Abstract
Social deficits are a hallmark feature of autism spectrum disorder (ASD) and related developmental syndromes. Although there is no standard treatment for social dysfunction, clinical studies have identified oxytocin as a potential therapeutic with prosocial efficacy. We have previously reported that peripheral oxytocin treatment can increase sociability and ameliorate repetitive stereotypy in adolescent mice from the C58/J model of ASD-like behavior. In the present study, we determined that prosocial oxytocin effects were not limited to the adolescent period, since C58/J mice, tested in adulthood, demonstrated significant social preference up to 2 weeks following subchronic oxytocin treatment. Oxytocin was also evaluated in adult mice with underexpression of the N-methyl-D-aspartate receptor NR1 subunit (encoded by Grin1), a genetic model of autism- and schizophrenia- like behavior. Subchronic oxytocin had striking prosocial efficacy in male Grin1 knockdown mice; in contrast, chronic regimens with clozapine (66 mg/kg/day) or risperidone (2 mg/kg/day) failed to reverse deficits in sociability. Neither the subchronic oxytocin regimen, nor chronic treatment with clozapine or risperidone, reversed impaired prepulse inhibition in the Grin1 knockdown mice. Overall, these studies demonstrate oxytocin can enhance sociability in mouse models with divergent genotypes and behavioral profiles, adding to the evidence that this neurohormone could have therapeutic prosocial efficacy across a spectrum of developmental disorders.
Keywords: autism spectrum disorders, clozapine, oxytocin, risperidone, schizophrenia, sociability
1. Introduction
Oxytocin is a neuropeptide hormone with a long-recognized role in maternal responses and mother-infant bonding. Clinical studies in subjects with autism spectrum disorder (ASD) have found that acute oxytocin can improve social function and decrease motor stereotypy and other forms of repetitive behavior (Andari et al., 2010; Guastella et al., 2010; Hollander et al., 2003, 2007). Further, Hall and colleagues (2012) observed that acute oxytocin could ameliorate indicators of social anxiety in male adolescents and adults with fragile X syndrome. One recent study using a 5-week regimen with intranasal oxytocin in young children (3 to 8 years in age) with ASD found improved social responsivity, although no concomitant reduction in abnormal repetitive behavior (Yatawara et al. 2015). These initial reports also suggest that oxytocin might not have the same potential for adverse events as found with more powerful psychoactive agents, such as risperidone or fluoxetine, used to treat co-morbid symptoms in ASD (Mahajan et al., 2012; West et al., 2009; Yatawara et al. 2015). However, not all clinical trials using intranasal application of oxytocin to ameliorate social deficits or other symptoms have proven successful (Anagnostou et al. 2012; Cacciotti-Saiji et al. 2015; Dadds et al. 2014), indicating the need for further investigation of oxytocin as a therapeutic agent.
Our research group has reported that peripheral administration of oxytocin can alleviate sociability deficits in two mouse models of autism-like behavior, the BALB/cByJ and C58/J inbred strains (Teng et al., 2013). Previous work has shown that BALB/cByJ and the related substrain, BALB/cJ, are characterized by a lack of social preference in a three-chambered choice task and by anxiety-like behavior in an elevated plus maze (Brodkin et al., 2004; Moy et al., 2007; Sankoorikal et al., 2006). Our previous study showed that, while acute oxytocin treatment did not reverse social deficits, a subchronic regimen of four injections, given across 8-9 days, led to significant sociability in adolescent BALB/cByJ mice, tested 24 hours following the final dose (Teng et al., 2013).
Our group has also investigated oxytocin effects in the C58/J inbred strain, which has low sociability in a three-chambered task, deficits in social transmission of food preference, and overt repetitive behavior (Moy et al., 2008b, 2014; Muehlmann et al., 2012; Ryan et al., 2010; Silverman et al., 2012). We found that a subchronic oxytocin regimen had prosocial effects in adolescent male and female C58/J mice, with increases in social preference emerging one or two weeks following treatment (Teng et al., 2013). Acute, but not subchronic, administration of oxytocin led to significant decreases in abnormal repetitive behavior.
In the present studies, we investigated whether oxytocin would exert prosocial effects in adult C58/J mice, similar to our findings in adolescents. Motivation for social affiliation and regulation of social interactions can differ between adolescents and adults (Spear 2011; see also Ernst et al., 2006). For example, Morales and Spear (2014) reported that, in a two-chamber test box, adolescent rats had higher levels of social interaction and a higher frequency of crossovers toward an unfamiliar social partner than adult rats. These data support a greater sensitivity to the rewarding aspects of novel social stimuli during adolescence, and raise the possibility that oxytocin might be most effective at this stage of development, while long-term social deficits in adults could be more recalcitrant to reversal.
These new studies also extended the evaluation of oxytocin to a third model of ASD-like behavior and synaptopathology, the Grin1 knockdown mouse. Although the mechanistic basis for ASD is not known, genetic analyses in human populations have implicated several genes important for synaptic function, including GRIN1, which encodes the obligatory NMDAR1 subunit of the N-methyl-D-aspartate (NMDA) receptor (Abrahams and Geschwind 2008; Voineagu et al., 2011; Zeidan-Chulia et al., 2014); however, not all studies have found an positive association between ASD and GRIN1 (e.g. Sanders et al. 2013; Tarabeux et al. 2011). There is growing evidence that alterations in NMDA receptor signaling play a role in ASD and other neurodevelopmental disorders (for recent reviews, see Burnashev et al. 2015; Lee et al. 2015), including reports that autism candidate genes, such as NEUROLIGIN-1 and SHANK3, serve as regulators of NMDA receptor function (Budreck et al. 2013; Duffney et al. 2013). Mice with reduced Grin1 expression recapitulate many ASD features, including overt social deficits, inappropriate social interaction, abnormal repetitive behavior, self-injurious responses, and impaired sensorimotor gating (Billingslea et al. 2014; Duncan et al., 2004, Finlay et al. 2015; Gandal et al., 2012, Milenkovic et al., 2014; Mohn et al., 1999, Moy et al., 2008a, 2012, 2014; Saunders et al. 2013). We determined the effects of oxytocin on social deficits, reduced prepulse inhibition, and hyperactivity in Grin1 knockdown mice. We also examined whether chronic regimens with atypical antipsychotics, initiated in early adolescence or young adulthood, have prosocial efficacy in the Grin1 knockdown model.
2. Methods and materials
2.1. Animals
C58/J mice were offspring of breeding pairs obtained from Jackson Laboratories (Bar Harbor, ME). Grin1neo/neo mice engineered with a neomycin resistance gene (neo) in intron 20 of the Grin1 locus and Grin1+/+ littermate controls were generated from heterozygous breeder pairs, as previously described (Mohn et al., 1999; Moy et al., 2012). Experimenters conducting the behavioral tests were blind to genotype.
Mice were maintained in groups of 2-4 animals per polycarbonate mouse cage, in a room under a 12-hour light/dark cycle (lights off at 7pm). ProLab RMH 3000 chow and water were provided ad libitum. All animal procedures were conducted in strict compliance with the animal welfare policies set by the National Institutes of Health and the University of North Carolina (UNC), and were approved by the UNC Institutional Animal Care and Use Committee.
2.2. Drug treatment regimens
2.2.1. Oxytocin
Oxytocin (Bachem, Torrance, CA) was dissolved in saline containing 0.002% glacial acetic acid. All injections were administered IP (intraperitoneal) in a volume of 10.0 ml/kg. For the subchronic regimen, mice were given four injections of vehicle or oxytocin (1.0 or 2.0 mg/kg) across 8-9 days, with at least 48 hr between each injection (i.e. mice were injected on sequential weekdays WFMW or WFTTh). Experimenters conducting the behavioral tests were blind to drug treatments.
2.2.2. Chronic clozapine and risperidone regimens in Grin1 mice
Chronic regimens with clozapine (30 days; 66 mg/kg/day) or risperidone (21 days; 2.0 mg/kg/day) were initiated with a preliminary ramping up of drug dose to minimize sedative or other side effects at the beginning of treatment. Doses were selected to reflect therapeutic dosage in humans, determined by clinical levels of dopamine D2 receptor occupancy (Kapur et al., 2003; Wadenberg et al., 2001).
Slow-release pellets were utilized for chronic clozapine administration because of difficulties in higher-dose drug solubility for osmotic minipumps (Kapur et al., 2003) and issues with variable plasma levels during administration in drinking water (Perez-Costas et al., 2008). At 11-14 weeks of age, mice were briefly anesthetized by isoflurane and implanted, using a trocar injector, with subcutaneous 30-day slow-release clozapine or sham tablets (Innovative Research of America, Sarasota, FL). The target dosage of 66 mg/kg/day was reached by incremental stages across 8 days, with 2-3 total pellet implants per subject. Following each implant, the trocar injection site was sealed using Tissuemend (Jeffers Inc., Dothan, AL).
For the initial acclimation to risperidone (Sigma-Aldrich, St. Louis, MO), adolescent mice (starting at age 33-38 days) received 3 IP injections of either saline vehicle containing 1% glacial acetic acid (adjusted to pH 5.5) or risperidone (0.3 mg/kg), with 2-3 days between each injection. One day following the third injection, mice were briefly anesthetized by isoflurane and implanted with a subcutaneous osmotic minipump (Model 1002; Alzet; Braintree Sci. Inc., Braintree, MA) containing either risperidone (2.0 mg/kg/day) or vehicle, for a 14-day delivery. At the end of the 14-day period, mice were again anesthetized, and the depleted 14-day pump was replaced by a new 7-day pump (Model 1007D) for the final phase of the 21-day regimen. This pump replacement allowed dosage to be adjusted for increased body weight during the chronic risperidone treatment.
2.3. C58/J inbred strain model
Oxytocin has persistent effects on social behavior in adolescent C58/J mice (Teng et al., 2013). In this study, we investigated whether social deficits in adult C58/J mice could also be reversed by oxytocin treatment. Subjects were male and female mice (7-8 of each sex per treatment group; 5-6 months of age at time of testing), treated using a subchronic regimen of vehicle or 1.0 mg/kg oxytocin. Mice were tested in the 3-chamber choice task at two time points, 24 hr and 2 wk post-treatment.
2.4. Grin1 knockdown model
2.4.1. Acute oxytocin effects on open field activity
Subjects were 7-9 male mice and 6-9 female mice of each genotype (Grin1+/+ and Grin1neo/neo) per treatment group, 8-11 months in age, taken from 12 litters. Each mouse was given 3 1-hr tests, one with vehicle pretreatment and one with each dose of oxytocin (0.5 and 1.0 mg/kg), with 1 week between each test. A balanced treatment design was used, so that order of treatments was balanced for genotype and sex across the 3 tests. Mice were placed into the activity chambers immediately after each treatment.
2.4.2. Acute oxytocin effects on sensorimotor gating
Subjects were 12-13 male mice and 10-13 female mice of each genotype (Grin1+/+ and Grin1neo/neo), 5-7 months of age, taken from 23 litters. The acoustic startle test was conducted 50 min following treatment with vehicle or oxytocin (1.0 mg/kg). Each mouse was given 2 sessions, one with vehicle pretreatment and one with oxytocin pretreatment, with 1 week between each session. A balanced treatment design was used, so that order of treatment was balanced by genotype and sex across the 2 tests.
2.4.3. Subchronic oxytocin regimen
Subjects were 8-9 male mice and 8-10 female mice of each genotype (Grin1+/+ and Grin1neo/neo) per treatment group (vehicle or 1.0 mg/kg oxytocin), tested at 3-5 months of age, taken from 29 litters. Each subject was tested in the 3-chamber choice task approximately 24 hr following the final treatment. Mice were further tested in an acoustic startle assay 48 hr post-treatment, and the marble-burying assay 4-5 days post-treatment. Because no genotype or treatment effects were observed in the female groups, an additional set of female Grin1 mice (6-8 of each genotype, 7 months in age, taken from 5 litters) were given subchronic treatment with a higher dose of oxytocin (2.0 mg/kg) and tested in the 3-chamber choice task.
2.4.4. Chronic clozapine
Subjects were 4-8 male mice and 4-6 female mice of each genotype per treatment group, 3-4 months of age at time of behavioral testing, taken from 23 litters. Mice were tested in the 3-chamber choice task 32-35 days following the final subcutaneous implant of a 30-day slow-release pellet. Mice were also tested in an acoustic startle assay at two time points, 15-16 days and 35-38 days following the final pellet implantation.
2.4.5. Chronic risperidone
Subjects were 6-9 male mice and 5-8 female mice of each genotype per treatment group, 60-70 days of age at time of behavioral testing, taken from 23 litters. Mice were tested in the 3-chamber choice task 23-26 days following the start of a 21-day chronic regimen (administered by osmotic minipump). Mice were also tested in an acoustic startle assay 24-27 days and a marble-burying task 28-29 days after initiation of the chronic regimen.
2.5. Behavioral testing procedures
2.5.1. Three-chamber social choice test
Social approach was assessed in a 3-chamber Plexiglas box (procedure modified from Moy et al., 2007). The test started with a 10-min habituation phase, with free exploration of the empty test box, followed by a 10-min test for sociability. During the sociability phase, the test mouse was given a choice between an unfamiliar stranger mouse (a sex-matched C57BL/6J adult), contained in a Plexiglas cage placed in one side chamber, or an empty Plexiglas cage in the opposite side chamber. Cages were drilled with holes to allow investigation of the stranger. Measures were taken of the time spent in each chamber, time spent in 5 cm proximity to each cage, and number of entries into each chamber, by an automated image tracking system (Ethovision, Noldus Information Technology, Wageningen, the Netherlands).
2.5.2. Open field test
Activity was assessed in a photocell-equipped automated open field (41 cm × 41 cm × 30 cm; Versamax System, AccuScan Instruments, Columbus, OH). Measures were taken of total distance traveled, rearing movements, and time spent in the center region of the chamber, for each 1-hr test.
2.5.3. Acoustic startle test
Mice were evaluated for acoustic startle responses with an SR-Lab system (San Diego Instruments). Each test session consisted of a 5-min habituation period, followed by 42 trials: no-stimulus trials, trials with the acoustic startle stimulus (40 ms; 120 dB) alone, and trials in which a prepulse stimulus (20 ms; either 74, 78, 82, 86, or 90 dB) had onset 100 ms before the onset of the startle stimulus. The different trial types were presented in blocks of 7, in randomized order within each block, with an average intertrial interval of 15 sec. Measures were taken of startle amplitude, defined as the peak response during a 65-msec sampling window following onset of the startle stimulus. PPI was calculated as 100 - [(response amplitude for prepulse stimulus and startle stimulus together / response amplitude for startle stimulus alone) × 100].
2.5.4. Marble-burying assay
Each subject was tested in a polycarbonate mouse cage located in a sound-attenuating chamber with ceiling light and fan. The cage contained 5 cm deep clean corncob bedding, with 20 black glass marbles (14 mm diameter) arranged in an equidistant 5 X 4 array on top of the bedding. Measures were taken of the number of marbles covered 2/3 or more by the bedding after a 30 min test.
2.6. Statistical analysis
Data were analyzed with one-way, two-way, or repeated measures analysis of variance (ANOVA), with factors treatment, sex, and genotype (dependent on experiment), using Statview software (SAS, Cary, NC). Repeated measures included side of social test box, test session, or prepulse sound level. Separate repeated measures ANOVAs were conducted for each sex to determine oxytocin effects on sociability in male and female mice. Within-treatment repeated measures ANOVAs were used to determine side preference in the social choice test. Fisher's protected least-significant difference (PLSD) tests were used for comparing group means only when a significant F value was determined by ANOVA. Significance was set at p<0.05.
3. Results
3.1. Prosocial oxytocin effects in adult C58/J mice
3.1.1. Sociability in male and female C58/J mice
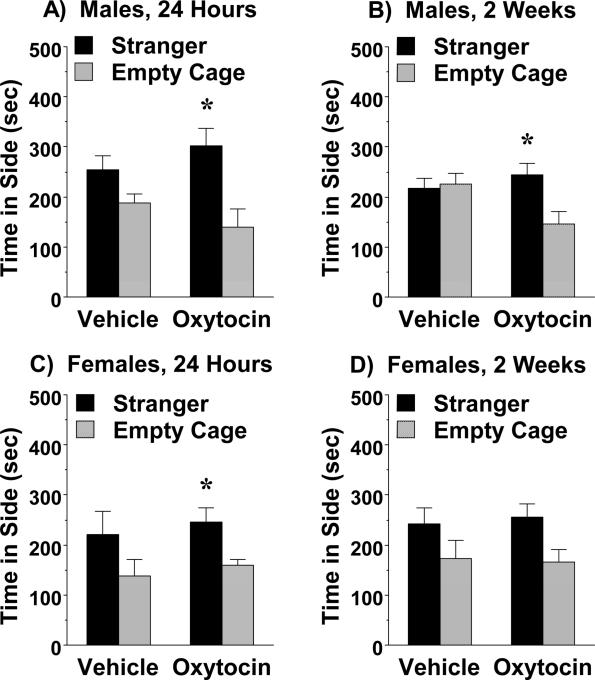
Previous work in our laboratory has shown that subchronic oxytocin has persistent prosocial effects in adolescent C58/J mice (Teng et al., 2013). In the present study, both male and female adult C58/J mice (ages 5-6 months) treated with oxytocin, but not vehicle, had significant preference for spending time in the stranger side of a 3-chamber box (Fig. 1). In the male mice, the prosocial oxytocin effects were evident at 24 hr and 2 wk post-treatment [within-treatment group post-hoc analyses following significant effect of side, F(1,14)=9.38, p=0.0084; determined by a 3-way repeated measures ANOVA, with factors treatment, side, and time point for testing] (Fig. 1A, B). In the male groups, there was a non-significant trend for a treatment x side interaction [F(1,14)=3.8, p=0.0717]. Female mice treated with oxytocin demonstrated significant preference for the stranger side only at the 24-hour time point [within-treatment group post-hoc analyses following significant effect of side, F(1,13)=11.40, p=0.005] (Fig. 1C).
Figure 1. Persistent prosocial effects of subchronic oxytocin in adult C58/J mice.
Subjects were tested for sociability in a 3-chamber choice task. The subchronic regimen consisted of 4 treatments with either vehicle or oxytocin (1.0 mg/kg, IP) across an 8-9 day period. Mice were tested at 24 hr, and again at 2 wk, following the final treatment. *p<0.05, within-group comparison to empty cage side.
3.1.2. Preference for social proximity in male and female C58/J mice
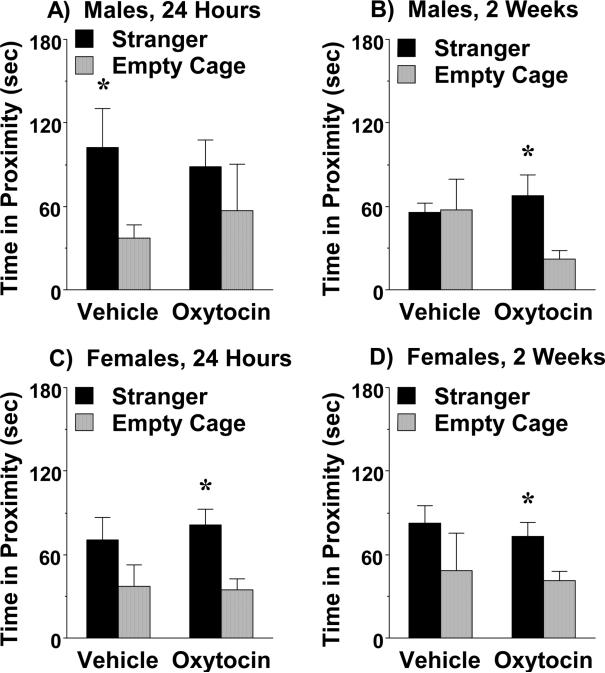
We have previously reported that adolescent C58/J male mice have positive sociability with the measure of sniffing directed towards a stranger mouse (Moy et al., 2008). The present study used a similar measure, proximity to each cage. In the first test, given 24 hr following the subchronic regimen, the C58/J male mice treated with vehicle, but not oxytocin, spent significantly more time in proximity to the stranger mouse than the empty cage (Fig. 2A). However, during second test, only the oxytocin-treated group demonstrated significant social preference [within-treatment group post-hoc analyses following significant effect of side, F(1,14)=6.66, p=0.0218] (Fig. 2B). In the female groups, mice treated with oxytocin demonstrated significant preference for the stranger side at both the 24-hr and 2-wk time points [within-treatment group post-hoc analyses following significant effect of side, F(1,13)=34.29, p<0.0001] (Fig. 2C, D). In contrast, vehicle-treated female mice failed to demonstrate positive sociability with the proximity measure in either test.
Figure 2. Effects of subchronic oxytocin on time spent in proximity to a stranger mouse.
Testing occurred at 24 hr, and again at 2 wk, following the final treatment. During the second test, only the oxytocin treatment groups had significant social preference. *p<0.05, within-group comparison to empty cage side.
3.1.3. Oxytocin effects on entries in the 3-chamber test
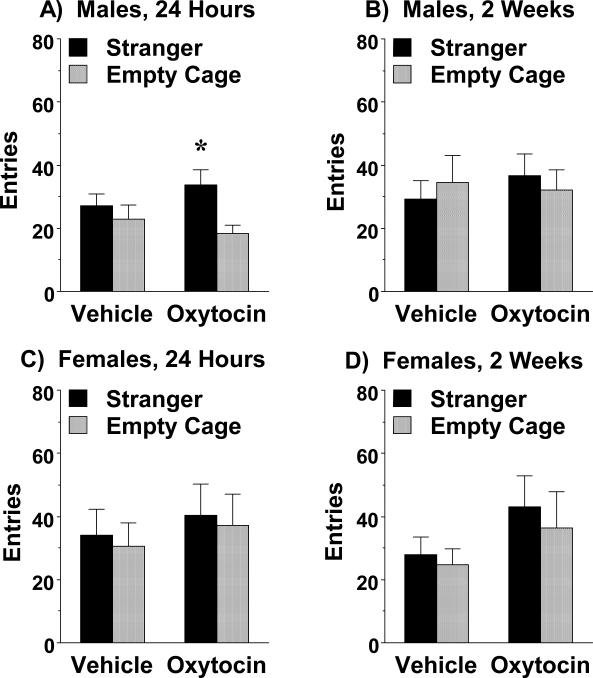
Subchronic oxytocin did not alter number of entries during the test in the C58/J groups, indicating prosocial effects were not due to general changes in activity or exploration (Fig. 3). Number of entries is not typically used as an index for social approach; however, it is notable that the male C58/J mice treated with oxytocin, but not vehicle, showed significantly more entries into the side containing the stranger mouse during the first social test [effect of side, F(1,14)=8.89, p=0.0099] (Fig. 3A).
Figure 3. Effects of subchronic oxytocin on side-chamber entries by C58/J mice.
Overall number of entries was not changed by oxytocin in either male or female mice. Testing occurred at 24 hr, and again at 2 wk, following the final treatment. *p<0.05, within-group comparison to empty cage side.
3.2. Acute oxytocin effects in Grin1+/+ and Grin1neo/neo mice
3.2.1. Acute oxytocin effects on hyperactivity in an open field
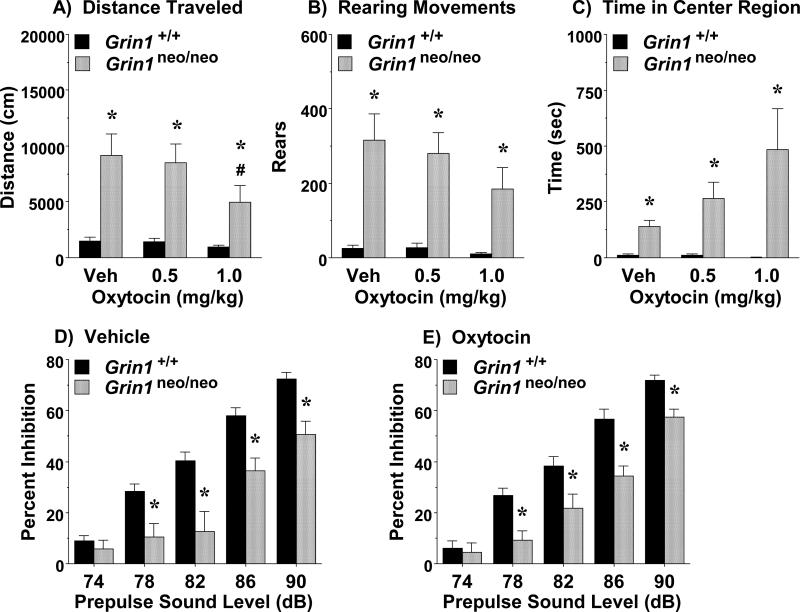
This study determined whether acute oxytocin could reduce the overt hyperactivity previously reported in Grin1 knockdown mice (Duncan et al., 2004; Mohn et al., 1998; Moy et al., 2014). Three-way repeated measures ANOVAs did not reveal significant effects of sex on performance in the open field; therefore, data for males and females were combined for analysis. In line with previous findings, the Grin1neo/neo mice had higher levels of activity than wild-type mice (Fig. 4A, B). Highly significant main effects of genotype were found for each measure [distance traveled, F(1,29)=14.04, p=0.0008; rearing movements, F(1,29)=21.07, p<0.0001; and center time, F(1,29)=13.16, p=0.0011 (Fig. 4C)]. In addition, a significant effect of treatment was revealed for distance traveled [F(2,58)=3.26, p=0.0456]. Post-hoc comparisons confirmed that the higher dose of oxytocin (1.0 mg/kg) led to a significant decrease in locomotor activity in the Grin1neo/neo mice. No effects of oxytocin on activity were observed in the wild-type group.
Figure 4. Effects of acute oxytocin on hyperactivity and sensorimotor gating deficits in Grin1neo/neo mice.
A-C) Vehicle (Veh) or oxytocin (0.5 or 1.0 mg/kg, IP) was administered immediately before a 1-hr open field test. D, E) Vehicle or oxytocin (1.0 mg/kg, IP) was administered 50 min before an acoustic startle test. *p<0.05, comparison to Grin1+/+ mice. #p<0.05, within-genotype comparison to vehicle (Panel A).
3.2.2. Acute oxytocin effects on sensorimotor gating
Previous studies have shown that oxytocin and oxytocin receptor agonists can rescue sensorimotor gating deficits in rodents (Feifel et al., 2012; Ring et al., 2010). In this study, we determined if acute administration of oxytocin could reverse impaired prepulse inhibition in the Grin1neo/neo mice. A 3-way repeated measures ANOVA did not indicate any significant effects of sex; therefore, data from males and females were combined. As shown in Fig. 4D and E, the Grin1neo/neo mice demonstrated deficits in prepulse inhibition at almost every decibel level, which were not reversed by acute treatment with oxytocin [main effect of genotype, F(1,46)=18.48, p<0.0001; genotype x decibel interaction, F(4,184)=7.59, p<0.0001; no significant effects of treatment].
3.3. Prosocial effects of subchronic oxytocin in Grin1neo/neo mice
3.3.1. Oxytocin effects on sociability in male Grin1 mice
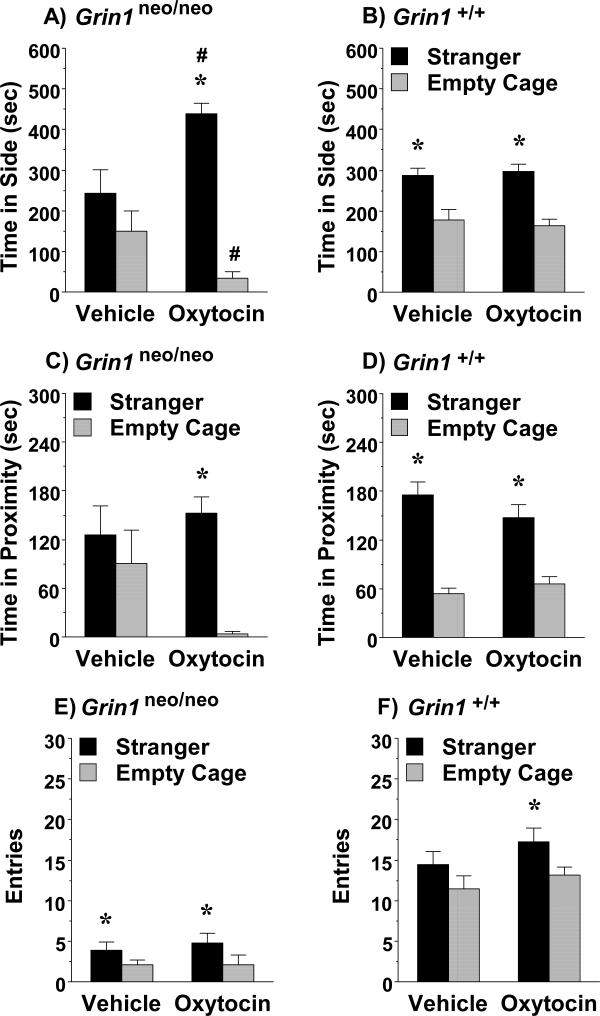
Subchronic oxytocin (1.0 mg/kg) led to a striking increase in sociability in male Grin1neo/neo mice, but not wild-type controls (Fig. 5). As shown in Fig. 5A, only the Grin1 knockdown mice treated with oxytocin had significant social preference 24 hr following the final injection. In contrast, male Grin1+/+ mice had similar positive sociability after either the vehicle or oxytocin regimen (Fig. 5B). A repeated measures ANOVA on time spent in each chamber indicated significant 2-way interactions between genotype and side [F(1,29)=5.92, p=0.0214], treatment and side [F(1,29)=10.04, p=0.0036], and a 3-way interaction between genotype, treatment, and side [F(1,29)=7.46, p=0.0106]. A similar 3-way interaction for genotype, treatment, and side emerged for the measure of proximity to each cage (Fig. 5C and D) [F(1,24)=4.99, p=0.0351; data missing for 5 mice tested before cage-zone tracking available].
Figure 5. Prosocial effects of subchronic oxytocin in male Grin1neo/neo mice.
Subjects were tested for sociability in a 3-chamber choice task. The subchronic regimen consisted of 4 treatments with either vehicle or oxytocin (1.0 mg/kg, IP) across an 8-9 day period. Grin1 mice were tested 24 hr following the final treatment. *p<0.05, within-group comparison to empty cage side. #p<0.05, comparison to vehicle-treated group (Panel A).
3.3.2. Lack of oxytocin effects on entries in male Grin1 mice
Subchronic oxytocin did not alter number of entries during the test in the Grin1 groups, indicating prosocial effects were not due to general changes in activity or exploration (Fig. 5). In the Grin1neo/neo mice, oxytocin did not rescue deficits in entry numbers (Fig. 5E) [main effect of genotype, F(1,29)=97.02, p<0.0001; and side, F(1,29)=21.46, p<0.0001]. In the Grin1+/+ mice, only the oxytocin-treated group showed more entries into the stranger side, versus the empty cage side (Fig. 5F) [within-treatment group post-hoc analyses following significant effect of side, F(1,14)=9.91, p=0.0071].
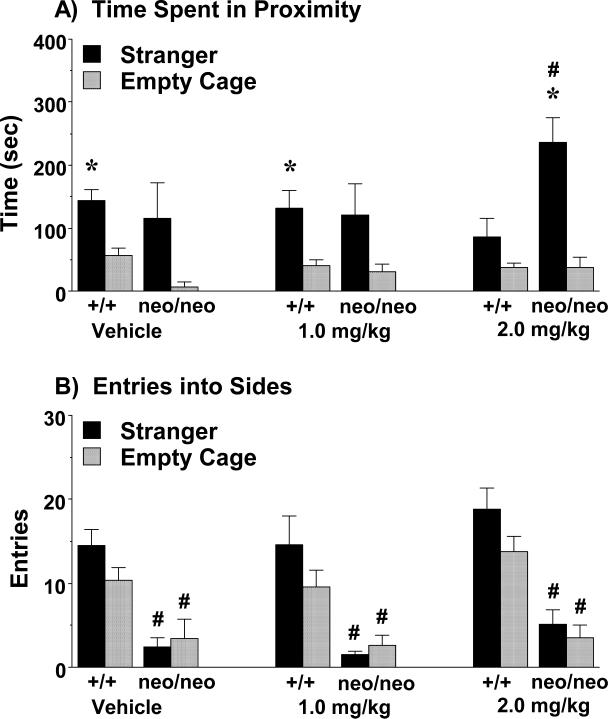
3.3.3. Oxytocin (1.0 and 2.0 mg/kg) effects on sociability in female Grin1 mice
In contrast to the results from the male Grin1 mice, there were no significant effects of genotype or treatment (1.0 mg/kg oxytocin) for measures of sociability in the female groups. Therefore, an additional set of female mice was tested with a higher dose of oxytocin, 2.0 mg/kg, using a subchronic regimen. Measures of proximity are presented in Figure 6A, since wild-type female mice (from either treatment group) did not have significant sociability by the measure of time spent in each side of the test box. As shown in Figure 6A, the Grin1+/+ and Grin1neo/neo mice treated with vehicle or with 1.0 mg/kg oxytocin spent similar amounts of time in proximity to the stranger mouse, although preference was only significant in the wild-type females [within-treatment group post-hoc analyses following significant effect of side, F(1,27)=23.11, p<0.0001; no effect of genotype or treatment; data missing for 4 mice tested before cage-zone tracking available]. In contrast, following treatment with the higher dose, the female Grin1neo/neo mice demonstrated overt social preference and spent significantly more time than the wild-type mice in proximity to the stranger mouse [main effect of genotype, F(1,12)=9.51, p=0.0095; effect of side, F(1,12)=17.49, p=0.0013, and genotype x side interaction, F(1,12)=6.42, p=0.0262].
Figure 6. Increased sociability in female Grin1neo/neo mice following subchronic treatment with 2.0 mg/kg oxytocin.
Subjects were tested for social preference in a 3-chamber choice task. The subchronic regimen consisted of 4 treatments with either vehicle, 1.0 mg/kg, or 2.0 mg/kg oxytocin (IP) across an 8-9 day period, with testing 24 hr following the final treatment. *p<0.05, within-group comparison to empty cage side. #p<0.05, comparison to Grin1 wild-type (+/+).
3.3.4. Lack of oxytocin effects on entries in female Grin1 mice
As observed in the male Grin1 groups, subchronic oxytocin did not alter number of entries during the test in the female mice, or reverse marked deficits in the Grin1neo/neo groups (Fig. 6B). Highly significant main effects of genotype were observed in the mice treated with 1.0 mg/kg oxytocin [F(1,31)=34.16, p<0.0001] and 2.0 mg/kg oxytocin [F(1,12)=25.7, p=0.0003].
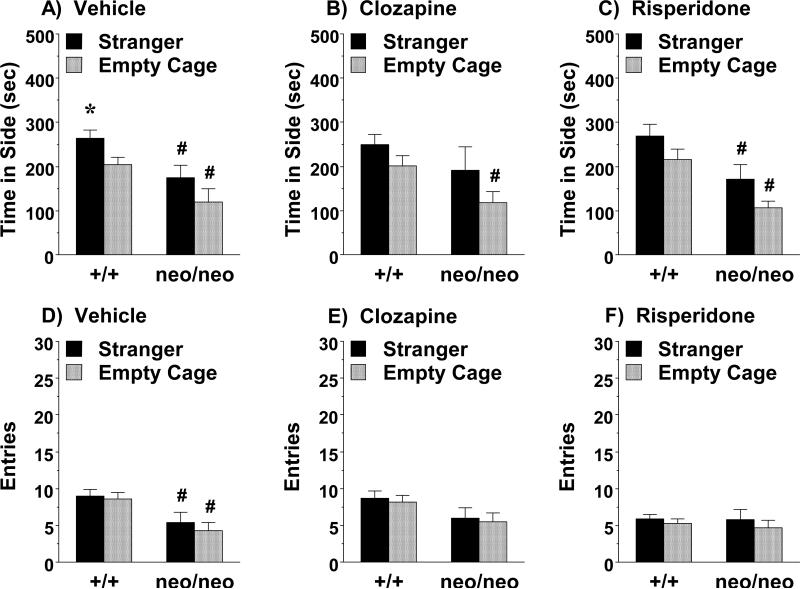
3.3.5. Lack of prosocial effects of chronic clozapine or risperidone in Grin1neo/neo mice
In a search for therapeutic agents with prosocial efficacy, our research group evaluated chronic regimens with two atypical antipsychotics, clozapine and risperidone. Overall, neither chronic regimen reversed social deficits in the Grin1neo/neo mice (Fig. 7). Separate repeated measures ANOVAs for clozapine and risperidone did not indicate any significant effects of treatment or sex; therefore, data were combined for vehicle groups, and for males and females. An overall repeated measures ANOVA for time spent in each side revealed highly significant effects of genotype [F(1,93)=32.88, p<0.0001] and side [F(1,93)=13.20, p=0.0005], but not treatment. Interestingly, neither the wild-type nor Grin1neo/neo groups demonstrated significant social preference following the chronic antipsychotic regimens (Fig. 7B and C). Further, the chronic clozapine and risperidone treatments failed to alleviate the low numbers of entries observed in the Grin1neo/neo groups [main effect of genotype, F(1,93)=6.55, p=0.0121; and side, F(1,93)=6.42, p=0.0129; but not treatment] (Fig. 7E and F).
Figure 7. Lack of significant prosocial effects of chronic clozapine or chronic risperidone in Grin1neo/neo mice.
Testing occurred following chronic treatment with either clozapine (acclimated 30-day regimen; 66 mg/kg/day) or risperidone (acclimated 21-day regimen; 2.0 mg/kg/day). *p<0.05, within-genotype comparison to empty cage side (Panel A). #p<0.05, comparison to wild-type (+/+) group.
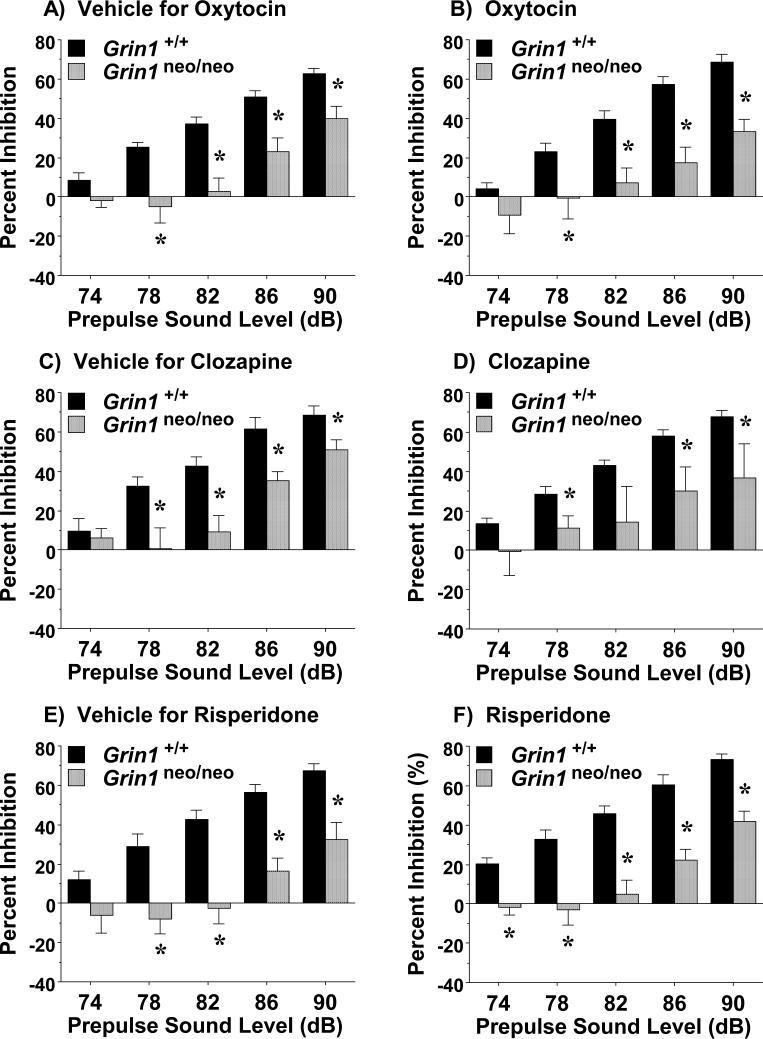
3.4. Failure to reverse sensorimotor gating deficits in Grin1neo/neo mice
On the day following the social approach test (48 hr following the final injection with oxytocin or vehicle), the Grin1 mice were further evaluated in an acoustic startle test. An overall repeated measures ANOVA for prepulse inhibition did not indicate any significant effects for sex; therefore, data for male and female mice were combined. As shown in Fig. 8A and B, the Grin1neo/neo mice had overt deficits in prepulse inhibition, which were not rescued by the subchronic oxytocin regimen [main effect of genotype, F(1,64)=31.64, p<0.0001; genotype x sound level interaction, F(4,246)= 5.87, p=0.0002; no effects of treatment].
Figure 8. No effects of subchronic oxytocin, or chronic clozapine or risperidone, on impaired sensorimotor gating in Grin1neo/neo mice.
A and B) Grin1 wild-type (+/+) and knockdown (neo/neo) mice were tested for prepulse inhibition of acoustic startle responses 48 hr after the final treatment in the subchronic regimen. C and D) Grin1 mice were tested on day 15-16 of the chronic clozapine regimen (66 mg/kg/day). Data were omitted from one male Grin1neo/neo mouse in the clozapine-treated group with extremely low startle amplitudes. E and F) Grin1 mice were tested 3-6 days following the chronic risperidone (21 day; 2.0 mg/kg/day) regimen. *p<0.05.
Similarly, chronic treatment with the antipsychotic drugs did not reverse sensorimotor gating deficits in the Grin1neo/neo mice. Repeated measures ANOVAs did not indicate any significant effects for sex; therefore, data for male and female mice were combined. As presented in Fig. 8C and D, the Grin1neo/neo mice in both the vehicle and clozapine groups had reduced prepulse inhibition, in comparison to wild-type [main effect of genotype, F(1,39)=14.56, p=0.0005; genotype x sound level interaction, F(4,156)=3.06, p=0.0184; no effects of treatment]. In the clozapine study, mice were given a re-test on days 36-38 of the acclimated chronic regimen; no significant effects of clozapine were observed at this additional time point (data not shown). A similar pattern was observed following chronic risperidone, i.e. Grin1neo/neo mice in both treatment groups had comparable deficits in prepulse inhibition (Fig. 8E, F) [main effect of genotype, F(1,52)=52.18, p<0.0001; genotype x sound level interaction, F(4,208)=5.63, p=0.0003; no effects of treatment].
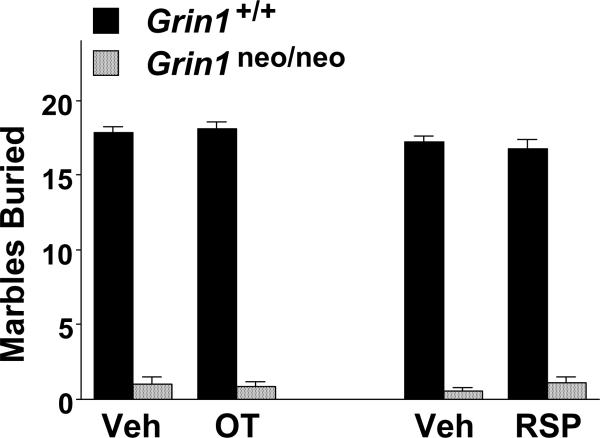
3.5. Failure to reverse deficits in marble-burying by Grin1neo/neo mice
We have previously reported that Grin1 knockdown mice have overt deficits in a marble-burying task (Moy et al. 2014). In the present study, Grin1 mice were tested for marble-burying 4-5 days after the final treatment in a subchronic oxytocin regimen. As shown in Fig. 9, oxytocin did not rescue deficits in the Grin1neo/neo mice. A 3-way ANOVA indicated a highly significant effect of genotype [F(1,50)=1755.56, p<0.0001], with no effects of treatment or sex. Similarly, chronic risperidone did not have significant effects in a marble-burying test, conducted 28-29 days after the first pump implant. Both the vehicle- and risperidone- treated Grin1neo/neo mice had profound deficits in marble-burying, in comparison to the wild-type mice [main effect of genotype, F(1,52)=1371.76, p<0.0001].
Figure 9. No effects of subchronic oxytocin or chronic risperidone treatment on marble-burying deficits in Grin1neo/neo mice.
The marble-burying assay was conducted 4-5 days following the end of the subchronic regimen of oxytocin or vehicle, or 7-8 days following a chronic risperidone (21 day; 2.0 mg/kg/day) regimen. Post-hoc tests were not conducted, due to number of zero scores in the Grin1neo/neo groups.
4. Discussion
The present studies demonstrated that oxytocin has prosocial effects in C58/J and Grin1 knockdown mice, two genetically-divergent mouse models of neurodevelopmental disorders. In C58/J, significant sociability was found up to 2 weeks following a subchronic oxytocin regimen, supporting the premise that repeated treatment with oxytocin can induce persistent alterations in neural circuitry underlying aspects of social perception, motivation, or reward. In the Grin1 knockdown model, subchronic oxytocin led to a striking increase in sociability in the Grin1neo/neo mice, without altering social approach in the wild-type group. In both models, the enhanced social approach was observed in adult mice, indicating that chronic social deficits maintained beyond adolescence are not recalcitrant to reversal. Together with our previous findings in BALB/cByJ (Teng et al., 2013), these results provide evidence that prosocial effects of oxytocin can be observed in genetically- and phenotypically- diverse mouse models of autism-relevant behaviors, suggesting oxytocin could have generalized efficacy across subtypes of the autism spectrum disorders.
In mice, the IP route of injection has been shown to induce a rapid increase in oxytocin levels of amygdala and hippocampus, measured by sequential microdialysates across a 2 hour period (Neumann et al. 2013). Our studies used a subchronic regimen with four IP injections of oxytocin. Sobota and colleagues (2015) found that a similar subchronic regimen with oxytocin increases social approach, reduces anxiety-like behavior, and decreases amygdalar activation in adolescent C57BL/6J mice. Other researchers have reported that, in adolescent rats, more extended regimens (10 IP injections of oxytocin) can lead to persistent increases in social preference and decreases in anxiety-like behavior (Bowen et al., 2011; Suraev et al., 2014). More recently, a study using mice with targeted disruption of Cntnap2, an ASD candidate gene, found increased sociability following a daily oxytocin regimen (from postnatal day 7 to 21) in adolescent knockout mice (Penagarikano et al., 2015). The investigators also showed that acute treatment with oxytocin had significant effects on social approach, but not on hyperactivity or perseverative responses, in the Cntnap2 model. These results are in contrast to the present findings, in which acute oxytocin significantly attenuated overt hyperactivity in Grin1 knockdown mice. It is notable that, in the C58/J model, we have previously reported that acute oxytocin significantly decreases abnormal repetitive behavior at a dose that does not reduce general locomotion (Teng et al., 2013).
Not all studies have reported positive effects from chronic oxytocin regimens in rodents. Bales et al. (2014) did not observe enhanced sociability in BTBR T+Itpr3tf/J mice following daily treatment with oxytocin across 30 days. In monogamous prairie voles, a 21-day regimen, from weaning age to puberty, led to decreased time spent by male voles in side-to-side contact with a familiar female partner (Bales et al., 2013). Huang and colleagues (2014) found that 7-to-21 day regimens of intranasal oxytocin in C57BL/6J led to decreased affiliative behavior, as well as decreased oxytocin receptor binding in brain regions implicated in social behavior and reward, including amygdala and nucleus accumbens. A similar reduction in oxytocin receptor binding has been reported following 15-day central infusion of oxytocin in C57BL/6 (Peters et al., 2014). These latter studies suggest that hyperstimulation of normal social circuitry with oxytocin might have detrimental consequences in wild-type or control mice. Although Sobota et al. (2015) were able to demonstrate prosocial effects of subchronic oxytocin in adolescent C57BL/6J mice, the oxytocin treatment did not reverse social deficits induced by the NMDA antagonist ketamine, in contrast to our present findings with the NMDA receptor knockdown mice.
The Grin1neo/neo mouse was originally proposed as a model of schizophrenia (Mohn et al., 1999). Recent clinical studies in subjects with schizophrenia have suggested that oxytocin could have therapeutic efficacy for deficits in emotion recognition, attribution bias, and other aspects of social cognition, following acute (Davis et al., 2013; Fischer-Shofty et al., 2013; Woolley et al., 2014) or chronic (Pedersen et al., 2011) treatment. In addition, 2-to-8 week regimens of intranasal oxytocin have been reported to alleviate more general positive and negative symptoms in schizophrenia (Feifel et al. 2010; see Pedersen 2014 for review), suggesting that oxytocin might have broader antipsychotic-like activity. Work in rodent models has demonstrated antipsychotic-like effects of acute oxytocin or oxytocin agonists on sensorimotor gating deficits (Feifel et al., 2012; Ring et al., 2010), although Huang et al. (2014) did not observe changes in prepulse inhibition following chronic oxytocin in C57BL/6J. In the present studies, neither acute nor subchronic oxytocin reversed impaired prepulse inhibition in Grin1neo/neo mice, providing evidence that antipsychotic-like oxytocin action is dependent upon the particular animal model.
We also evaluated the effects of chronic antipsychotic treatment on social deficits in the Grin1 knockdown model. Currently, only two drugs have FDA approval for treatment of ASD, risperidone and aripiprazole. While these drugs have been found to have some benefits against irritability in ASD, there is still no standard treatment for impaired social behavior (Chadman 2014). Similarly, atypical antipsychotics generally have modest-to-poor efficacy against social deficits in schizophrenia (Penn et al., 2009; Roberts et al., 2010). Studies in the BTBR T+Itpr3tf/J and Cntnap2−/− mouse models of ASD-like behavior have shown that acute risperidone does not reverse sociability deficits (Chadman 2011; Gould et al., 2011; Penagarikano et al., 2011; Silverman et al., 2010). Further, Mielnik et al. (2014) did not observe increased sociability following acute clozapine in mice with deficient NMDA receptor function. However, the question remained whether chronic antipsychotic intervention, initiated in early adolescence or young adulthood, could alleviate the severity of social impairment. In the present study, we found that chronic clozapine or risperidone failed to rescue social deficits in Grin1neo/neo mice. Further, although acute treatment with these agents can ameliorate impaired sensorimotor gating in the Grin1 model (Duncan et al., 2006a,b), chronic exposure did not alter performance in the acoustic startle task in either wild-type or knockdown mice.
Overall, these results point to a unique and selective prosocial efficacy for oxytocin. Together with our previous findings in BALB/cByJ, we have demonstrated that a subchronic oxytocin regimen can lead to persistent enhancement of sociability across three models with divergent genotypes and behavioral profiles, suggesting the possibility of generalized therapeutic benefits across the autism spectrum disorders, as well as other neurodevelopmental disorders characterized by social impairment. Further studies with this panel of models could identify common abnormalities in signaling pathways underlying deficits in social approach, as well as the mechanism of action for adaptive changes in brain with subchronic oxytocin intervention.
highlights.
- Persistent prosocial oxytocin effects were observed in adult C58/J mice.
- Oxytocin increased sociability in C58/J up to 2 weeks following a subchronic regimen.
- Subchronic oxytocin reversed overt social deficits in Grin1 knockdown mice.
Acknowledgments
We sincerely thank Rebecca Dye and Tamara T. Davis for their valuable contributions to this research. Support for this project was provided by the Department of Defense (AR1002312P1, AR1002312P2, and AR100231P3), the National Institute for Mental Health (RO1 MH080069 and K01 MH094406), and the National Institute of Child Health & Human Development (P30 HD03110; U54 HD079124). Dr. Teng was supported by an Autism Speaks Translational Postdoctoral Fellowship (#7952). These funding sources were not involved in the study design, the collection, analysis or interpretation of data, writing of the report, or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9(5):341–55. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostou E, Soorya L, Chaplin W, Bartz J, Halpern D, Wasserman S, Wang AT, Pepa L, Tanel N, Kushki A. Intranasal oxytocin versus placebo in the treatment of adults with autism spectrum disorders: a randomized controlled trial. Mol Autism. 2012;3:16. doi: 10.1186/2040-2392-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci USA. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM, Conley OG, Lee MH, Guoynes CD, Downing GM, Yun CR, Solomon M, Jacob S, Mendoza SP. Chronic intranasal oxytocin causes long-term impairments in partner preference formation in male prairie voles. Biol Psychiatry. 2013;74(3):180–8. doi: 10.1016/j.biopsych.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, Solomon M, Jacob S, Crawley JN, Silverman JL, Larke RH, Sahagun E, Puhger KR, Pride MC, Mendoza SP. Long-term exposure to intranasal oxytocin in a mouse autism model. Transl Psychiatry. 2014;4:e480. doi: 10.1038/tp.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingslea EN, Tatard-Leitman VM, Anguiano J, Jutzeler CR, Suh J, Saunders JA, Morita S, Featherstone RE, Ortinski PI, Gandal MJ, Lin R, Liang Y, Gur RE, Carlson GC, Hahn CG, Siegel SJ. Parvalbumin cell ablation of NMDA-R1 causes increased resting network excitability with associated social and self-care deficits. Neuropsychopharmacology. 2014;39(7):1603–13. doi: 10.1038/npp.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen MT, Carson DS, Spiro A, Arnold JC, McGregor IS. Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats. PloS One. 2011;6(11):e27237. doi: 10.1371/journal.pone.0027237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodkin ES, Hagemann A, Nemetski SM, Silver LM. Social approach–avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Res. 2004;1002:151–157. doi: 10.1016/j.brainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Budreck EC, Kwon OB, Jung JH, Baudouin S, Thommen A, Kim HS, Fukazawa Y, Harada H, Tabuchi K, Shigemoto R, Scheiffele P, Kim JH. Neuroligin-1 controls synaptic abundance of NMDA-type glutamate receptors through extracellular coupling. Proc Natl Acad Sci USA. 2013;110(2):725–30. doi: 10.1073/pnas.1214718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev N, Szepetowski P. NMDA receptor subunit mutations in neurodevelopmental disorders. Curr Opin Pharmacol. 2015;20:73–82. doi: 10.1016/j.coph.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Cacciotti-Saija C, Langdon R, Ward PB, Hickie IB, Scott EM, Naismith SL, Moore L, Alvares GA, Redoblado Hodge MA, Guastella AJ. A double-blind randomized controlled trial of oxytocin nasal spray and social cognition training for young people with early psychosis. Schizophr Bull. 2015. 2015;41(2):483–93. doi: 10.1093/schbul/sbu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadman KK. Fluoxetine but not risperidone increases sociability in the BTBR mouse model of autism. Pharmacol Biochem Behav. 2011;97:586–594. doi: 10.1016/j.pbb.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Chadman KK. Making progress in autism drug discovery. Expert Opin Drug Discov. 2014;9(12):1389–91. doi: 10.1517/17460441.2014.962511. [DOI] [PubMed] [Google Scholar]
- Dadds MR, MacDonald E, Cauchi A, Williams K, Levy F, Brennan J. Nasal oxytocin for social deficits in childhood autism: a randomized controlled trial. J Autism Dev Disord. 2014;44(3):521–31. doi: 10.1007/s10803-013-1899-3. [DOI] [PubMed] [Google Scholar]
- Davis MC, Lee J, Horan WP, Clarke AD, McGee MR, Green MF, Marder SR. Effects of single dose intranasal oxytocin on social cognition in schizophrenia. Schizophr Res. 2013;147(2-3):393–7. doi: 10.1016/j.schres.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Duffney LJ, Wei J, Cheng J, Liu W, Smith KR, Kittler JT, Yan Z. Shank3 deficiency induces NMDA receptor hypofunction via an actin-dependent mechanism. J Neurosci. 2013;33(40):15767–78. doi: 10.1523/JNEUROSCI.1175-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Lieberman JA, Koller BH. Effects of haloperidol, clozapine, and quetiapine on sensorimotor gating in a genetic model of reduced NMDA receptor function. Psychopharmacology. 2006a;184:190–200. doi: 10.1007/s00213-005-0214-1. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Lieberman JA, Koller BH. Typical and atypical antipsychotic drug effects on locomotor hyperactivity and deficits in sensorimotor gating in a genetic model of NMDA receptor hypofunction. Pharmacology Biochem Behav. 2006b;85:481–491. doi: 10.1016/j.pbb.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Perez A, Eddy DM, Zinzow WM, Lieberman JA, Snouwaert JN, Koller BH. Deficits in sensorimotor gating and tests of social behavior in a genetic model of reduced NMDA receptor function. Behav Brain Res. 2004;153:507–519. doi: 10.1016/j.bbr.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Macdonald K, Nguyen A, Cobb P, Warlan H, Galangue B, Minassian A, Becker O, Cooper J, Perry W. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry. 2010;68:678–680. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- Feifel D, Shilling PD, Belcher AM. The effects of oxytocin and its analog, carbetocin, on genetic deficits in sensorimotor gating. Eur Neuropsychopharmacol. 2012;22(5):374–8. doi: 10.1016/j.euroneuro.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay JM, Dunham GA, Isherwood AM, Newton CJ, Nguyen TV, Reppar PC, Snitkovski I, Paschall SA, Greene RW. Effects of prefrontal cortex and hippocampal NMDA NR1-subunit deletion on complex cognitive and social behaviors. Brain Res. 10. 2015;1600:70–83. doi: 10.1016/j.brainres.2014.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Shofty M, Brüne M, Ebert A, Shefet D, Levkovitz Y, Shamay-Tsoory SG. Improving social perception in schizophrenia: the role of oxytocin. Schizophr Res. 2013;146(1-3):357–62. doi: 10.1016/j.schres.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Anderson RL, Billingslea EN, Carlson GC, Roberts TP, Siegel SJ. Mice with reduced NMDA receptor expression: more consistent with autism than schizophrenia? Genes Brain Behav. 2012;11(6):740–50. doi: 10.1111/j.1601-183X.2012.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+ tf/J mouse social behavior. J Neurochem. 2011;116:291–303. doi: 10.1111/j.1471-4159.2010.07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella A J, Einfeld S, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, Hickie IB. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67:692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, McCarthy BE, Parker KJ, Reiss AL. Effects of intranasal oxytocin on social anxiety in males with fragile X syndrome. Psychoneuroendocrinology. 2012;37(4):509–18. doi: 10.1016/j.psyneuen.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, Anagnostou E, Wasserman S. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BR, Mosovich S. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger's disorders. Neuropsychopharmacology. 2003;28:193–198. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- Huang H, Michetti C, Busnelli M, Managò F, Sannino S, Scheggia D, Giancardo L, Sona D, Murino V, Chini B, Scattoni ML, Papaleo F. Chronic and acute intranasal oxytocin produce divergent social effects in mice. Neuropsychopharmacology. 2014;39(5):1102–14. doi: 10.1038/npp.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J Pharmacol Exper Therap. 2003;305:625–631. doi: 10.1124/jpet.102.046987. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Choi SY, Kim E. NMDA receptor dysfunction in autism spectrum disorders. Curr Opin Pharmacol. 2015;20:8–13. doi: 10.1016/j.coph.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Mahajan R, Bernal MP, Panzer R, Whitaker A, Roberts W, Handen B, Anagnostou E, Veenstra-VanderWeele J, Autism Speaks Autism Treatment Network Psychopharmacology Committee Clinical practice pathways for evaluation and medication choice for attention-deficit/hyperactivity disorder symptoms in autism spectrum disorders. Pediatrics. 2012;130:S125–S138. doi: 10.1542/peds.2012-0900J. [DOI] [PubMed] [Google Scholar]
- Mielnik CA, Horsfall W, Ramsey AJ. Diazepam improves aspects of social behaviour and neuron activation in NMDA receptor-deficient mice. Genes Brain Behav. 2014;13(7):592–602. doi: 10.1111/gbb.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milenkovic M, Mielnik CA, Ramsey AJ. NMDA receptor-deficient mice display sexual dimorphism in the onset and severity of behavioural abnormalities. Genes Brain Behav. 2014;13(8):850–62. doi: 10.1111/gbb.12183. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Morales M, Spear LP. The effects of an acute challenge with the NMDA receptor antagonists, MK-801, PEAQX, and ifenprodil, on social inhibition in adolescent and adult male rats. Psychopharmacology (Berl) 2014;231(8):1797–807. doi: 10.1007/s00213-013-3278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, Crawley JN, Duncan GE, Bodfish JW. Development of a mouse test for repetitive, restricted behaviors: Relevance to autism. Behav Brain Res. 2008a;188:178–194. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nikolova VD, Riddick NV, Baker LK, Koller BH. Preweaning sensorimotor deficits and adolescent hypersociability in Grin1 knockdown mice. Developmental Neuroscience. 2012;34:159–173. doi: 10.1159/000337984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, Crawley JN, Magnuson TR. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008b;191:118–129. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, West LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of ten inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Riddick NV, Nikolova VD, Teng BL, Agster KL, Nonneman RJ, Young NB, Baker LK, Nadler JJ, Bodfish JW. Repetitive behavior profile and supersensitivity to amphetamine in the C58/J mouse model of autism. Behavioural Brain Research. 2014;259:200–214. doi: 10.1016/j.bbr.2013.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlmann AM, Edington G, Mihalik AC, Buchwald Z, Koppuzha D, Korah M, Lewis MH. Further characterization of repetitive behavior in C58 mice: developmental trajectory and effects of environmental enrichment. Behav Brain Res. 2012;235:143–149. doi: 10.1016/j.bbr.2012.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Pedersen CA. Schizophrenia and alcohol dependence: Diverse clinical effects of oxytocin and their evolutionary origins. Brain Res. 2014;1580:102–123. doi: 10.1016/j.brainres.2014.01.050. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Gibson CM, Rau SW, Salimi K, Smedley KL, Casey RL, Leserman J, Jarskog LF, Penn DL. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophr Res. 2011;132:50–53. doi: 10.1016/j.schres.2011.07.027. [DOI] [PubMed] [Google Scholar]
- Peñagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, Sonnenblick LI, Gruver R, Almajano J, Bragin A, Golshani P, Trachtenberg JT, Peles E, Geschwind DH. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell 30. 2011;147(1):235–46. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peñagarikano O, Lázaro MT, Lu XH, Gordon A, Dong H, Lam HA, Peles E, Maidment NT, Murphy NP, Yang XW, Golshani P, Geschwind DH. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015;7(271):271ra8. doi: 10.1126/scitranslmed.3010257. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn DL, Keefe RS, Davis SM, Meyer PS, Perkins DO, Losardo D, Lieberman JA. The effects of antipsychotic medications on emotion perception in patients with chronic schizophrenia in the CATIE trial. Schizophr Res. 2009;115(1):17–23. doi: 10.1016/j.schres.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Costas E, Guidetti P, Melendez-Ferro M, Kelley JJ, Roberts RC. Neuroleptics and animal models: feasibility of oral treatment monitored by plasma levels and receptor occupancy assays. J Neural Transm. 2008;115(5):745–53. doi: 10.1007/s00702-007-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Slattery DA, Uschold-Schmidt N, Reber SO, Neumann ID. Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology. 2014;42:225–36. doi: 10.1016/j.psyneuen.2014.01.021. [DOI] [PubMed] [Google Scholar]
- Ring RH, Schechter LE, Leonard SK, Dwyer JM, Platt BJ, Graf R, Grauer S, Pulicicchio C, Resnick L, Rahman Z, Sukoff Rizzo SJ, Luo B, Beyer CE, Logue SF, Marquis KL, Hughes ZA, Rosenzweig-Lipson S. Receptor and behavioral pharmacology of WAY-267464, a non-peptide oxytocin receptor agonist. Neuropharmacology. 2010;58(1):69–77. doi: 10.1016/j.neuropharm.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Roberts DL, Penn DL, Corrigan P, Lipkovich I, Kinon B, Black RA. Antipsychotic medication and social cue recognition in chronic schizophrenia. Psychiatry Res. 2010;178(1):46–50. doi: 10.1016/j.psychres.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Young NB, Crawley JN, Bodfish JW, Moy SS. Social deficits, stereotypy and early emergence of repetitive behavior in the C58/J inbred mouse strain. Behav Brain Res. 2010;208:178–188. doi: 10.1016/j.bbr.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, He X, Willsey AJ, Ercan-Sencicek AG, Samocha KE, Cicek AE, Murtha MT, Bal VH, Bishop SL, Dong S, Goldberg AP, Jinlu C, Keaney JF, 3rd, Klei L, Mandell JD, Moreno-De-Luca D, Poultney CS, Robinson EB, Smith L, Solli-Nowlan T, Su MY, Teran NA, Walker MF, Werling DM, Beaudet AL, Cantor RM, Fombonne E, Geschwind DH, Grice DE, Lord C, Lowe JK, Mane SM, Martin DM, Morrow EM, Talkowski ME, Sutcliffe JS, Walsh CA, Yu TW, Autism Sequencing Consortium. Ledbetter DH, Martin CL, Cook EH, Buxbaum JD, Daly MJ, Devlin B, Roeder K, State MW. Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron. 2015;87(6):1215–33. doi: 10.1016/j.neuron.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankoorikal GMV, Kaercher KA, Boon CJ, Lee JK, Brodkin ES. A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biol Psychiatry. 2006;59:415–423. doi: 10.1016/j.biopsych.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Saunders JA, Tatard-Leitman VM, Suh J, Billingslea EN, Roberts TP, Siegel SJ. Knockout of NMDA receptors in parvalbumin interneurons recreates autism-like phenotypes. Autism Res. 2013;6(2):69–77. doi: 10.1002/aur.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Smith DG, Rizzo SJS, Karras MN, Turner SM, Tolu SS, Bryce DK, Smith DL, Fonseca K, Ring RH, Crawley JN. Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism. Sci Transl Med. 2012;4:131–151. doi: 10.1126/scitranslmed.3003501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35(4):976–89. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobota R, Mihara T, Forrest A, Featherstone RE, Siegel SJ. Oxytocin reduces amygdala activity, increases social interactions, and reduces anxiety-like behavior irrespective of NMDAR antagonism. Behav Neurosci. 2015;129(4):389–98. doi: 10.1037/bne0000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: emerging convergences across laboratory animal and human data. Dev Cogn Neurosci. 2011;1(4):392–400. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suraev AS, Bowen MT, Ali SO, Hicks C, Ramos L, McGregor I. Adolescent exposure to oxytocin, but not the selective oxytocin receptor agonist TGOT, increases social behavior and plasma oxytocin in adulthood. Horm Behav. 2014;65(5):488–96. doi: 10.1016/j.yhbeh.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Tarabeux J, Kebir O, Gauthier J, Hamdan FF, Xiong L, Piton A, Spiegelman D, Henrion É , Millet B, S2D team. Fathalli F, Joober R, Rapoport JL, DeLisi LE, Fombonne É , Mottron L, Forget-Dubois N, Boivin M, Michaud JL, Drapeau P, Lafrenière RG, Rouleau GA, Krebs MO. Rare mutations in N-methyl-D-aspartate glutamate receptors in autism spectrum disorders and schizophrenia. Transl Psychiatry. 2011;1:e55. doi: 10.1038/tp.2011.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng B, Nonneman RJ, Agster KL, Nikolova VD, Davis TT, Riddick NV, Baker LK, Pedersen CA, Jarstfer MB, Moy SS. Prosocial effects of oxytocin in two mouse models of autism spectrum disorders. Neuropharmacology. 2013;72:187–196. doi: 10.1016/j.neuropharm.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, Mill J, Cantor RM, Blencowe BJ, Geschwind DH. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474(7351):380–4. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadenberg M-LG, Soliman A, VanderSpek SC, Kapur S. Dopamine D2 receptor occupancy is a common mechanism underlying animal models of antipsychotics and their clinical effects. Neuropsychopharmacology. 2001;25:633–641. doi: 10.1016/S0893-133X(01)00261-5. [DOI] [PubMed] [Google Scholar]
- West L, Brunssen SH, Waldrop J. Review of the evidence for treatment of children with autism with selective serotonin reuptake inhibitors. J Spec Pediatr Nurs. 2009;14(3):183–91. doi: 10.1111/j.1744-6155.2009.00196.x. [DOI] [PubMed] [Google Scholar]
- Woolley JD, Chuang B, Lam O, Lai W, O'Donovan A, Rankin KP, Mathalon DH, Vinogradov S. Oxytocin administration enhances controlled social cognition in patients with schizophrenia. Psychoneuroendocrinology. 2014;47:116–25. doi: 10.1016/j.psyneuen.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, Guastella AJ. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Molecular Psychiatry. 2015 doi: 10.1038/mp.2015.162. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidán-Chuliá F, de Oliveira BH, Salmina AB, Casanova MF, Gelain DP, Noda M, Verkhratsky A, Moreira JC. Altered expression of Alzheimer's disease-related genes in the cerebellum of autistic patients: a model for disrupted brain connectome and therapy. Cell Death Dis 22. 2014;5:e1250. doi: 10.1038/cddis.2014.227. [DOI] [PMC free article] [PubMed] [Google Scholar]