Abstract
Background
Recent epidemiologic evidence suggests that higher circulating vitamin D does not protect against prostate cancer and, in fact, may increase the risk of developing this malignancy. However, few studies have examined the most clinically relevant outcome, prostate cancer mortality.
Methods
We examined pre-diagnostic serum 25-hydroxy-vitamin D (25(OH)D) and prostate cancer survival in a cohort of 1,000 cases in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study. During 23 years of follow-up, 363 men died from their disease. Cox proportional hazards models were used to estimate the hazard ratio (HR) and 95% CI of death from prostate cancer by season-specific quintile of 25(OH)D. Multivariable models were adjusted for age, physical activity, cigarettes per day, and family history of prostate cancer.
Results
Men with higher serum 25(OH)D were less likely to die from their prostate cancer (Q5 vs. Q1 HR=0.72, 95% CI=0.52 – 0.99, p-trend=0.006). This finding was independent of stage or grade at diagnosis, and appeared restricted to men who survived longer (survived <3.3 years: Q5 vs. Q1 HR=0.95, 95% CI=0.61 – 1.50, p-trend=0.53; survived ≥3.3 years: Q5 vs. Q1 HR=0.53, 95% CI=0.34 – 0.85, p-trend=0.0002).
Conclusions
In this population of men diagnosed with prostate cancer, higher serum 25(OH)D years prior to diagnosis was associated with longer prostate cancer survival.
Impact
In light of inconsistent evidence regarding the role of vitamin D in the development of prostate cancer, the present findings regarding the most clinically relevant prostate cancer outcome, disease-specific mortality, could have important public health implications.
Keywords: Vitamin D, Prostatic Neoplasms, Mortality, Survival Analysis, Prospective Cohort
Introduction
It has been hypothesized that vitamin D may prevent many types of malignancies including prostate cancer. The primary circulating form of vitamin D is 25-hydroxyvitamin D (25(OH)D), which is considered the best indicator of an individual's vitamin D status (1), and is converted to the hormonally active form, 1-25-dihydroxyvitamin D (1,25(OH)2D), by 1-α-hydroxylase (1, 2). Laboratory studies have demonstrated that 1,25(OH)2D has many anti-carcinogenic actions, including being anti-inflammatory, anti-angiogenic, and pro-apoptotic (1, 3). Because vitamin D is synthesized in the skin in response to exposure to UVB radiation, early ecologic studies considered it as a possible explanation for the higher incidence or mortality of some cancers at latitudes receiving less sunlight (4). Evidence from epidemiologic studies with measured circulating 25(OH)D concentration is inconsistent with respect to the role of vitamin D in prostate cancer, however. A recent meta-analysis concluded that men with higher serum 25(OH)D had a higher risk of developing cancer of the prostate than men with lower serum 25(OH)D (5), although this analysis did not differentiate between more and less aggressive disease. Two recent studies suggest that the association differs by aggressiveness, with one reporting a possible U-shaped relation with plasma 25(OH)D (6), and the other indicating higher 25(OH)D might be associated with an increased risk of low-grade, but a decreased risk of high-grade, disease (7).
Prostate cancer is the most commonly diagnosed cancer among men in the US, but has one of the most favorable survival rates, with 94% of patients surviving for at least 15 years (8). Thus, prostate cancer mortality is perhaps a more clinically relevant outcome for risk association analyses. Yet few studies have examined serum 25(OH)D in relation to this endpoint, and those that have produced conflicting results. Three studies examined pre-diagnostic 25(OH)D concentrations comparing incident cases of lethal prostate cancer to cancer-free controls; one found an inverse association (9) while two others showed no association (10, 11). Serum 25(OH)D concentrations at the time of prostate cancer diagnosis was also unrelated to subsequent mortality in two studies (12, 13), and inversely in one (14). Most of these studies were relatively small, however (9, 12-14), leaving unsettled the issue of whether higher vitamin D status can improve prostate cancer survival.
In the present analysis, we examined the association between pre-diagnostic serum 25(OH)D and prostate cancer mortality in a cohort of 1,000 men diagnosed with the malignancy in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study.
Materials and Methods
Study Population
The ATBC Study was a randomized, double-blind, placebo-controlled, primary prevention trial conducted to determine the effects of supplementation with α-tocopherol and β-carotene on cancer incidence (15). Caucasian male smokers (n=29,133) from southwestern Finland were recruited between 1985 and 1988. Men were between 50-69 years old at baseline and smoked at least 5 cigarettes per day as part of the enrollment criteria. Men were ineligible if they had previously had cancer or another serious illness at enrollment, or if they reported current use of supplements containing vitamin E (>20mg), vitamin A (>20,000 IU), or β-carotene (>6mg). Men who were enrolled in the trial were assigned to one of four groups based on a 2×2 factorial design: 1) α-tocopherol (dl-α-tocopheryl acetate, 50mg/day), 2) β-carotene (20 mg/day), 3) both supplements, or 4) placebo. Trial participants were supplemented for 5-8 years, until death, or until the trial ended on April 30, 1993. Although the intervention trial ended, follow-up is ongoing through the Finnish Cancer Registry and the Register of Causes of Death and for this analysis is complete through December 31, 2012. The ATBC Study was approved by institutional review boards at both the U.S. National Cancer Institute and the Finnish National Public Health Institute, and written informed consent was obtained from all participants. At enrollment, participants completed questionnaires about general risk factors, smoking, and medical history, as well as a food-frequency questionnaire (16). Participants also underwent physical examination by registered nurses to measure their height and weight, and to collect an overnight fasting blood sample.
Exposure and Outcome Assessment
Previously, 1,000 prostate cancer cases were selected as part of a case-control set for which 25(OH)D was measured. Details of the case-control selection have been published (11). For the present analysis, these 1,000 cases were treated as a cohort with participants entering at the time of prostate cancer diagnosis. Follow-up time was calculated from the date of diagnosis to date of death from prostate cancer, death from another cause, or the censoring date (December 31, 2012), whichever was earliest. Cause of death was ascertained by linkage with the Register of Causes of Death. Cases were defined as death from prostate cancer as the underlying cause (n=363); men with all other causes of death were censored at their death date.
25(OH)D was measured by Heartland Assays, Inc. (Ames, IA) using the DiaSorin Liaison 25(OH)D TOTAL assay (17). Each batch of samples contained four or six blinded quality control (QC) samples from our study; inter- and intrabatch CVs were 7.1 and 10.1%, respectively. Additional details of the laboratory and quality control methods have been published previously (18).
Statistical Analysis
All statistical analyses were conducted using SAS v. 9.2 (Cary, NC). Survival time was estimated by quintiles of 25(OH)D using the Kaplan-Meier method. Cox proportional hazards models were used to estimate the hazard ratio (HR) and 95% CI of death from prostate cancer by season-specific quintile of 25(OH)D. Quintile cutpoints were determined separately for men whose blood was collected in the winter (November – May) and in the summer (June – October) based on the control distribution in the original case-control study in each season. The cutpoints were as follows: winter: Q1 ≤16, Q2 >16 - ≤24, Q3 >24 - ≤33, Q4 >33 - ≤46, Q5 >46 nmol/L; summer: Q1 ≤26, Q2 >26 - ≤36, Q3 >36 - ≤49, Q4 >48 - ≤60, Q5 >60 nmol/L. The trend across categories was assessed by entering an ordinal variable into the model, treating it as a continuous variable, and assessing its statistical significance using the Wald test.
Factors that are known or hypothesized to be associated with prostate cancer survival or circulating 25(OH)D concentration were considered as potential confounding variables and were included in the multivariable model. The final multivariable models were adjusted for: age at diagnosis with prostate cancer (years, continuous), physical activity (yes = at least light or moderate occupational activity or moderate leisure time activity), and family history of prostate cancer (yes, no, missing). Further adjustment for stage at diagnosis, trial supplement assignment, or time from blood collection to diagnosis with prostate cancer did not alter the results, and therefore are not included in the final model. Analyses were conducted stratifying by survival time (< median, ≥ median, i.e., 3.3 years), disease aggressiveness at diagnosis, and time between blood collection and prostate cancer diagnosis (< median, ≥ median, i.e. 12.5 years).
Results
Characteristics of the cohort of cases by prostate cancer survival status are shown in Table 1. The cases that died of prostate cancer were older at blood collection but younger at the time of prostate cancer diagnosis, were less physically active, and were more likely to have a family history of prostate cancer. There were no differences in vitamin D dietary intake or supplement use by prostate cancer survival status. Characteristics of the cohort by season-specific quintile of 25(OH)D are shown in Table 2. Men with higher serum 25(OH)D tended to be older at the time of blood collection and prostate cancer diagnosis, were more physically active and more likely to have a family history of prostate cancer, and had higher dietary and supplemental vitamin D intake and serum alpha-tocopherol and beta-carotene.
Table 1. Selected baseline characteristics [medians (interquartile range) or number (percent)] for prostate cancer cases by survivor status, ATBC Study.
| Characteristic | Survivors (n=637) | Deceased (n=363) | p-value |
|---|---|---|---|
|
| |||
| Age at blood collection (years) | 57 (53 – 61) | 58 (55 – 63) | < 0.0001 |
| Age at diagnosis (years) | 70 (67 - 74) | 68 (64 – 73) | < 0.0001 |
| Height (cm) | 174 (170 – 178) | 173 (170 – 177) | 0.19 |
| Weight (kg) | 78.2 (71.1 – 87.6) | 77.5 (70.7 – 85.5) | 0.27 |
| BMI (kg/m2) | 26.0 (23.9 – 28.6) | 25.8 (23.9 – 28.3) | 0.61 |
| Cigarettes per day | 20 (15 – 25) | 20 (15 – 25) | 0.02 |
| Physically active | 138 (21.7) | 57 (15.7) | 0.02 |
| History of diabetes | 13 (2.0) | 10 (2.8) | 0.47 |
| Family history of prostate cancer | 30 (4.7) | 22 (5.1) | 0.003 |
| Energy intake (kcal/day) | 2,616 (2,184 – 3,130) | 2,582 (2,162 – 3,218) | 0.65 |
| Dietary vitamin D (IU/day) | 4.7 (3.2 – 6.7) | 4.9 (3.2 – 7.0) | 0.56 |
| Dietary calcium (mg/day) | 1,329 (1,004 – 1,708) | 1,352 (1,003 – 1,739) | 0.77 |
| Ethanol intake (g/day) | 10.8 (2.5 – 24.7) | 9.9 (2.0 – 22.9) | 0.28 |
| Supplemental vitamin D use | 51 (8.0) | 27 (7.4) | 0.93 |
| Supplemental calcium use | 76 (11.9) | 40 (11.0) | 0.84 |
| Serum cholesterol (mmol/L) | 6.2 (5.4 – 6.9) | 6.2 (5.4 – 7.0) | 0.88 |
| Serum alpha-tocopherol (mg/L) | 11.4 (9.8 – 13.3) | 11.4 (9.7 – 13.6) | 0.74 |
| Serum beta-carotene (μg/L) | 185 (118 – 269) | 176 (123 – 271) | 0.99 |
| Serum retinol (μg/L) | 587 (509 – 672) | 592 (522 – 666) | 0.51 |
| Serum 25(OH)D (nmol/L) | 35.2 (22.8 – 49.3) | 32.3 (22.6 – 50.7) | 0.32 |
Table 2. Selected baseline characteristics [medians (interquartile range) or number (percent)] by season-specific quintile of serum 25(OH)D, ATBC Study.
| Season-Specific Quintile of Serum 25(OH)D | p-value | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristic | Q1 (n=166) | Q2 (n=203) | Q3 (n=206) | Q4 (n=187) | Q5 (n=237) | |
|
| ||||||
| Age at blood collection (years) | 58 (54 – 62) | 57 (53 – 61) | 56 (53 – 62) | 57 (53 – 61) | 59 (55 – 63) | 0.02 |
| Age at diagnosis (years) | 69 (66 – 73) | 69 (65 – 73) | 69 (66 – 73) | 69 (65 – 73) | 70 (67 – 74) | 0.02 |
| Height (cm) | 173 (169 – 177) | 173 (170 – 178) | 174 (170 – 179) | 174 (170 – 178) | 174 (171 – 178) | 0.61 |
| Weight (kg) | 75.6 (69.0 – 84.3) | 77.2 (71.0 – 88.0) | 77.7 (70.5 – 86.7) | 80.0 (71.4 – 87.8) | 78.5 (72.1 – 86.2) | 0.23 |
| BMI (kg/m2) | 25.8 (23.6 – 28.2) | 26.1 (23.6 – 28.8) | 26.0 (23.9 – 28.3) | 26.1 (24.5 – 28.3) | 25.7 (24.2 – 28.4) | 0.50 |
| Cigarettes per day | 20 (13 – 30) | 20 (15 – 25) | 20 (15 – 25) | 20 (15 – 25) | 20 (14 – 25) | 0.44 |
| Physically active | 18 (10.8) | 42 (20.7) | 40 (19.4) | 42 (22.5) | 53 (22.4) | 0.06 |
| History of diabetes | 7 (4.2) | 5 (2.5) | 3 (1.5) | 2 (1.1) | 5 (2.1) | 0.35 |
| Family history of prostate cancer | 11 (6.6) | 6 (3.0) | 3 (1.5) | 15 (8.0) | 17 (7.2) | 0.005 |
| Energy intake (kcal/day) | 2,600 (2,104 – 3,261) | 2,643 (2,150 – 3,197) | 2,614 (2,206 – 3,124) | 2,613 (2,125 – 3,130) | 2582 (2,201 – 3,160) | 0.99 |
| Dietary vitamin D (IU/day) | 3.5 (2.4 – 4.9) | 4.1 (2.9 – 6.2) | 4.9 (3.3 – 6.5) | 4.9 (3.6 – 6.7) | 5.9 (4.2 – 8.4) | <0.0001 |
| Dietary calcium (mg/day) | 1,326 (974 – 1,825) | 1,329 (966 – 1,725) | 1,306 (1,006 – 1,708) | 1,377 (1.044 – 1.703) | 1,351 (1,003 – 1,710) | 0.85 |
| Ethanol intake (g/day) | 9.3 (1.6 – 22.9) | 9.7 (1.9 – 24.5) | 12.1 (2.3 – 27.1) | 10.6 (3.4 – 24) | 11.8 (3.3 – 22.9) | 0.66 |
| Supplemental vitamin D use | 1 (0.6) | 9 (4.4) | 18 (8.7) | 18 (9.6) | 32 (13.5) | 0.0005 |
| Supplemental calcium use | 15 (9.0) | 17 (8.4) | 24 (11.7) | 21 (11.2) | 39 (16.5) | 0.11 |
| Serum cholesterol (mmol/L) | 6.2 (5.4 – 6.8) | 6.2 (5.4 – 6.9) | 6.2 (5.4 – 6.8) | 6.3 (5.5 – 7.1) | 6.1 (5.4 – 6.8) | 0.71 |
| Serum alpha-tocopherol (mg/L) | 10.6 (8.6 – 12.9) | 11.1 (9.8 – 13.3) | 11.3 (9.8 – 13.3) | 11.8 (10.2 – 13.9) | 11.8 (10.3 – 13.4) | <0.0001 |
| Serum beta-carotene (μg/L) | 147 (89 – 241) | 169 (116 – 249) | 186 (120 – 259) | 196 (130 – 279) | 213 (139 – 311) | <0.0001 |
| Serum retinol (μg/L) | 575 (497 – 634) | 578 (506 – 666) | 588 (512 – 682) | 603 (515 – 684) | 595 (531 – 686) | 0.21 |
| Serum 25(OH)D (nmol/L) | 14.2 (10.5 – 18.4) | 22.7 (20.0 – 29.9) | 30.8 (27.3 – 39.8) | 41.3 (37.4 – 50.3) | 64.1 (53.9 – 74.3) | - |
| Time from baseline to diagnosis (years) | 11.5 (8.1 – 14.5) | 12.7 (8.0 – 15.1) | 12.7 (9.0 – 15.8) | 12.8 (8.2 – 15.0) | 12.8 (9.4 – 15.7) | 0.19 |
| Time from diagnosis to death or end of follow-up (years) | 3.9 (1.8 – 8.2) | 4.7 (2.2 – 9.5) | 6.6 (2.8 – 10.2) | 6.6 (2.6 – 10.6) | 6.3 (2.4 – 10.8) | 0.0003 |
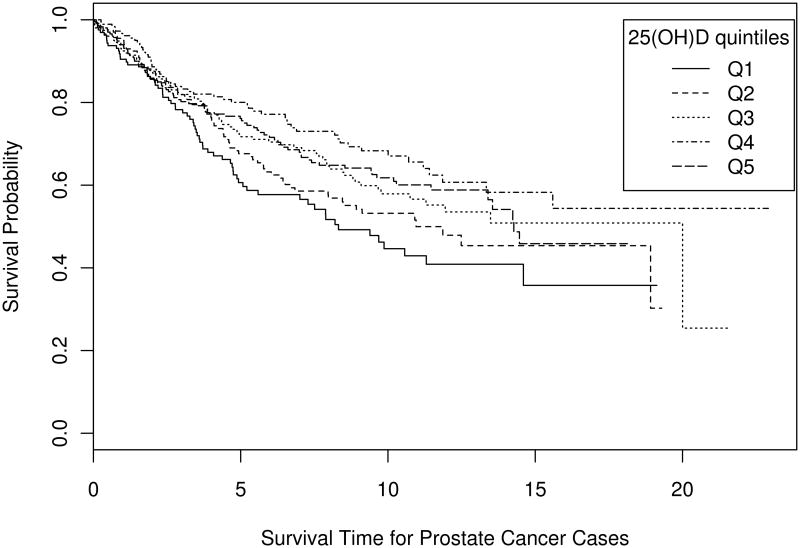
After adjustment for age at diagnosis, men with prostate cancer who had higher serum 25(OH)D were less likely to die from their disease than men with the lowest serum 25(OH)D (Q5 vs. Q1 HR=0.69, 95% CI=0.50 – 0.95, p-trend=0.003; Table 3). There was very little attenuation of these findings with multivariable adjustment (Q5 vs. Q1 HR=0.72, 95% CI=0.52 – 0.99, p-trend=0.006; Table 3). In the crude Kaplan-Meier analysis, prostate cancer-specific survival appeared better for men with higher 25(OH)D, consistent with the data in Table 3 (log-rank p-value = 0.07; Figure 1).
Table 3. Association between season-specific quintile of serum 25(OH)D at baseline and mortality among men diagnosed with prostate cancer, ATBC Study.
| Season-Specific Quintile of Serum 25(OH)D | # Prostate Cancer Deaths | Person-Years | HR (95% CI)* | HR (95% CI)† |
|---|---|---|---|---|
| Q1 | 69 | 875 | 1.0 (ref) | 1.0 (ref) |
| Q2 | 79 | 1,231 | 0.81 (0.59 – 1.12) | 0.84 (0.61 – 1.17) |
| Q3 | 76 | 1,433 | 0.69 (0.50 – 0.96) | 0.71 (0.51 – 0.99) |
| Q4 | 55 | 1,368 | 0.52 (0.37 – 0.75) | 0.54 (0.38 – 0.77) |
| Q5 | 83 | 1,629 | 0.69 (0.50 – 0.95) | 0.72 (0.52 – 0.99) |
| p-trend | 0.003 | 0.006 |
Adjusted for age at prostate cancer diagnosis
Adjusted for age at prostate cancer diagnosis, cigarettes smoked per day, physical activity, and family history of prostate cancer
Figure 1. Prostate cancer survival by season-specific quintile of serum 25(OH)D at ATBC Study enrollment.
The inverse association between serum 25(OH)D concentration and prostate cancer mortality appeared restricted to men who lived longer (Table 4). Among men who lived <3.3 years after being diagnosed, 25(OH)D was unrelated to mortality (Q5 vs. Q1 HR=0.95, 95% CI=0.61 – 1.50; p=0.53), whereas among men who lived ≥3.3 years, there was an inverse association (Q5 vs. Q1 HR=0.53, 95% CI=0.34 – 0.85, p=0.0002; Table 4). We observed no effect modification when stratifying by disease aggressiveness or time between blood collection and diagnosis, or when men diagnosed within 2 years of blood collection were excluded (data not shown).
Table 4. Survival time-stratified association between season-specific quintile of serum 25(OH)D at baseline and mortality among men diagnosed with prostate cancer, ATBC Study.
| Survived <3.3 years | Survived ≥3.3 years | |||
|---|---|---|---|---|
| Season-Specific Quintile of Serum 25(OH)D | # Prostate Cancer Deaths | HR (95% CI)* | # Deaths | HR (95% CI)* |
| Q1 | 34 | 1.0 (ref) | 35 | 1.0 (ref) |
| Q2 | 36 | 0.81 (0.51 – 1.30) | 43 | 0.87 (0.55 – 1.36) |
| Q3 | 36 | 0.84 (0.52 – 1.34) | 40 | 0.61 (0.38 – 0.96) |
| Q4 | 30 | 0.72 (0.44 – 1.18) | 25 | 0.39 (0.23 – 0.66) |
| Q5 | 45 | 0.95 (0.61 – 1.50) | 38 | 0.53 (0.34 – 0.85) |
| p-trend | 0.53 | 0.0002 | ||
Adjusted for age at prostate cancer diagnosis, cigarettes smoked per day, physical activity, and family history of prostate cancer
Discussion
In this large prospective examination of men diagnosed with prostate cancer, we found higher serum 25(OH)D up to 20 years prior to diagnosis was related to statistically significantly increased prostate cancer survival. The finding was independent of stage or grade at diagnosis, and was stronger among men who survived longer. Because the association was unchanged when we excluded from the analysis men diagnosed within 2 years of blood collection, reverse causality is an unlikely explanation for it. Similarly, the stronger association among cases that lived longer with their prostate cancer reduces the possibility of lead-time bias.
The previous literature regarding vitamin D status and prostate cancer mortality is limited and has yielded mixed results, with some studies showing an inverse relation (9, 14) and others reporting no association (10-12, 19). As with most studies of lethal prostate cancer, sample size is an important concern; i.e., all of the null studies, including a previous analysis in the present cohort based on 276 deaths, had insufficient power (i.e., 6% - 41%) to detect an association of the magnitude of their reported OR or HR for higher circulating 25(OH)D, leaving it uncertain whether they were truly null (10-12, 19). The present findings of a beneficial role for higher vitamin D status and survival are, however, consistent with the two studies showing inverse associations between 25(OH)D and either incident prostate cancer that resulted in death (9), or mortality after prostate cancer diagnosis (14). Differing study designs and assessment of 25(OH)D status at varying times relative to the prostate cancer diagnoses likely also contributed to the divergent findings. For example, three studies examined circulating 25(OH)D at the time of diagnosis in relation to disease-specific mortality (12, 14, 19), and one only enrolled men with stage 4 disease (12), thus potentially (and likely) entailing an element of reverse causality, with both primary tumors and distant metastases influencing vitamin D status. These studies contrast with those examining prospective, pre-diagnostic 25(OH)D concentrations in men diagnosed with prostate cancer that would prove lethal and prostate cancer-free controls (9-11), whereas the present analysis focused on disease-specific survival among men with prostate cancer. Additional investigations are clearly needed.
A recent meta-analysis of 21 studies including nearly 12,000 cases and 14,000 controls examining circulating 25(OH)D in relation to prostate cancer incidence reported that higher 25(OH)D was associated with an increased risk of developing prostate cancer (5), and there is evidence from biomarker and genetic studies that this may pertain to high stage and high grade disease (11, 20). That the directionality of the association between circulating 25(OH)D and prostate cancer risk may be opposite that of prostate cancer mortality would on the surface seem counter-intuitive. However, the direct association with survival in the present analysis was independent of stage and grade at diagnosis, and although higher stage and grade are associated with poorer prognosis, they are not perfect predictors of survival. One possible explanation for the findings is that vitamin D influences prostate tumor metastasis through mechanisms unrelated to stage or grade at diagnosis. For example, one study found that vitamin D prevented metastasis of androgen independent prostate tumors in rats (21), studies of prostate cancer cell lines have shown vitamin D inhibition of metastasis through decreased motility, chemotaxis and invasion (22, 23). Another possible explanation for the present findings is that higher vitamin D being associated with elevated risk of non-lethal prostate cancer leads to an enrichment of non-lethal disease in the higher 25(OH)D quintiles and a spurious inverse association with lethal disease. This potential source of bias could not, however, explain the inverse association observed with incidence of disease that proved to be lethal compared to cancer free controls in one study (9).
The present analysis has many important strengths including our relatively large number of prostate cancer deaths, measurement of 25(OH)D in prospectively-collected serum in one laboratory, and detailed information on potential confounding factors. Although our findings and those from some previous studies suggest that years prior to diagnosis and earlier in the natural history of prostate cancer may be the important etiologic window to capture the 25(OH)D-survival relation, we did not have measurements of 25(OH)D for the cases at multiple time points to examine this further. Additional studies with vitamin D data before and after prostate cancer diagnosis could address this issue.
In this population of men diagnosed with prostate cancer, higher serum 25(OH)D prior to diagnosis was associated with improved prostate cancer survival. Our findings that higher 25(OH)D reduces the most clinically relevant prostate cancer outcome, disease-specific mortality, if true, could have important public health implications, including whether vitamin D supplementation should be considered for men diagnosed with prostate cancer. Additional large studies of circulating 25(OH)D and prostate cancer survival, and possibly clinical studies of supplementation among prostate cancer patients,are needed to corroborate the present findings before such recommendations could be made.
Acknowledgments
Financial Support: The ATBC Study is supported by the Intramural Research Program of the National Cancer Institute at the National Institutes of Health (U.S. Public Health Service), and by U.S. Public Health Service Contract Numbers awarded to D. Albanes: N01-CN-45165, N01-RC-45035, N01-RC-37004, HHSN261201000006C, HHSN261200800001E, and HHSN261201500005C.
Footnotes
Conflicts of Interest: The authors have no conflicts to disclose.
References
- 1.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States) Cancer Causes Control. 2005;16:83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–8. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79:362–71. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz GG, Hulka BS. Is vitamin D deficiency a risk factor for prostate cancer? (Hypothesis) Anticancer research. 1990;10:1307–11. [PubMed] [Google Scholar]
- 5.Xu Y, Shao X, Yao Y, Xu L, Chang L, Jiang Z, et al. Positive association between circulating 25-hydroxyvitamin D levels and prostate cancer risk: new findings from an updated meta-analysis. Journal of cancer research and clinical oncology. 2014;140:1465–77. doi: 10.1007/s00432-014-1706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schenk JM, Till CA, Tangen CM, Goodman PJ, Song X, Torkko KC, et al. Serum 25-hydroxyvitamin D concentrations and risk of prostate cancer: results from the Prostate Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev. 2014;23:1484–93. doi: 10.1158/1055-9965.EPI-13-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristal AR, Till C, Song X, Tangen CM, Goodman PJ, Neuhauser ML, et al. Plasma vitamin D and prostate cancer risk: results from the Selenium and Vitamin E Cancer Prevention Trial. Cancer Epidemiol Biomarkers Prev. 2014;23:1494–504. doi: 10.1158/1055-9965.EPI-14-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–71. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 9.Shui IM, Mucci LA, Kraft P, Tamimi RM, Lindstrom S, Penney KL, et al. Vitamin-D Related Genetic Variation, Plasma Vitamin D, and Risk of Lethal Prostate Cancer: A Prospective Nested Case-Control Study. J Natl Cancer Inst. 2012 doi: 10.1093/jnci/djs189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shui IM, Mondul AM, Lindstrom S, Tsilidis KK, Travis RC, Gerke T, et al. Cancer. 2015. Circulating vitamin D, vitamin D-related genetic variation, and risk of fatal prostate cancer in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albanes D, Mondul AM, Yu K, Parisi D, Horst RL, Virtamo J, et al. Serum 25-Hydroxy Vitamin D and Prostate Cancer Risk in a Large Nested Case-Control Study. Cancer Epidemiol Biomarkers Prev. 2011;20:1850–60. doi: 10.1158/1055-9965.EPI-11-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta D, Trukova K, Popiel B, Lammersfeld C, Vashi PG. The association between pre-treatment serum 25-hydroxyvitamin D and survival in newly diagnosed stage IV prostate cancer. PLoS One. 2015;10:e0119690. doi: 10.1371/journal.pone.0119690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holt SK, Kwon EM, Peters U, Ostrander EA, Stanford JL. Vitamin D pathway gene variants and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18:1929–33. doi: 10.1158/1055-9965.EPI-09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tretli S, Hernes E, Berg JP, Hestvik UE, Robsahm TE. Association between serum 25(OH)D and death from prostate cancer. Br J Cancer. 2009;100:450–4. doi: 10.1038/sj.bjc.6604865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.he alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. The ATBC Cancer Prevention Study Group. Ann Epidemiol. 1994;4:1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 16.Pietinen P, Hartman AM, Haapa E, Rasanen L, Haapakoski J, Palmgren J, et al. Reproducibility and validity of dietary assessment instruments. II. A qualitative food frequency questionnaire. Am J Epidemiol. 1988;128:667–76. doi: 10.1093/oxfordjournals.aje.a115014. [DOI] [PubMed] [Google Scholar]
- 17.Ersfeld DL, Rao DS, Body JJ, Sackrison JL, Jr, Miller AB, Parikh N, et al. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin Biochem. 2004;37:867–74. doi: 10.1016/j.clinbiochem.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Gallicchio L, Helzlsouer KJ, Chow WH, Freedman DM, Hankinson SE, Hartge P, et al. Circulating 25-hydroxyvitamin D and the risk of rarer cancers: Design and methods of the Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172:10–20. doi: 10.1093/aje/kwq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holt SK, Kolb S, Fu R, Horst R, Feng Z, Stanford JL. Circulating levels of 25-hydroxyvitamin D and prostate cancer prognosis. Cancer Epidemiol. 2013;37:666–70. doi: 10.1016/j.canep.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mondul AM, Shui IM, Yu K, Travis RC, Stevens VL, Campa D, et al. Genetic variation in the vitamin d pathway in relation to risk of prostate cancer--results from the breast and prostate cancer cohort consortium. Cancer Epidemiol Biomarkers Prev. 2013;22:688–96. doi: 10.1158/1055-9965.EPI-13-0007-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lokeshwar BL, Schwartz GG, Selzer MG, Burnstein KL, Zhuang SH, Block NL, et al. Inhibition of prostate cancer metastasis in vivo: a comparison of 1,23-dihydroxyvitamin D (calcitriol) and EB1089. Cancer Epidemiol Biomarkers Prev. 1999;8:241–8. [PubMed] [Google Scholar]
- 22.Krishnan AV, Shinghal R, Raghavachari N, Brooks JD, Peehl DM, Feldman D. Analysis of vitamin D-regulated gene expression in LNCaP human prostate cancer cells using cDNA microarrays. Prostate. 2004;59:243–51. doi: 10.1002/pros.20006. [DOI] [PubMed] [Google Scholar]
- 23.Tokar EJ, Webber MM. Cholecalciferol (vitamin D3) inhibits growth and invasion by up-regulating nuclear receptors and 25-hydroxylase (CYP27A1) in human prostate cancer cells. Clinical & experimental metastasis. 2005;22:275–84. doi: 10.1007/s10585-005-8393-z. [DOI] [PubMed] [Google Scholar]