Abstract
Melanoma differentiation-associated gene 5 (MDA-5, IFIH1), a cytosolic innate pattern recognition receptor, functions as a first line of defense against viral infection by sensing double-stranded RNA (dsRNA). Ectopic expression of MDA-5 has been shown to induce cancer cell death, but the mechanism of action by which MDA-5 exerts these cytotoxic effects is unclear. Here, we demonstrate that ectopic expression of MDA-5 via replication incompetent adenovirus (Ad.Mda-5) initiates multiple signaling cascades, culminating in cytotoxicity and type I interferon (IFN) production in mouse and human prostate cancer cells. This intrinsic dual activity of MDA-5 required the adaptor protein IFN-β promoter stimulator 1 (IPS-1, MAV) and could be functionally uncoupled. MDA-5 lacking N-terminal caspase-recruitment domains (CARDs) engaged an intracellular death program in cancer cells, but was unable to efficiently stimulate expression of IFN-β. In contrast to cancer cells susceptible to MDA-5-mediated cytotoxicity, normal cells were highly resistant and instead developed a robust type I IFN response. Strikingly, intratumoral delivery of Ad.Mda-5 led to regression of pre-established prostate cancers and development of long-lasting antitumor immune memory, which was primarily attributed to the activation of tumor-reactive cytotoxic T lymphocytes and/or natural killer cells. Using the CARDs-truncated MDA-5 mutant, silencing of IPS-1, and antibody blockade of the IFN-α/β-receptor, we further demonstrate that type I IFN signaling was crucial for in situ MDA-5-induced protective antitumor immunity. Therefore, deliberately targeting the evolutionarily conserved MDA-5-IPS-1 antiviral pathway in tumors can provoke parallel tumoricidal and immunostimulatory effects that bridge innate and adaptive immune responses for the therapeutic treatment of cancer.
Keywords: Innate pattern recognition receptor, melanoma differentiation associated gene-5, type I interferon, cell death, antitumor immunity, tumor microenvironment
Introduction
Innate pattern recognition receptors (PRRs), such as RIG-I-like receptor and toll-like receptors, are specialized for recognizing pathogen-associated molecular patterns and play essential roles in host immunity (1). Melanoma differentiation associated gene-5 (mda-5) or Helicard, initially identified by our group as a type I interferon (IFN)-inducible gene in human melanoma by subtraction hybridization (2), is a cytoplasmic RIG-I-like receptor and now considered as a first line of defense against viral infection by sensing viral double-stranded RNA (dsRNA) (3). The MDA-5 protein consists of two N-terminal tandem caspase-recruitment domains (CARDs), the central DExD/H-box motif helicase domain, and C-terminal domain. Upon recognition of viral dsRNA, MDA-5 binds to the adapter protein IFN-β promoter stimulator 1 (IPS-1), which is tethered to the outer mitochondrial membrane (4). This interaction via CARD domains triggers a complex signaling cascade involving transcription factors, NF-κB and IFN regulatory factor 3 (IRF-3) in particular, resulting in expression of the type I IFNs and IFN-stimulated genes to initiate antiviral immune responses (5–8). MDA-5 can also execute its antiviral activity by inducing death of virus-infected cells in a type I IFN-independent manner (9, 10). In this context, cleavage of the MDA-5 protein and subsequent nuclear translocation of the helicase domain further accelerates DNA fragmentation (11).
Our early work found that ectopic expression of mda-5 gene induces death of cancer cells (12, 13). We and others recently showed that activation of MDA-5 via intracellular delivery of polyinosinic:polycytidylic acid or poly(I:C), a synthetic mimetic of viral dsRNA, stimulates intrinsic cell death program involving the pro-apoptotic molecules NOXA and Caspases in cancer cells, but not healthy cells (14–16). While MDA-5-induced susceptibility of cancer cells to apoptosis shares the features observed during the elimination of virus-infected cells upon MDA-5 activation, the underlying basis of MDA-5 action in engaging the cancer cell death pathway remains less understood. Given the critical role of MDA-5 in priming type I IFN response and antiviral immunity, the therapeutic potential of MDA-5 in enhancing immune activation against established tumors has yet to be determined.
In the present study, we have investigated the biological effects of MDA-5, via ectopic expression of human or mouse MDA-5 protein, on selective induction of prostate cancer cell death and activation of type I IFN response. We have provided new insight into the structural domains of MDA-5 that confer direct tumoricidal or type I IFN-promoting effect upon its expression in cancer cells. Moreover, we demonstrate for the first time the superior antitumor efficacy of in situ MDA-5 therapy in eradicating established prostate cancers. The mechanistic studies reveal that this enhanced tumor control is mediated primarily by engaging type I IFN response in the tumor site via MDA-5-IPS-1 axis, which results in systemic mobilization of both innate and adaptive components of the immune system.
Materials and methods
Mice
Male wild-type (WT) C57BL/6 mice were obtained from National Cancer Institute (Bethesda, MD). IPS-1-deficient mice (IPS-1−/−), athymic nude mice were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were maintained under specific pathogen-free conditions. All experiments and procedures involving mice were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Cell lines and reagents
TRAMP-C2 cell line was derived from a prostate tumor that arose in a TRAMP (Transgenic Adenocarcinoma of Mouse Prostate) mouse on a C57BL/6 background (17, 18). TRAMP-C2 cells-expressing ovalbumin (C2-OVA) were generated in our laboratory (19). Human prostate adenocarcinoma line LNCaP and normal human prostate epithelial cell line RWPE-1 (CRL-11609) were from the American Type Culture Collection (ATCC). Cell line authentication was performed using short tandem repeat profiling DNA analysis and electrophoresis automatic sequencing by DDC Medical (Fairfield, OH). Mouse bone marrow-derived dendritic cells (BMDCs) were prepared as we previously described (20).
Production of recombinant virus
Human mda-5 (GenBank # NM_022168) and mouse mda-5 (GenBank accession # NM_027835) cDNAs were obtained by RT-PCR from THP1-derived macrophages and poly(I:C)-stimulated BMDCs, respectively. The replication-deficient adenovirus (Ad) encoding human influenza hemagglutinin (HA)-tagged human MDA-5 (hMDA-5), mouse MDA-5 (mMDA-5), CARD domains truncated forms of mouse or human MDA-5 (amino acid 252-1025, mMDA-5HC or hMDA-5HC retaining the helicase and CTD domains), 1st CARD domain truncated mouse MDA-5 (amino acid 101-1025, mMDA-5ΔC1), 2nd CARD domain truncated mouse MDA-5 (amino acid 101-200 deleted, mMda-5ΔC2) and control virus Ad.CMV were constructed using AdEasy™ system and packaged in HEK293A cells as previously described (18). Lentiviruses (LV) encoding mouse IPS-1 shRNA for gene knockdown (LV-IPS-1/KD, GCAACCAGACTGGACCAAATA) and scrambled control shRNA (LV-SC) were packaged using Phoenix cells as previously described (21).
Cell viability assays
Cell death was assessed using the Annexin V-FITC apoptosis detection kit (Roche Molecular Biochemicals, Germany) and analyzed by FACSCaliber flow cytometer (Becton Dickinson Biosciences, Franklin Lakes, NJ). Cell proliferation after Ad infection was measured using MTT assay as previously described (19).
Tumor studies
TRAMP-C2 or C2-OVA tumor cells (2×106) were injected subcutaneously (s.c.) in the right flank area of 6–8 weeks-old male C57BL/6 mice. 9–10 days after tumor implantation (approximately 3–5 mm in diameter), mice were randomly divided into groups and treated intratumorally (i.t.) with 1×108 p.f.u. of Ads. in 50 μL of PBS every 3 days for a total of 5 doses. LNCaP cells (1×107/mL) in 50 μL cold PBS were mixed with an equal volume of Matrigel and injected s.c. into the right flanks of male athymic nude mice. In some experiments, CD8+, CD4+ T cells, NK cells were depleted by intraperitoneal (i.p.) injection of 2.43, GK1.5, or PK136 antibodies, respectively, as described previously (18). For IFN-γ neutralization or blockade of type I IFN-α/β receptor, 200 μg anti-IFN-γ (clone XMG1.2) or 1 mg anti-IFNAR1 (clone MAR1-5A3) antibodies from Bio X Cell (West Lebanon, NH) were administrated i.p. five times at 3-days intervals, beginning one day prior to therapy.
Flow cytometric analysis and intracellular cytokine staining
Tumor tissues were processed by enzymatic digestion into single cell suspension as we previously described (18). Splenocytes from C2-OVA tumor bearing mice were stimulated with 1 μg/mL OVA254–267 peptide for 4 days. Tumor-infiltrating leukocytes from tumor tissues, freshly isolated or peptide-stimulated splenocytes were stained with antibodies to CD8a, NK1.1, CD3, and analyzed with a flow cytometer. Intracellular staining of IFN-γ and Granzyme B was performed as previously described (22).
qRT-PCR
Real time PCR was performed using TaqMan primers and carboxyfluorescein (FAM)-labeled probe sets from Life Technologies as we described previously (21). Target gene expression was normalized to Bactin and analyzed using the 2(−ΔΔCT) relative quantification method.
Cytotoxic activities of CTL and NK
The cytolytic activity of cytotoxic T cell (CTL) and natural killer (NK) cells was determined based on lactate dehydrogenase (LDH) release assay using a CytoTox 96 Non-Radioactive Cytotoxicity Assay Kit (Promega, Madison, WI).
Statistical analysis
Experiments were repeated two or three times. Quantitative data are expressed as mean ± S.D. for all figure panels in which error bars are shown. Statistical significance between groups within experiments was determined by the Student’s t-test or ANOVA test. A p value of less than 0.05 was considered statistically significant.
Other materials and methods
More details on reagents, cell viability assays, RT-PCR and qRT-PCR, immunofluorescence and confocal microscopy, and cytolytic assays are described in the Extended Experimental Procedures.
Results
Ectopic expression of MDA-5 triggers prostate cancer cell death and type I IFN production
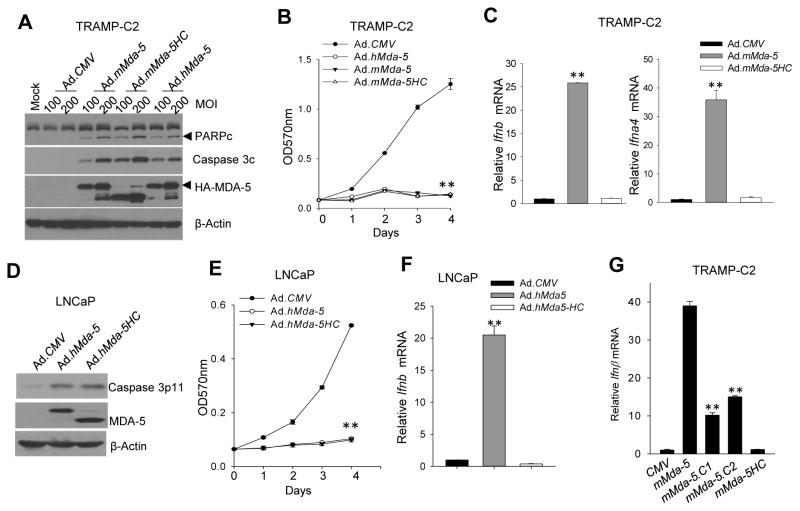
The proteolytic cleavage and separation of the N-terminal CARD domains from helicase domain of MDA-5 has been shown to accelerate apoptotic stimuli, such as virus infection and FasL treatment (11). To confirm the pro-apoptotic effect of ectopic expression of MDA-5, we infected mouse prostate cancer cells (i.e., TRAMP-C2) with an adenovirus encoding human MDA-5, mouse MDA-5, or mMDA-5HC (i.e., the CARD domains-truncated form of mouse MDA-5). Both mouse and human homologues of MDA-5 as well as mMDA-5HC were comparable in engaging intracellular death pathway, indicated by the cleavage of Caspase 3 and its main target poly (ADP-ribose) polymerase (PARP) (Fig 1A). Expressed full length MDA-5 appeared to undergo spontaneous MOI-dependent cleavage. Induction of cell death and growth inhibition of TRAMP-C2 tumor cells was confirmed by Annexin V staining (Supplementary figure S1A) and MTT assays (Fig. 1B), respectively. Infection of TRAMP-C2 cells with control Ad.CMV at a MOI up to 500 had no effect on cell viability (data not shown). Consistent with an established role of MDA-5 in type I IFN response, overexpression of full length mMDA-5, not mMDA-5HC, strongly induced upregulation of IFN-β and IFN-α4 in TRAMP-C2 tumor cells (Fig. 1C). Similar results were obtained in human prostate cancer LNCaP cells upon infection with Ad.hMda-5 or Ad.hMda-5HC (Fig. 1D–F, supplementary figure S1B). These results indicate that ectopic expression of MDA-5 in cancer cells provokes a ligand-free, cell death signaling and type I IFN response, which share the similar features as previously reported in viral-infected cells. Moreover, the CARD domains are required for stimulation of type I IFN signaling, but dispensable for killing of cancer cells.
Figure 1. Ectopic expression of MDA-5 results in cancer cell death and concomitant type I IFN production.
A. Adenoviral infection of mouse TRAMP-C2 tumor cells to express full length mouse MDA-5 (mMDA-5), human MDA5 (hMDA-5), or the CARDs-truncated mMDA-5 (mMDA-5HC) stimulated intrinsic cell death pathway, indicated by cleaved PARP (PARPc) and Caspase 3 (Caspase 3c, Asp175). B. Overexpression of MDA-5 or mMDA-5HC suppressed proliferation of TRAMP-C2 cells, analyzed by MTT assays. C. Deletion of CARD domains reduces the capacity of MDA-5 to induce IFN-β production. TRAMP-C2 cells were infected with indicated adenoviruses at a MOI of 200 for 90 min, followed by qRT-PCR analyses of the mRNA levels of IFN-β or IFN-α4 (**, p<0.01 vs CMV). D-F. Overexpression of full-length or CARD-truncated human MDA-5 in LNCaP cells activates Caspase 3 (D) and inhibits proliferation (E). Upregulation of the Ifnb gene was only observed in Ad.hMDA-5 infected cells (F). G. CARDs domains are essential for MDA-5-initiated type I IFN production. Deletion of either the first CARD (i.e., mMDA-5ΔC1) or the second CARD (i.e., mMDA-5ΔC2) impaired MDA-5-induced elevation of the Ifnb gene in TRAMP-C2 cells. **, p<0.01, vs mMDA-5. The experiments were repeated three times with similar results.
Taking into consideration that MDA-5 contains two N-terminal CARD domains, we constructed two additional truncated mouse MDA-5 mutants lacking either the first CARD (i.e., mMDA-5ΔC1) or the second CARD (i.e., mMDA-5ΔC2) to further dissect their roles in MDA-5-induced type I IFN response. As shown in Fig. 1G, absence of either CARD significantly reduced MDA-5 overexpression-triggered activation of the Ifnb gene in TRAMP-C2 cells. In agreement with analysis of mMDA-5HC, both mMDA-5ΔC1 and mMDA-5ΔC2 efficiently induced Caspase 3 activation and cancer cell death (Supplementary figure S1C–D).
IPS-1 is required for MDA-5-induced cancer cell death and IFN-β production
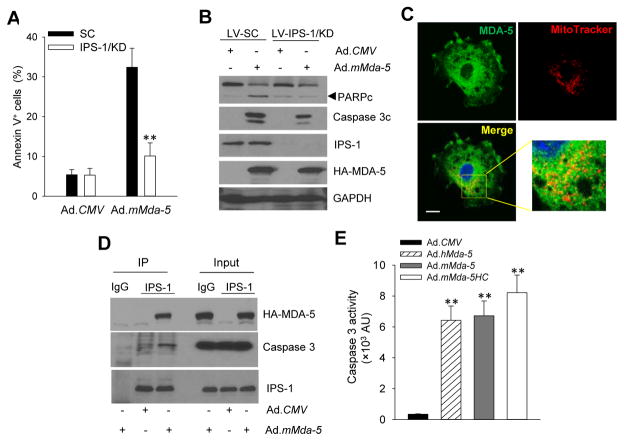
Given the documented role of the adaptor protein IPS-1 in RIG-I-like receptor-induced type I IFN response (6), we examined the potential involvement of IPS-1 in MDA-5 expression-induced cancer cell death and type I IFN production. Lentiviral-mediated IPS-1 knockdown in TRAMP-C2 cells conferred substantial protection from MDA-5-induced cell death, as shown by reduced frequency of Annexin V+ cells (Fig. 2A) as well as decreased levels of the cleaved Caspase 3 or PARP (Fig. 2B).
Figure 2. IPS-1 is required for MDA-5-induced apoptosis in cancer cells.
A–B. Lentiviral shRNA-mediated knockdown (KD) of IPS-1 rendered TRAMP-C2 cells resistant to MDA5-induced apoptosis, assayed by Annexin V staining (A) and immunoblotting of cleaved PARP or Caspase 3 (B). Cells infected with lentiviral-encoded scrambled shRNA (LV-SC) were used as controls. C. Presence of expressed HA-tagged MDA-5 in mitochondria, as shown by confocal microscopy analysis of its co-localization with MitoTracker. Scale bar, 10 μm. D. MDA-5 directly associates with the adapter protein IPS-1 and promotes the IPS-1 interaction with Caspase 3. TRAMP-C2 cells were infected with Ad.mMda-5 or Ad.CMV. Cell lysates were immunoprecipitated with anti-IPS-1 antibodies, followed by immunoblotting with anti-HA antibodies (for HA-tagged MDA-5) or anti-Caspase 3 antibodies. E. Expression of MDA-5 or mMDA-5HC activates Caspase 3, with its enzymatic activity measured in cell extracts 48 h after infection. AU, arbitrary units. The experiments were repeated three times with similar results (**, p<0.01).
The mitochondrial localization of IPS-1, disruption of mitochondria membrane potential, and cell death are important cellular responses to viral infection (23, 24). Confocal microscopy imaging showed a major cytoplasmic localization of ectopically expressed MDA-5 in Ad.mMda-5 infected TRAMP-C2 cells. MDA-5 protein lacks putative mitochondrial targeting sequence based on conventional prediction algorithms (TargetP, MitoProt). However, a small portion of HA-tagged MDA-5 were clearly visible in the mitochondria compartment when co-staining with the mitochondrial marker MitoTracker (Fig. 2C), which suggests potential involvement of mitochondria-associated MDA-5 in induction of cell death. The active form of Caspase 3 has also been observed in the mitochondria, mostly translocated from the cytosol (25). Indeed, co-immunoprecipitation assays showed that ectopically expressed MDA-5 directly interacted with intracellular IPS-1, which appreciably increased IPS-1 association with Caspase 3 (Fig. 2D). Additionally, expression of full length MDA-5 or CARDs-truncated MDA-5 mutant caused similar enzymatic activation of Caspase 3 in TRAMP-C2 cells (Fig. 2E), which is consistent with their tumoricidal activities.
IPS-1 mediates MDA-5 overexpression-induced type I IFN response
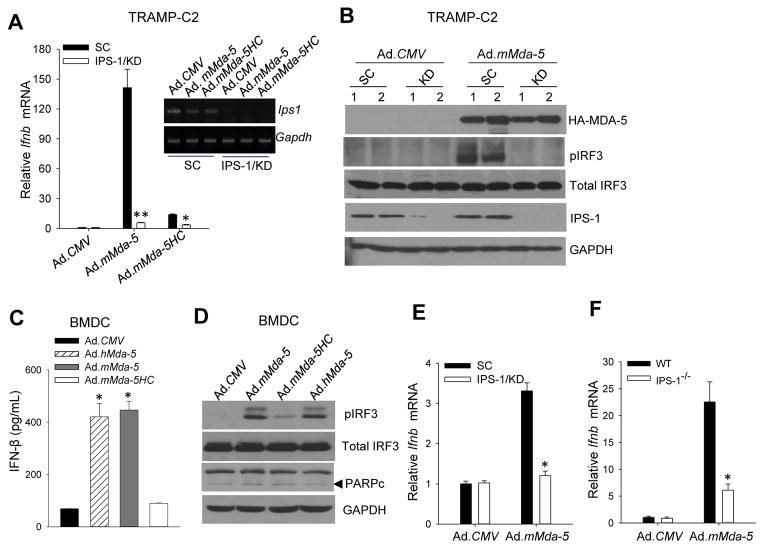
A Previous study reported that deficiency of IPS-1 abolished type I IFN response in various cell types upon viral infection (26). We found that genetically ablating IPS-1 in TRAMP-C2 tumor cells nearly completely blocked MDA-5 overexpression-induced IFN-β production. However, MDA-5HC failed to stimulate IFN-β production in TRAMP-C2 cells, suggesting the CARD domain is essential for MDA-5-IPS-1 signaling-triggered type I IFN response (Fig. 3A). Recruitment of transcription factor IRF3 to IPS-1 is required for its activation to coordinately regulate the type I IFNs during viral infection (5, 27). Immunoblotting analysis showed that MDA-5 overexpression in TRAMP-C2 induced the phosphorylation of IRF3, which was inhibited by IPS-1 knockdown, indicating that MDA-5-induced type I IFN production in cancer cells is dependent on IPS-1-IRF3 signaling pathway (Fig. 3B).
Figure 3. MDA-5 induces IFN-β production in cancer cells and normal cells via IPS-1-IRF3 signaling pathway.
A–B. Stable knockdown of IPS-1 (KD) in TRAMP-C2 tumor cells abolished MDA-5-induced IFN-β expression (A) and IRF3 phosphorylation (B). C–D. MDA-5 expression-triggered type I IFN response is intact in normal cells. Primary BMDCs were subjected to ELISA assays for IFN-β production (C) or immunoblotting analyses for phosphorylation of IRF3 and cleaved PARP (D) after infection with indicated viruses. E–F. IPS-1-dependent IFN-β expression upon MDA-5 expression in normal cells. IPS-1-knockdown BMDCs (E) or IPS-1−/− BMDCs (F) were infected with Ad.CMV or Ad.mMda-5, followed by qRT-PCR analysis of IFN-β expression. LV-scrambled BMDCs (SC) or wild-type (WT) BMDCs were used as controls, respectively. The results are presented as fold over Ad.CMV-infected control cells. Data are means ± S.D. (n =3) and representative of three experiments (*, p<0.05; **, p<0.01).
We next examined the effects of MDA-5 overexpression in dendritic cells (DCs), which play a pivotal role in responding to viral infection and in shaping host immunity (28). In contrast to cancer cells, ectopic expression of MDA-5 or MDA-5HC had little effect on viability of BMDCs (Supplementary Fig. S2). However, both human and mouse MDA-5, but not CARDs-truncated MDA-5 (i.e., mMDA-5HC), induced high production of IFN-β (Fig. 3C). As expected, overexpression of either MDA-5 or MDA-5HC had no effect on PARP cleavage, while only full length MDA-5 was able to induce phosphorylation of IRF3 in BMDCs (Fig. 3D). Furthermore, MDA-5 overexpression-induced IFN-β production was substantially suppressed in either IPS-1 genetically ablated BMDCs (Fig. 3E) or IPS-1 deficient BMDCs (Fig. 3F), suggesting that MDA-5-initiated IPS-1-IRF3-type I IFN signaling pathway was identical and functional in both cancer cells and host myeloid cells.
Previous studies showed that intracellular delivery of synthetic dsRNA poly(I:C) using cationic liposome polyethylenimine (pIC-PEI) induces type I IFN-independent apoptosis in cancer cells via activation of MDA-5 (14–16). The concurrent upregulation of anti-apoptotic molecule Bcl-xL in normal cells is believed to render these cells less susceptible to pIC-PEI induced apoptosis (15). Similarly, primary BMDCs or normal human prostate epithelial cell line RWPE-1 were resistant to MDA-5 expression-induced apoptosis as compared with mouse (i.e., TRAMP-C2) or human (i.e., LNCaP) prostate cancer cells (Supplementary Fig. S3A and S3B). We showed that pIC-PEI transfection only modestly induced MDA-5 expression when comparing with Ad.mMda-5, however, pIC-PEI transfection enhanced the cleavage of PARP (Supplementary Fig. S3A and S3B) in normal cells. This is further confirmed by Annexin V staining of BMDCs (Supplementary Fig. S3C), indicating the onset of apoptosis in these normal cells following pIC-PEI treatment. Additionally, we did not see upregulation of Bcl-xL or MCL1 in pIC-PEI transfected normal cells (i.e., BMDCs. Supplementary Fig. S3D) as previously reported (15).
Interestingly, ectopic expression of MDA-5 stimulated much higher levels of IRF3 activation in mouse TRAMP-C2 tumor cells than did pIC-PEI (Supplementary Fig. S4A). Consistently, MDA-5 overexpression induced much higher magnitude and more sustained production of IFN-β as compared with pIC-PEI transfection (Supplementary Fig. S4B). A similar enhanced IRF3 activation and IFN-β induction were also observed in Ad.Mda-5-infected BMDCs (Supplementary Fig. S4C and S4D).
In situ MDA-5 therapy potentiates an antitumor immune response to prostate cancer
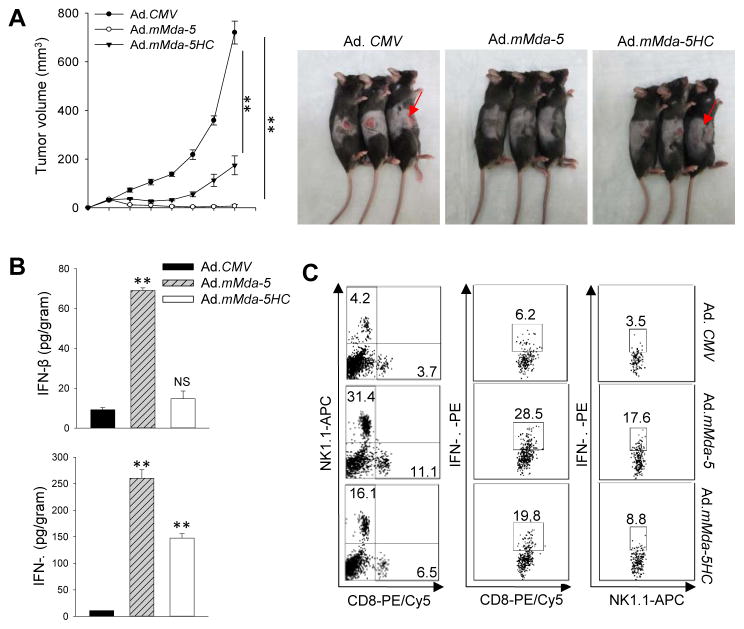
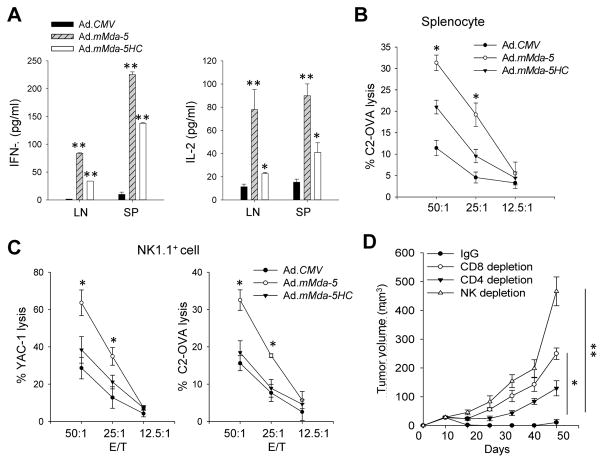
To test the hypothesis that tumoricidal activity of MDA-5 and concurrent induction of type I IFNs drives antitumor immunity in vivo, we exploited OVA-expressing TRAMP-C2 tumor line (C2-OVA) that permits immunomonitoring of antigen-specific immune response (19). Mice established with C2-OVA tumors (average size 50 mm3) received i.t. treatment with Ad.CMV, Ad.mMda-5, and Ad.mMda-5HC. While Ad.CMV had little effect, Ad.mMda-5HC that is impaired in inducing type I IFNs partially delayed tumor growth. However, four out of five mice that had been treated with Ad.mMda-5 rejected their tumors (Fig. 4A). TUNEL assays showed that Ad.mMda-5 therapy caused significantly increased tumor cell death that positively correlated with enhanced tumor infiltration by NK1.1+ and CD8+ cells as compared with Ad.CMV or Ad.mMda-5HC (Supplementary Fig. S5A). Ad.mMda-5 treatment induced the highest levels of IFN-β, IFN-γ (Fig. 4B), MCP-1 and IL-12 (Supplementary Fig. S5B) in the tumors, whereas Ad.mMda-5HC only resulted in moderate upregulation of IFN-γ expression. Flow cytometric analyses further confirmed the markedly increased frequencies of tumor-infiltrating NK cells and CD8+ T cells after MDA-5 therapy, which is consistent with the observation of high levels of MCP-1, a chemoattractant known to regulate the recruitment of T lymphocytes and NK cells (29, 30), as well as IL-12, which enhances the cytotoxic function of these antitumor effector cells (31). This elevated immune infiltration after Ad.mMda-5 therapy was also associated with elevated activation of OVA-specific CD8+ T and NK cells, as determined by intracellular staining for IFN-γ (Fig. 4C) and Granzyme B (Supplementary Fig. S5C).
Figure 4. In situ MDA-5 therapy eradicates mouse prostate cancer and potentiates innate and adaptive immune activation in tumor sites.
A. C57BL/6 mice established with C2-OVA tumors (n=5) were treated intratumorally with adenoviruses for a total of five doses. Ad.mMda-5 therapy resulted in regression of tumors compared to those receiving Ad.CMV or Ad.mMda-5HC. Photographs of treated tumors at the end of the study are also shown. B. Elevation of IFN-β and IFN-γ levels in C2-OVA tumors following Ad.mMda-5 therapy, as determined by tissue ELISA assays. C. MDA-5 therapy induces increased tumor infiltration by CD8+ T cells and NK1.1+ cells as well as enhanced IFN-γ production in these infiltrating cells, assayed by intracellular cytokine staining and FACS analyses. Data are representative of three independent experiments (**, p<0.01; NS, not significant).
The therapeutic activity of MDA-5 depends on immune activation involving CD8+ T cells and NK cells
Upon stimulation with tumor cell lysates, splenocytes or draining lymph node cells from Ad.mMda-5-treated mice showed enhanced production of IFN-γ and IL-2 (Fig. 5A). The increased frequency of OVA-specific IFN-γ-producing CD8+ CTLs was similarly shown in the spleens from Ad.mMda-5-treated mice (Supplementary Fig. S6A), suggesting systemic activation of tumor/antigen-reactive T cells by local MDA-5 therapy. Furthermore, splenocytes from mice receiving Ad.mMda-5 displayed higher levels of cytotoxicity against the C2-OVA cells compared to those from Ad.CMV or Ad.mMda-5HC-treated mice (Fig. 5B). Enhanced cytolytic activity of NK cells, derived from Ad.mMda-5-treated mice, was also shown when either C2-OVA cells or YAC-1 cells were used as targets (Fig. 5C). To provide direct evidence and identify the immune cell subsets responsible for antitumor potency of MDA-5 therapy, we carried out in vivo depletion of leukocyte populations prior to treatment. Depletion of CD4+ cells modestly reduced the therapeutics effect, whereas the lack of CD8+ cells or NK cells ablated the antitumor activity of Ad.mMda-5 (Fig. 5D).
Figure 5. In situ MDA-5 therapy generates a potent tumor-reactive CTL and NK cell response.
A. Lymph node (LN) cells or splenocytes (SP) from treated C2-OVA tumor bearing mice were stimulated with tumor cell lysates, followed by ELISA analyses of IFN-γ or IL-2 levels in the media. B. Increased cytotoxicity of effector T cells from Ad.mMda-5-treated mice. Pooled splenocytes (n=3) were cocultured with inactivated C2-OVA cells for 6 days in the presence of IL-2 (40 IU/mL). The cytolytic activity of stimulated T cells was determined using a LDH release assay with live C2-OVA cells as targets. C. Cytotoxicity of NK cells was analyzed by mixing NK1.1+ cells sorted from the spleens (n=3) as effectors with C2-OVA or YAC-1 cells (1×104) as targets at varying effector to target (E:T) ratios. Data shown are the mean percentage of killing from triplicates ± SD and are representative of two independent experiments. D. CD8+ cells and NK1.1+ cells are involved in the therapeutic activity of Ad.mMda-5. C2-OVA tumor-baring mice (n=5) were depleted of effector cell subsets with antibodies prior to MDA-5 therapy (*, p<0.05; **, p<0.01).
In the C2-OVA tumor model, approximately 80 % of mice receiving in situ MDA-5 therapy showed tumor regression. These tumor-free mice were protected from tumor re-challenge two months later with either C2-OVA or parental TRAMP-C2 cells. Despite no tumor development in these mice upon secondary challenge, we showed strong induction of IFN-γ in the spleens or splenocytes stimulated with MHC I-restricted OVA254–267 peptide (Supplementary Fig. S6B and S6C), indicating the establishment of long-term protective immune memory.
In situ MDA-5 therapy induces immune-mediated inhibition of human prostate cancer
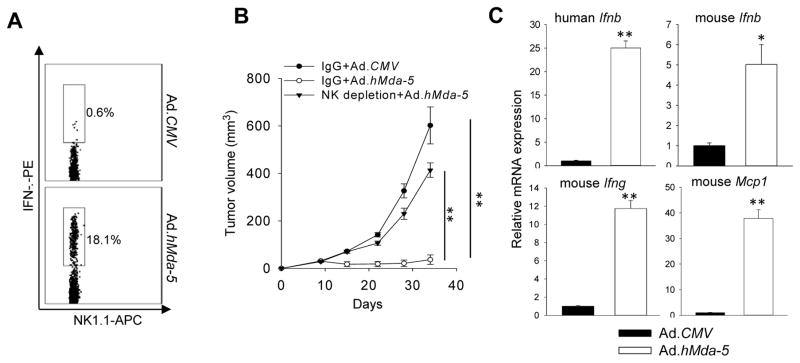
Since NK cells are involved in the antitumor activity of MDA-5 therapy in immune competent mice, we further examined its potential role for controlling human prostate cancer (i.e., LNCaP) in athymic nude mice, which have a functional NK cell compartment. As predicted, Ad.hMda-5 therapy induced NK cell activation, as shown by intracellular cytokine staining for IFN-γ-expression in tumor-infiltrating NK cells (Fig. 6A). These tumor-bearing mice, when depleted of NK cells, failed to respond to Ad.hMda-5 treatment, resulting in outgrowth of human prostate cancer (Fig. 6B). Intriguingly, qRT-PCR analyses showed that both mouse and human forms of IFN-β were up-regulated in the LNCaP tumors, suggesting that administration of human MDA-5 elicits type I IFN response in human prostate cancer cells as well as in the stromal compartment and both of which may contribute to the functional activation of immune effector cells. Similar to the observation in mouse prostate tumor model, Ad.hMda-5 also induced IFN-γ and MCP-1 production in the tumor site (Fig. 6C).
Figure 6. In situ MDA-5 therapy suppresses human prostate cancer via NK cell activation.
A. Activation of NK cells by Ad.hMda-5 therapy, indicated by intracellular IFN-γ staining of CD3−NK1.1+ cells isolated from LNCaP tumors in athymic nude mice. B. Depletion of NK1.1+ cells abolished the therapeutic activity of hMDA-5. Starting one day before treatment, mice (n=5) were given PK136 (200 μg/ml) or control IgG at weekly intervals. C. Elevation of human Ifnb, mouse Ifnb, Ifng, and Mcp1 gene expression in LNCaP tumors after Ad.hMda-5 therapy. Data shown are representative of two independent experiments (*, p<0.05; **, p<0.01).
Type I IFN pathway in the tumor sites is essential for antitumor efficacy of in situ MDA-5 therapy
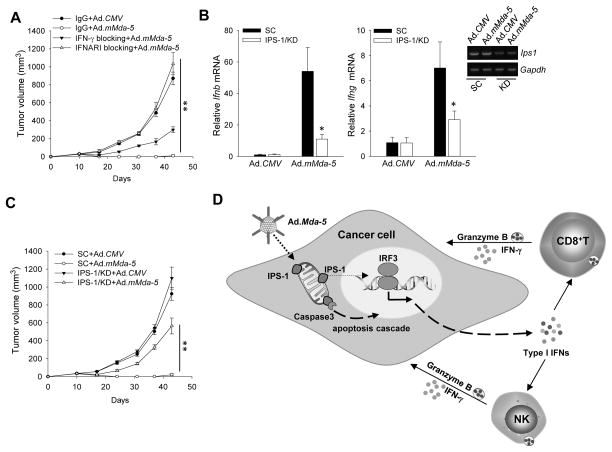
Considering the robust induction of IFN-β in the tumor environment following MDA-5 treatment, we sought to define the contribution of type I IFN response to MDA-5 expression-generated antitumor immunity. In vivo blockade of IFN-β function using antibodies against IFN-α/β receptor subunit 1 (IFNAR-1) abolished the therapeutic effect of Ad.mMda-5 treatment in C2-OVA tumor model, whereas neutralization of endogenous IFN-γ only partially attenuated the antitumor activity of MDA-5 (Fig. 7A). Antibody blockade of IFNAR-1 did not affect Ad.mMda5-induced IFN-β production, but significantly reduced the IFN-β level in the tumor site (Supplementary Fig. S7).
Figure 7. Type I IFN pathway is crucial for in situ MDA-5 therapy-induced protective antitumor immunity.
A. Type I IFN signaling and IFN-γ are critical for tumor elimination by Ad.mMda-5. Antibody blockade of the IFN-α/β receptor (IFNAR-1) or neutralization of IFN-γ dampened the antitumor activity of Ad.mMda-5 in C2-OVA tumor-bearing mice (n=5). B. Downregulation of IPS-1 expression in tumors by administration of a lentivirus encoding shRNA for gene silencing attenuated MDA-5-induced immune activation, indicated by reduced levels of IFN-β or IFN-γ in tumors. C. Silencing of IPS-1 abrogated the antitumor potency of Ad.mMda-5. Data shown are representative of two independent experiments (*, p<0.05; **, p<0.01). D. Targeting the MDA-5-IPS-1 pathway in tumors to potentiate protective antitumor immunity. Overexpression of MDA-5, a sensor for viral dsRNA, in cancer cells triggers activation of IRF-3 and Caspase 3 in an IPS-1 dependent manner. The interaction between MDA-5 and its downstream adapter protein IPS-1 results in production of type I IFNs and concomitant cancer cell death. The type I IFNs promote the activation and effector functions of natural killer (NK) cells and/or cytotoxic T lymphocytes (CTLs). Collaborative action of tumor-reactive innate and adaptive immune cells leads to effective tumor eradication.
Considering that IPS-1 mediates type I IFN production by ectopic expression of MDA-5, we down-regulated the IPS-1 levels in the tumor sites by intra/peritumoral injection of lentivirus-encoding IPS-1 shRNA (Fig. 7B). IPS-1 silencing resulted in reduced production of both IFN-β and IFN-γ in the tumor sites (Fig. 7B), suggesting that IPS-1 is critical for Ad.mMda-5-induced type I IFN response and immune activation. As a result, IPS-1 silencing profoundly impaired the therapeutic efficacy of MDA-5 therapy (Fig. 7C).
Discussion
The critical role of the innate PRR MDA-5 has been well recognized in the host antiviral response by sensing cytoplasmic dsRNA and initiating a well-synchronized signaling cascade resulting in upregulation of type I IFNs and an array of IFN-stimulated genes. Emerging evidence shows that ectopic expression of MDA-5 (2, 12, 13) or viral dsRNA mimetic (14–16, 32) directly induces a type I IFNs-independent tumoricidal effect in human cancer cells (15). Considering the therapeutic potential of MDA-5, the current study provides new insight into the molecular basis of MDA-5 function in provoking cancer cell death and type I IFN signaling. Moreover, we dissect the impact of these two features of MDA-5 on its therapeutic capacity in immune competent mouse model and demonstrate, for the first time, that MDA-5 activation elicited type I IFN signaling and consequent priming of innate and adaptive immune responses are essential for the antitumor potency of MDA-5-targeted therapy.
Our data show that ectopic expression of mouse or human MDA-5 in prostate cancer cells triggers two distinct and functionally independent pathways, i.e., induction of cell death and production of type I IFNs. Both mouse and human MDA-5 are comparable in engaging these two cellular processes, probably due to their sequence homology and evolutionarily conservative function in the host viral defense. Intriguingly, while tandem CARD domain-truncated MDA-5 (i.e., mouse or human MDA-5HC, mMDA-5ΔC1, or mMDA-5ΔC2) is fully competent in inducing cancer cell death, it lacks the ability to stimulate type I IFN production (Fig. 1), indicating that the CARDs are essential for MDA-5-initiated signaling to upregulate type I IFN genes (e.g., Ifna4, Ifnb) and, more importantly, these two functional features of MDA-5 may be uncoupled due to the cleavage and release of CARDs from MDA-5, which has been previously reported in viral-infected cells (9). Indeed, the CARDs were recently suggested to provide a polymerization-dependent signaling platform for recruiting the adaptor protein IPS-1 and amplifying the downstream signaling cascade (33). We also make an interesting finding that IPS-1 mediates MDA-5 overexpression induced Caspase 3-dependent cell death program as well as the induction of type I IFNs in cancer cells because silencing of IPS-1 impairs both pathways (Fig. 2). Confocal imaging and co-immunoprecipitation data indicate that at least a small portion of ectopically expressed MDA-5 resides in mitochondria organelle and is associated with IPS-1. Furthermore, MDA-5 expression in cancer cells clearly promotes the interaction of IPS-1 with Caspase 3, resulting in enhanced activation of Caspase 3. However, additional molecular studies are required to define the C-terminal domain required for activation of the Caspase 3 pathway and to understand how IPS-1 differentially regulates these two MDA-5-initiated cellular responses in cancer cells.
We demonstrate that MDA-5 overexpression-induced cell death is specific for cancer cells (e.g., mouse or human prostate cancer), not normal cells (e.g., prostate epithelial cells, primary myeloid DCs), which is generally consistent with our previous finding in pIC-PEI -transfected human melanoma (14) and human pancreatic cancer (16) cells. However, ectopic expression of MDA-5 appears to more efficiently induce type I IFN production in both prostate cancer cells and normal cells. In addition, pIC-PEI transfection displays some cytotoxic effects on normal cells compared to adenovirus-mediated infection for MDA-5 overexpression that exhibits little normal cell toxicity, which may be caused by the activation of multiple PRRs (e.g., RIG-I, TLR3 or TLR5) in response to the pIC-PEI stimulation(34). Furthermore, compared to pIC-PEI, MDA-5 overexpression induces stronger activation of IRF3 as well as much more robust and sustained IFN-β production that may potentially support immune-mediated tumor destruction.
A striking finding of our study is that, in addition to its direct tumoricidal effect, tumor-targeted MDA-5 therapy potentiates highly potent antitumor immune responses and demonstrates superior antitumor activity (Fig. 4 and 5). In situ MDA-5 therapy using a non-replicative adenovirus (i.e., Ad.mMda-5) results in a cure of 80 % of syngeneic mouse prostate cancers established in immunocompetent mice. Generation of protective antitumor immunity is further indicated by the observation that these tumor-free mice resist secondary tumor challenge. In situ MDA-5 therapy-induced immune activation is also supported by elevation of type I IFN (i.e., IFN-β) and Th1 cytokine IFN-γ in the tumor microenvironment (TME) associated with increased frequencies in functionally activated, tumor-infiltrating cytotoxic CD8+ CTLs and NK cells. Furthermore, depletion of immune cell subsets reveals that both CD8+ T cells and NK cells are primarily responsible for the therapeutic inhibition of established TRAMP-C2 tumors, suggesting that mobilizing the innate and adaptive arms of the immune systems is a major antitumor mechanism underlying in situ MDA-5 therapy in immune competent mice.
Type I IFNs are critical for functional activation of NK cells and antigen-presenting cells (e.g., DCs) as well as cross-priming of CD8+ CTLs in immune surveillance or tumor immunotherapy (35–37). We have provided compelling evidence supporting a crucial role of type I IFN pathway in MDA-5 therapy-induced antitumor immunity. First, deletion of CARDs that are critical for type I IFN production in cancer cells substantially reduced the antitumor efficacy of MDA-5, which coincides with decreased activation and infiltration of cytotoxic effector cells in the TME (Fig. 4). Second, antibody blockade of receptor for type I IFNs attenuates the MDA-5 therapy-induced antitumor response (Fig. 7A). Lastly, disruption of MDA-5-triggered type I IFN production via silencing of IPS-1 in the TME also similarly abolished the antitumor effect of MDA-5 (Fig. 7C). Therefore, the intrinsic function of MDA-5 in stimulating the type I IFN pathway, compared to its pro-apoptotic activity in cancer cells, may be more essential in MDA-5 therapy-potentiated tumor eradication in vivo, at least in immune competent host (Fig. 7D). Given the ubiquitous expression of MDA-5 and induction of type I IFN in cancer cells as well as in the stroma after MDA-5 therapy, it is of particularly germane to understand the relative contribution of tumor or host cell-derived IPS-1-type I IFN pathway to overall antitumor immunity.
Collectively, we demonstrate that ectopic expression of MDA-5 selectively induces two distinct pathways, i.e., cancer cell death and type I IFN response, both of which is mediated by the adaptor molecule IPS-1. In situ MDA-5-targeted therapy generates superior antitumor immune response that is critically dependent on type I IFN response, highlighting a previously unappreciated role of MDA-5 in antitumor immunity. Since the immunosuppressive tumor microenvironment presents a major challenge for successful cancer immunotherapy, strategically engaging this ancient viral sensing MDA-5-IPS-1 pathway (38) in the TME represents a novel approach to efficiently reprograming or restoring protective antitumor immunity for cancer treatment.
Supplementary Material
Acknowledgments
The present study was supported in part by National Institutes of Health (NIH) Grants CA175033, CA154708 (X-Y.W), Department of Defense W81XWH-11-0480/0481, W81XWH-13-0409/0455 (P.B.F., X-Y.W), the National Foundation for Cancer Research (P.B.F). Flow cytometry facility was supported in part by NCI Cancer Center Support Grant to VCU Massey Cancer Center P30CA16059. X-Y.W. is the Mary Anderson Harrison Distinguished Professor in Cancer Research in the VCU Massey Cancer Center. P.B.F. holds the Thelma Newmeyer Corman Chair in Cancer Research in the VCU Massey Cancer Center.
Abbreviations
- Ad
adenovirus
- MDA-5
melanoma differentiation associated protein-5
- IPS-1
interferon-β promoter stimulator 1
- TME
tumor microenvironment
Footnotes
The authors disclose no potential conflict of interest
Author contribution
X.Y. and X.Y.W designed research; X.Y., H.W., X.L., C. G., F.Y. performed research. X.Y., X.Y.W and P.B.F analyzed data. X.Y., X.Y.W., and P.B.F. wrote the manuscript.
References
- 1.Meylan E, Tschopp, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 2.Kang DC, Gopalkrishnan RV, Wu Q, Jankowsky E, Pyle AM, Fisher PB. mda-5: An interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc Natl Acad Sci U S A. 2002;99:637–42. doi: 10.1073/pnas.022637199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–5. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 4.Wu B, Peisley A, Richards C, Yao H, Zeng X, Lin C, et al. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell. 2013;152:276–89. doi: 10.1016/j.cell.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 5.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–82. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, et al. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nature immunology. 2005;6:981–8. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 7.Bowie AG, Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signalling. Nat Rev Immunol. 2008;8:911–22. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeuchi O, Akira S. MDA5/RIG-I and virus recognition. Curr Opin Immunol. 2008;20:17–22. doi: 10.1016/j.coi.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Barral PM, Morrison JM, Drahos J, Gupta P, Sarkar D, Fisher PB, et al. MDA-5 is cleaved in poliovirus-infected cells. Journal of virology. 2007;81:3677–84. doi: 10.1128/JVI.01360-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuo RL, Kao LT, Lin SJ, Wang RY, Shih SR. MDA5 plays a crucial role in enterovirus 71 RNA-mediated IRF3 activation. PloS one. 2013;8:e63431. doi: 10.1371/journal.pone.0063431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovacsovics M, Martinon F, Micheau O, Bodmer JL, Hofmann K, Tschopp J. Overexpression of Helicard, a CARD-containing helicase cleaved during apoptosis, accelerates DNA degradation. Current biology : CB. 2002;12:838–43. doi: 10.1016/s0960-9822(02)00842-4. [DOI] [PubMed] [Google Scholar]
- 12.Kang DC, Gopalkrishnan RV, Lin L, Randolph A, Valerie K, Pestka S, et al. Expression analysis and genomic characterization of human melanoma differentiation associated gene-5, mda-5: a novel type I interferon-responsive apoptosis-inducing gene. Oncogene. 2004;23:1789–800. doi: 10.1038/sj.onc.1207300. [DOI] [PubMed] [Google Scholar]
- 13.Lin L, Su Z, Lebedeva IV, Gupta P, Boukerche H, Rai T, et al. Activation of Ras/Raf protects cells from melanoma differentiation-associated gene-5-induced apoptosis. Cell Death Differ. 2006;13:1982–93. doi: 10.1038/sj.cdd.4401899. [DOI] [PubMed] [Google Scholar]
- 14.Tormo D, Checinska A, Alonso-Curbelo D, Pérez-Guijarro E, Cañón E, Riveiro-Falkenbach E, et al. Targeted Activation of Innate Immunity for Therapeutic Induction of Autophagy and Apoptosis in Melanoma Cells. Cancer Cell. 2009;16:103–14. doi: 10.1016/j.ccr.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Besch R, Poeck H, Hohenauer T, Senft D, Häcker G, Berking C, et al. Proapoptotic signaling induced by RIG-I and MDA-5 results in type I interferon-independent apoptosis in human melanoma cells. The Journal of Clinical Investigation. 2009;119:2399–411. doi: 10.1172/JCI37155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhoopathi P, Quinn BA, Gui Q, Shen XN, Grossman SR, Das SK, et al. Pancreatic cancer-specific cell death induced in vivo by cytoplasmic-delivered polyinosine-polycytidylic acid. Cancer Res. 2014;74:6224–35. doi: 10.1158/0008-5472.CAN-14-0819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325–30. [PubMed] [Google Scholar]
- 18.Yu X, Guo C, Yi H, Qian J, Fisher PB, Subjeck JR, et al. A multifunctional chimeric chaperone serves as a novel immune modulator inducing therapeutic antitumor immunity. Cancer research. 2013;73:2093–103. doi: 10.1158/0008-5472.CAN-12-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao P, Sun X, Chen X, Wang Y, Foster BA, Subjeck J, et al. Secretable chaperone Grp170 enhances therapeutic activity of a novel tumor suppressor, mda-7/IL-24. Cancer Res. 2008;68:3890–8. doi: 10.1158/0008-5472.CAN-08-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yi H, Yu X, Gao P, Wang Y, Baek SH, Chen X, et al. Pattern recognition scavenger receptor SRA/CD204 down-regulates Toll-like receptor 4 signaling-dependent CD8 T-cell activation. Blood. 2009;113:5819–28. doi: 10.1182/blood-2008-11-190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu X, Yi H, Guo C, Zuo D, Wang Y, Kim HL, et al. Pattern recognition scavenger receptor CD204 attenuates Toll-like receptor 4-induced NF-kappaB activation by directly inhibiting ubiquitination of tumor necrosis factor (TNF) receptor-associated factor 6. The Journal of biological chemistry. 2011;286:18795–806. doi: 10.1074/jbc.M111.224345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian J, Yi H, Guo C, Yu X, Zuo D, Chen X, et al. CD204 suppresses large heat shock protein-facilitated priming of tumor antigen gp100-specific T cells and chaperone vaccine activity against mouse melanoma. J Immunol. 2011;187:2905–14. doi: 10.4049/jimmunol.1100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott I, Norris KL. The mitochondrial antiviral signaling protein, MAVS, is cleaved during apoptosis. Biochemical and biophysical research communications. 2008;375:101–6. doi: 10.1016/j.bbrc.2008.07.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lei Y, Moore CB, Liesman RM, O'Connor BP, Bergstralh DT, Chen ZJ, et al. MAVS-mediated apoptosis and its inhibition by viral proteins. PloS one. 2009;4:e5466. doi: 10.1371/journal.pone.0005466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chandra D, Tang DG. Mitochondrially localized active caspase-9 and caspase-3 result mostly from translocation from the cytosol and partly from caspase-mediated activation in the organelle. Lack of evidence for Apaf-1-mediated procaspase-9 activation in the mitochondria. The Journal of biological chemistry. 2003;278:17408–20. doi: 10.1074/jbc.M300750200. [DOI] [PubMed] [Google Scholar]
- 26.Daffis S, Suthar MS, Szretter KJ, Gale M, Jr, Diamond MS. Induction of IFN-beta and the innate antiviral response in myeloid cells occurs through an IPS-1-dependent signal that does not require IRF-3 and IRF-7. PLoS pathogens. 2009;5:e1000607. doi: 10.1371/journal.ppat.1000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu S, Cai X, Wu J, Cong Q, Chen X, Li T, et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science. 2015;347:aaa2630. doi: 10.1126/science.aaa2630. [DOI] [PubMed] [Google Scholar]
- 28.Schmid MA, Diamond MS, Harris E. Dendritic cells in dengue virus infection: targets of virus replication and mediators of immunity. Frontiers in immunology. 2014;5:647. doi: 10.3389/fimmu.2014.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamilton ST, Scott GM, Naing Z, Rawlinson WD. Human cytomegalovirus directly modulates expression of chemokine CCL2 (MCP-1) during viral replication. The Journal of general virology. 2013;94:2495–503. doi: 10.1099/vir.0.052878-0. [DOI] [PubMed] [Google Scholar]
- 30.Antonelli LR, Gigliotti Rothfuchs A, Goncalves R, Roffe E, Cheever AW, Bafica A, et al. Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. The Journal of clinical investigation. 2010;120:1674–82. doi: 10.1172/JCI40817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13:722–8. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glas M, Coch C, Trageser D, Dassler J, Simon M, Koch P, et al. Targeting the cytosolic innate immune receptors RIG-I and MDA5 effectively counteracts cancer cell heterogeneity in glioblastoma. Stem cells. 2013;31:1064–74. doi: 10.1002/stem.1350. [DOI] [PubMed] [Google Scholar]
- 33.Berke IC, Yu X, Modis Y, Egelman EH. MDA5 assembles into a polar helical filament on dsRNA. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18437–41. doi: 10.1073/pnas.1212186109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cubillos-Ruiz JR, Engle X, Scarlett UK, Martinez D, Barber A, Elgueta R, et al. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. The Journal of Clinical Investigation. 2009;119:2231–44. doi: 10.1172/JCI37716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swann JB, Hayakawa Y, Zerafa N, Sheehan KC, Scott B, Schreiber RD, et al. Type I IFN contributes to NK cell homeostasis, activation, and antitumor function. Journal of immunology. 2007;178:7540–9. doi: 10.4049/jimmunol.178.12.7540. [DOI] [PubMed] [Google Scholar]
- 36.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nature reviews Immunology. 2006;6:836–48. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 37.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarkar D, Desalle R, Fisher PB. Evolution of MDA-5/RIG-I-dependent innate immunity: independent evolution by domain grafting. Proc Natl Acad Sci U S A. 2008;105:17040–5. doi: 10.1073/pnas.0804956105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.