Abstract
The protein phosphatase 2 (PP2A) holoenzyme consists of a catalytic subunit, a scaffold subunit, and a regulatory subunit. Based on loss of function analysis using PP2A catalytic inhibitors or inhibition via tumor viral antigens, limited studies suggest that PP2A is a putative tumor suppressor. However, PP2A has also been shown to facilitate the activation of oncogenic signaling pathways when associated with specific regulatory subunits. In this study, we investigated the possible oncogenic role of PP2A in pancreatic cancer. We found a striking increase in the expression of PR55α (PPP2R2A), a PP2A regulatory subunit, in pancreatic cancer cells compared to normal pancreatic epithelial cells. Consistently, PR55α expression was markedly elevated in pancreatic ductal adenocarcinoma tissues compared to adjacent normal pancreatic tissues (P<0.0001) and correlated with poor survival of pancreatic cancer patients (P<0.0003). RNAi-mediated depletion of PR55α in pancreatic cancer cell lines resulted in diminished phosphorylation of both AKT and ERK1/2 (MAPK3/1) and decreased protein levels of β-catenin (CTNNB1). Accordingly, pancreatic cancer cells with reduced PR55α expression exhibited significantly impaired properties of transformation, including attenuated cell growth, clonogenicity, mobility, and anchorage-independent growth. Moreover, orthotopic implantation of PR55α-depleted pancreatic cancer cells into nude mice resulted in markedly reduced tumorigenicity (P<0.001) and distant metastases. Together, these results suggest that PR55α promotes pancreatic cancer development by sustaining hyperactivity of multiple oncogenic signaling pathways, including AKT, ERK, and Wnt. These studies also provide a basis for exploring PR55α as a diagnostic or therapeutic target in pancreatic cancer.
Keywords: pancreatic cancer, PP2A, PR55α, oncogenic signaling, tumor xenograft mouse model
INTRODUCTION
PP2A, a family of heterotrimeric holoenzymes, accounts for the majority of serine/threonine phosphatase activity in human cells (1,2). Each PP2A holoenzyme consists of one catalytic subunit (C), one scaffolding subunit (A) and one regulatory subunit (1,2). There are two PP2A catalytic subunits, Cα/Cβ, and two PP2A scaffold subunits, Aα/Aβ. Cα and Cβ share 97% sequence identity while Aα and Aβ share 87% sequence identity (3). The PP2A regulatory subunits are classified into four distinct families, B/PR55, B’/PR61, B”/PR72 and PTP/PR53, and each family contains at least four members (1). Despite their recognition of similar sequence motifs within the A-subunits, there is no sequence homology among the PP2A regulatory subunits (3). The specific function of each PP2A holoenzyme is determined by its regulatory subunit, which controls the substrate specificity, cellular localization and enzymatic activity of the holoenzyme (1).
PP2A plays essential roles in many fundamental cellular functions, including cell proliferation, migration and survival (1,2). Evidence supports an important role for PP2A in cellular transformation and tumorigenesis: (i) several viral oncoproteins can function as PP2A regulatory subunits to induce transformation (4-6), (ii) PP2A regulates several oncogenic signaling cascades including AKT, ERK1/2 and Wnt/β-catenin (1) and (iii) PP2A regulates the tumor suppressing signaling pathway ARF/MDM2/p53, which plays a key role in blocking transformation and tumorigenesis of many cancer types (2). However, due to the structural complexity of PP2A holoenzymes and the wide range of substrates that PP2A acts on, the role of PP2A in oncogenic transformation remains largely undefined. Based on loss of function analysis using PP2A catalytic inhibitors (e.g. okadaic acid) or viral oncoproteins that inhibit PP2A activity, a very limited number of studies have described PP2A as a putative tumor suppressor (4-8). However, several different studies indicate that PP2A can also facilitate activation of oncogenic signaling pathways when associated with specific regulatory subunits (9-11).
The PR55 family of PP2A regulatory subunits consists of 4 isoforms (α/β/γ/δ). While PR55β and PR55γ are primarily expressed in brain tissues, PR55α and, to a lesser extent, PR55δ are expressed almost ubiquitously (12-14). PP2A/PR55 associates/regulates several core-signaling pathways, including ARF/MDM2/p53, PKCζ/Par3/Par6, PI3K/AKT, Raf/MEK/ERK, TGFBR1/TGF-β and Wnt/β-catenin (1,15). While deregulation of these pathways contributes critically to cellular transformation (16,17), the role of PP2A/PR55 to the pathogenesis remains largely undefined.
PP2A/PR55α has been reported to promote ERK1/2 phosphorylation/activation through dephosphorylation of KSR-Ser392, Raf-Ser259 and Raf Ser295 (9,18). KSR is a scaffold that facilitates the Ras/Raf/MEK/ERK cascade and Raf kinase is an upstream activator of ERK1/2. While dephosphorylation of KSR-Ser392 and Raf-Ser295 prevents the sequestration/inhibition of these proteins by 14-3-3 (9), dephosphorylation of Raf-Ser259 by PP2A/PR55α directly activates Raf (18). Therefore, through activation of KSR and Raf, PP2A/PR55α positively regulates ERK1/2 phosphorylation. On the other hand, PP2A/PR55γ shows the opposite effect on the Raf/MEK/ERK cascade via dephosphorylation/inhibition of c-Src that activates Raf (19).
PR55α is involved in the regulation of AKT activation, which requires phosphorylation of AKT-Thr308/Ser473 (20). In FL5.12 murine pro-B-cell lymphoid cells, PP2A/PR55α has been shown to inhibit AKT by dephosphorylation of AKT-Thr308 but not AKT-Ser473 (21). PR55α is also reported to positively regulate Wnt/β-catenin pathway. Beta-catenin is a key component of the Wnt signaling pathway and its intracellular level is primarily regulated by ubiquitination/proteasome degradation. Upon phosphorylation by casein kinase I and GSK3α/β at Ser33/Ser37/Thr41 residues, β-catenin forms a complex with E3-ubiquitin ligase β-TrCP, leading to proteasome-mediated degradation (22). PP2A/PR55α dephosphorylates β-catenin-Ser33/Ser37/Thr41, stabilizing β-catenin protein and activating Wnt signaling (10,23).
In this report, we examined PR55α expression in human and murine pancreatic cancer cells/tissues and investigated the influence of PP2A/PR55α on the oncogenic signalings mediated by AKT, ERK1/2 and β-catenin in pancreatic cancer cells. We also examined the impact of PR55α on transformed phenotypes, tumorigenicity and metastatic potential of pancreatic cancer cells. Results in this report demonstrate that PP2A/PR55α is required for sustaining the hyperactivities of multiple oncogenic signaling pathways and maintaining the malignant properties of pancreatic cancer cells.
MATERIALS AND METHODS
Cell culture
Human pancreatic cancer cell lines AsPC-1, Capan-1, CD-18/HPAF and L3.6 were recently obtained from the ATCC and resuscitated from early passage liquid nitrogen stocks. Cells were cultured for less than two months before reinitiating culture and routinely inspected microscopically for stable phenotype. ATCC uses morphology, karyotyping, and PCR based approaches to confirm the identity of human cell lines. HPNE cells are primary human pancreatic ductal cells immortalized using human telomerase and contain no transformed properties (24). Aliquots of HPNE cells were stored at −150°C to ensure that cells used for experiments were passaged for fewer than 6 months. The cells were routinely inspected for identity by morphology and growth curve analysis and validated to be mycoplasma free.
Antibodies
Antibodies are listed in Supplementary Material.
Tissue microarray (TMA) and immunohistochemistry
The clinical specimen for immunohistochemistry analysis (IHC) was a commercial TMA (OD-CT-DgPan01-006) (US Biomax). The TMA included 72 cases of human pancreatic ductal adenocarcinoma (PDAC) and matched normal adjacent tissues (NAT). Among those, 38 cases were documented with patients’ survival data. The TMA was analyzed for PR55α expression by IHC using a PR55α specific antibody (14-27 or 100C1) at 1:100 dilution, as described previously (25). The PR55α immunostaining intensity was evaluated by a UNMC pathologist who was blinded to the clinical information.
Immunoblotting
Immunoblotting was performed as described previously (26).
siRNA transfection
siRNA duplexes were purchased from Dharmacon. Non-targeting Control-siRNA were designed by the manufacturer to contain at least 4 mismatches to any human, rat or mouse genes. SMARTpool siRNA targeting PPP2R2A (PR55α) contains four siRNA targeting multiple sites on PR55α. Cells were transfected with 100 nM siRNA using DharmaFECT-1 (Dharmacon). The siRNA sequences are included in Supplementary Materials.
Growth kinetics
Growth kinetics was performed as described previously (27).
Clonogenic survival assay
Clonogenic survival assay was performed as described previously (28).
Wound-healing assay
Wound-healing assay was performed as described previously (29).
shRNA lentiviral vectors
Lentiviral vectors (pGIPZ) expressing non-silencing Control-shRNA or PR55α-shRNA were obtained from Dharmacon. Four PR55α-shRNAs targeting various regions of the PR55α gene were used for study. The shRNA sequences and lentiviral infection is described in Supplementary Materials.
Soft-agar assay
Soft-agar assay was performed as described previously (27).
Animal models
All in vivo experiments were approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee.
Genetically engineered mouse models of PDAC: The triple transgenic KPC mice (KrasG12D; Trp53R172H/+; Pdx1-Cre) were originally acquired from the NCI Mouse Models of Human Cancers Consortium (Frederick, MD). The composite mouse strain with targeted expression of mutant KrasG12D and Trp53R172H/+ in the mouse pancreas was generated and maintained by exploiting pancreas-specific Pdx1-Cre (KPC), which develops spontaneous PDAC.
Xenograft model of human pancreatic cancer: 6-week-old female athymic mice (Harlan) were divided into four groups (n=5 per group): a control group, which bore Control-shRNA-transduced tumor cells, and three examining groups, which bore PR55α-shRNA-transduced with tumor cells. Additional routine detail is described in Supplementary Materials.
Statistical analysis
Histo-score was evaluated by multiplying the values of the IHC-staining intensity and the percent of immunoreactive cells. The survival period was defined as the duration from the time of surgery until the last clinical follow-up or date of death. Survival curves were generated by Kaplan-Meier method using Prism Graphpad. All other statistical analyses were done using SigmaPlot.
RESULTS
PR55α is overexpressed in human pancreatic cancer cells
Aberrant activation of oncogenic signaling pathways contributes essentially to tumorigenesis and changes in protein phosphorylations have been a major cause for this activation. Since PP2A is a major cellular serine/threonine phosphatase, we investigated its contribution to the activation of oncogenic pathways in pancreatic cancer cells.
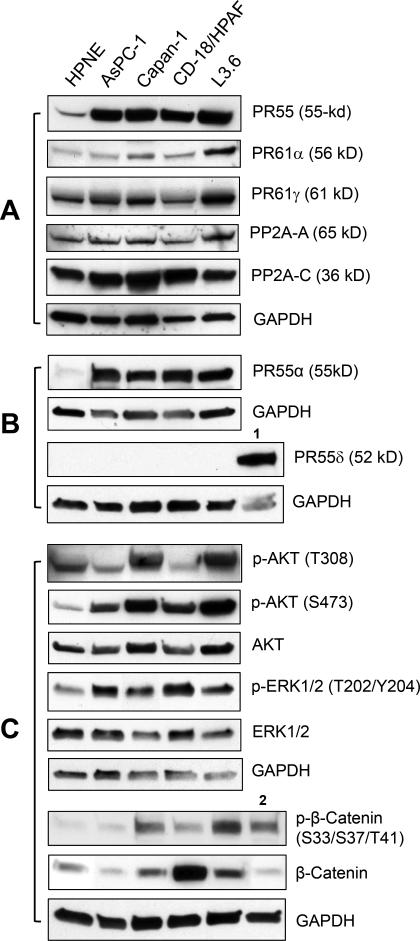
We analyzed the expression of PP2A subunits in a panel of human pancreatic cancer cell lines along with normal human pancreatic cells as controls. As shown in Fig. 1A, with an antibody recognizing all four isoforms (α, β, γ, δ) of the PR55 regulatory subunit, immunoblotting detected an average of 6-fold increase in PR55 expression in the pancreatic cancer cells compared to HPNE cells, which are normal human pancreatic ductal cells immortalized with telomerase (30). We also examined the expression of the catalytic and scaffold subunits of PP2A, as well as another two PP2A regulatory subunits PR61α and PR61γ. The latter were suggested as tumor suppressors by previous studies (31-33). As shown in Fig. 1A, the expressions of these PP2A subunits were not particularly associated with the malignant potential of these pancreatic cells.
Figure 1.
Analysis of PP2A subunits and PP2A downstream targets in normal and malignant pancreatic cells. A, expressions of PP2A regulatory subunits (PR55, PR61α and PR61γ), scaffold subunit (PP2A-A) and catalytic subunit (PP2A-C) were assessed by immunoblotting in the indicated cell lines. B, expressions of PR55α and PR55δ were determined by immunoblotting. The level of GAPDH was assessed for protein loading control. 1, mouse brain lysate. C, the cells were analyzed for the phosphorylation and total protein of AKT, ERK1/2 and β-catenin by immunoblotting. 2, HEK293 cell lysate.
Among PR55 isoforms, the α and, to a lesser extent, the δ isoforms were reported to be expressed almost ubiquitously (6), whereas the β and γ isoforms are primarily expressed only in brain tissues (12-14). We therefore analyzed the expression of PR55α and δ in the indicated pancreatic cancer cells. As shown in Fig. 1B, the PR55α expression pattern in these cells was the same as detected with the antibody recognizing all PR55 isoforms (PR55α vs. PR55). In contrast, no PR55δ expression was detected in the panel of pancreatic cell lines, while PR55δ expression was detected in mouse brain tissue, used as positive control (Fig. 1B). These results suggest that only the PR55α is expressed in these pancreatic cell lines.
PR55α overexpression in pancreatic cancer cells is associated with overall increases in the activities of oncogenic signaling pathways
We next assessed the phosphorylation of AKT and ERK1/2, which are known targets of PP2A, in the human pancreatic cell lines. Results in Fig. 1C showed an overall increase in phosphorylation of AKT-S473 and ERK1/2-T202/Y204, indicative of activation of these proteins (34,35), in the pancreatic cancer cells compared to normal HPNE control. These increases in protein phosphorylation were not accompanied by changes in total levels of the corresponding proteins (Fig. 1C).
Since a full-activation of AKT requires phosphorylation of both Ser473 and Thr308 (20), we also analyzed the panel for AKT-T308 phosphorylation. Compared to the normal HPNE control, AKT-Thr308 phosphorylation was higher in Capan-1 and L3.6 cells, but was lower in AsPC-1 and CD18/HPAF cells (Fig. 1C), implicating a lack of correlation between PR55α expression and AKT-Thr308 phosphorylation in the pancreatic cancer cells.
We also analyzed β-catenin protein in these cells. β-catenin is a PP2A target and plays a key role in Wnt signaling (1). As shown in Fig. 1C, compared to HPNE control, a large increase in β-catenin expression was detected in CD-18/HPAF cells, while only a subtle increase of β-catenin level was detected in L3.6 cells. On the other hand, β-catenin expression in AsPC-1 and Capan-1 cells was found to be similar to that of normal HPNE cells. Since previous studies described a negative regulation of β-catenin protein stability through a phosphorylation of β-catenin-S33/S37/T41 (22), we analyzed this phosphorylation in these cells, using HEK293 cells as a positive control (36). Fig. 1C revealed an elevated β-catenin-S33/S37/T41 phosphorylation in Capan-1, CD-18/HPAF and L3.6 pancreatic cancer cells compared to that found in HPNE control.
Collectively, these data suggest that PR55α overexpression in pancreatic cancer cells is correlated with an overall increase in the activities of oncogenic signaling pathways, including ERK1/2, AKT and Wnt.
PR55α is overexpressed in human PDAC tissues
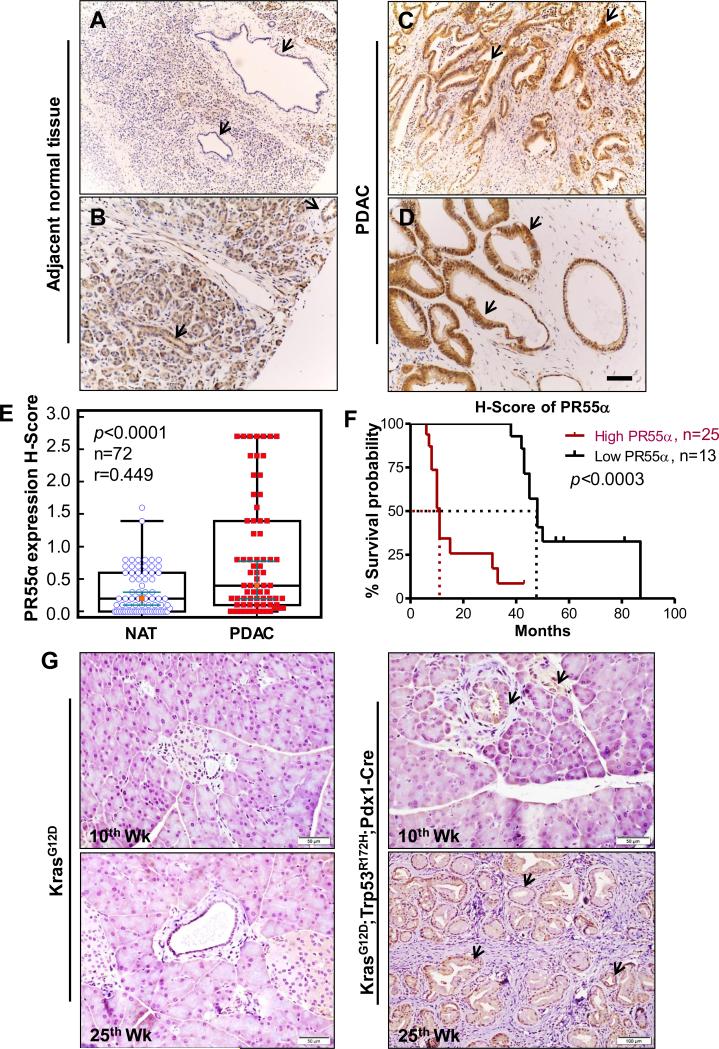
To validate the detection of PR55α overexpression in pancreatic cancer cells by immunoblotting, we assessed PR55α expression by IHC in a TMA of 72 cases of human PDAC with NAT specimens. As shown in Fig. 2A-E, IHC detected specific staining for PR55α with moderate to intense staining in the cytoplasm of the majority of PDAC tissues, whereas the corresponding NAT were negative or weakly positive for PR55α staining. Among TMA samples, 67% (48/72) of cases of PDAC tissues had intense to moderately high levels of PR55α expression, whereas weak or no PR55α expression was detected in their normal counterparts. The analysis also showed that 19% (14/72) of cases were negative or showed a similar pattern of PR55α expression in the tumor and corresponding NAT and its matching tumor tissues. In addition, 14% (10/72) of cases showed much higher PR55α expression in the normal tissues than PDAC tissues. The higher PR55α level in these NATs was in part due to the involvement of the neoplastic transformation process, as they are obtained from areas close to tumors. Overall, IHC analysis indicates that there is a significant increase in PR55α expression in pancreatic tumor samples compared to their paired normal tissue samples (P<0.0001).
Figure 2.
PR55α is overexpressed in human PDAC specimens. NAT and PDAC tissues from patients were analyzed for PR55α expression by IHC. A-D, representative images show normal (A-B) and PDAC tissues (C-D) from the same patient probed for PR55α protein expression by IHC. Arrows point to pancreatic ducts. A, a negative staining for PR55α expression. B, a moderate staining for PR55α expression. C-D, a strong staining for PR55α expression. Scale bar represents 100-μm. E, box plot depicting a summary of quantification of PR55α expression in NAT and PDAC tissues. F, Kaplan-Meier survival analysis was plotted for the PDAC cases that were high (N=25) and low (N=13) in PR55α protein expression. An H-Score of ≥0.2 was considered a high level of PR55α expression. Red and black lines denote the cases expressing high and low PR55α, respectively. G, PR55α is overexpressed in mouse pancreatic tumor cells. The floxed KPC mice (KrasG12D; Trp53R172H/+; Pdx1-Cre), a model of pancreatic cancer progression, were sacrificed at 10 and 25 weeks of age, respectively, along with their littermate control LSL-KrasG12D mice (KrasG12D). Pancreata of these mice were analyzed for PR55α expression by IHC with a mouse specific anti-PR55α antibody. Arrows point to pancreatic ducts. Arrows: positive IHC staining for PR55α expression.
To understand the clinical significance of PR55α expression, we performed a Kaplan-Meier survival analysis on 38 cases of PDAC for which patient survival data was available. The clinical characteristics of these cases are described in Supplementary Table S1. In the 38 tumor samples, the H score for PR55α expression ranges from 0-2.7, while the mean H-score for their matched NATs is 0.2 (Fig. 2E). To assess the relationship between PR55α expression and overall patient survival in these samples, we used the mean H score of NATs (0.2) as a threshold to divide the tumor samples into low and high PR55α expressing cohorts, as described previously (37). As shown in Fig. 2F, a high PR55α expression level (H-Score of PR55α expression ≥0.2) was significantly associated with an overall poor survival of pancreatic cancer patients (P<0.0003). The median survival for patients with high PR55α expression was only 11 months, whereas the median survival for patients with low PR55α expression was 48 months (Fig. 2F). This result suggests that a high level of PR55α expression is predictive of poor survival in patients with pancreatic cancer.
PR55α overexpression is detected in mouse PDAC
To verify the association of PR55α expression with pancreatic cancer development, we analyzed PR55α protein expression in pancreata of KPC triple transgenic mice (KrasG12D; Trp53R172H/+; Pdx1-Cre). The KPC mouse is a well-validated and clinically relevant spontaneous murine model of PDAC (38,39). At about 10 weeks of age, KPC mice start to develop a spectrum of premalignant lesions named Pancreatic Intraepithelial Neoplasias (PanINs) that ultimately progress to carcinomas with a moderate- to well-differentiated ductal morphology, which is similar to that commonly observed in human PDAC (38). As a littermate control, LSL-KrasG12D mice that do not develop PDAC were included in the studies. Results of this study are consistent with that obtained from the human pancreatic TMA analysis described in Fig. 2A-E, revealing a marked increase in PR55α expression in PanINs developed in the KPC mice at both 10 and 25 weeks of age compared to the pancreatic ducts of littermate control mice (Fig. 2G).
Decrease of PR55α expression by siRNA diminishes oncogenic signalings in pancreatic cancer cells
Using specific siRNA, we examined the impact of PR55α expression on the activities of oncogenic signalings targeted by PP2A.
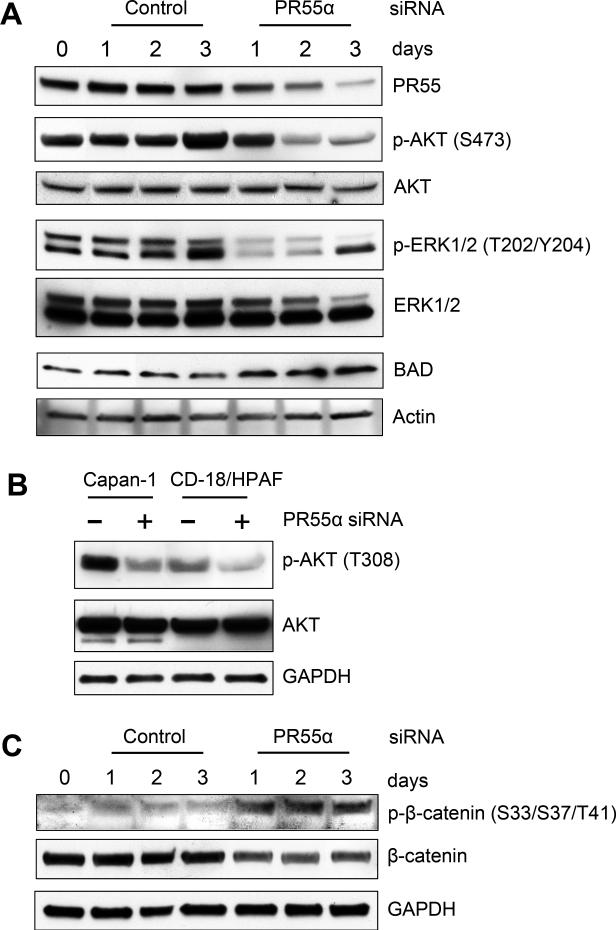
As shown in Fig. 3A, PR55α-siRNA transfected CD-18/HPAF cells exhibited a marked decrease in total PR55 protein expression compared to Control-siRNA transfected cells. Associating with this decrease in PR55 levels in the PR55α-siRNA transfected cells, phosphorylation of AKT-S473 and ERK1/2-T202/Y204 was markedly reduced, indicative of decreased AKT and ERK1/2 activities (Fig. 3A). On day 3 post transfection, there was an increase in phosphorylation of ERK2, but not ERK1, in both Control- and PR55α-siRNA transfected cells, which is likely a non-specific effect of the transient transfection method (Fig. 3A). To validate the inhibition of AKT and ERK1/2 signaling in these cells, we analyzed their common downstream target BAD, a pro-apoptotic protein whose stability is normally inhibited by the AKT and ERK1/2 pathways (40,41). As shown in Fig. 3A and Supplementary Fig. S1A, BAD protein expression was upregulated by 2-3 fold in the PR55α-siRNA transfected cells compared to control cells.
Figure 3.
Decrease of PR55α protein expression by siRNA diminishes AKT, ERK1/2 and Wnt signalings in CD-18/HPAF pancreatic cancer cells. Cells were transfected with PR55α-siRNA or control-siRNA and incubated for the indicated times. A, the transfected cells were analyzed by immunoblotting for phosphorylation and/or levels of PR55, AKT, ERK1/2, BAD and Actin. B, the indicated cells were transfected with PR55α- or Control-siRNA, incubated for 3 days and analyzed for p-AKT (T308), AKT and GAPDH. C, CD-18/HPAF cells transfected with PR55α-siRNA or control-siRNA were analyzed for levels of p-β-catenin (S33/S37/T41), β-catenin and Actin by immunoblotting.
We also assessed the effect of PR55α on AKT-T308 phosphorylation, which is also involved in AKT activation (35). Fig. 3B showed that transfection of PR55α-siRNA resulted in a decrease in AKT-T308 phosphorylation in both Capan-1 and CD-18/HPAF cells.
We next examined the influence of PR55α expression on β-catenin. As demonstrated previously, β-catenin level is mainly regulated through proteasomal degradation that is generally facilitated by the phosphorylation of β-catenin-Ser33/Ser37/Thr41 (22). As shown in Fig. 3C and Supplementary Fig. S1B, decrease of PR55α expression by siRNA in CD-18/HPAF cells led to an increase in phosphorylation β-catenin-Ser33/Ser37/Thr41 and a concomitant decrease in β-catenin protein expression.
Together, these results provide direct evidence suggesting a vital role for PR55α in the maintenance of hyperactive AKT, ERK1/2 and Wnt signalings in pancreatic cancer cells.
Decrease of PR55α expression in pancreatic cancer cells suppresses cell growth
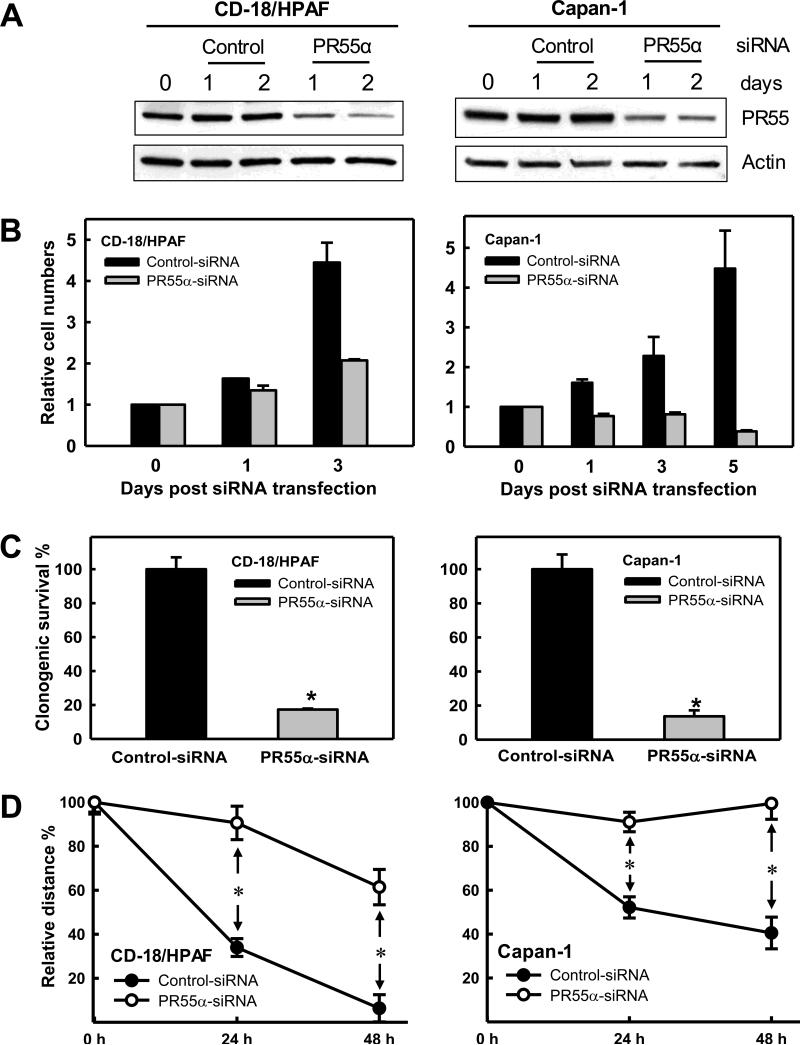
We tested the effect of PR55α on growth kinetics of pancreatic cancer cells using siRNA. As shown in Fig. 4A, transfection of PR55α-siRNA significantly decreased PR55α expression in both CD-18/HPAF and Capan-1 cells, whereas Control-siRNA had no noticeable effect on PR55α expression in these cells. Consequently, decrease of PR55α expression by siRNA suppressed growth of both CD-18/HPAF and Capan-1 cells compared to Control-siRNA transfected cells (Fig. 4B).
Figure 4.
Effect of PR55α expression on growth kinetics and mobility of pancreatic cancer cells. A, indicated cells were transfected with Control-siRNA or PR55α-siRNA and analyzed for PR55α and actin levels by immunoblotting. B, Control-siRNA or PR55α-siRNA transfected cells were incubated for the indicated times and counted. Results depict the relative number of cells in each sample and represent the mean±s.d. of two sets of experiments in duplicates. C, cells (3×104) were transfected with Control-siRNA or PR55α-siRNA, and incubated for 10 days in growth medium to form colonies. The colonies were visualized by crystal violet staining and quantified using the ImageJ analytical program. The results are shown as mean±s.d. of two set of experiments in duplicate samples. *, P=<0.001 (n=4), significant difference between the PR55α-siRNA transfected and Control-siRNA transfected cells. D, cells were transfected with Control-siRNA or PR55α siRNA, incubated for 48h and examined for mobility using wound-healing motility assay (29). Wounds were photographed and measured at 0h, 24h and 48h after wounding. Cell migration was assessed as percentage wound coverage over time. The line graphs depict the percentage wound coverage and are shown as mean±s.d. of two sets of experiments in duplicate samples. *, P=<0.001 (n=4), significant difference between the Control-siRNA transfected and PR55α-siRNA transfected cells.
Using a clonogenic assay (28), we validated the impact of PR55α on pancreatic cancer cell growth. For this study, pancreatic cancer cells were transfected with PR55α-siRNA and incubated for 10 days to allow colony formation. Consistent with the result in Fig. 4B, decrease of PR55α expression by siRNA also markedly inhibited the clonogenicity of pancreatic cancer cells compared to Control-siRNA transfected cells (Fig. 4C and Supplementary Fig. S2A).
Collectively, these results suggest a requirement for PR55α in sustaining the hyper-proliferative state of pancreatic cancer cells.
Decrease of PR55α expression impedes migration of pancreatic cancer cells
PP2A plays an essential role in the regulation of cell mobility and invasion (42). We therefore tested the effect of PR55α on mobility of pancreatic cancer cells using a wound-healing assay (29). As shown in Fig. 4D and Supplementary Fig. S3, transfection of PR55α-siRNA markedly reduced the mobility of CD-18/HPAF, Capan-1 and AsPC-1 cells, suggesting that PR55α is essential for the migratory and invasive advantages of pancreatic cancer cells.
Decrease of PR55α expression inhibits anchorage-independent growth of pancreatic cancer cells
Anchorage-independent growth capability, the most important property of tumor cells, is conjointly driven by multiple oncogenic signaling pathways, including AKT, ERK1/2 and Wnt (43). As Fig. 3 showed that PR55α is required for maintaining the hyperactivities of these signaling pathways in pancreatic cancer cells, we assessed the effect of PR55α on anchorage-independent growth of pancreatic cancer cells.
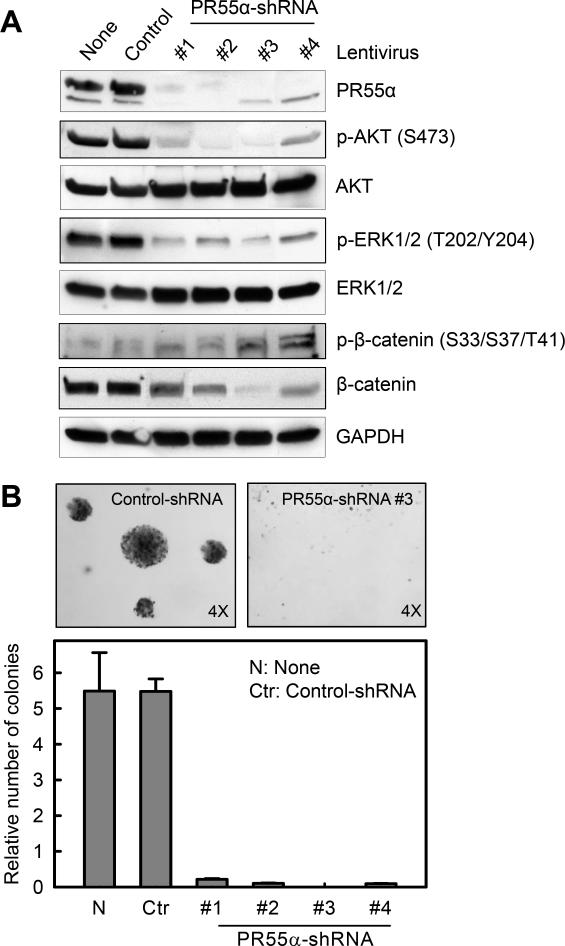
For this study, we developed a series of pancreatic cancer cells (CD-18/HPAF) stably expressing shRNA targeting four different regions of PR55α. As a control, cells stably transduced with non-silencing Control-shRNA were included in the study. As shown in Fig. 5A, transduction with each PR55α-shRNA markedly reduced PR55α expression in CD-18/HPAF cells. In contrast, Control-shRNA transduced cells showed no effect on PR55α expression in CD-18/HPAF cells compared to un-transduced cells (Fig. 5A).
Figure 5.
shRNA knockdown of PR55α diminishes activities of oncogenic targets of PP2A and suppresses anchorage-independent growth of pancreatic cancer cells. CD-18/HPAF cells were stably transduced with shRNA targeting several regions of PR55α (#1, #2, #3 and #4) or non-targeting Control-shRNA. A, the transduced cells were analyzed for phosphorylation and/or level of PR55α, AKT, ERK1/2, β-catenin and GAPDH by immunoblotting. B, effect of PR55α knockdown on anchorage-independent growth was examined using a soft-agar assay. Upper panels: representative images of soft-agar assay. Lower panel: colonies in soft-agar were counted and shown as mean±s.d. of two separate experiments in duplicates.
We next analyzed the effect of PR55α-shRNA on AKT, ERK1/2 and β-catenin. Consistent with the effect of PR55α-siRNA shown in Fig. 3A, decrease of PR55α expression in CD-18/HPAF cells by each of the four PR55α-shRNAs also resulted in a marked reduction in phospho-AKT-S473 and phospho-ERK1/2-T202/Y204 with no noticeable change in protein levels (Fig. 5A). The stable knockdown of PR55α also markedly reduced the steady-state levels of β-catenin protein, along with minor increases in β-catenin-S33/S37/T41 phosphorylation in these cells. In contrast, none of these changes were observed in the Control-shRNA transduced cells (Fig. 5A).
Using a soft-agar assay (27), we tested the effect of PR55α on anchorage-independent growth. As shown in Fig. 5B, decrease of PR55α expression by each of the PR55α-shRNAs almost completely inhibited the ability of CD-18/HPAF cells to form colonies in soft-agar. These results suggest a requirement for PR55α for anchorage-independent growth of pancreatic cancer cells.
Knockdown of PR55α expression by shRNA impairs pancreatic cancer cell growth and metastasis in vivo
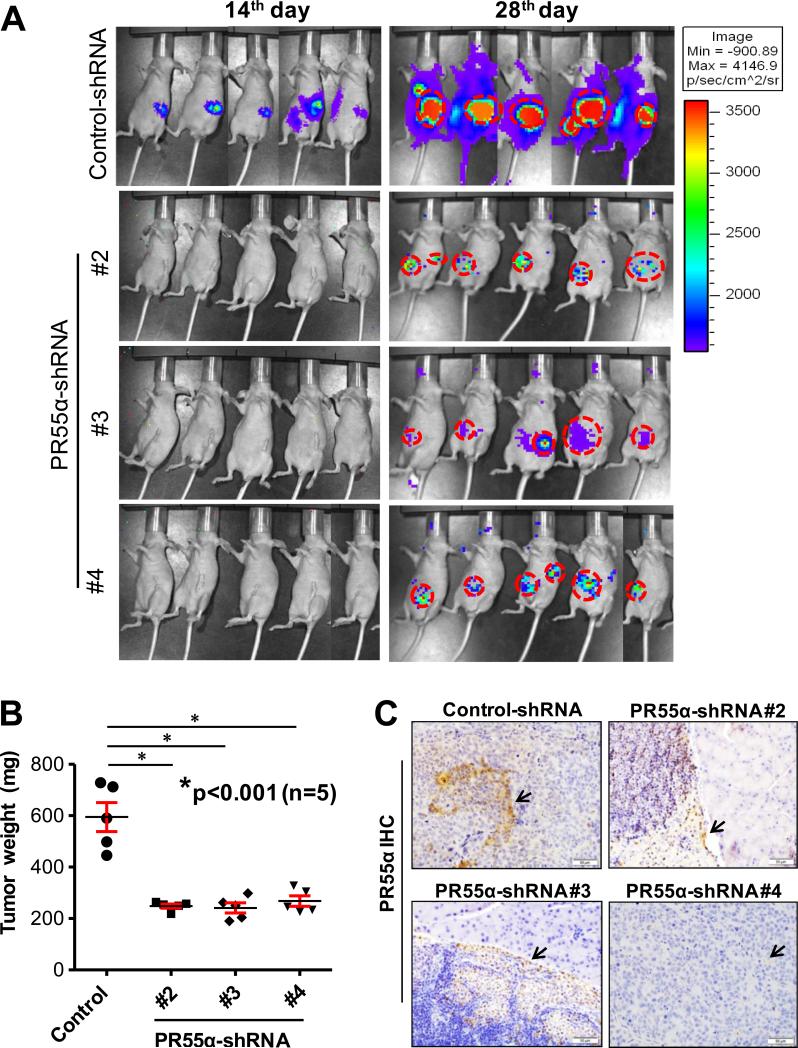
Since decrease of PR55α expression using siRNA or shRNA resulted in suppression of signaling pathways that are normally required for hyper-proliferative, survival, migratory and anchorage independent properties of pancreatic cancer cells (Fig. 3-5), we further evaluated the effect of PR55α on the tumorigenic and metastatic potential of pancreatic cancer cells in vivo. Nude mice were orthotopically implanted with CD-18/HPAF cells stably expressing Control- and PR55α-shRNAs and monitored for tumor growth for five consecutive weeks using in vivo bioluminescence imaging. As shown in Fig. 6A and Supplementary Fig. S4A, PR55α knockdown tumor cells grew much slower than control cells in the pancreata of mice. Based on the weights of tumor xenografts obtained at 5 weeks after implantation, there was a marked reduction in the size of tumor formed by the PR55α silenced pancreatic cancer cells compared with the control cells (Fig. 6B and Supplementary Fig. S4A). We also analyzed the xenograft tissues by IHC/H&E staining, and confirmed the persistent silencing of PR55α in the tumor cells expressing PR55α-shRNA (Fig. 6C and Supplementary Fig. S4B). Remarkably, the tumor growth inhibitory effect was paralleled with a decreased metastatic potential in the PR55α knockdown cells. Compared to control group, the mice implanted with the PR55α knockdown cells showed a marked reduction in the incidence of metastasis to distant organs (liver, spleen, small intestine, diaphragm, peritoneum, cecum and mesenteric lymph node) (Table 1). For instance, a significant decline in liver metastasis was observed in the mice implanted with PR55α knockdown cells compared to those implanted with control cells (P<0.02). These observations are consistent with the in vitro data showing a suppression of AKT/ERK/Wnt signalings in pancreatic cancer cells by PR55α knockdown (Fig. 3 and Fig. 5), and suggest that an essential role for PR55α in tumor growth and metastasis of pancreatic cancer.
Figure 6.
Decrease of PR55α expression in pancreatic cancer cells suppresses tumor growth and metastases. Luciferase expressing CD-18/HPAF cells (CD-18/HPAF-Luc) (5×105), which had been stably transduced with Control-shRNA and PR55α-shRNA (#2, #3 and #4), were orthotopically implanted into the pancreata of athymic mice and monitored for tumor growth and metastases for 5-weeks using a bioluminescent imaging system. A, images of tumor-bearing mice at the indicated days post implantation. B, box-plot depicts the average pancreas weight of the mice implanted with Control- or PR55α-shRNA transduced cells. *, P<0.001 (n=5), significant reduction of pancreas weight in the groups of mice implanted with PR55α-shRNA-transduced cells compared to control group. C, PR55α expression in the pancreatic tumor xenograft tissues were analyzed by IHC. Arrows: positive IHC staining for PR55α expression.
Table 1.
Incidence of metastasis to various organs developed by the implanted CD-18/HPAF-Luc cells transduced with Control-shRNA or PR55α-shRNA was analyzed and quantified in the sacrificed mice.
| Groups | Site of metastases | ||||||
|---|---|---|---|---|---|---|---|
| Liver | Spleen | Intestine | Diaphragm | Peritoneum | Cecum | Mesenteric lymph node | |
| Control shRNA | 4/5 (80%) | 3/5 (60%) | 1/5 (20%) | 1/5 (20%) | 1/5 (20%) | 2/5 (40%) | 1/5 (20%) |
| PR55α shRNA#2 | 0/5* | 1/5 (20%) | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| PR55α shRNA#3 | 0/5* | 2/5 (40%) | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| PR55α shRNA#3 | 0/5* | 2/5 (40%) | 1/5 (20%) | 0/5 | 0/5 | 0/5 | 0/5 |
NOTE:
P<0.02, significant different from the Control-shRNA transduced cells.
DISCUSSION
The role of PP2A in tumorigenesis remains largely undefined and much controversial information has been obtained on this function of PP2A. PP2A was initially suggested as a putative tumor suppressor, based on loss of function analyses using PP2A catalytic inhibitors or viral oncoproteins that inhibit PP2A (4-8). Furthermore, studies focusing on the PR61 family of the PP2A regulatory subunits further supported PP2A as a tumor suppressor (1), indicating that PP2A/PR61 can inhibit Wnt signaling, stabilize p53 protein and suppress BCL2 anti-apoptotic activity (1). On the other hand, different studies also challenged the notion that PP2A holoenzymes are all acting as tumor suppressors. These studies show that PP2A, when associated with specific regulatory subunits, can facilitate activation of oncogenic signaling pathways (1). Among those, PP2A/PR55α has been demonstrated to activate ERK1/2 and Wnt pathways (9,10,23). Consistently, two cancer-related PP2A-Aα point mutants, E64G in breast cancer and E64D in lung cancer, are defective in binding to PR61, but can bind normally to PR55α (44,45). These paradoxical observations re-emphasize the complexity and diversity of PP2A functions, which are attributed to the large number of non-conserved PP2A regulatory subunits (1). Thus, the evidence now indicate that PP2A not only can function as a tumor suppressor, but can also act as a tumor promoter, depending on its regulatory subunit.
This report shows that the expression of PP2A regulatory subunit PR55α is detected at much higher levels in pancreatic cancer cell lines relative to normal pancreatic ductal cells (see Fig. 1). Consistently, PR55α expression is also markedly increased in both human and murine PDAC tissues compared to normal pancreatic tissues (see Fig. 2). Furthermore, Kaplan-Meier analysis reveals a significant inverse-correlation between PR55α protein expression and overall survival of pancreatic cancer patients, suggesting that high PR55α expression in pancreatic cancer denotes a trend in poor survival of pancreatic cancer patients. Supporting this suggestion, the in vitro and in vivo studies in this report reveal that PR55α is required for sustaining hyperactivities of oncogenic signaling pathways, including AKT, ERK1/2 and Wnt, and maintaining both transformed phenotypes and malignant potential of pancreatic cancer cells (see Fig. 3-6 and Table 1).
PP2A/PR55α has been reported to promote ERK1/2 phosphorylation/activation through the dephosphorylation of KSR-Ser392, Raf-Ser259 and Raf-Ser295 (9,18). While dephosphorylation of KSR-Ser392 and Raf-Ser295 releases these proteins from sequestration/inhibition by 14-3-3 (9), dephosphorylation of Raf-Ser259 directly activates Raf (18). Results in this report are consistent with these previous reports, showing that PR55α expression is required for sustaining ERK1/2 activation in pancreatic cancer cells (see Fig. 3 and 5).
The mechanism by which PP2A/PR55α regulates AKT still remains largely unclear; this is due to the complexity of the AKT regulatory mechanisms. AKT can be activated through both PI3K-dependent and PI3K-independent mechanisms (46). With the PI3K-dependent mechanism, AKT is first recruited to the plasma membrane by PIP3, and subsequently phosphorylated by mTOR/Rictor (mTORC2) at Ser473 and then by PDK1 at Thr308, leading to the full-activation of AKT (20,46). Through PI3K-independent mechanisms, AKT can be directly regulated by numerous kinases, including PKA, ACK1, TNK2, IKKε, TBK1, Src, PTK6, ATM and DNA-PK, as well as several phosphatases, including PP2A, PTEN, PHLPPs and INPP4B (46,47). A previous study in FL5.12 murine pro-B-cell lymphoid cells shows that PP2A/PR55α can directly dephosphorylate AKT-Thr308 but not AKT-Ser473 (21). However, results in this report show that PR55α is apparently required for the maintenance of both AKT-Ser473 and AKT-Thr308 phosphorylation in human pancreatic cancer cells (see Fig. 3A-B and 5A). The discordance between our result and previous observations might be due to difference in cell type or tumor type. It also suggests that the regulation of AKT phosphorylation involves multiple signaling mechanisms, which are differentially targeted by the PR55α linked PP2A.
Since PP2A/PR55α has been shown to increase β-catenin protein stability by dephosphorylating β-catenin-Ser33/Ser37/Thr41 (10,23), we have examined this regulation in pancreatic cancer cells using both siRNA (transient knockdown) and shRNA (stable knockdown). While both transient and stable knockdown of PR55α result in a marked decrease in β-catenin protein level in pancreatic cancer cells, a better correlation between the change in PR55α expression and β-catenin-S33/S37/T41 phosphorylation is observed in the cells with PR55α transient knockdown (see Fig. 3C versus Fig. 5). In the PR55α stable knockdown cells, we observed only a subtle, if any, increase in β-catenin-S33/S37/T41 phosphorylation compared to control cells. The minimal change in β-catenin-S33/S37/T41 phosphorylation in the stable knockdown cells can probably be attributed to the adaptation of these cells to the low level of PR55α, which has kept β-catenin at a very low steady-state level in the cells.
While PR55α has been demonstrated by in vitro studies to have a modulatory effect on the phosphorylation of key signaling players such as ERK1/2, AKT and Wnt (21,23,48), its in vivo function in many cancers, including pancreatic cancer, remains poorly understood. Using an orthotopic mouse model of pancreatic cancer, the present study have demonstrated that inhibition of PR55α expression leads to a significant suppression in tumor growth and metastasis (see Fig. 6, Table 1 and Supplementary Fig. S4). Thus, these in vivo obtained results are consistent with the in vitro modulatory effects of PR55α on the proliferation, survival, motility and anchorage independence observed in pancreatic cancer cells (see Fig. 3-5), suggesting a critical role for PR55α in pancreatic tumor growth and metastasis. Future studies will be needed to determine whether the level of PR55α expression is associated with the staging and prognosis of pancreatic cancer. Additionally, a global genetic approach is needed to identify additional targets of PR55α that are involved in pancreatic cancer development and maintenance. Nevertheless, studies in this report provide direct evidence that PR55α is an attractive therapeutic target for pancreatic cancer.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Keith Johnson for critical discussion and Tom Dao for assistance with Live-Cell Microscopy (funded by NIH COBRE Grant P30GM106397). We also thank Kavita Mallya for technical help.
Financial support: This work was supported, in parts, by Pilot Project Funding from 5P30GM106397 to YY, U54CA163120 to SKB and P50CA127297 to SKB, MO and YY.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
REFERENCES
- 1.Eichhorn PJ, Creyghton MP, Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim Biophys Acta. 2009;1795(1):1–15. doi: 10.1016/j.bbcan.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Seshacharyulu P, Pandey P, Datta K, Batra SK. Phosphatase: PP2A structural importance, regulation and its aberrant expression in cancer. Cancer Lett. 2013;335(1):9–18. doi: 10.1016/j.canlet.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janssens V, Longin S, Goris J. PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail). Trends Biochem Sci. 2008;33(3):113. doi: 10.1016/j.tibs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Bikel I, Montano X, Agha ME, Brown M, McCormack M, Boltax J, et al. SV40 small t antigen enhances the transformation activity of limiting concentrations of SV40 large T antigen. Cell. 1987;48(2):321–30. doi: 10.1016/0092-8674(87)90435-1. [DOI] [PubMed] [Google Scholar]
- 5.Pallas DC, Shahrik LK, Martin BL, Jaspers S, Miller TB, Brautigan DL, et al. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990;60(1):167–76. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- 6.Skoczylas C, Fahrbach KM, Rundell K. Cellular targets of the SV40 small-t antigen in human cell transformation. Cell Cycle. 2004;3(5):606–10. [PubMed] [Google Scholar]
- 7.Fujiki H, Suganuma M. Tumor promotion by inhibitors of protein phosphatases 1 and 2A: the okadaic acid class of compounds. Adv Cancer Res. 1993;61:143–94. doi: 10.1016/s0065-230x(08)60958-6. [DOI] [PubMed] [Google Scholar]
- 8.Moreno CS, Ramachandran S, Ashby DG, Laycock N, Plattner CA, Chen W, et al. Signaling and transcriptional changes critical for transformation of human cells by simian virus 40 small tumor antigen or protein phosphatase 2A B56gamma knockdown. Cancer Res. 2004;64(19):6978–88. doi: 10.1158/0008-5472.CAN-04-1150. [DOI] [PubMed] [Google Scholar]
- 9.Ory S, Zhou M, Conrads TP, Veenstra TD, Morrison DK. Protein Phosphatase 2A Positively Regulates Ras Signaling by Dephosphorylating KSR1 and Raf-1 on Critical 14-3-3 Binding Sites. Curr Biol. 2003;13(16):1356–64. doi: 10.1016/s0960-9822(03)00535-9. [DOI] [PubMed] [Google Scholar]
- 10.Bajpai R, Makhijani K, Rao PR, Shashidhara LS. Drosophila Twins regulates Armadillo levels in response to Wg/Wnt signal. Development. 2004;131(5):1007–16. doi: 10.1242/dev.00980. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto K, Li H, Jensen MR, Zhang T, Taya Y, Thorgeirsson SS, et al. Cyclin G recruits PP2A to dephosphorylate Mdm2. Molecular Cell. 2002;9(4):761–71. doi: 10.1016/s1097-2765(02)00504-x. [DOI] [PubMed] [Google Scholar]
- 12.Mayer RE, Hendrix P, Cron P, Matthies R, Stone SR, Goris J, et al. Structure of the 55-kDa regulatory subunit of protein phosphatase 2A: evidence for a neuronal-specific isoform. Biochemistry. 1991;30(15):3589–97. doi: 10.1021/bi00229a001. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt K, Kins S, Schild A, Nitsch RM, Hemmings BA, Götz J. Diversity, developmental regulation and distribution of murine PR55/B subunits of protein phosphatase 2A. Eur J Neurosci. 2002;16(11):2039–48. doi: 10.1046/j.1460-9568.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhao J-Q, Xie S-S, Liu W-B, Xiao Y-M, Zeng X-M, Deng M, et al. Molecular Cloning of the Genes Encoding the PR55/Bβ/δ Regulatory Subunits for PP-2A and Analysis of Their Functions in Regulating Development of Goldfish, Carassius auratus. Gene Regul Syst Bio. 2010;4:135–48. doi: 10.4137/GRSB.S6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sontag JM, Sontag E. Regulation of cell adhesion by PP2A and SV40 small tumor antigen: An important link to cell transformation. Cell Mol Life Sci. 2006;63(24):2979–91. doi: 10.1007/s00018-006-6300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain S, Singhal S, Lee P, Xu R. Molecular genetics of hepatocellular neoplasia. Am J Transl Res. 2010;2(1):105–18. [PMC free article] [PubMed] [Google Scholar]
- 17.Macgregor-Das AM, Iacobuzio-Donahue CA. Molecular Pathways in Pancreatic Carcinogenesis. J Surg Oncol. 2013;107(1):8–14. doi: 10.1002/jso.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams DG, Coffee RL, Zhang H, Pelech S, Strack S, Wadzinski BE. Positive Regulation of Raf1-MEK1/2-ERK1/2 Signaling by Protein Serine/Threonine Phosphatase 2A Holoenzymes. J Biol Chem. 2005;280(52):42644–54. doi: 10.1074/jbc.M502464200. [DOI] [PubMed] [Google Scholar]
- 19.Eichhorn PJ, Creyghton MP, Wilhelmsen K, van Dam H, Bernards R. A RNA interference screen identifies the protein phosphatase 2A subunit PR55gamma as a stress-sensitive inhibitor of c-SRC. PLoS Genet. 2007;3(12):e218. doi: 10.1371/journal.pgen.0030218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D, et al. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17(3):313–25. doi: 10.1038/sj.onc.1201947. [DOI] [PubMed] [Google Scholar]
- 21.Kuo YC, Huang KY, Yang CH, Yang YS, Lee WY, Chiang CW. Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55alpha regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. J Biol Chem. 2008;283(4):1882–92. doi: 10.1074/jbc.M709585200. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang W, Yang J, Liu Y, Chen X, Yu T, Jia J, et al. PR55 alpha, a regulatory subunit of PP2A, specifically regulates PP2A-mediated beta-catenin dephosphorylation. J Biol Chem. 2009;284(34):22649–56. doi: 10.1074/jbc.M109.013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee KM, Yasuda H, Hollingsworth MA, Ouellette MM. Notch 2-positive progenitors with the intrinsic ability to give rise to pancreatic ductal cells. Lab Invest. 2005;85(8):1003–12. doi: 10.1038/labinvest.3700298. [DOI] [PubMed] [Google Scholar]
- 25.Pandey P, Seshacharyulu P, Das S, Rachagani S, Ponnusamy MP, Yan Y, et al. Impaired expression of protein phosphatase 2A subunits enhances metastatic potential of human prostate cancer cells through activation of AKT pathway. Br J Cancer. 2013;108(12):2590–600. doi: 10.1038/bjc.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan Y, Cao PT, Greer PM, Nagengast ES, Kolb RH, Mumby MC, et al. Protein phosphatase 2A has an essential role in the activation of gamma-irradiation-induced G2/M checkpoint response. Oncogene. 2010;29(30):4317–29. doi: 10.1038/onc.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Batra SK, Castelino-Prabhu S, Wikstrand CJ, Zhu X, Humphrey PA, Friedman HS, et al. Epidermal growth factor ligand-independent, unregulated, cell-transforming potential of a naturally occurring human mutant EGFRvIII gene. Cell Growth Differ. 1995;6(10):1251–9. [PubMed] [Google Scholar]
- 28.Cai Z, Chattopadhyay N, Liu WJ, Chan C, Pignol J-P, Reilly RM. Optimized digital counting colonies of clonogenic assays using ImageJ software and customized macros: Comparison with manual counting. Int J Radiat Biol. 2011;87(11):1135–46. doi: 10.3109/09553002.2011.622033. [DOI] [PubMed] [Google Scholar]
- 29.Seshacharyulu P, Ponnusamy MP, Rachagani S, Lakshmanan I, Haridas D, Yan Y, et al. Targeting EGF-receptor(s) - STAT1 axis attenuates tumor growth and metastasis through downregulation of MUC4 mucin in human pancreatic cancer. Oncotarget. 2015;6(7):5164–81. doi: 10.18632/oncotarget.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee KM, Choi KH, Ouellette MM. Use of exogenous hTERT to immortalize primary human cells. Cytotechnology. 2004;45(1-2):33–8. doi: 10.1007/10.1007/s10616-004-5123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto H, Hinoi T, Michiue T, Fukui A, Usui H, Janssens V, et al. Inhibition of the Wnt Signaling Pathway by the PR61 Subunit of Protein Phosphatase 2A. J Biol Chem. 2001;276(29):26875–82. doi: 10.1074/jbc.M100443200. [DOI] [PubMed] [Google Scholar]
- 32.Ruvolo PP, Clark W, Mumby M, Gao F, May WS. A functional role for the B56 alpha-subunit of protein phosphatase 2A in ceramide-mediated regulation of Bcl2 phosphorylation status and function. J Biol Chem. 2002;277(25):22847–52. doi: 10.1074/jbc.M201830200. [DOI] [PubMed] [Google Scholar]
- 33.Nobumori Y, Shouse GP, Wu Y, Lee KJ, Shen B, Liu X. Characterization of B56γ tumor-associated mutations reveals mechanisms for inactivation of B56γ-PP2A. Mol Cancer Res. 2013;11(9):995–1003. doi: 10.1158/1541-7786.MCR-12-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wortzel I, Seger R. The ERK Cascade: Distinct Functions within Various Subcellular Organelles. Genes Cancer. 2011;2(3):195–209. doi: 10.1177/1947601911407328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13(22):2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 36.Liu C, Li Y, Semenov M, Han C, Baeg G-H, Tan Y, et al. Control of β-Catenin Phosphorylation/Degradation by a Dual-Kinase Mechanism. Cell. 2002;108(6):837–47. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 37.Galizia G, Lieto E, Ferraraccio F, Orditura M, De Vita F, Castellano P, et al. Determination of Molecular Marker Expression Can Predict Clinical Outcome in Colon Carcinomas. Clin Cancer Res. 2004;10(10):3490–99. doi: 10.1158/1078-0432.CCR-0960-03. [DOI] [PubMed] [Google Scholar]
- 38.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7(5):469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 39.Lakshmanan I, Seshacharyulu P, Haridas D, Rachagani S, Gupta S, Joshi S, et al. Novel HER3/MUC4 oncogenic signaling aggravates the tumorigenic phenotypes of pancreatic cancer cells. Oncotarget. 2015;6(25):21085–99. doi: 10.18632/oncotarget.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91(2):231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 41.Pucci B, Indelicato M, Paradisi V, Reali V, Pellegrini L, Aventaggiato M, et al. ERK-1 MAP kinase prevents TNF-induced apoptosis through bad phosphorylation and inhibition of Bax translocation in HeLa Cells. J Cell Biochem. 2009;108(5):1166–74. doi: 10.1002/jcb.22345. [DOI] [PubMed] [Google Scholar]
- 42.Basu S. PP2A in the regulation of cell motility and invasion. Curr Protein Pept Sci. 2011;12(1):3–11. doi: 10.2174/138920311795659443. [DOI] [PubMed] [Google Scholar]
- 43.Zavoral M, Minarikova P, Zavada F, Salek C, Minarik M. Molecular biology of pancreatic cancer. World J Gastroenterol. 2011;17(24):2897–908. doi: 10.3748/wjg.v17.i24.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen W, Arroyo JD, Timmons JC, Possemato R, Hahn WC. Cancer-associated PP2A Aalpha subunits induce functional haploinsufficiency and tumorigenicity. Cancer Res. 2005;65(18):8183–92. doi: 10.1158/0008-5472.CAN-05-1103. [DOI] [PubMed] [Google Scholar]
- 45.Ruediger R, Ruiz J, Walter G. Human Cancer-Associated Mutations in the Aα Subunit of Protein Phosphatase 2A Increase Lung Cancer Incidence in Aα Knock-In and Knockout Mice. Mol Cell Biol. 2011;31(18):3832–44. doi: 10.1128/MCB.05744-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faes S, Dormond O. PI3K and AKT: Unfaithful Partners in Cancer. Int J Mol Sci. 2015;16(9):21138. doi: 10.3390/ijms160921138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao Y, Hung M-C. Physiological regulation of Akt activity and stability. Am J Transl Res. 2010;2(1):19–42. [PMC free article] [PubMed] [Google Scholar]
- 48.Jayadeva G, Kurimchak A, Garriga J, Sotillo E, Davis AJ, Haines DS, et al. B55α PP2A Holoenzymes Modulate the Phosphorylation Status of the Retinoblastoma-related Protein p107 and Its Activation. J of Biol Chem. 2010;285(39):29863–73. doi: 10.1074/jbc.M110.162354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.