Abstract
Inactivation of the p53 tumor suppressor by mutation or overexpression of negative regulators occurs frequently in cancer. Since p53 plays a key role in regulating proliferation or apoptosis in response to DNA damaging chemotherapies, strategies aimed at reactivating p53 are increasingly being sought. Strategies to reactivate wild-type p53 include the use of small molecules capable of releasing wild-type p53 from key, cellular negative regulators, such as Hdm2 and HdmX. Derivatives of the Hdm2 antagonist Nutlin-3 are in clinical trials. However, Nutlin-3 specifically disrupts Hdm2-p53, leaving tumors harboring high levels of HdmX resistant to Nutlin-3 treatment. Here we identify CTX1, a novel small molecule that overcomes HdmX-mediated p53 repression. CTX1 binds directly to HdmX to prevent p53-HdmX complex formation, resulting in the rapidly induction of p53 in a DNA damage-independent manner. Treatment of a panel of cancer cells with CTX1 induced apoptosis or suppressed proliferation and importantly, CTX1 demonstrates promising activity as a single agent in a mouse model of circulating primary human leukemia. CTX1 is a small molecule HdmX inhibitor that demonstrates promise as a cancer therapeutic candidate.
Keywords: p53, Targeted Therapy, HdmX, Drug Development, Drug Screening
Introduction
DNA-damaging chemotherapy is the first-line therapy for most types of cancer. Unfortunately, side effects from this approach are significant and often dose limiting as DNA damage leads to toxicity on normal cells. As one of the main pathways responsible for the anti-cancer activity of DNA damaging chemotherapy involves the activation of p53, strategies to activate p53 and subsequently p53-dependent cancer cell death without DNA damage are highly valuable. The stabilization and subsequent activation of p53 protein is well known to lead to either cell cycle arrest and/or apoptosis (1–5). Though p53 is mutated in approximately 50% of all human cancers, wild-type p53 is extremely common in some malignancies such as Acute Myeloid Leukemia (AML) and melanoma (6–9). Malignancies in which p53 is often wild-type are particularly attractive targets for p53 activation strategies. Though p53 is wild-type in certain cancers, it is generally accepted that it is functionally inactivated. In these cases the inactivation of p53 often occurs through one or both of its negative regulators, Hdm2 and HdmX.
Hdm2 and HdmX are highly homologous proteins that directly bind p53 and impair p53 functional activity. Hdm2 exhibits E3 Ubiquitin ligase activity and leads to p53 ubiquitination and subsequent proteasomal degradation. This activity helps to maintain low basal levels of p53. Hdm2, similar to HdmX, can inhibit p53 transcriptional activity by interacting with the N-terminal domain of p53. In normal cells, a defined balance in the relative levels of Hdm2 and HdmX, either individually or cooperatively, regulate p53 functions, keeping target gene transcription at levels that do not cause apoptosis or cell cycle arrest (10, 11). However, in cancer overexpression of one or both of these proteins can lead to impaired p53 function and cancer development and/or progression. It is important to note that HdmX and Hdm2 regulate p53-independent anti-tumor pathways as well leading to activity of Hdm2/HdmX inhibitors in p53 deficient cells as well (12–14).
In order to induce p53 in a non-genotoxic manner, investigators have focused for a number of years on the development of potent small molecule inhibitors of the Hdm2/p53 interaction (15). For example, the small molecule nutlin-3 has been identified as an inhibitor of Hdm2 and p53 (16). Nutlin compounds mimic the critical amino acids within the p53 alpha-helix that bind within the hydrophobic pocket of Hdm2 where p53 normally resides, and show p53-specific antitumor properties (17). Studies surrounding nutlin-3 have proven successful against malignancies such as B-cell chronic lymphocytic leukemia, multiple myeloma and AML (18–20). Currently there are several clinical trials in progress to evaluate nutlin-3 derivatives as anti-cancer agents. Of note, in addition to strategies targeting wild-type p53, several complementary approaches have been developed for cases in which p53 is mutant or deficient (21–23).
Though there was initial excitement in targeting the Hdm2/p53 interaction as a therapeutic strategy, this initial excitement over selective Hdm2 inhibitors, has been dampened by observations that HdmX which also inhibits p53 activity, is overexpressed in a relatively large percentage of cancers. In these relatively frequent cases the HdmX/p53 complex can prevent the optimal induction of p53-dependent anti-tumor activity by Hdm2 inhibitors such as nutlin-3 (24). In order to identify novel small molecules that can overcome HdmX-mediated p53 suppression, we performed a compound library screen. Our screen led to the identification of the small molecule, CTX1, which exhibits potential as a cancer therapeutic that can bind HdmX, induce p53 and kill cancer cells without the necessity of DNA damage. Importantly, CTX1 exhibits potent activity even as a single agent and demonstrated superior efficacy to nutlin-3 in a mouse AML model system.
Materials and Methods
Chemicals
CTX1 was obtained from the Development Therapeutics Program at the National Cancer Institute. CTX-1 biotin was synthesized in our laboratory. Doxorubicin, trypan blue and nutlin-3 were obtained from Sigma. Recombinant GST-FXR, GST-p53, and GST-HdmX were purchased from Abnova.
Cell Lines
All cell lines were obtained from ATCC except OCI-AML3 which were obtained from DSMZ. Upon receiving the cell lines, frozen stocks were prepared within 1–2 passages and new stocks were thawed frequently to keep the original condition. The cell lines were passaged for less than 6 months after receipt or resuscitation. They were also routinely authenticated based on growth rate, morphology and viability and were frequently confirmed to be mycoplasma free. The generation of the MCF7 shp53 cells and IMR90 cells were previously described as well as the validation of the stable knockdown of p53 and overexpression of HdmX and Hdm2 in these cells (24). Cells were cultured in RPMI-1640 media (Invitrogen) supplemented with 10% FBS (Invitrogen), penicillin G (100μg/ml) and streptomycin (100μg/ml). The OCI-AML3 HDMX overexpressing cells were generated by stable transfection of plvx-mcherry-HA-HDMX and isolation of m-cherry expressing cells by flow sorting (BD FACSAria).
Cell cycle analysis
Cells were treated as described and fixed at −20°C in 90% methanol. Cells were washed in PBS, treated with RNase A (final concentration 0.5μg/ml) (Sigma) and stained with propidium iodide (Sigma) (50μg/ml). The cells were kept at room temperature for 60 minutes and analyzed by flow cytometry on a BD XL cytometer. The cell cycle was modelled using the Modfit software (Verity House).
Western blot analysis
Western blot analysis was performed with p21, p53, p-ser15 p53, p-H2AX, PARP (Santa Cruz), HdmX (Bethyl) and β-actin antibodies (Sigma). Cells were treated as indicated and washed in PBS. Cells were centrifuged and lysed with a Triton containing lysis buffer. Protein lysates (50 μg per lane) were electrophoresed on SDS-polyacrylamide gels and then transferred to PVDF membranes (Millipore) using a wet transfer apparatus (Bio-Rad). The membranes were blocked, incubated with the indicated primary antibodies at 4°C overnight, and then the appropriate HRP conjugated secondary antibody. Protein bands were visualized by autoradiography after incubation with enhanced chemiluminescence reagent (Millipore).
Biotin-Immunoprecipitation
Briefly, Binding/wash buffer (Tris-buffered saline containing 0.1% Tween-20 detergent) containing 2μM CTX1-biotin was incubated with 150ng of either recombinant HdmX and/or P53 for 30 minutes. The samples were desalted (to remove unincorporated biotin) using Zeba Desalt Spin columns (Thermo Scientific). The sample was mixed with prewashed Streptavidin Magnetic Beads (Pierce) and incubated at room temperature for 1 hour with mixing. The beads were washed, the bound antigen was eluted with sample buffer, and western blot was performed.
P53 protein Immunoprecipitation
P53 antibody was coupled to magnetic beads with borate buffer using the Direct Magnetic IP/Co-IP Kit (Thermo) according to the manufacturer's instructions. Next CTX1, Nutlin-3, 9-aminoacridine or DMSO (5uM final concentration) were added to individual tubes containing 100ng of recombinant HdmX. The mixture was incubated at 4°C for 90 minutes under mixing. 100ng of recombinant p53 was added to each tube and it was incubated for another 2 hours at 4°C. The antibody coupled beads were then added for 2 hours at room temperature on a mixer. The beads were washed twice with IP lysis buffer and the bound protein was eluted with sample buffer and assessed by western blot.
Cell based Immunoprecipitaitons
Exponentially growing OCI cells cultures were treated with 3 μM CTX1, 8 μM Nutlin and or 15 μM RO-5963 for 4.5hrs. Whole-cell extracts were generated using modified RIPA lysis buffer 25 mM Tris (pH 8.0), 100 mM NaCl, 0.5 mM EDTA, 0.50% NP-40 and complete protease mixture tablet (Roche). Protein extracts (~750 μg) were precleared and immune precipitation was performed using the direct magnetic IP kit (Pierce/Thermo Scientific) as per the manufacturer's protocol. For the immunoprecipitation, mouse monoclonal anti-p53 (Santa Cruz) and rabbit polyclonal anti-HDMX (Bethyl Laboratories) were used. Immune complexes were then collected, proteins were eluted and subjected to Western blotting with the indicated antibodies.
Spectrophotometric Analysis
UV-VIS spectral scanning or wave scan measurement methods were adopted (Genesys 10S UV-VIS Spectrophotometer, Thermo Scientific) in this study. Briefly either CTX1 or 9AA compounds were mixed individually with recombinant HdmX protein in a 2:1 molar ratio in normal saline. Sample were scanned with or without passing through a de-salting column (Thermo Scientific) to ensure bound Protein-Compound complex were separated from unbound compound. The Absorbance of various samples were measured over a specified wavelength range of 200–700nm.
Biacore Study
Surface plasmon resonance (SPR) was performed on a Biacore T100 instrument (GE Healthcare) at 25 degree C using PBSP+ (GE Healthcare) plus 5% DMSO as running buffer. S series sensor CM5 sensorchip (GE Healthcare) was used to immobilize HdmX. CTX1 with increasing concentrations (156.3nM, 312.5nM, 625nM, 1.25uM, 2.5uM, and 5uM 1.56uM) was injected over the surface at 30uL/min for 2min. 2M GnHCl was used for regeneration of the surface. Data analysis and determination of affinity constants were made using Biaevaluation3 software (GE Healthcare). The concentration series was fitted using a 1:1 kinetics model.
Elisa
The ELISA was done as previously described (25). Briefly, recombinant p53 was bound to an ELISA plate (96-well format), and recombinant HdmX or Hdm2 were pre-incubated with the indicated doses of CTX1 and Nutlin-3. The HdmX and Hdm2 were added to the wells containing p53, and incubated for 1 hour. After extensive washing, the amount of HdmX or Hdm2 bound to p53 was quantified using anti-Hdmx or anti-Hdm2.
Library screen
Approximately 5000 MCF7 cells stably overexpressing HdmX and expressing a p21/ConA-/βgal reporter were seeded in 96 well plates. The wells were treated with approximately 20,000 compounds at 10μM from libraries from 3 sources (Enamine, Prestwick, and the National Cancer Institute) for 72 hours. Next the reported induction was measured using the Beta-Glo Reagent (Promega) according to the manufacturer's protocol (Promega).
Cell growth and death measurements
Cell growth was assessed using the MTT assay (Trevigen) according to the manufacturer's protocol. Cell death was assessed using Trypan blue staining by counting at least 200 cells from two independent fields and/or using Annexin V staining (Santa Cruz) according to manufacturer's suggestions. All cell death experiment values represent means +/− standard deviations from at least two independent experiments
Mouse Xenograft
6 week old female NOD/SCID/IL2Rγ−/− mice (Jackson Laboratory) were injected into the tail vein with 5×106 cells primary human AML cells (n=5 per group). Drug treatment was started 2 days after tumor cell injection. Nutlin-3 was given by oral gavage (200mg/kg) and CTX1 was injected i.p. (30mg/kg) five days a week for 3 weeks. Flow cytometry was performed on bone marrow cells isolated from the mouse femur using a human specific CD45PE antibody (BD Biosciences) using a BD XL cytometer to confirm AML infiltration into the bone marrow. The Case Western Reserve University Animal Research Committee approved all of the animal protocols used in this study.
Results
Screening for novel HdmX inhibitors
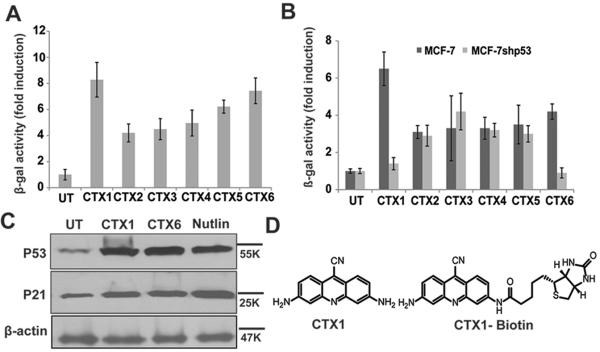
To identify activators of p53 in cells overexpressing HdmX, a cell-based screen was employed. In setting up a screening protocol we looked for compounds capable of inducing p53-dependent transcriptional activity of a p53-dependent reporter greater than 4-fold in breast cancer cells in which p53 activity is inhibited through stable overexpression of HdmX. We screened over 20,000 small molecules from a number of compound collections from Prestwick, NCI and Enamine. From this screen 6 compounds exhibited a reproducible induction of the p53 reporter (Fig. 1A). Next the p53-dependence of reporter activation by the positive compounds was assessed using the HdmX-overexpressing reporter cells stably infected with shRNA directed against p53 or a control shRNA (Fig. 1B). Two compounds, CTX1 and CTX6, exhibited strong p53-dependent reporter activation in the presence of high levels of HdmX. As the disruption of the HdmX/p53 interaction leads to a rapid induction of p53 protein, we next tested the ability of CTX1 and CTX6 to induce p53 and its transcriptional target p21 (Fig. 1C). Both compounds were found to induce p53 and p21 protein expression. Due to the higher activity of CTX1 on inducing p53-reported activation, our studies have focused on this compound (Fig. 1D).
Figure 1. Identification of novel HdmX inhibitors.
A. Identification of novel compounds that induce a p21/ConA-β-gal reporter in MCF7 cells. MCF7 cells were treated for 24hr with the indicated compounds (2μM) and β-gal activity was measured. B. CTX1 and CTX6 exhibit p53-dependent activation of the reporter construct. MCF7 and MCF7shp53 cells were treated with the indicated compounds and β-gal activity was measured as described in fig1a. C. CTX1 and CTX6 induce p53 and p21. MCF7 cells were treated with CTX1 (2μM), CTX6 (3μM), or nutlin-3 (10μM) for 6hr and a western blot was performed. D. Structure of CTX1 and CTX1-biotin. Error bars represent standard deviation.
CTX1 rapidly induces p53 in a non-DNA damage dependent fashion
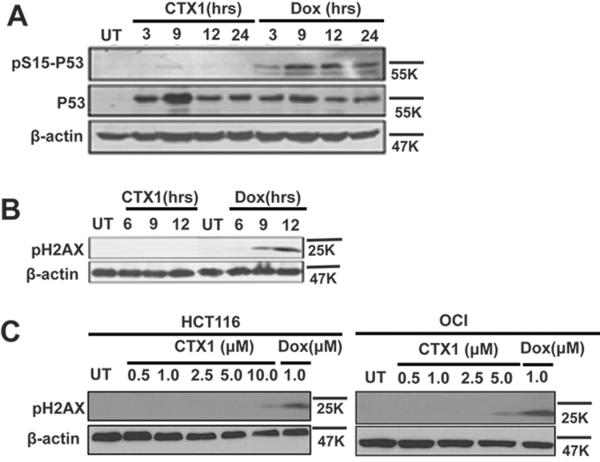
As the compound library screen was aimed at identifying compounds that induce p53 by overcoming HdmX-mediated suppression, we predicted that unlike the vast majority of chemotherapeutics, CTX1 can induce p53 independently of DNA damage. CTX1, unlike doxorubicin (a well-known DNA damage agent) does not induce measurable DNA damage as measured by common markers of DNA damage (phosphorylation of γ-H2AX or p53 (ser-15)) at doses necessary for p53 induction (Fig. 2A–C). Therefore, CTX1-mediated p53 induction was found to be independent of genotoxic stress. Of note, CTX1 induces p53 rapidly (ex. 2 hours) supporting its direct role in stabilizing the p53 protein as opposed to induction by DNA damage or transcriptional mechanisms (Fig. 2A and FigS1).
Figure 2. CTX1 rapidly induces p53 independent of DNA damage.
A–B. MCF7 cells were treated with CTX1 (3μM) or Doxorubicin (1μM) for the indicated times (hours) and western analysis was performed for markers of DNA damage (p-p53 (Ser15) and p-H2AX). C. HCT116 cells (left panel) or OCI cells (right panel) were treated with the indicated doses of CTX1 or Doxorubicin for 9 hours and western analysis was performed using the indicated antibodies.
CTX1 exhibits specificity in targeting HdmX
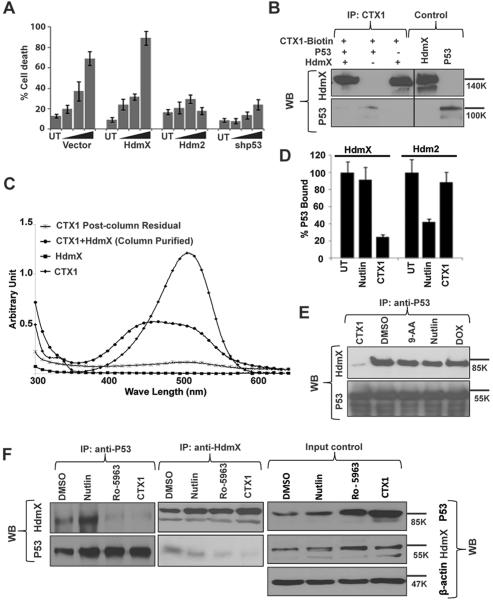
To determine whether CTX1 is specific for overcoming p53 suppression due to HdmX, Hdm2, or both, we utilized a fibroblast cell model system in which p53 was suppressed by HdmX or Hdm2 overexpression. In addition as HdmX inhibition should partially lead to cell death through p53 induction, we also used cells in which p53 expression was knocked down. This same cell model system was previously shown to define the specificity of the Hdm2 inhibitor, nutlin-3, for Hdm2 (24, 26). CTX1 induced significant p53-dependent cell death preferentially in HdmX-expressing cells (Vector or HdmX) as compared to cells in which p53 is inactivated by Hdm2 overexpression or p53 is knocked down using p53 targeting shRNA (Fig. 3A). This study demonstrates CTX1 shows specificity in targeting HdmX over its homologue Hdm2. In addition, this model system further supports the ability of CTX1 to overcome HdmX-mediated p53 suppression. To further confirm the ability of CTX1 to overcome HdmX-mediated suppression of cell killing, we employed a leukemia cell model system. Consistent with the fibroblast cell model, OCI-AML3 (OCI) cells overexpressing HdmX were found to exhibit a similar sensitivity to CTX1 mediated cell killing as parental cells in contrast to the Hdm2 inhibitor, nutlin-3, that as expected demonstrates reduced activity in the presence of high HdmX levels (Fig. S2A–B).
Figure 3. CTX1 specifically targets and directly binds to HdmX.
A. CTX1 preferentially kills cells transformed by HdmX and not Hdm2 or shp53. IMR90 cells overexpressing the indicated constructs were treated with increasing doses of CTX1 (1, 2.5 and 5μM) and assessed for cell death at 72hr by trypan blue staining. B. CTX1 binds HdmX and to a lesser extent p53. Recombinant p53, HdmX, and/or biotin-CTX1 were incubated in vitro and streptavidin beads were used to pull down the protein complex. The bound protein was eluted and analyzed by western blot with the indicated antibodies. C. Spectral studies suggest CTX1 and HdmX directly interact. CTX1 and HdmX alone represent the spectral pattern of both agents without purification. The CTX1+HdmX sample and CTX1 Postcolumn Residual samples underwent size exclusion chromatography to remove unbound CTX1. D–E. CTX1 disrupts the interaction of recombinant HdmX/p53 but not Hdm2/p53 by ELISA and co-immunoprecipitation. The indicated drugs were used as controls. F. CTX1 disrupts the interaction of HdmX/p53 in cells. Immunoprecipitations were performed as indicated using lysate from OCI cells treated with the indicated drugs or a DMSO control. Error bars represent standard deviation.
CTX1 directly interacts with HdmX and modulates p53-HdmX binding
In order to explore how CTX1 leads to p53 induction, we assessed whether or not CTX1 can directly interact with HdmX and/or p53. In order to test for direct binding, we synthesized a biotin-conjugated version of CTX1 (Fig. 1D). The CTX1-biotin compound exhibits similar activity in cell killing to the parent compound (Fig. S2B). We found that CTX1 can directly interact with GST-HdmX, but not unrelated proteins such as GST-FXR by immunoprecipitation (Fig. 3B and Fig. S3A). In addition, GST-HdmX can bind at least weakly with p53. Biotin alone was confirmed not to bind with HdmX demonstrating the CTX1 component of the CTX1-biotin conjugate is responsible for this interaction (Fig. S3A).
In addition to testing the binding of CTX1 and HdmX through immunoprecipitations using biotin-tagged CTX1, we also confirmed the interaction of HdmX with non-tagged CTX1 using two biophysical based approaches. First, we took advantage of the fact that CTX1, but not HdmX exhibits a specific absorbance pattern that is detectable by a spectrophotometer. After co-incubation of CTX1 with HdmX (and removal of free CTX1 by size exclusion chromatography), not only does HdmX now exhibit an absorbance pattern, but there is also a clear spectral shift of the absorbance pattern as compared to free CTX1 (Fig 3C). This spectral shift is highly suggestive of an HdmX/CTX1 interaction. In addition size exclusion chromatography performed on CTX1 alone as a control demonstrates the absorbance seen with the HdmX/CTX1 sample is not due to residual non-bound CTX1 (Post-column Residual control, Fig. 3C).
Interestingly, CTX1 is an acridine containing molecule and other acridine containing compounds such as 9-aminoacridine (9-AA) have previously been shown to rapidly induce p53 in a non-DNA damage dependent fashion possibly related to its ability to intercalate in DNA (27). Interestingly, we did not detect binding of 9-AA to HdmX using the same spectral studies suggesting a distinct mechanism of action (Fig. S3B).
Using another biophysical approach, we also demonstrated HdmX and CTX1 binding using surface plasmon resonance (SPR). HdmX demonstrated strong binding to CTX1 (Kd 450nM) but not nutlin-3 (Kd 5.1μM) (Fig. S3C). Again 9-AA did not demonstrate any binding to HdmX using SPR at doses up to 12.5μM (data not shown).
Besides assessing for interactions of CTX1 and HdmX, we assessed for the ability of CTX1 to directly impair the interaction of p53 and HdmX that could result in the observed stabilization of p53 protein. Utilizing an ELISA assay, we found that CTX1 disrupted HdmX/p53, but not Hdm2/p53 interactions (Fig. 3D). In contrast the Hdm2 inhibitor, nutlin-3, disrupts the interaction between p53 and Hdm2, but not p53 and HdmX. To further confirm the ability of CTX1 to disrupt HdmX and p53 binding, we performed co-immunoprecipitation assays using both purified recombinant HdmX and p53 as well as native protein in OCI cells (Fig. 3E–F). Utilizing these assays, we further demonstrate that CTX1, but not 9-AA or a standard DNA damaging agent, doxorubicin, impair the binding of HdmX and p53. As a control, we also found that the compound RO-0596 which has been reported to impair HdmX/p53 binding also disrupts binding in our immunoprecipitations (28). This result suggests that CTX1's ability to impair HdmX/p53 binding is specific and is not simply due to its acridine moiety.
CTX1 impairs cancer cell growth
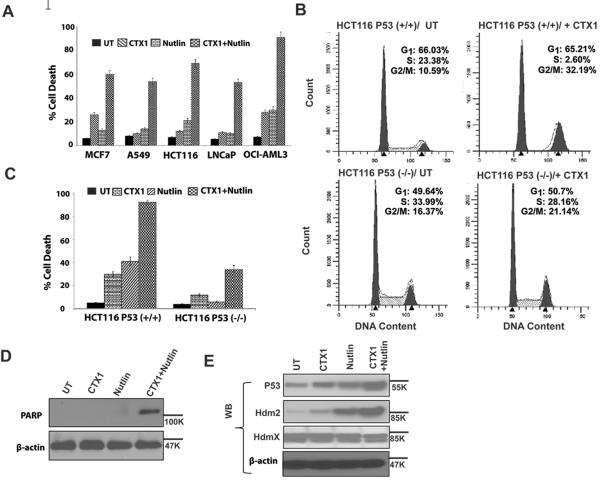
In order to explore the effects of CTX1 on cancer cell growth and survival, we tested the ability of CTX1 to inhibit the growth and/or kill a panel of wild-type p53 cancer cell lines including HCT116, Hela, A549, MCF7, LNCaP, OCI-AML3 (OCI), and MOLM-13. In addition, mutant p53 cell lines (HT29, DLD1 and K562) and p53 deficient cells (HCT116 p53−/−, A549 shp53, and Jurkat) were tested (HdmX and p53 status of cell lines are summarized in Table 1). CTX1 was tested alone as well as at lower doses in combination with the commonly used Hdm2 inhibitor, nutlin-3. These studies revealed that low doses of CTX1 and nutlin-3 led to cooperative killing when combined with nutlin-3. In addition, while CTX1 can clearly cause death of p53 deficient cancer cells, testing of isogenic cells (HCT116 and A549) demonstrated a modest increase in killing of p53 expressing cells. Of note, HdmX can lead to induction of both p53 dependent and independent pathways. Also of note, the leukemia cell lines tested demonstrated an increased sensitivity to CTX1-mediated cell killing as compared to the solid tumor cell types (Fig. 4A–C, Table 2, and Fig. S4). As CTX1 can induce cell killing in p53 mutant cell lines, we also investigated whether or not it can stabilize mutant p53 levels. As seen in Supplementary figure 4C, CTX1 can induce p53 protein levels in the p53 mutant cell line, HT29. Besides cell lines, we have also found that CTX1 exhibits potent activity (LD50 ~ 1μM) as a single agent on primary AML patient samples in a similar fashion to AML cell lines (Table 2).
Table 1. p53 and HdmX statuses of cell lines used.
HdmX mRNA expression values were obtained from the Cancer Cell Line Encyclopedia Project (http://www.broadinstitute.org/ccle/home) (38).
| p53 status HdmX log mRNA expression | ||
|---|---|---|
| OCI | wild-type | 9.12 |
| Jurkat | null | 9.41 |
| Hela | wild-type | unknown |
| LnCAP | wild-type | 7.54 |
| HCT116 | wild-type | 7.31 |
| Molm-13 | wild-type | 7.77 |
| K562 | mutant | 8.15 |
| HT29 | mutant | 7.24 |
| DLD1 | mutant | 6.45 |
| MCF7 | wild-type | 7.79 |
| A549 | wild-type | 6.51 |
Figure 4. CTX1 induces apoptosis and growth arrest of cancer cells.
A and C. The combination of CTX1 and nutlin-3 lead to significant apoptosis. The indicated cell lines were treated with CTX1 3μM (MCF7), 4μM (A549), 2μM (HCT116), 4μM (LNCaP) 0.75μM (OCI); nutlin-3 (5μM), or a combination for 72hr and cell death was assessed by trypan blue exclusion. B. CTX1 preferentially impairs the growth of cells expressing wild-type p53. HCT116 p53+/+ or p53-null cells were treated with CTX1 (2μM) and cell cycle analysis was performed using PI staining at 16hr D–E. CTX1 and nutlin-3 cooperate to induce p53, Hdm2 and PARP cleavage. OCI cells were treated for 24hr (D) and 2hr (E) with the indicated compounds and western analysis was performed. Error bars represent standard deviation.
Table 2.
LD50 of CTX1 on cancer cell lines
| LD50 μM | |
|---|---|
| OCI | 0.97 |
| Jurkat | 1 |
| Hela | 8.3 |
| LnCAP | 2.9 |
| HCT116 | 2.81 |
| HCT116 p53−/− | 5.65 |
| Molm-13 | 1.11 |
| K562 | 1.01 |
| HT29 | 4.05 |
| DLD1 | 7.4 |
| MCF7 | 4.29 |
| A549 | 5.32 |
| A549 shp53 | 7.7 |
| AML, PT 1 | 1.25 |
| AML, PT 2 | 0.75 |
| AML, PT 3 | 1.26 |
Cell cycle analysis of paired p53-expressing and p53-deficient cancer cell lines (HCT116 and A549) also demonstrates that CTX1 mediates growth inhibition partially through a p53-dependent pathway. For example, after 16hr of treatment with CTX1 in HCT116 p53-wild-type cells there is a decrease in S phase from 23% to 3% while HCT116 p53-null cells exhibit a reduction in S phase from 34% to 28% (Fig. 4B and Fig. S4A). While both HCT116 and A549 p53-wild-type cells exhibit a dramatic reduction in S phase, HCT116 also see a modest accumulation of cells in the G2/M phase. Interestingly other agents have also been reported to preferentially accumulate HCT116 cells in G2/M as compared to A549 cells suggesting differences in cell cycle regulatory pathways (29).
To further characterize the mechanism of cell killing Annexin-V staining was done to assess for apoptosis in HCT116 p53-wildtype and p53-null cells. The combination of low doses of CTX1 and nutlin-3 led to a significant enhancement of apoptosis in p53-wildtype, but not p53-null cells (Fig. 4C and Fig. S4B).
In agreement with the synergistic and additive induction of cell death when combining CTX1 and Hdm2 inhibition, we also observed a modest increase in p53 protein induction, the p53 target protein Hdm2 and a marker of apoptosis, PARP cleavage, in cells treated with the combination regimen (Fig. 4D–E).
In addition to p53 induction, we also assessed the regulation of Hdm2 and Hdmx in response to CTX1 alone as these proteins are known to be induced and downregulated by nutlin-3 respectively. We observed that the expression of these proteins were not markedly impacted by CTX1 alone, however, Hdm2 induction was enhanced by CTX1 (likely due to enhanced p53 induction) (Fig 4E).
CTX1 exhibits high in vivo activity
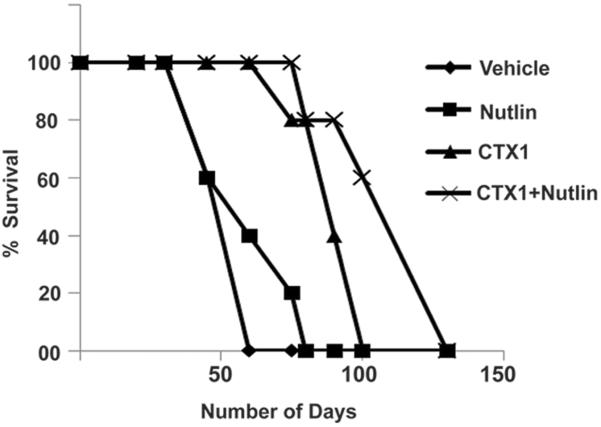
As CTX1 represents one of the few examples of a compound that can induce p53 and kill cancer cells in a genotoxic-independent fashion, we performed mouse efficacy studies in order to begin to explore its clinical potential. We utilized a highly aggressive AML model system for this study as this is a disease unlike most malignancies in which wild-type p53 status is extremely common and new therapeutics are urgently needed. The ability of CTX1 (30mg/kg i.p.), nutlin-3 (200mg/kg p.o.) or the combination to impact the growth of primary human AML cells (wild-type p53) in immunodeficient mice was assessed. This model system closely mimics the human disease as it utilizes a primary patient sample and the leukemic cells circulate in the mouse and proliferate in the bone marrow. Utilizing a primary human AML sample, CTX1 even as a single agent significantly enhanced the survival of mice in this model system (Fig 5). Of note this model system is clinically important as there are no existing therapeutics that are efficacious in this patient population. While all of the vehicle mice succumbed to disease by 60 days after cell injection, mice treated with CTX1 alone or in combination with nutlin-3 had a significantly increased survival time (p<0.0001 log rank test).
Figure 5. CTX1 demonstrates significant anti-cancer activity in vivo.
NSG mice (n=5 per group) were injected with primary human AML cells by the tail vein (5×106 cells in 100μl of media) and mice were treated with CTX1 (30mg/kg ip) or Nutlin-3 (200mg/kg po) and assessed for survival. Log rank test, p<0.0001.
Importantly, CTX1 exhibited significant anti-cancer activity alone as well as in combination with nutlin-3. There was a significant survival advantage of CTX1 treatment as compared to nutlin-3 in this mouse model using a standard dosing regimen for nutlin-3 while further studies are necessary to identify optimal CTX1 dosing. This work suggests that CTX1 may have potential even as a single agent. In addition to efficacy, the mouse studies also demonstrate the potential safety of CTX1. The mice treated with CTX1 alone or in combination with nutlin-3 gained weight in a similar fashion to the vehicle treated mice and did not show any obvious signs of toxicity. This study shows the potential of CTX1 as a leukemia therapeutic and that HdmX inhibitors alone may be a promising therapeutic strategy. Overall, we have identified a novel HdmX inhibitor that is a promising potent anti-cancer agent with apparent low toxicity that is worthy of further development.
Discussion
P53 is a major regulator of cancer cell growth and drug resistance. While many cancer cells exhibit p53 mutations to inactivate p53, another mechanism to regulate p53 function is through the up-regulation of negative regulators such as HdmX and Hdm2. By targeting these negative regulators of p53, it is possible to directly activate p53 without the necessity of DNA damage. Our objective was to identify a small molecule inhibitor that could overcome HdmX-mediated suppression of p53. Since HdmX is overexpressed in a large number of cancers and Hdm2 inhibitors such as nutlin-3 do not show significant efficacy on cancer cells that overexpress HdmX, the development of small molecules targeting HdmX is important. Targeting HdmX is also known to have anti-cancer properties that are independent of p53. Another potential advantage of HdmX inhibition, is that it has been predicted to be safer than Hdm2 inhibition as the lack of the mouse homologue, Mdm2 but not Mdmx, leads to significant p53-dependent toxicities in normal adult tissues (30).
While there are numerous studies demonstrating the potential for small molecules capable of inhibiting Hdm2/p53 interactions there are extremely few reported small molecules which have been shown to be capable of inhibiting HdmX/p53 and none that have demonstrated both cell and animal efficacy. It is not clear that the compounds reported previously as Hdmx/p53 inhibitors are suitable for clinical development (reviewed in (28)). SJ-172550 is a compound recently described as an HdmX/p53 inhibitor, but the authors concluded that the compound was not suitable for further development as it forms cysteine adducts with Hdm2 and HdmX (31, 32). Another small molecule, WK298, was reported to disrupt HdmX/p53 interactions but does not exhibit any cellular activity (33). Finally, RO-5963 is a dual Hdm2 and HdmX inhibitor though it exhibits weaker activity against the Hdm2/p53 interaction in cells (15, 28). This molecule exhibits a unique mechanism of action distinct from CTX1 as it has been reported to impair HdmX/p53 binding by stabilizing HdmX/Hdm2 heterodimers, however, the in vivo activity of this agent has not been described. Besides small molecule inhibitors, a stapled p53 helix and peptide inhibitors have also been reported (25, 34). Therefore, the identification of CTX1 that demonstrates both in vitro and mouse in vivo anti-cancer efficacy is important for the potential clinical targeting of the HdmX mediated p53 suppression in patients. Besides direct inhibitors of Hdmx/p53, other investigators have taken alternative and potentially complementary approaches to induce p53 in a non-genotoxic manner. For example, NSC207895 is a compound that modulates HdmX transcription and other groups have developed E3 ubiquitin ligase inhibitors (28, 35, 36).
The identification of CTX1 as an HdmX/p53 inhibitor was unexpected as CTX1 contains an acridine ring structure which is found in many other well-known compounds tested as anti-cancer agents that can induce DNA damage. Interestingly, however, there are also several acridine containing compounds that like CTX1 can induce p53 in a non-DNA damage dependent fashion. For example, quinacrine and 9-aminoacridine (9-AA) have been shown to exhibit this property and their anti-cancer activities have been attributed to a combination of p53 induction and NFkB inhibition (27, 37). Though CTX1 shares some structural similarities with 9-AA, the mechanisms of p53 induction do not appear to completely overlap as 9-AA was not found to be capable of disrupting HdmX/p53 interactions or to interact with HdmX.
Though CTX1 can disrupt HdmX/p53 interactions, induce p53, and cause p53-dependent cell death, it clearly also can induce cell death through additional pathways. These p53-independent activities of CTX1 fit well with the fact that HdmX (as well as Hdm2) are known to exhibit many p53-independent anti-tumor pathways (12–14). It will be interesting to see if some of these p53-independent pathways overlap with those reported for other non-DNA damaging acridine agents such as 9-AA. In addition, these p53-independent pathways suggest CTX1 may have utility for p53 deficient tumors as well.
Though the in vitro activity of CTX1 is strongly enhanced by concurrent Hdm2 inhibition using an agent such as nutlin-3, CTX1 alone is a promising lead anti-cancer agent. The potential of CTX1 as a single agent can be seen from the efficacy of CTX1 in a circulating AML mouse model system. In these studies CTX1 alone showed significant efficacy that was higher than nutlin-3 using a standard nutlin-3 dosing regimen. Of note the standard AML therapeutic cytarabine also does not demonstrate efficacy in this aggressive disease model. CTX1 further was well tolerated in mice and did not show any overt evidence of toxicities. Overall, we identified a novel potent small molecule inhibitor, CTX1, which is capable of binding Hdmx, overcoming HdmX-mediated p53 suppression in a non-genotoxic manner and inducing cancer cell death particularly in combination with an Hdm2 inhibitor. CTX1 exhibits anti-cancer both in vitro and in vivo and therefore has potential to be developed into a novel targeted therapeutic.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by the National Institute of Health award R43CA139791 (to D.N. Wald and M.K. Agarwal).
Abbreviations
- AML
Acute Myeloid Leukemia
- NSG
Nod/Scid IL-2γR −/− mice
- SPR
surface plasmon resonance
- OCI
OCI-AML3
- 9-AA
9-aminoacridine
Footnotes
Conflicts of Interest: Mukesh Agarwal and David Wald submitted a provisional patent application on CTX1
References
- 1.Dumont P, Leu JI, Della Pietra AC, 3rd, George DL, Murphy M. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat Genet. 2003;33:357–65. doi: 10.1038/ng1093. [DOI] [PubMed] [Google Scholar]
- 2.Leu JI, Dumont P, Hafey M, Murphy ME, George DL. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol. 2004;6:443–50. doi: 10.1038/ncb1123. [DOI] [PubMed] [Google Scholar]
- 3.Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, et al. p53 has a direct apoptogenic role at the mitochondria. Molecular cell. 2003;11:577–90. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 4.Chipuk JE, Bouchier-Hayes L, Kuwana T, Newmeyer DD, Green DR. PUMA couples the nuclear and cytoplasmic proapoptotic function of p53. Science. 2005;309:1732–5. doi: 10.1126/science.1114297. [DOI] [PubMed] [Google Scholar]
- 5.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–31. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 6.Volkenandt M, Schlegel U, Nanus DM, Albino AP. Mutational analysis of the human p53 gene in malignant melanoma. Pigment Cell Res. 1991;4:35–40. doi: 10.1111/j.1600-0749.1991.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 7.Lubbe J, Reichel M, Burg G, Kleihues P. Absence of p53 gene mutations in cutaneous melanoma. The Journal of investigative dermatology. 1994;102:819–21. doi: 10.1111/1523-1747.ep12381544. [DOI] [PubMed] [Google Scholar]
- 8.Boyapati A, Kanbe E, Zhang DE. p53 alterations in myeloid leukemia. Acta Haematol. 2004;111:100–6. doi: 10.1159/000074489. [DOI] [PubMed] [Google Scholar]
- 9.Saha MN, Qiu L, Chang H. Targeting p53 by small molecules in hematological malignancies. Journal of hematology & oncology. 2013;6:23. doi: 10.1186/1756-8722-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson MW, Berberich SJ. Constitutive mdmx expression during cell growth, differentiation, and DNA damage. DNA Cell Biol. 1999;18:693–700. doi: 10.1089/104454999314971. [DOI] [PubMed] [Google Scholar]
- 11.Wu X, Bayle JH, Olson D, Levine AJ. The p53-mdm-2 autoregulatory feedback loop. Genes & development. 1993;7:1126–32. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Zhang R. p53-independent activities of MDM2 and their relevance to cancer therapy. Current cancer drug targets. 2005;5:9–20. doi: 10.2174/1568009053332618. [DOI] [PubMed] [Google Scholar]
- 13.de Lange J, Teunisse AF, Vries MV, Lodder K, Lam S, Luyten GP, et al. High levels of Hdmx promote cell growth in a subset of uveal melanomas. American journal of cancer research. 2012;2:492–507. [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q, Lozano G. Molecular pathways: targeting Mdm2 and Mdm4 in cancer therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19:34–41. doi: 10.1158/1078-0432.CCR-12-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wade M, Li YC, Wahl GM. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nature reviews Cancer. 2013;13:83–96. doi: 10.1038/nrc3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vassilev LT. Small-molecule antagonists of p53-MDM2 binding: research tools and potential therapeutics. Cell cycle. 2004;3:419–21. [PubMed] [Google Scholar]
- 17.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 18.Coll-Mulet L, Iglesias-Serret D, Santidrian AF, Cosialls AM, de Frias M, Castano E, et al. MDM2 antagonists activate p53 and synergize with genotoxic drugs in B-cell chronic lymphocytic leukemia cells. Blood. 2006;107:4109–14. doi: 10.1182/blood-2005-08-3273. [DOI] [PubMed] [Google Scholar]
- 19.Kojima K, Konopleva M, Samudio IJ, Shikami M, Cabreira-Hansen M, McQueen T, et al. MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood. 2005;106:3150–9. doi: 10.1182/blood-2005-02-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Secchiero P, Barbarotto E, Tiribelli M, Zerbinati C, di Iasio MG, Gonelli A, et al. Functional integrity of the p53-mediated apoptotic pathway induced by the nongenotoxic agent nutlin-3 in B-cell chronic lymphocytic leukemia (B-CLL) Blood. 2006;107:4122–9. doi: 10.1182/blood-2005-11-4465. [DOI] [PubMed] [Google Scholar]
- 21.Rana S, Gupta K, Gomez J, Matsuyama S, Chakrabarti A, Agarwal ML, et al. Securinine induces p73-dependent apoptosis preferentially in p53-deficient colon cancer cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2010;24:2126–34. doi: 10.1096/fj.09-148999. [DOI] [PubMed] [Google Scholar]
- 22.Issaeva N, Bozko P, Enge M, Protopopova M, Verhoef LG, Masucci M, et al. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nature medicine. 2004;10:1321–8. doi: 10.1038/nm1146. [DOI] [PubMed] [Google Scholar]
- 23.Mandinova A, Lee SW. The p53 pathway as a target in cancer therapeutics: obstacles and promise. Science translational medicine. 2011;3:64rv1. doi: 10.1126/scitranslmed.3001366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patton JT, Mayo LD, Singhi AD, Gudkov AV, Stark GR, Jackson MW. Levels of HdmX expression dictate the sensitivity of normal and transformed cells to Nutlin-3. Cancer research. 2006;66:3169–76. doi: 10.1158/0008-5472.CAN-05-3832. [DOI] [PubMed] [Google Scholar]
- 25.Noguchi T, Oishi S, Honda K, Kondoh Y, Saito T, Kubo T, et al. Affinity-based screening of MDM2/MDMX-p53 interaction inhibitors by chemical array: identification of novel peptidic inhibitors. Bioorganic & medicinal chemistry letters. 2013;23:3802–5. doi: 10.1016/j.bmcl.2013.04.094. [DOI] [PubMed] [Google Scholar]
- 26.Hu B, Gilkes DM, Farooqi B, Sebti SM, Chen J. MDMX overexpression prevents p53 activation by the MDM2 inhibitor Nutlin. The Journal of biological chemistry. 2006;281:33030–5. doi: 10.1074/jbc.C600147200. [DOI] [PubMed] [Google Scholar]
- 27.Gurova KV, Hill JE, Guo C, Prokvolit A, Burdelya LG, Samoylova E, et al. Small molecules that reactivate p53 in renal cell carcinoma reveal a NF-kappaB-dependent mechanism of p53 suppression in tumors. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:17448–53. doi: 10.1073/pnas.0508888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graves B, Thompson T, Xia M, Janson C, Lukacs C, Deo D, et al. Activation of the p53 pathway by small-molecule-induced MDM2 and MDMX dimerization. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:11788–93. doi: 10.1073/pnas.1203789109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoeferlin LA, Oleinik NV, Krupenko NI, Krupenko SA. Activation of p21-Dependent G1/G2 Arrest in the Absence of DNA Damage as an Antiapoptotic Response to Metabolic Stress. Genes Cancer. 2011;2:889–99. doi: 10.1177/1947601911432495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ringshausen I, O'Shea CC, Finch AJ, Swigart LB, Evan GI. Mdm2 is critically and continuously required to suppress lethal p53 activity in vivo. Cancer cell. 2006;10:501–14. doi: 10.1016/j.ccr.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Bista M, Smithson D, Pecak A, Salinas G, Pustelny K, Min J, et al. n the mechanism of action of SJ-172550 in inhibiting the interaction of MDM4 and p53. PloS one. 2012;7:e37518. doi: 10.1371/journal.pone.0037518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reed D, Shen Y, Shelat AA, Arnold LA, Ferreira AM, Zhu F, et al. Identification and characterization of the first small molecule inhibitor of MDMX. The Journal of biological chemistry. 2010;285:10786–96. doi: 10.1074/jbc.M109.056747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Popowicz GM, Czarna A, Wolf S, Wang K, Wang W, Domling A, et al. Structures of low molecular weight inhibitors bound to MDMX and MDM2 reveal new approaches for p53-MDMX/MDM2 antagonist drug discovery. Cell cycle. 2010;9:1104–11. doi: 10.4161/cc.9.6.10956. [DOI] [PubMed] [Google Scholar]
- 34.Bernal F, Wade M, Godes M, Davis TN, Whitehead DG, Kung AL, et al. A stapled p53 helix overcomes HDMX-mediated suppression of p53. Cancer cell. 2010;18:411–22. doi: 10.1016/j.ccr.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Ma X, Ren S, Buolamwini JK, Yan C. A small-molecule inhibitor of MDMX activates p53 and induces apoptosis. Molecular cancer therapeutics. 2011;10:69–79. doi: 10.1158/1535-7163.MCT-10-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herman AG, Hayano M, Poyurovsky MV, Shimada K, Skouta R, Prives C, et al. Discovery of Mdm2-MdmX E3 ligase inhibitors using a cell-based ubiquitination assay. Cancer discovery. 2011;1:312–25. doi: 10.1158/2159-8290.CD-11-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang W, Ho WC, Dicker DT, MacKinnon C, Winkler JD, Marmorstein R, et al. Acridine derivatives activate p53 and induce tumor cell death through Bax. Cancer Biol Ther. 2005;4:893–8. doi: 10.4161/cbt.4.8.2134. [DOI] [PubMed] [Google Scholar]
- 38.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–7. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.