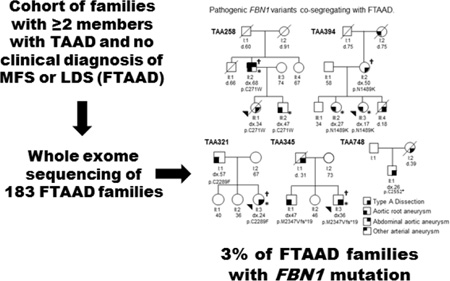
Abstract
Marfan syndrome (MFS) due to mutations in FBN1 is a known cause of thoracic aortic aneurysms and acute aortic dissections (TAAD) associated with pleiotropic manifestations. Genetic predisposition to TAAD can also be inherited in families in the absence of syndromic features, termed familial TAAD (FTAAD), and several causative genes have been identified to date. FBN1 mutations can also be identified in FTAAD families, but the frequency of these mutations has not been established. We performed exome sequencing of 183 FTAAD families and identified pathogenic FBN1 variants in five (2.7%) of these families. We also identified eight additional FBN1 rare variants that could not be unequivocally classified as disease-causing in six families. FBN1 sequencing should be considered in individuals with FTAAD even without significant systemic features of MFS.
Keywords: familial thoracic aortic aneurysm and dissection, FBN1, Marfan syndrome
Graphical Abstract
INTRODUCTION
Genetic predisposition to thoracic aortic aneurysms and dissections can occur in individuals with syndromic features, as in Marfan syndrome (MFS), or in the absence of syndromic features, termed familial thoracic aortic aneurysms and dissections. Individuals with MFS have pleiotropic involvement of the cardiovascular, skeletal, ocular, integument, and pulmonary systems due to FBN1 mutations (1–3). Although patients with TAAD due to underlying FBN1 mutations with limited involvement of other organ systems have been reported (4–6), these patients are not typically referred for FBN1 sequencing analysis.
Approximately 20% of TAAD patients without a diagnosis of MFS report a family history of aortic disease (7, 8). Mutations in several genes that disrupt smooth muscle cell function and transcription growth factor beta signalling have been described in these patients; in particular, mutations in TGFBR2, TGFBR1, SMAD3, and TGFB2 are each responsible for 1–3% of FTAAD without significant features of MFS or Loeys Dietz syndrome (LDS) (9–11). We sought to determine the frequency of FBN1 mutations in patients with FTAAD in whom clinical features did not lead to a diagnosis of MFS or prompt FBN1 testing.
MATERIALS AND METHODS
The study protocol was approved by the UTHealth Institutional Review Board and the study participants gave informed consent. Families with ≥2 members with TAAD, but without a clinical diagnosis of MFS or LDS were enrolled in the study. Blood or saliva samples and medical information were obtained from affected family members, although not all affected members were available (e.g., died of an acute aortic dissection) or had been evaluated by a geneticist. Phenotypic characterisation of the vascular disease was previously described (10). Phenotypic features beyond the vascular system were assessed by a clinical geneticist or cardiologist familiar with genetic aortic syndromes. Aortic measurements were reviewed by a cardiologist familiar with thoracic aortic disease and Z-scores were calculated based on normal values for age, gender, and body size (12).
A total of 183 unrelated families underwent exome sequencing using DNA from one or multiple affected members and data were analysed as previously described (10). FBN1 rare variants that disrupted the protein (missense, nonsense, frameshift variants, and variants disrupting the donor and acceptor splice sites) were identified and described based on the RefSeq code NM_000138.4. Sanger DNA sequencing was performed to confirm the FBN1 variants identified by exome sequencing and co-segregation with TAAD in the family.
RESULTS
To identify additional genes for FTAAD, we pursued exome sequencing of 183 families and identified thirteen heterozygous rare variants in FBN1 (Table 1). Based on established criteria of pathogenicity of FBN1 variants in MFS (13, 14), five of these variants were classified as pathogenic and co-segregated with TAAD in the families with available samples (Figure 1). A nonsense (c.7656C>A; p.Cys2552Ter) and frameshift mutation (c.7039_7040delAT; p.Met2347Valfs*19) were identified in two families (TAA748 and TAA345). Three missense variants that disrupt amino acids in the EGF-like domains are predicted to be pathogenic: c.813C>G (p.Cys271Trp) in family TAA258 and c.6866G>T (p.Cys2289Phe) in family TAA321 disrupt highly conserved cysteine residues important for folding of the domain, and c.4467T>A (p.Asn1489Lys) in family TAA394 disrupts an amino acid critical for calcium binding to the domain.
Table 1.
Computational and predicted functional effect of FBN1 variants identified in patients with FTAAD.
| Family ID | TAA258 | TAA321 | TAA345 | TAA394 | TAA748 | TAA289 | TAA289 | TAA620 | TAA662 | TAA662 | TAA682 | TAA698 | TAA760 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nucleotide change |
c.813 C>G |
c.6866 G>T |
c.7039_7040 delAT |
c.4467 T>A |
c.7656 C>A |
c.185 G>A |
c.6596 G>A |
c.2093 C>T |
c.8121 C>A |
c.3797 A>T |
c.3428 G>A |
c.4270 C>G |
c.2207 A>G |
| Amino acid substitution |
p.C271W | p.C2289F | p.M2347V fs*19 |
p.N1489K | p.C2552* | p.R62H | p.G2199D | p.P698L | p.D2707E | p.Y1266F | p.G1143D | p.P1424A | p.N736S |
| Variant type | missense | missense | deletion | missense | nonsense | missense | missense | missense | missense | missense | missense | missense | missense |
| Exon | 7 | 55 | 57 | 36 | 61 | 2 | 53 | 16 | 64 | 30 | 27 | 34 | 18 |
| Domain | EGF-like #4 |
EGF-like #35 |
TGFBP 7 | EGF-like #26 |
EGF-like #40 |
4-cysteine motif |
EGF-like #33 |
TB3 | - | EGF-like #20 |
EGF-like #13 |
EGF-like #24 |
EGF-like #7 |
| phyloPa | 6.519 | 7.8 | ․ | 0.731 | 1.67 | 3.619 | 7.818 | 9.869 | 1.286 | 1.748 | 7.275 | 6.523 | 7.698 |
| phastConsb | 1 | 1 | ․ | 0.997 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| GERPc | 4.48 | 5.8 | ․ | 2.15 | 3.84 | 3.26 | 6.07 | 6.06 | 5.38 | 2.28 | 5.6 | 5.81 | 4.79 |
| CADDd | 25.3 | 35 | ․ | 24.5 | 49 | 28.8 | 34 | 26.3 | 16.97 | 16.71 | 32 | 23.3 | 24.6 |
| FATHMMe | D | D | ․ | D | ․ | D | D | D | T | D | D | D | D |
| LRTf | D | D | ․ | D | D | N | D | D | D | N | D | D | D |
| Mutation Assessorg |
H | H | ․ | M | ․ | M | N | L | N | N | M | L | M |
| Mutation Tasterh |
D | D | ․ | D | A | D | D | D | D | D | D | D | D |
| Polyphen2i | D | D | ․ | P | ․ | D | D | D | B | B | D | P | P |
| SIFTj | D | D | ․ | D | ․ | D | D | T | T | T | D | D | D |
| ExAC All MAFk |
0.0000082 | 0 | 0 | 0 | 0 | 0.0000584 | 0 | 0.0000082 | 0 | 0.0000989 | 0 | 0.0001812 | 0 |
| Variant classification |
Pathogenic | Pathogenic | Pathogenic | Pathogenic | Pathogenic | Uncertain significance |
Uncertain significance |
Likely benign |
Uncertain significance |
Uncertain significance |
Uncertain significance |
Uncertain significance |
Likely benign |
PhyloP score: evolutionary conservation score, higher score indicates more conserved site;
PhastCons: evolutionary conservation score ranges from 0 to 1, with 1 being most conserved;
GERP: evolutionary conservation score, higher score indicates more conserved site (17);
CADD: (18);
FATHMM: D=damaging, T=tolerated, (17);
LRT: D=deleterious, N=neutral, (19);
Mutation Assessor: H=functional, high, M=functional, medium, L=non-functional, low, N=non-functional, neutral, (20);
Mutation Taster: A=disease-causing automatic, D=disease-causing, (21);
PolyPhen-2 HVAR: D=probably damaging, P=possibly damaging, B=benign, (22);
SIFT: D=damaging, T=tolerated, (23);
MAF: minor allele frequency in all populations in the Exome Aggregation Consortium (ExAC) database.
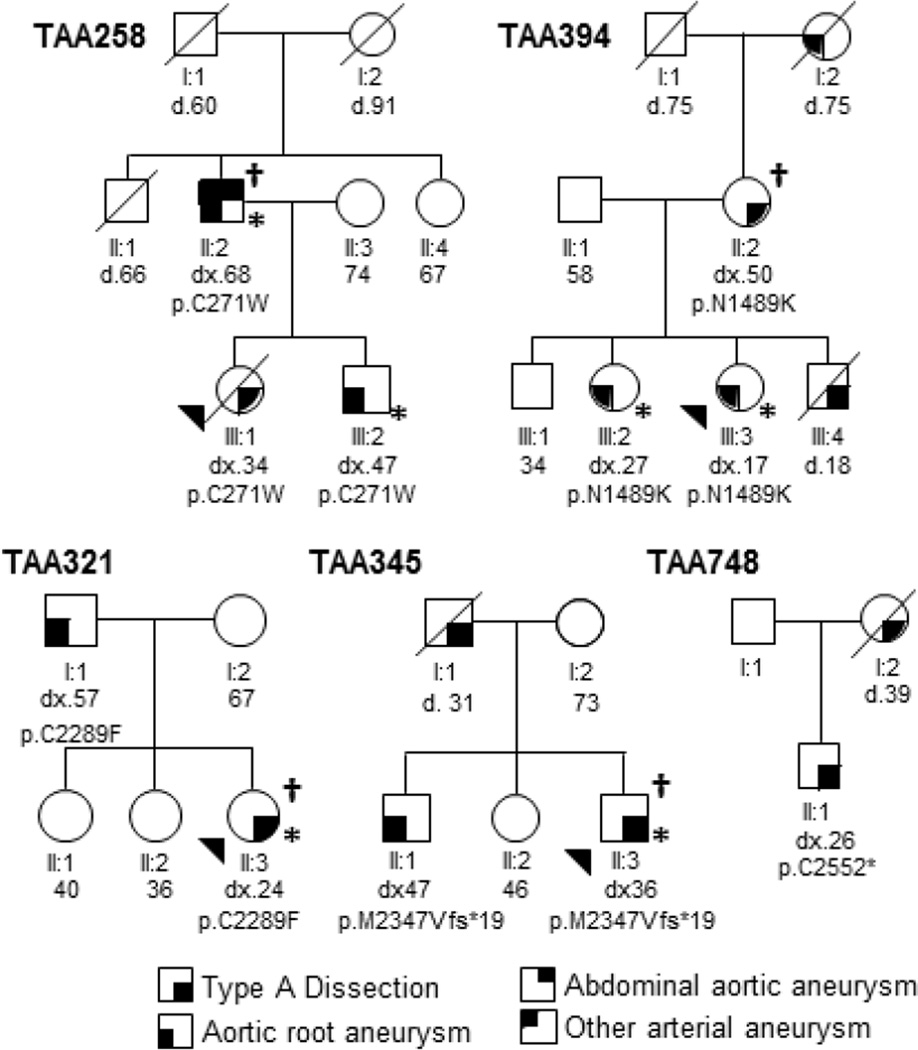
Figure 1. Segregation of pathogenic FBN1 variants with TAAD.
Circles represent females, squares represent males, and an arrowhead indicates the proband. A diagonal line through a symbol indicates the individual is deceased, with their age of death shown below the symbol. Age at onset or diagnosis of TAAD (dx) is shown below each individual. Symbols used to represent disease diagnoses are indicated in the figure key. Individuals marked with asterisk were evaluated by a clinical geneticist, and individuals marked with the symbol † underwent exome sequencing.
Affected members of these families did not have sufficient systemic features of MFS to raise clinical suspicion and pursue FBN1 sequencing. The proband of family TAA258 (III:1) died from a type A aortic dissection. After her death, her 68-year-old father was screened and found to have a 4.4 cm aortic root (Z-score 2.08) and dilatation of the abdominal aorta and bilateral iliac arteries. He is 193 cm tall and has mild mitral valve prolapse and pes planus. The proband’s brother has mild aortic root dilatation (4.1 cm, Z-score 2.24) with effacement of the sinotubular junction and mild mitral regurgitation at the age of 47. He is 188 cm tall and has a systemic score of six based on the revised Ghent criteria (Table 2).
Table 2.
Clinical characteristics of individuals with pathogenic FBN1 variantsa.
| Characteristics | TAA258- II:2 |
TAA258- III:2 |
TAA321- III:3 |
TAA345 II:3 |
TAA394- III:2 |
TAA394- III:3 |
|---|---|---|---|---|---|---|
| Age at exam, years | 74 | 47 | 25 | 45 | 29 | 17 |
| Height, cm | 193 | 188 | 142 | 210 | 175 | 175 |
| Body surface area, m2 | 2.34 | 1.94 | 1.67 | ․ | 1.84 | 1.66 |
| Cardiovascular | ||||||
| Aortic root aneurysm (z score) | 5.1 cm (2.98) | 4.1 cm (2.24) | ․ | ․ | 4.1 cm (4.22) | 4.6 cm (6.87) |
| Aortic dissection | − | − | Type A | Type A | − | − |
| Mitral valve prolapse | + (mild) | − | − | − | + (mild) | − |
| Ocular | ||||||
| Ophthalmology exam | + | + | + | + | + | + |
| Myopia | − | + | − | − | + | + |
| Ectopia Lentis | − | − | − | − | − | − |
| Systemic | ||||||
| Wrist sign | − | + | − | − | − | − |
| Thumb sign | − | + | − | + | − | + |
| Pectus carinatum | − | − | − | − | − | − |
| Pectus excavatum | − | − | − | − | − | − |
| Chest asymmetry | − | − | − | − | − | − |
| Hindfoot deformity | − | − | − | − | − | − |
| Pes Planus | + | + | − | − | − | − |
| Pneumothorax | − | − | − | + | − | − |
| Dural ectasia | ․ | ․ | ․ | ․ | ․ | ․ |
| Protrusio acetabuli | ․ | ․ | ․ | ․ | ․ | ․ |
| Reduced upper to lower segment ratio | − | + | − | ․ | − | − |
| Increased arm span to height ratio | − | + | − | + | − | − |
| Scoliosis | − | − | − | − | + | − |
| Thoracolumbar kyphosis | − | − | − | − | − | − |
| Skin striae | − | − | − | − | + | + |
| Reduced elbow extension | − | − | − | − | − | − |
| Dolichocephaly | − | − | − | − | − | + |
| Enophthalmos | − | − | − | − | − | − |
| Down slanting palpebral fissures | − | − | − | − | − | − |
| Malar hypoplasia | − | − | − | − | − | − |
| Retrognathia | − | − | − | − | − | − |
| Systemic score | 1 | 6 | 0 | 3 | 3 | 3 |
Symbols “+” indicate characteristic is present, “− “ absent, and “․ “ unknown.
The proband of family TAA394 (III:3) was diagnosed with aortic root dilatation (4.2 cm; Z-score 5.07) during pregnancy at the age of 17. She underwent a cesarean section followed by a valve-sparing aortic root replacement due to rapid aortic enlargement. She has arachnodactyly, skin striae, and myopia. Her brother (III:4) died at the age of 18 from aortic rupture due to a motor vehicle accident. Her mother had a type A aortic dissection at the age of 50, and her older sister has aortic root dilatation (4.1 cm; Z-score 4.22), myopia, scoliosis and skin striae. Both her mother and sister carry the FBN1 variant; DNA sample was not available from her brother.
The proband of TAA321 (II:3) had an ascending aorta rupture at the age of 24. She is 142.5 cm tall and has joint laxity and arachnodactyly. Her father also carries the FBN1 variant and was diagnosed with aortic root enlargement (4.3 cm) at the age of 56 after the proband’s dissection.
Individual II:3 of family TAA345 presented with a type A dissection at the age of 36. He has increased arm span:height ratio, positive thumb sign and spontaneous pneumothorax. His brother (II:1) also carries the FBN1 variant and is 210 cm tall and had surgical repair of a 6.5 cm aortic root aneurysm at the age of 30; he has no history of eye problems or clinical diagnosis of MFS by his report.
The proband of family TAA748 is Hispanic and had surgery for aortic root aneurysm and type A dissection at the age of 26. He is 183 cm tall and has joint laxity and arachnodactyly. No other MFS features were reported by his referring physician. His mother died of an aortic dissection at the age of 39. No samples from family members were available for FBN1 analysis.
Five other FBN1 variants are in EGF-like domains that do not disrupt an amino acid in a manner established to cause MFS (Table 1). A p.Tyr1266Phe and a p.Asp2707Glu substitution were identified in the proband of TAA662, who had a type B aortic dissection and 4.3 cm aortic root dilatation at age 58 years. The proband reported symptoms of myopia and caved-in chest and no family history of MFS. There are no additional affected family members available, but analysis of unaffected relatives indicated that these variants are on different alleles. FBN1 c.3428G>A (p.Gly1143Asp) was identified in a 53-year-old man with aortic root aneurysm and has not been reported previously; samples from affected relatives are not available. The proband has no other features of MFS or family history of MFS. FBN1 c.6596G>A (p. Gly2199Asp), as well as c.185G>A (p.Arg62His), were identified in an African American man with thoracic aortic dissection and a family history of aortic dissection without skeletal features of MFS or lens dislocation. Samples from additional family members are not available for analysis. FBN1 variants p.Pro1424Ala and p.Asn736Ser did not segregate with aortic disease; additionally, the probands had ascending aortic aneurysms associated with bicuspid aortic valve and did not have features of MFS or family history of MFS. FBN1 p.Pro698Leu located in a transforming growth factor binding protein-like domain did not co-segregate with aortic disease; the proband had a type A dissection, ascending aortic dilatation (not aortic root), and other risk factors (bicuspid aortic valve and hypertension) but no features of MFS or family history of MFS.
DISCUSSION
The frequency of pathogenic FBN1 variants in patients with FTAAD is 3% (5/183). Of the five pathogenic variants, two are null mutations, one of which has been reported in patients with MFS. Three of the variants are missense mutations that disrupt conserved amino acid residues in the EGF-like domains, predicted to be deleterious based on multiple computational programs, and co-segregate with TAAD in the families. The p.Cys271Trp substitution has been reported previously in a patient with MFS (15). The p.Asn1489Lys substitution has been reported in a 56 year old woman with a type B aortic dissection and aortic root aneurysm and no skeletal features of the MFS (6). These findings highlight the variable clinical presentations of FBN1 mutations, but the underlying mechanisms of this fundamental genetic phenomenon are not understood.
The affected individuals had no lens dislocation and systemic scores that ranged from zero to six as determined by a geneticist. Assessment of other family members may help raise the suspicion of MFS, but these members may not be readily available. Our group and others have reported FBN1 mutations in patients with FTAAD or incomplete MFS (4, 5, 16). We also reported a lack of skeletal features in Hispanic MFS patients (23), and one of the probands in this report is Hispanic. Thus, the variable presence of MFS systemic features indicate that sequencing of FBN1 as part of a gene panel should be pursued in probands with familial TAAD and no significant systemic manifestations suggestive of MFS.
Acknowledgments
We are grateful to the patients and families for their participation. This work was supported by R01HL109942 and UL1 RR024148 from the National Institutes of Health, and the John Ritter Foundation, Vivian L. Smith Foundation and Richard T. Pisani Fund (D.M.M). Exome sequencing was provided by the University of Washington Center for Mendelian Genomics and was funded by the National Human Genome Research Institute and the National Heart, Lung and Blood Institute grant 1U54HG006493 to Drs. Debbie Nickerson, Jay Shendure and Michael Bamshad. We thank the Exome Aggregation Consortium and the groups that provided exome variant data for comparison.
Footnotes
Conflict of Interest Statement: The authors declare no conflict of interest.
A full list of contributing groups can be found at http://exac.broadinstitute.org/about.
References
- 1.Dietz HC, Cutting GR, Pyeritz RE, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352(6333):337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- 2.Milewicz DM, Pyeritz RE, Crawford ES, et al. Marfan syndrome: defective synthesis, secretion, and extracellular matrix formation of fibrillin by cultured dermal fibroblasts. J Clin Invest. 1992;89(1):79–86. doi: 10.1172/JCI115589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pyeritz RE. The Marfan syndrome. Annu Rev Med. 2000;51:481–510. doi: 10.1146/annurev.med.51.1.481. [DOI] [PubMed] [Google Scholar]
- 4.Milewicz DM, Michael K, Fisher N, et al. Fibrillin-1 (FBN1) mutations in patients with thoracic aortic aneurysms. Circulation. 1996;94(11):2708–2711. doi: 10.1161/01.cir.94.11.2708. [DOI] [PubMed] [Google Scholar]
- 5.Francke U, Berg MA, Tynan K, et al. A Gly1127Ser Mutation in An Egf-Like Domain of the Fibrillin-1 Gene Is A Risk Factor for Ascending Aortic-Aneurysm and Dissection. American Journal of Human Genetics. 1995;56(6):1287–1296. [PMC free article] [PubMed] [Google Scholar]
- 6.Brautbar A, LeMaire SA, Franco LM, et al. FBN1 mutations in patients with descending thoracic aortic dissections. Am J Med Genet A. 2010;152A(2):413–416. doi: 10.1002/ajmg.a.32856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biddinger A, Rocklin M, Coselli J, et al. Familial thoracic aortic dilatations and dissections: a case control study. J Vasc Surg. 1997;25(3):506–511. doi: 10.1016/s0741-5214(97)70261-1. [DOI] [PubMed] [Google Scholar]
- 8.Coady MA, Davies RR, Roberts M, et al. Familial patterns of thoracic aortic aneurysms. Arch Surg. 1999;134(4):361–367. doi: 10.1001/archsurg.134.4.361. [DOI] [PubMed] [Google Scholar]
- 9.Pannu H, Fadulu V, Chang J, et al. Mutations in transforming growth factor-beta receptor type II cause familial thoracic aortic aneurysms and dissections. Circulation. 2005;112(4):513–520. doi: 10.1161/CIRCULATIONAHA.105.537340. [DOI] [PubMed] [Google Scholar]
- 10.Regalado ES, Guo DC, Villamizar C, et al. Exome Sequencing Identifies SMAD3 Mutations as a Cause of Familial Thoracic Aortic Aneurysm and Dissection With Intracranial and Other Arterial Aneurysms. Circ Res. 2011;109(6):680–686. doi: 10.1161/CIRCRESAHA.111.248161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boileau C, Guo DC, Hanna N, et al. TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat Genet. 2012;44(8):916–921. doi: 10.1038/ng.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devereux RB, de SG, Arnett DK, et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons >/=15 years of age. Am J Cardiol. 2012;110(8):1189–1194. doi: 10.1016/j.amjcard.2012.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loeys BL, Dietz HC, Braverman AC, et al. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47(7):476–485. doi: 10.1136/jmg.2009.072785. [DOI] [PubMed] [Google Scholar]
- 14.Khau Van KP, Baux D, Pallares-Ruiz N, et al. Missense mutations of conserved glycine residues in fibrillin-1 highlight a potential subtype of cb-EGF-like domains. Hum Mutat. 2010;31(1):E1021–E1042. doi: 10.1002/humu.21131. [DOI] [PubMed] [Google Scholar]
- 15.Collod-Beroud G, Le Bourdelles S, Ades L, et al. Update of the UMD-FBN1 mutation database and creation of an FBN1 polymorphism database. Hum Mutat. 2003;22(3):199–208. doi: 10.1002/humu.10249. [DOI] [PubMed] [Google Scholar]
- 16.Villamizar C, Regalado ES, Fadulu VT, et al. Paucity of skeletal manifestations in hispanic families with FBN1 mutations. Eur J Med Genet. 2010;53(2):80–84. doi: 10.1016/j.ejmg.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shihab HA, Gough J, Cooper DN, et al. Predicting the functional, molecular, and phenotypic consequences of amino acid substitutions using hidden Markov models. Hum Mutat. 2013;34(1):57–65. doi: 10.1002/humu.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kircher M, Witten DM, Jain P, et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014;46(3):310–315. doi: 10.1038/ng.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chun S, Fay JC. Identification of deleterious mutations within three human genomes. Genome Res. 2009;19(9):1553–1561. doi: 10.1101/gr.092619.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reva B, Antipin Y, Sander C. Predicting the functional impact of protein mutations: application to cancer genomics. Nucleic Acids Res. 2011;39(17):e118. doi: 10.1093/nar/gkr407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarz JM, Rodelsperger C, Schuelke M, et al. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7(8):575–576. doi: 10.1038/nmeth0810-575. [DOI] [PubMed] [Google Scholar]
- 22.Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7(4):248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi Y, Sims GE, Murphy S, et al. Predicting the functional effect of amino acid substitutions and indels. PLoS ONE. 2012;7(10):e46688. doi: 10.1371/journal.pone.0046688. [DOI] [PMC free article] [PubMed] [Google Scholar]