clinics, Inc. (DCI) (all to R.H. Weiss). Partial support was provided by NIEHS R01 ES002710 and NIEHS Superfund Program P42 ES004699 (to B.D. Hammock.)
Footnotes
Conflict of Interest
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Escudier B, Kataja V Group EGW. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2010;21(Suppl 5):v137–v139. doi: 10.1093/annonc/mdq206. [DOI] [PubMed] [Google Scholar]
- 2.Cohen HT, McGovern FJ. Renal-cell carcinoma. The New England journal of medicine. 2005;353:2477–2490. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 3.Luo J, Margolis KL, Adami HO, Lopez AM, Lessin L, Ye W, et al. Body size, weight cycling, and risk of renal cell carcinoma among postmenopausal women: the Women's Health Initiative (United States) American journal of epidemiology. 2007;166:752–759. doi: 10.1093/aje/kwm137. [DOI] [PubMed] [Google Scholar]
- 4.Haggstrom C, Rapp K, Stocks T, Manjer J, Bjorge T, Ulmer H, et al. Metabolic factors associated with risk of renal cell carcinoma. PloS one. 2013;8:e57475. doi: 10.1371/journal.pone.0057475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nature reviews Urology. 2010;7:245–257. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunt JD, van der Hel OL, McMillan GP, Boffetta P, Brennan P. Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. International journal of cancer Journal international du cancer. 2005;114:101–108. doi: 10.1002/ijc.20618. [DOI] [PubMed] [Google Scholar]
- 7.Weiss RH, Lin PY. Kidney cancer: identification of novel targets for therapy. Kidney international. 2006;69:224–232. doi: 10.1038/sj.ki.5000065. [DOI] [PubMed] [Google Scholar]
- 8.Belldegrun AS, Klatte T, Shuch B, LaRochelle JC, Miller DC, Said JW, et al. Cancer-specific survival outcomes among patients treated during the cytokine era of kidney cancer (1989–2005): a benchmark for emerging targeted cancer therapies. Cancer. 2008;113:2457–2463. doi: 10.1002/cncr.23851. [DOI] [PubMed] [Google Scholar]
- 9.Wettersten HI, Hakimi AA, Morin D, Bianchi C, Johnstone ME, Donohoe DR, et al. Grade-Dependent Metabolic Reprogramming in Kidney Cancer Revealed by Combined Proteomics and Metabolomics Analysis. Cancer research. 2015;75:2541–2552. doi: 10.1158/0008-5472.CAN-14-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganti S, Taylor SL, Abu Aboud O, Yang J, Evans C, Osier MV, et al. Kidney tumor biomarkers revealed by simultaneous multiple matrix metabolomics analysis. Cancer research. 2012;72:3471–3479. doi: 10.1158/0008-5472.CAN-11-3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molina AM, Motzer RJ. Clinical practice guidelines for the treatment of metastatic renal cell carcinoma: today and tomorrow. The oncologist. 2011;16(Suppl 2):45–50. doi: 10.1634/theoncologist.2011-S2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutson TE, Davis ID, Machiels JP, De Souza PL, Rottey S, Hong BF, et al. Efficacy and safety of pazopanib in patients with metastatic renal cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:475–480. doi: 10.1200/JCO.2008.21.6994. [DOI] [PubMed] [Google Scholar]
- 13.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schutz G, et al. Regorafenib (BAY 73–4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. International journal of cancer Journal international du cancer. 2011;129:245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 14.Chang YS, Adnane J, Trail PA, Levy J, Henderson A, Xue D, et al. Sorafenib (BAY 43–9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer chemotherapy and pharmacology. 2007;59:561–574. doi: 10.1007/s00280-006-0393-4. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Michaelson MD, Rosenberg J, Bukowski RM, Curti BD, George DJ, et al. Sunitinib efficacy against advanced renal cell carcinoma. The Journal of urology. 2007;178:1883–1887. doi: 10.1016/j.juro.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 16.Shepard DR, Garcia JA. Toxicity associated with the long-term use of targeted therapies in patients with advanced renal cell carcinoma. Expert review of anticancer therapy. 2009;9:795–805. doi: 10.1586/era.09.29. [DOI] [PubMed] [Google Scholar]
- 17.Wu S, Chen JJ, Kudelka A, Lu J, Zhu X. Incidence and risk of hypertension with sorafenib in patients with cancer: a systematic review and meta-analysis. The lancet oncology. 2008;9:117–123. doi: 10.1016/S1470-2045(08)70003-2. [DOI] [PubMed] [Google Scholar]
- 18.Mross K, Frost A, Steinbild S, Hedbom S, Buchert M, Fasol U, et al. A phase I dose-escalation study of regorafenib (BAY 73–4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:2658–2667. doi: 10.1158/1078-0432.CCR-11-1900. [DOI] [PubMed] [Google Scholar]
- 19.Eisen T, Joensuu H, Nathan PD, Harper PG, Wojtukiewicz MZ, Nicholson S, et al. Regorafenib for patients with previously untreated metastatic or unresectable renal-cell carcinoma: a single-group phase 2 trial. The lancet oncology. 2012;13:1055–1062. doi: 10.1016/S1470-2045(12)70364-9. [DOI] [PubMed] [Google Scholar]
- 20.Folkman J. Tumor angiogenesis: therapeutic implications. The New England journal of medicine. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 21.Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annual review of pharmacology and toxicology. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Progress in lipid research. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 23.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Z, Xu F, Huse LM, Morisseau C, Draper AJ, Newman JW, et al. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circulation research. 2000;87:992–998. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- 25.Webler AC, Michaelis UR, Popp R, Barbosa-Sicard E, Murugan A, Falck JR, et al. Epoxyeicosatrienoic acids are part of the VEGF-activated signaling cascade leading to angiogenesis. American journal of physiology Cell physiology. 2008;295:C1292–C1301. doi: 10.1152/ajpcell.00230.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pozzi A, Macias-Perez I, Abair T, Wei S, Su Y, Zent R, et al. Characterization of 5,6- and 8,9-epoxyeicosatrienoic acids (5,6- and 8,9-EET) as potent in vivo angiogenic lipids. The Journal of biological chemistry. 2005;280:27138–27146. doi: 10.1074/jbc.M501730200. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Wei X, Xiao X, Hui R, Card JW, Carey MA, et al. Arachidonic acid epoxygenase metabolites stimulate endothelial cell growth and angiogenesis via mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways. The Journal of pharmacology and experimental therapeutics. 2005;314:522–532. doi: 10.1124/jpet.105.083477. [DOI] [PubMed] [Google Scholar]
- 28.Cheng LM, Jiang JG, Sun ZY, Chen C, Dackor RT, Zeldin DC, et al. The epoxyeicosatrienoic acid-stimulated phosphorylation of EGF-R involves the activation of metalloproteinases and the release of HB-EGF in cancer cells. Acta pharmacologica Sinica. 2010;31:211–218. doi: 10.1038/aps.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panigrahy D, Edin ML, Lee CR, Huang S, Bielenberg DR, Butterfield CE, et al. Epoxyeicosanoids stimulate multiorgan metastasis and tumor dormancy escape in mice. The Journal of clinical investigation. 2012;122:178–191. doi: 10.1172/JCI58128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang D, Dubois RN. Epoxyeicosatrienoic acids: a double-edged sword in cardiovascular diseases and cancer. The Journal of clinical investigation. 2012;122:19–22. doi: 10.1172/JCI61453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu JY, Stephen Lee KS, et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc Natl Acad Sci U S A. 2013;110:6530–6535. doi: 10.1073/pnas.1304321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inoue H, Kauffman M, Shacham S, Landesman Y, Yang J, Evans CP, et al. CRM1 blockade by selective inhibitors of nuclear export attenuates kidney cancer growth. The Journal of urology. 2013;189:2317–2326. doi: 10.1016/j.juro.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nithipatikom K, Endsley MP, Isbell MA, Falck JR, Iwamoto Y, Hillard CJ, et al. 2-arachidonoylglycerol: a novel inhibitor of androgen-independent prostate cancer cell invasion. Cancer research. 2004;64:8826–8830. doi: 10.1158/0008-5472.CAN-04-3136. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Carlson ME, Kuchel GA, Newman JW, Watkins BA. Dietary DHA reduces downstream endocannabinoid and inflammatory gene expression and epididymal fat mass while improving aspects of glucose use in muscle in C57BL/6J mice. International journal of obesity. 2015 doi: 10.1038/ijo.2015.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Analytical chemistry. 2009;81:8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nording ML, Yang J, Georgi K, Hegedus Karbowski C, German JB, Weiss RH, et al. Individual variation in lipidomic profiles of healthy subjects in response to omega-3 Fatty acids. PloS one. 2013;8:e76575. doi: 10.1371/journal.pone.0076575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 38.Skovseth DK, Kuchler AM, Haraldsen G. The HUVEC/Matrigel assay: an in vivo assay of human angiogenesis suitable for drug validation. Methods in molecular biology. 2007;360:253–268. doi: 10.1385/1-59745-165-7:253. [DOI] [PubMed] [Google Scholar]
- 39.Guo D, Jia Q, Song HY, Warren RS, Donner DB. Vascular endothelial cell growth factor promotes tyrosine phosphorylation of mediators of signal transduction that contain SH2 domains. Association with endothelial cell proliferation. The Journal of biological chemistry. 1995;270:6729–6733. doi: 10.1074/jbc.270.12.6729. [DOI] [PubMed] [Google Scholar]
- 40.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer research. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 41.Ivanov SV, Kuzmin I, Wei MH, Pack S, Geil L, Johnson BE, et al. Down-regulation of transmembrane carbonic anhydrases in renal cell carcinoma cell lines by wild-type von Hippel-Lindau transgenes. Proc Natl Acad Sci U S A. 1998;95:12596–12601. doi: 10.1073/pnas.95.21.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurban G, Hudon V, Duplan E, Ohh M, Pause A. Characterization of a von Hippel Lindau pathway involved in extracellular matrix remodeling, cell invasion, and angiogenesis. Cancer research. 2006;66:1313–1319. doi: 10.1158/0008-5472.CAN-05-2560. [DOI] [PubMed] [Google Scholar]
- 43.Donadio JV, Jr, Bergstralh EJ, Offord KP, Spencer DC, Holley KE. A controlled trial of fish oil in IgA nephropathy. Mayo Nephrology Collaborative Group. The New England journal of medicine. 1994;331:1194–1199. doi: 10.1056/NEJM199411033311804. [DOI] [PubMed] [Google Scholar]
- 44.Hwang SH, Wecksler AT, Zhang G, Morisseau C, Nguyen LV, Fu SH, et al. Synthesis and biological evaluation of sorafenib- and regorafenib-like sEH inhibitors. Bioorganic & medicinal chemistry letters. 2013;23:3732–3737. doi: 10.1016/j.bmcl.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schebb NH, Ostermann AI, Yang J, Hammock BD, Hahn A, Schuchardt JP. Comparison of the effects of long-chain omega-3 fatty acid supplementation on plasma levels of free and esterified oxylipins. Prostaglandins & other lipid mediators. 2014;113–115:21–29. doi: 10.1016/j.prostaglandins.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu JY, Park SH, Morisseau C, Hwang SH, Hammock BD, Weiss RH. Sorafenib has soluble epoxide hydrolase inhibitory activity, which contributes to its effect profile in vivo. Molecular cancer therapeutics. 2009;8:2193–2203. doi: 10.1158/1535-7163.MCT-09-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morisseau C, Inceoglu B, Schmelzer K, Tsai HJ, Jinks SL, Hegedus CM, et al. Naturally occurring monoepoxides of eicosapentaenoic acid and docosahexaenoic acid are bioactive antihyperalgesic lipids. J Lipid Res. 2010;51:3481–3490. doi: 10.1194/jlr.M006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.