Abstract
The role of insulin-like growth factors (IGFs) in prostate cancer development is not fully understood. To investigate the association between circulating concentrations of IGFs (IGF-I, IGF-II, IGFBP-1, IGFBP-2, IGFBP-3) and prostate cancer risk, we pooled individual participant data from 17 prospective and two cross-sectional studies, including up to 10,554 prostate cancer cases and 13,618 control participants. Conditional logistic regression was used to estimate the odds ratios (ORs) for prostate cancer based on the study-specific fifth of each analyte. Overall, IGF-I, IGF-II, IGFBP-2, and IGFBP-3 concentrations were positively associated with prostate cancer risk (Ptrend all ≤ 0.005), and IGFBP-1 was weakly inversely associated with risk (Ptrend = 0.05). However, heterogeneity between the prospective and cross-sectional studies was evident (Pheterogeneity = 0.03), unless the analyses were restricted to prospective studies (with the exception of IGF-II, Pheterogeneity = 0.02). For prospective studies, the OR for men in the highest versus the lowest fifth of each analyte was 1.29 (95% confidence interval=1.16-1.43) for IGF-I, 0.81 (0.68-0.96) for IGFBP-1, and 1.25 (1.12-1.40) for IGFBP-3. These associations did not differ significantly by time-to-diagnosis or tumor stage or grade. After mutual adjustment for each of the other analytes, only IGF-I remained associated with risk. Our collaborative study represents the largest pooled analysis of the relationship between prostate cancer risk and circulating concentrations of IGF-I, providing strong evidence that IGF-I is highly likely to be involved in prostate cancer development.
Keywords: prostate cancer, insulin-like growth factor, insulin-like growth factor binding protein, pooled analysis
Introduction
Insulin-like growth factors (IGFs) and their associated binding proteins (IGFBPs) are involved in the regulation of cell proliferation, differentiation and apoptosis and there has been considerable interest in their role in the development of prostate cancer. Previous individual prospective studies and our 2008 pooled analysis of individual participant data from 3,700 men with prostate cancer in the Endogenous Hormones and Prostate Cancer Collaborative Group (EHPCCG) have indicated that men with high IGF-I concentrations have an elevated risk for the disease (1). However, there were insufficient data (both in terms of numbers of cases and the range of analytes studied) to provide reliable estimates of risk for overall prostate cancer in relation to concentrations of other IGF-axis biomarkers (IGF-II, IGFBP-1 and IGFBP-2), either individually or in combination. Furthermore, previous studies did not have sufficient numbers of advanced or high-grade disease to determine whether circulating IGF concentrations influence prostate cancer initiation or progression, or both (2)(3). The possible role of reverse causality in explaining the observed association between IGF-I and prostate cancer risk also requires further investigation, with preclinical tumours potentially influencing IGF concentrations at blood draw in both cross-sectional screening studies and prospective studies, particularly in those with a short time lag between blood collection and diagnosis and a relatively high proportion of clinically detected advanced cases.
The EHPCCG (now expanded as the Endogenous Hormones, Nutritional Biomarkers and Prostate Cancer Collaborative Group, EHNBPCCG) was established to conduct collaborative re-analyses of individual data on the relationships between prediagnostic circulating concentrations of sex hormones and IGFs and subsequent risk for prostate cancer (1, 4). In the current report we examine the role of circulating IGFs using individual participant data on up to 10,554 men with prostate cancer from up to 19 studies of IGF-I and IGFBP-3, as well as on other IGF-axis analytes including IGF-II, IGFBP-1 and IGFBP-2 (2, 3, 5-21), and investigate whether the association of IGFs with risk differs by tumour characteristics and with time from blood collection to diagnosis.
Methods
Data collection
The EHNBPCCG is described in detail elsewhere (1, 4). Studies were eligible for the current collaborative individual participant meta-analysis if they had data on circulating levels of IGF-I, IGF-II, IGFBP-1, IGFBP-2 or IGFBP-3 and subsequent prostate cancer risk. Studies were identified through searches using the search terms “insulin-like growth factor”, and “prostate cancer” on computerized bibliographic systems, including PubMed, Web of Science, Cochrane Library, and CancerLit, through the reference lists of publications identified in this search, and through correspondence with study investigators. Further details of data collection and processing are provided in Supplementary Methods.
Individual participant data were available from 19 studies by the dataset closure for these analyses on November 8th, 2012; Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBC) (5); Baltimore Longitudinal Study of Aging (BLSA) (6), British United Provident Association Study (BUPA) (7), the Cardiovascular Health Study (CHS) (8), the CLUE 1 Study (9), European Prospective Investigation into Cancer and Nutrition (EPIC) (10), European Randomized Study of Screening for Prostate Cancer (ERSPC) (11), Health Professionals Follow-up Study (HPFS) (12), Japan Collaborative Cohort Study (JACC) (13), Kaiser Permanente Medical Care Programme (KPMCP) (14), Melbourne Collaborative Cohort Study (MCCS) (15), Multiethnic Cohort (MEC) (16), Northern Sweden Health and Disease Cohort (NSHDC) (17), Prostate Cancer Prevention Trial (PCPT) (3), Physicians’ Health Study (PHS) (18), Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) (19), the Prostate Testing for Cancer and Treatment (ProtecT) feasibility study (20) and main study (2), and the SUpplémentation en VItamines et Minéraux AntioXydants (SU.VI.MAX) trial (21). In total, these 19 studies included data on IGF-I and IGFBP-3 from up to 10554 prostate cancer cases and 13618 control participants, representing more than 98% of the worldwide data. Of these studies, 11 also provided data on circulating IGF-II and 6 provided data on IGFBP-1 and IGFBP-2. The characteristics of these studies and the assay methods are shown in Supplementary Tables S1 and S2, respectively. Most of the studies are case-control studies nested within traditional prospective cohort studies, with some variation in the case mix of these studies according to the prevalence of prostate-specific antigen (PSA) testing within that population during follow-up. Four of the studies (ERSPC, PCPT, PLCO and ProtecT) are observational investigations using data from trials that included organized screening for prostate cancer, and have distinct characteristics. In three of these trials, men with a raised PSA or abnormal digital rectal examination at recruitment-screening were excluded, and the eligible cases were diagnosed during subsequent follow-up (for ERSPC and PCPT the majority being diagnosed at the end of the study, 4 and 7 years after recruitment, respectively), with the majority of cases being detected either through PSA-screening (ERSPC and PLCO) or by routine end of study biopsy (PCPT). The ProtecT studies include participants from a trial of different prostate cancer treatments, in which (mostly asymptomatic) men were screened with PSA and those with PSA ≥3 ng/mL were offered a diagnostic biopsy; men diagnosed at this time were included as cases for the observational study of biomarkers and prostate cancer. The data from ProtecT are reported here because on average the blood was collected several years before the cancer would have been diagnosed in an unscreened population (22), although the study is cross-sectional rather than prospective.
Details of recruitment, informed consent and ethics approvals are provided in the original publications (2, 3, 5-21). Information sought about prostate cancer included date of diagnosis and stage and grade of disease. In order to provide a common definition across studies, prostate cancer was defined as being early stage if it was TNM stage <T2 with no reported lymph node involvement or metastases, or stage I; other localized stage if it was TNM stage T2 with no reported lymph node involvement or metastases, stage II, or equivalent (i.e. a tumour which does not extend beyond the prostate capsule); advanced stage if it was tumor-node-metastasis (TNM) stage T3 or T4 and/or N1+ and/or M1, stage III–IV, or equivalent (i.e. a tumour extending beyond the prostate capsule and/or lymph node involvement and/or distant metastases); or stage unknown. Aggressive disease was categorized as “no” for TNM stage ≤T3 with no reported lymph node involvement or metastases or equivalent, “yes” for TNM stage T4 and/or N1+ and/or M1 and/or stage IV disease or death from prostate cancer, or unknown. Prostate cancer was defined as low-intermediate grade if the Gleason sum was <8 or equivalent (i.e. extent of differentiation good, moderate or poor), high grade if the Gleason sum ≥8 or equivalent (i.e. undifferentiated), or grade unknown.
Statistical analyses
The methods of analysis were similar to those described previously by this collaborative group (1, 4, 23 and see Supplementary Methods). Concentrations of IGFs were positively skewed, therefore, log-transformed concentrations were used for all parametric analyses.
For each IGF analyte, men were categorized into fifths of its distribution, with cut-points defined by the study-specific quintiles of the distribution within control participants to allow for any systematic differences between the studies in assay methods and blood sample types (1). The main method of analysis was logistic regression conditioned on the matching variables within each study. To provide a summary measure of the odds ratio (for subgroup analyses) and to calculate a P for trend, the categorical variable representing the fifths of the IGF analyte was replaced with a continuous variable that was scored as 0, 0.25, 0.5, 0.75, and 1; because the mid-points of the lowest and highest fifths are the 10th and 90th percentiles of the study-specific IGF concentration, a unit increase in this variable can be taken to represent an 80 percentile increase in the study-specific concentration of IGF. To examine the effects of potential confounders (other than the matching criteria, controlled for by design), the logistic regression analyses were repeated including additional variables that were found to be associated with prostate cancer risk in this analysis, which included age at blood collection, body mass index (BMI), height, marital status, educational status, and cigarette smoking.
For each IGF analyte, heterogeneity in linear trends between studies was assessed by comparing the χ2 values for models with and without a (study) × (linear trend) interaction term. Tests for heterogeneity for case-defined factors were obtained by fitting separate models for each subgroup and assuming independence of the ORs using a method analogous to a meta-analysis. Tests for heterogeneity for non-case defined factors were assessed with a χ2-test of interaction between subgroup and the continuous trend test variable. A χ2-test of interaction was also used to determine whether risks by study-specific thirds of one analyte varied according to the study-specific third of another analyte.
All tests of statistical significance were 2-sided, and statistical significance was set at the 5% level. All statistical tests were carried out with Stata Statistical Software, Release 12 (StataCorp, LP, College Station, Texas).
Results
The 19 studies included approximately 10,500 case patients with prostate cancer and 13,600 control participants (Table 1). All the studies had data available on circulating IGF-I concentrations (10,554 cases) and 18 studies had data on IGFBP-3 concentration (9359 cases). Data were available from 11 studies for IGF-II (5523 cases) and 6 studies for IGFBP-1 (2490 cases) and IGFBP-2 (4952 cases) (Supplementary Figures S1 to S3). The mean age at baseline across the studies ranged from 54 to 72 years (Table 1). Geometric mean concentrations of all the analytes, with the exception of IGFBP-1, for most of the studies were higher for cases than for controls (Supplementary Table S3). Blood collection preceded prostate cancer diagnosis by an average of 5.2 years, though there was wide variation between the studies with more than 95% of cases in the cross-sectional ProtecT study being diagnosed within the first 3 years of follow-up, whereas for ATBC, BLSA and BUPA more than 80% of cases were diagnosed ≥7 after blood collection. On average, cases were 68 years of age at diagnosis and were diagnosed after 1994 (Table 2). The majority of cases with information on stage and grade of disease had localised (early or other localised) disease (ranging from 62% to 98% of cases across studies) and low-intermediate grade tumours (83% to 100% of cases).
Table 1.
Participant characteristics by study and case-control statusa
| Study (Year, Reference) | Case- control status |
Number | Age at recruitment (y) |
BMI (kg/m2) |
Married or cohabiting (%) |
Higher education (%) |
Current smokers (%) |
Intake of alcohol (g/d) |
Family history of prostate cancer (%) |
|---|---|---|---|---|---|---|---|---|---|
| Prospective Studies | |||||||||
| ATBC (2003)(5) | Case | 100 | 58.9 (4.6) | 26.5 (4.5) | 83.0 | 5.0 | 100.0 | 17.1 (23.9) | 7.5 |
| Control | 311 | 58.0 (4.5) | 26.5 (3.9) | 81.4 | 5.5 | 100.0 | 17.2 (19.6) | 4.2 | |
| BLSA (2000)(6) | Case | 72 | 64.4 (8.9) | 25.5 (3.0) | 94.8 | 63.9 | 5.6 | N/A | N/A |
| Control | 111 | 64.7 (9.4) | 26.4 (3.7) | 83.5 | 57.7 | 7.2 | N/A | N/A | |
| BUPA (2006)(7) | Case | 140 | 54.5 (6.2) | 25.0 (2.4) | N/A | N/A | 15.0 | 21.0 (16.9) | N/A |
| Control | 419 | 54.6 (6.2) | 25.4 (2.9) | N/A | N/A | 18.9 | 19.2 (16.7) | N/A | |
| CHS (2005)(8) | Case | 174 | 72.5 (4.4) | 26.8 (3.5) | 87.4 | 17.3 | 8.6 | N/A | N/A |
| Control | 174 | 72.4 (4.4) | 26.7 (4.1) | 83.3 | 14.5 | 13.8 | N/A | N/A | |
| CLUE 1 (2001)(9) | Case | 30 | 58.5 (9.1) | N/A | 93.3 | 16.7 | 16.7 | N/A | N/A |
| Control | 60 | 58.4 (8.9) | N/A | 90.0 | 1.7 | 26.7 | N/A | N/A | |
| EPIC Phase 1 ( 2007) (11) | Case | 630 | 60.9 (6.2) | 26.7 (3.5) | 87.5 | 25.4 | 23.5 | 20.7 (24.5) | N/A |
| Control | 630 | 60.9 (6.2) | 27.1 (3.6) | 89.2 | 23.1 | 27.8 | 20.5 (23.9) | N/A | |
| EPIC Phase 2 (2012)(10) | Case | 1107 | 58.6 (6.2) | 26.5 (3.4) | 88.4 | 25.7 | 22.8 | 20.1 (24.1) | N/A |
| Control | 1107 | 58.6 (6.2) | 26.8 (3.6) | 88.4 | 24.5 | 24.8 | 19.5 (21.3) | N/A | |
| ERSPC (2004)(12) | Case | 197 | 61.8 (4.4) | N/A | N/A | N/A | N/A | N/A | 18.7 |
| Control | 197 | 61.8 (4.4) | N/A | N/A | N/A | N/A | N/A | 16.2 | |
| HPFS Phase 1 (2005)(13) | Case | 682 | 65.3 (7.4) | 25.9 (3.6) | 93.4 | 100.0 | 4.8 | 11.9 (14.7) | 14.2 |
| Control | 682 | 65.1 (7.4) | 26.0 (3.5) | 93.0 | 100.0 | 3.9 | 11.4 (15.0) | 10.3 | |
| HPFS Phase 2 (2010)(14) | Case | 629 | 62.0 (7.8) | 25.9 (3.2) | 91.7 | 100.0 | 3.8 | 12.4 (16.3) | 14.8 |
| Control | 629 | 62.0 (7.8) | 26.1 (3.6) | 93.0 | 100.0 | 3.0 | 12.2 (16.8) | 10.8 | |
| JACC (2010)(15) | Case | 39 | 68.9 (6.1) | 22.3 (2.6) | 100.0 | 10.0 | 50.0 | 17.0 (19.2) | 2.6 |
| Control | 98 | 68.2 (5.5) | 22.4 (2.7) | 91.9 | 2.7 | 37.5 | 13.7 (17.5) | 0.0 | |
| KPMCP (1998)(16) | Case | 45 | 71.5 (5.1) | 25.7 (2.5) | 86.8 | 7.5 | 20.0 | 18.7 (30.3) | N/A |
| Control | 218 | 71.9 (4.5) | 25.8 (3.1) | 82.8 | 5.4 | 17.8 | 14.9 (22.9) | N/A | |
| MCCS (2006)(17) | Case | 554 | 60.9 (6.4) | 27.2 (3.5) | 80.2 | 22.0 | 9.6 | 19.0 (24.4) | N/A |
| Control | 1048 | 58.3 (7.2) | 27.2 (3.7) | 81.0 | 22.2 | 13.2 | 21.0 (25.8) | N/A | |
| MEC (2010)(18) | Case | 386 | 68.7 (7.1) | 26.7 (4.1) | 78.1 | 33.2 | 14.4 | 23.0 (42.6) | 14.0 |
| Control | 769 | 68.5 (7.2) | 26.9 (4.1) | 78.9 | 32.0 | 11.9 | 21.5 (37.9) | 8.3 | |
| NSHDC (2000, 2004)(19, 20) |
Case | 281 | 58.0 (4.5) | 26.1 (2.8) | 86.6 | 13.4 | 18.8 | 7.6 (6.0) | N/A |
| Control | 569 | 58.0 (4.4) | 26.6 (3.7) | 80.1 | 12.3 | 21.2 | 7.5 (6.0) | N/A | |
| PCPT (2013)(3) | Case | 1032 | 63.3 (5.5) | 27.4 (4.2) | 87.3 | 38.4 | 6.8 | 9.7 (16.1) | 21.0 |
| Control | 1032 | 63.3 (5.5) | 27.6 (4.0) | 87.8 | 36.9 | 7.6 | 8.9 (13.7) | 20.9 | |
| PHS (1998, 2002, 2010) | Case | 756 | 58.6 (8.1) | 24.7 (2.5) | N/A | 100.0 | 9.1 | 7.1 (6.2) | N/A |
| Control | 756 | 58.4 (8.0) | 24.7 (2.5) | N/A | 100.0 | 8.9 | 7.2 (6.3) | N/A | |
| PLCO (2007)(24) | Case | 728 | 65.1 (4.8) | 27.1 (3.6) | 87.1 | 43.7 | 6.9 | 16.6 (30.8) | 10.8 |
| Control | 886 | 64.9 (4.7) | 27.4 (2.5) | 86.7 | 42.2 | 8.8 | 16.2 (29.3) | 6.1 | |
| SU.VI.MAX (2005) (26) | Case | 100 | 55.1 (4.6) | 25.7 (3.1) | 93.9 | 35.4 | 13.3 | 25.2 (20.7) | 13.8 |
| Control | 400 | 55.0 (4.6) | 25.4 (2.9) | 87.2 | 35.2 | 13.2 | 28.1 (20.1) | 5.2 | |
| Cross-sectional Studies | |||||||||
| ProtecT -Feasibility Phase (2004)(25) |
Case | 282 | 61.6 (4.9) | 26.5 (3.1) | N/A | N/A | 9.9 | 21.6 (21.9) | 6.6 |
| Control | 774 | 61.6 (5.1) | 26.5 (3.6) | N/A | N/A | 10.8 | 23.5 (23.8) | 4.0 | |
| ProtecT (2012)(2) | Case | 2590 | 61.8 (5.1) | 26.9 (3.5) | N/A | N/A | 13.7 | 24.0 (25.3) | 8.4 |
| Control | 2748 | 61.6 (5.1) | 26.9 (3.7) | N/A | N/A | 13.9 | 24.3 (24.9) | 5.2 |
Values are mean (SD) unless otherwise indicated, percentages exclude men with missing values. Numbers are for men with an IGF-I measurement and in completed matched case-control sets for analysis.
Abbreviations: ATBC, Alpha-Tocopherol Beta-Catotene Cancer Prevention Study; BLSA, Baltimore Longitudinal Study of Aging; BMI, body mass index; BUPA, British United Provident Association Study; CHS, Cardiovascular Health Study; CLUE, Campaign Against Cancer and Stroke (“Give Us a Clue to Cancer”) Study; EPIC, European Prospective Investigation into Cancer and Nutrition; ERSP, European Randomized Study of Screening for Prostate Cancer; HPFS, Health Professionals Follow-up Study; JACC, Japan Collaborative Cohort Study; KPMCP, Kaiser Permanente Medical Care Program; MCCS, Melbourne Collaborative Cohort Study; MEC, Multiethnic Cohort; N/A, data not available for this study; NSHDC, Northern Sweden Health and Disease Cohort; PCPT, Prostate Cancer Prevention Trial; PHS, Physicians Health Study; PLCO, Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; ProtecT, Prostate Testing for Cancer and Treatment Study; PSA, prostate-specific antigen; SU.VI.MAX, Supplémentation en Vitamines et Minéraux Antioxydants.
Table 2.
Characteristics of case participants with prostate cancer (N = 10554)a
| Study | Time from blood collection diagnosis (%) |
Age at diagnosis (%) | Diagnosis year (%) | Disease stage (%) | Disease grade (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||||||||
| <3 y | 3-6 y | ≥7 y | <60 y | 60-69 y | ≥70 y | Before 1990 |
1990- 1995 |
1995- Onward |
Localisedb | Advancedb | Aggressive diseaseb |
Unavailable | Low- intermediateb |
Highb | Unavailable | |
| Prospective Studies | ||||||||||||||||
| ATBC | 0.0 | 7.0 | 93.0 | 0.0 | 60.0 | 40.0 | 0.0 | 9.0 | 91.0 | 69.7 | 30.3 | 15.2 | 1.0 | 100.0 | 0.0 | 3.0 |
| BLSA | 0.0 | 18.1 | 81.9 | 2.8 | 29.2 | 68.1 | 34.7 | 52.8 | 12.5 | 72.2 | 27.8 | 19.4 | 50.0 | 83.3 | 16.7 | 25.0 |
| BUPA | 2.9 | 14.3 | 82.9 | 17.1 | 50.7 | 32.1 | 45.7 | 42.9 | 11.4 | N/A | N/A | N/A | 100.0 | N/A | N/A | 100.0 |
| CHS | 46.6 | 51.1 | 2.3 | 0.0 | 8.6 | 91.4 | 0.0 | 62.1 | 37.9 | 76.1 | 23.9 | 7.7 | 32.8 | 99.3 | 0.7 | 18.4 |
| CLUE 1 | 3.3 | 6.7 | 90.0 | 10.0 | 50.0 | 40.0 | 100.0 | 0.0 | 0.0 | 78.9 | 21.1 | 10.5 | 36.7 | 92.0 | 8.0 | 16.7 |
| EPIC Phase 1 | 42.4 | 53.0 | 4.6 | 19.2 | 63.0 | 17.8 | 0.0 | 1.0 | 99.0 | 68.6 | 31.4 | 18.2 | 30.3 | 88.9 | 11.1 | 25.6 |
| EPIC Phase 2 | 5.2 | 47.1 | 47.7 | 17.6 | 59.0 | 23.4 | 0.0 | 0.4 | 99.6 | 74.5 | 25.5 | 16.8 | 20.9 | 85.7 | 14.3 | 30.4 |
| ERSPC | 0.0 | 100.0 | 0.0 | 5.1 | 69.0 | 25.9 | 0.0 | 0.0 | 100.0 | 94.4 | 5.6 | 0.5 | 0.0 | 97.4 | 2.6 | 1.5 |
| HPFS Phase 1 | 45.3 | 54.4 | 0.3 | 13.6 | 38.3 | 48.1 | 0.0 | 11.3 | 88.7 | 82.9 | 17.1 | 3.6 | 43.3 | 89.9 | 10.1 | 9.7 |
| HPFS Phase 2 | 0.5 | 29.4 | 70.1 | 11.9 | 35.9 | 52.1 | 0.0 | 0.2 | 99.8 | 96.6 | 3.4 | 1.1 | 10.2 | 91.6 | 8.4 | 10.8 |
| JACC | 17.9 | 53.8 | 28.2 | 0.0 | 33.3 | 66.7 | 5.1 | 51.3 | 43.6 | N/A | N/A | N/A | 100.0 | N/A | N/A | 100.0 |
| KPMCP | 17.8 | 17.8 | 64.4 | 0.0 | 6.7 | 93.3 | 100.0 | 0.0 | 0.0 | 61.9 | 38.1 | 28.6 | 53.3 | 100.0 | 0.0 | 77.8 |
| MCCS | 26.2 | 35.7 | 38.1 | 12.5 | 54.5 | 33.0 | 0.0 | 15.2 | 84.8 | 90.5 | 9.5 | 1.8 | 1.4 | 86.2 | 13.8 | 2.0 |
| MEC | 79.8 | 17.4 | 2.8 | 7.3 | 36.3 | 56.5 | 0.0 | 0.0 | 100.0 | N/A | N/A | N/A | 100.0 | 99.7 | 0.3 | 4.9 |
| NSHDC | 26.7 | 49.8 | 23.5 | 17.1 | 77.6 | 5.3 | 0.0 | 10.3 | 89.7 | 81.0 | 19.0 | 13.3 | 0.7 | 97.2 | 2.8 | 74.7 |
| PCPT | 11.6 | 27.2 | 61.1 | 1.6 | 50.8 | 47.6 | 0.0 | 0.4 | 99.6 | 98.3 | 1.7 | 0.6 | 2.4 | 95.2 | 4.8 | 2.4 |
| PHS | 7.1 | 16.7 | 76.2 | 12.4 | 45.5 | 42.1 | 24.3 | 51.6 | 24.1 | 85.0 | 15.0 | 8.1 | 5.4 | 89.9 | 10.1 | 3.4 |
| PLCO | 59.6 | 40.0 | 0.4 | 5.5 | 56.0 | 38.5 | 0.0 | 0.0 | 100.0 | 87.6 | 12.4 | 2.7 | 0.0 | 94.1 | 5.9 | 0.5 |
| SU.VI.MAX | 14.0 | 38.0 | 48.0 | 34.0 | 66.0 | 0.0 | 0.0 | 0.0 | 100.0 | N/A | N/A | N/A | 100.0 | 89.4 | 10.6 | 6.0 |
| Cross-sectional Studies | ||||||||||||||||
| ProtecT feas. | 95.7 | 3.9 | 0.4 | 29.8 | 68.4 | 1.8 | 0.0 | 0.0 | 100.0 | 81.5 | 18.5 | 0.4 | 3.9 | 93.6 | 6.4 | 0.7 |
| ProtecT main | 99.6 | 0.4 | 0.0 | 30.7 | 65.8 | 3.4 | 0.0 | 0.0 | 100.0 | 90.0 | 10.0 | 1.0 | 10.0 | 94.3 | 5.7 | 0.1 |
Data are for percentages of case patients among those with a known value for the characteristic and an IGF-I measurement and are in completed matched case-control sets for analysis. Percentages may not add up to 100 because of rounding. Stage and grade of disease are unavailable for some case patients, and the percentages shown are among case patients with known information as well as those with unknown information.
As a percentage of those with known stage or grade. A tumour was categorised as advanced stage if it was tumor-node-metastasis (TNM) stage T3 or T4 and/or N1+ and/or M1, stage III–IV, or the equivalent; localized if it was TNM stage T0 or T1 or T2 with no reported lymph node involvement or metastases, stage 0–II, or the equivalent, or stage unknown. Individuals with aggressive disease include men who have advanced stage prostate cancer who had tumours that were TNM stage T4 and/or N1+ and/or M1 and/or stage IV disease, and men who had died from prostate cancer. Prostate cancer was defined as high grade if the Gleason sum was at least 8 or the equivalent (undifferentiated), low-intermediate grade if the Gleason sum was less than 8 or the equivalent (extent of differentiation good, moderate or poor), or grade unknown.
For expansion of study names see Table 1. Abbreviations: N/A, data not available for this study.
IGF-I and IGF-II were strongly correlated with IGFBP-3 (r = 0.6 for both), and IGF-I and IGF-II were moderately positively correlated (r = 0.4) (Supplementary Table S4). These correlations were similar after additional adjustment for BMI (data not shown). All of the IGF analytes were correlated with SHBG; positive correlations were observed with IGFBP-1 (r = 0.3) and IGFBP-2 (r = 0.4), while inverse associations with SHBG were seen for IGF-I (r = −0.1), IGF-II (r = −0.3) and IGFBP-3 (r = −0.3). IGFBP-1 and IGFBP-2 were also positively correlated with testosterone concentrations (r = 0.3 for both). These correlations with SHBG and testosterone were weakened slightly by additional adjustment for BMI (data not shown).
Associations between circulating IGF concentrations and prostate cancer risk
Figure 1 shows the ORs by fifths of the IGF analytes and Figure 2 and Supplementary Figures S1-S4 show the relationships of the analytes with prostate cancer risk for the individual studies, together with overall estimates and tests for heterogeneity between studies and by study design. The relationships of the analytes with risk subdivided by clinical and other characteristics are shown in Figure 3 and Supplementary Figures S5-S12.
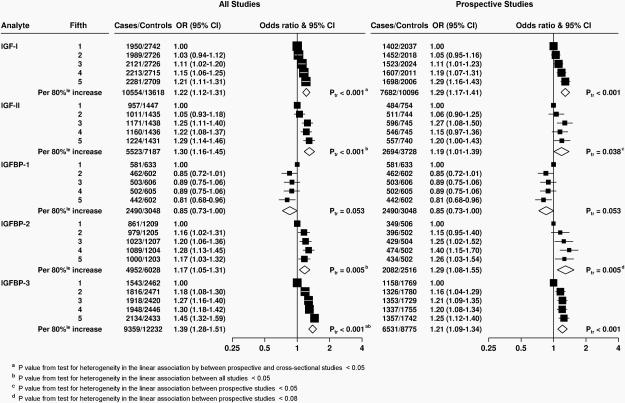
Figure 1. Odds ratios (95% confidence intervals) for prostate cancer associated with study-specific fifths of concentrations of selected insulin-like growth factors and their binding proteins in all studies and then restricted to prospective studies.
Estimates are from logistic regression conditioned on the matching variables within each study and without mutual adjustment for the other analytes. Ptrend was calcuated by replacing the fifths of concentration with a continuous variable that was scored 0, 0.25, 0.5, 0.75 and 1 in the conditional logistic regression model. 80%le = 80 percentile; CI = confidence interval; Ptr = Ptrend.
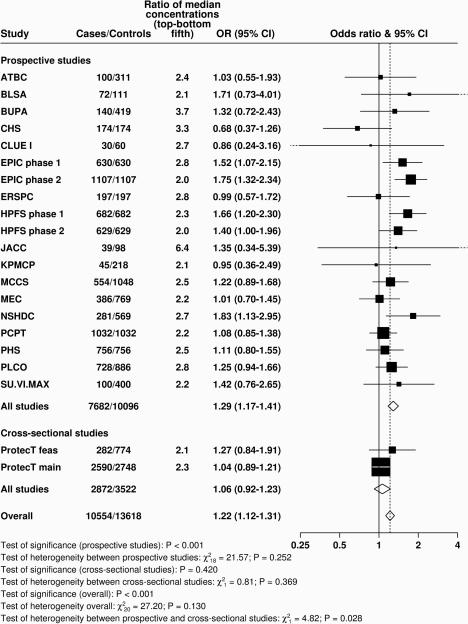
Figure 2. Study-specific odds ratios (95% confidence intervals) for prostate cancer associated with an 80 percentile increase in IGF-I.
Estimates are from logistic regression conditioned on the matching variables within each study and without mutual adjustment for the other analytes. Heterogeneity in linear trends between studies was tested by comparing the X2 values for models with and without a (studies) × (linear trend) interaction term. For expansion of study names see Table 1.
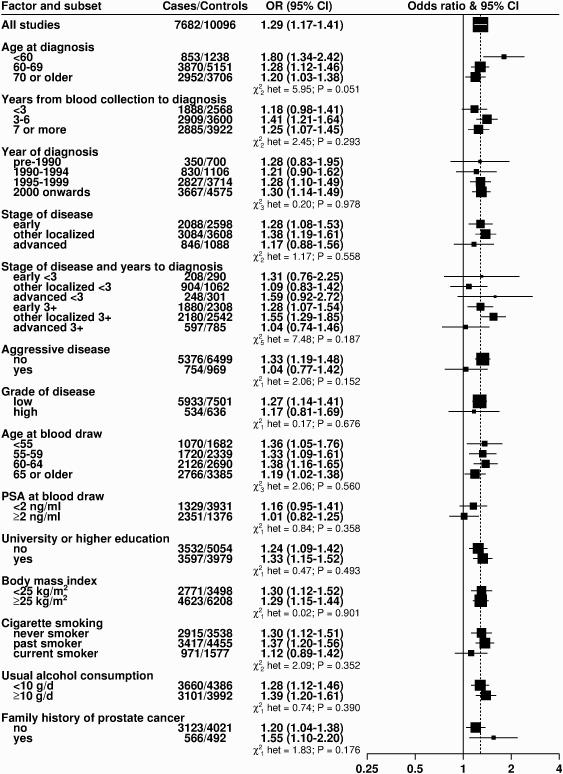
Figure 3. Odds ratios (95% confidence intervals) for prostate cancer associated with an 80 percentile increase in IGF-I in prospective studies, subdivided by various factors.
Estimates are from logistic regression conditioned on the matching variables within each study and without mutual adjustment for the other analytes. Odds ratios are for risk of prostate cancer overall, unless otherwise specified. Tests for heterogeneity for case-defined factors were obtained by fitting separate models for each subgroup and assuming independence of the ORs using a method analogous to a meta-analysis Tests for heterogeneity for non-case defined factors were assessed with a χ2-test of interaction between subgroup and the continuous trend test variable.
Concentrations of IGF-I and IGFBP-3 were positively associated with risk in a linear dose-response relationship (Ptrend <0.001, Figure 1 and Supplementary Table S5). However, there was evidence of heterogeneity in the linear trend in risk between the prospective and cross-sectional studies (i.e. ProtecT studies) for both analytes (Figure 2 and Supplementary Figure S4; Pheterogeneity by study design ≤0.03). The OR for men in the highest versus the lowest fifth of IGF-I for all studies combined was 1.21 (95% CI 1.11-1.31), but was 1.29 (1.16-1.43) when restricted to the prospective studies, with no evidence of heterogeneity between the prospective studies. There was no association with IGF-I in the ProtecT cross-sectional studies (1.06, 0.92-1.23). For IGFBP-3, the OR for prostate cancer in the highest fifth was 1.45 (1.32-1.59) for all studies combined (Pheterogeneity by study design <0.001). When restricted to prospective studies only, the corresponding OR was 1.25 (1.12-1.40), with no evidence of heterogeneity between studies. In addition, there was no evidence of statistical heterogeneity in the linear associations with IGF-I or IGFBP-3 by other factors such as time-to-diagnosis and stage and grade of disease (Pheterogeneity for both analytes all ≥0.05, Figure 3 and Supplementary Figure S1). The only exception was for age at diagnosis, for which there was some borderline significant weakening of the linear trend of IGF-I with risk by increasing age at diagnosis; with a stronger association for men diagnosed before the age of 60 years (OR for 80 percentile increase in IGF-I = 1.80, 1.34-2.42, Pheterogeneity/trend by age at diagnosis = 0.05/0.04, Figure 3). We also assessed overall risk for prostate cancer in relation to deciles of IGF-I and IGFBP-3 concentrations for the prospective studies: results were consistent with a linear trend for both analytes (Ptrend <0.001 for both, Supplementary Table S6). The risk in the highest versus lowest decile was 1.43 (1.24-1.65) for IGF-I and 1.39 (1.19-1.63) for IGFBP-3.
IGF-II concentration was positively associated with prostate cancer risk (OR in highest versus lowest fifth = 1.29 [95% CI 1.14-1.46], Ptrend <0.001, Figure 1); however, there was heterogeneity in the association with risk between all studies combined (Pheterogeneity = 0.005, Supplementary Figure S1), which persisted when the analyses were restricted to prospective studies only (Pheterogeneity = 0.02). There was also some evidence for heterogeneity in the association of IGF-I and prostate cancer risk by grade of disease (Pheterogeneity by grade = 0.03); the OR associated with an 80% increase in IGF-II was 1.19 (0.99-1.43) for low-intermediate grade disease and 0.49 (0.23-1.05) for high-grade disease (Supplementary Figure S7).
The risk of prostate cancer was lower for men with the highest levels of IGFBP-1 concentration (OR for highest versus lowest fifth = 0.81, 95% CI 0.68-0.96, Ptrend = 0.05). There was no evidence of heterogeneity between studies (data were available for IGFBP-1 from prospective studies only, Pheterogeneity = 0.9, Supplementary Figure S2) or by any of the participant or tumour characteristics (Supplementary Figure S6, Pheterogeneity for all ≥0.19).
IGFBP-2 was associated with an increased risk of prostate cancer with an OR for the highest versus lowest fifth of 1.17, 95% CI 1.03-1.32 (Ptrend = 0.005). However, there was evidence of heterogeneity among all studies (Pheterogeneity = 0.03, Supplementary Figure S3) and also borderline heterogeneity among both prospective and cross-sectional studies (Pheterogeneity ≤0.07), with a significantly elevated risk in the PCPT study but no association in the other prospective studies. Correspondingly, there was some evidence of a difference in trends in risk with IGFBP-2 concentration by tumour stage at diagnosis and by PSA at blood draw, as well as by BMI (Pheterogeneity ≤0.02, Supplementary Figure S10).
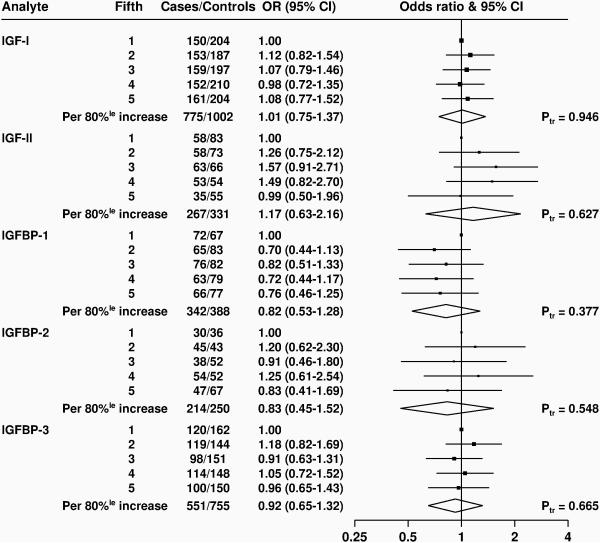
Figure 4 and Supplementary Figure S13 show analyses of the risk for aggressive prostate cancer in study-specific fifths of concentration for all IGF analytes; there were no statistically significant associations with any of the analytes, although there were relatively few cases with aggressive disease, with fewer than 350 aggressive cases with data on IGF-II, IGFBP-1 or IGFBP-2
Figure 4. Odds ratios (95% confidence intervals) for aggressive prostate cancer associated with study-specific fifths of concentrations of selected insulin-like growth factors and their binding proteins.
Estimates are from logistic regression conditioned on the matching variables within each study and without mutual adjustment for the other analytes.
Adjustment for confounders and mutual adjustment for other biomarkers
Adjustment for potential confounders made no appreciable difference to the associations with prostate cancer risk for any of the analytes (Supplementary Figures S14-S15). Additional adjustment for family history of prostate cancer in the studies for which data were available also made no material difference to the odds ratios. In the prospective studies, after mutual adjustment for each of the other IGF analytes and testosterone and SHBG separately, IGF-I remained associated with prostate cancer, whereas after adjustment for IGF-I, only the association of IGFBP-2 with risk remained (Supplementary Table S7). After adjustment for IGF-I, the association with risk for an 80 percentile increase in analyte concentration was 1.09 (95% CI 0.91-1.30) for IGF-II, 0.90 (0.76-1.06) for IGFBP-1, 1.35 (1.13-1.62) for IGFBP-2, and 1.09 (0.96-1.24) for IGFBP-3. After adjustment for IGF-II, IGFBP-1, IGFBP-2 and IGFBP-3, the ORs for an 80 percentile difference of IGF-I were 1.19 (1.00-1.42), 1.33 (1.11-1.58), 1.36 (1.13-1.63) and 1.18 (1.04-1.33), respectively.
The molar ratio of IGF-I to IGFBP-3 was not associated with prostate cancer risk (OR for an 80 percentile increase was 1.02, 95% CI 0.92-1.13, Ptrend = 0.67; data not shown).
The joint effects of IGF-I and IGFBP-3 in relation to prostate cancer risk
We also examined the joint effects of IGF-I and IGFBP-3 in relation to prostate cancer and found no interaction (Pinteraction = 0.6) (Supplementary Table S8).
Discussion
The results of this large collaborative analysis of individual participant data confirm moderate positive associations between prediagnostic circulating concentrations of IGF-I and prostate cancer risk. However, there was evidence of heterogeneity in the association between prospective and cross-sectional studies and, when ProtecT was excluded, men with high IGF-I concentrations had a 29% higher risk compared to those with low concentrations. These analyses include nearly all (>98%) of the published worldwide prospective data on IGFs and prostate cancer risk. The results from one small nested case-control study of IGF-I and IGFBP-3 in relation to prostate cancer risk (96 cases and 416 matched controls, OR for IGF-I = 1.26, 95% CI 0.66-2.41; OR for IGFBP-3 = 1.35, 0.15-6.59), which were unavailable for this re-analysis are compatible with our findings and their inclusion would not have materially altered our summary relative risk estimates (25). The large numbers of cases and corresponding matched controls make it possible not only to estimate prostate cancer risk associated with IGFs with greater precision but also to examine risks at extremes of the distribution. Results from an analysis of risk in relation to deciles of IGF-I and IGFBP-3 provide no suggestion that the association is anything but linear. In the prospective studies, risk for prostate cancer was approximately 40% higher in men with IGF-I or IGFBP-3 concentrations in the highest tenth of the distribution than in men with concentrations in the lowest tenth.
One goal of the collaborative group is to assemble sufficient data to examine associations between IGF levels and prostate cancer risk by tumour subtype, with the differences in study designs (prospective versus cross-sectional studies) and case mix (predominantly due to differences in PSA-testing leading to different proportions of advanced and high-grade disease across the studies) potentially providing useful insights into the role of IGFs in prostate cancer development. It has previously been suggested that the null findings from three large studies with predominantly screen-detected early disease (ERSPC, PCPT and ProtecT) (2, 3, 11) indicate that circulating IGF-I might not be associated with very early stage screen-detected disease and might instead be important for the progression of the disease. Evidence from a large study of protein expression in prostate tumour tissue has also suggested that activation of the IGF-pathway (either through increased IGF-IR expression or the loss/inactivation of PTEN and consequent constitutive activation of the IGF-I/PI3K/Akt pathway) is associated with progression of prostate cancer to lethal disease (26). However, it may be that for the subset of tumours with PTEN loss, because the IGF/PI3K/Akt pathway is constitutively activated, the actual concentration of circulating IGF-I may be less important for prostate cancer risk than for tumours without reduced/absent PTEN expression. In this large collaborative analysis, with more than three times as many cases as the previous collaborative analysis (1), we found no evidence that the associations of IGF-I with incident prostate cancer differed markedly by tumour stage and grade, although neither the association of IGF-I nor any other analyte with risk for aggressive disease was statistically significant. These findings suggest that IGF-I may have a role not just in the growth and progression of existing prostate tumours but also in the earlier stages of tumour development. There was some evidence that the association of IGF-I with prostate cancer risk was stronger for men diagnosed at an earlier age but this may be a chance finding given the many significance tests conducted.
The interpretation of our current findings for low or intermediate grade and localised prostate cancer is challenging as these will include both screen-detected and clinically detected tumours, and some that will never progress and some that, with time, will progress to become aggressive disease that is difficult to treat. Because of the large size of the collaboration, we were able to examine early T1 (screen-detected) localised disease from other localised (T2) disease and found an association of IGF-I with risk for both localised subtypes, further suggesting that IGF-I is not just a marker of early progression (2). We also found that the association of IGF-I with risk did not differ by time from blood collection to diagnosis, suggesting that the existence of pre-clinical tumours at blood draw, and hence reverse causality, is unlikely to explain the association of IGF-I with risk or the heterogeneity by study design.
The role of IGFs in prostate carcinogenesis is given some support by results from experimental studies, which identified a number of cancer promoting properties of IGF-I, including mitotic and anti-apoptotic effects (27) and by findings from an agnostic pathway analysis in a large study of common prostate cancer susceptibility polymorphisms that identified the IGF pathway as being related to prostate cancer risk (28). However, there may be other explanations for the apparent associations between IGFs and risk. The relationship between circulating IGFs and benign prostatic hypertrophy (BPH) is not well-established (29-31) , but should higher IGF-I concentrations be associated with an increased risk of BPH then the apparent association of IGF-I with prostate cancer risk might be partly due to increased detection of tumours among men undergoing examinations and PSA-testing because of the symptoms associated with BPH. To understand fully this potential confounding by BPH, more large prospective studies of IGFs and BPH are required. Residual confounding by other factors is unlikely to explain the results given the few established risk factors for prostate cancer and the similar results from the multivariable model after adjusting for a range of potential confounders.
The majority of circulating IGF-I (99%) is bound to IGFBPs (32). The largest fraction of IGF-I is bound to IGFBP-3, which is also strongly positively associated with prostate cancer risk in the current analysis. This finding is difficult to interpret with respect to the possible independent role, if any, of IGFBP-3 in prostate cancer aetiology because of the complex interrelationships between the IGF-axis analytes. It has been suggested both that elevated IGBP-3 levels may have adverse effects because of its role in prolonging the half-life of IGF-I in serum (33) and that IGFBP-3 might influence risk via IGF-I independent mechanisms (34). However, after mutual adjustment of IGF-I and IGFBP-3 in our analyses, only associations of IGF-I with prostate cancer remained. Given the moderate inter-correlations, it is possible that this mutual adjustment may represent statistical over-adjustment (2, 35) and equally in terms of explanatory biological pathways, adjusting IGFBP-3 for IGF-I may represent over-adjustment if the main effect of IGFBP-3 on risk is via its regulation of IGF-I levels in the circulation. Nonetheless, taken at face value our results suggest that association of IGFBP-3 and risk for prostate cancer may be simply due to its correlation with IGF-I.
There has been interest in the possible role of IGF-II in prostate carcinogenesis because, like IGF-I, IGF-II functions as a growth factor. Results from individual prospective studies have been generally null (3, 7, 16, 21), but interest in the role of IGF-2 has been reactivated by the finding of a higher risk for prostate cancer in men with high circulating IGF-2 concentration in the ProtecT study (2) and the identification of a common prostate cancer susceptibility allele in the region of the IGF-II gene (36). Based on over 5000 cases, our findings suggest a moderate association of IGF-II with prostate cancer risk but interpretation of these findings is difficult because of the heterogeneity in the association between individual studies. Our findings also suggest that raised IGF-II levels may be associated with an increased risk of PSA-screen detected disease, with the strongest associations being observed among men who had a high PSA at baseline, who were diagnosed with low-intermediate grade disease and who were diagnosed after the introduction of PSA-testing. However, many of the studies with IGF-II measurements were small (7 had IGF-II measurements on fewer than 250 men with prostate cancer) and the variation between studies and by grade may be due to chance findings in individual studies. A number of studies have suggested that IGF-II levels may serve as a tumour marker rather than an aetiological risk factor, with circulating levels increasing as disease progresses, consistent with the loss of imprinting of the IGF-II gene during the development of the disease (37). In the current analyses, however, we found no evidence of reverse causality, with similar associations across different durations of follow-up. More data are required to investigate the role of IGF-II, if any, in the development of prostate cancer.
There are relatively few published data on circulating IGFBP-1 in relation to prostate cancer risk (16, 17) and this is the first report on findings from a collaborative analysis of individual participant data. The current analysis includes both published (16, 17) and unpublished (CLUE, EPIC, HPFS and PHS) data. IGFBP-1 binds with IGF-I in the circulation, though only to a relatively small proportion compared to IGFBP-3, and is only weakly negatively correlated with IGF-I levels. It has been hypothesised that IGFBP-1 has a role in fine-tuning the availability of IGF-I to tissues because IGFBP-1 binds IGF-I with a higher affinity than that of the IGF-I receptor and reduces free IGF-I levels resulting in the inhibition of IGF-I receptor signalling (38). Our finding of a possible reduction in risk of prostate cancer in men with high IGFBP-1 concentrations is of particular interest given IGFBP-1 levels vary substantially in response to diet and obesity (39, 40), and may therefore be a modifiable risk factor. However, the association with IGFBP-1 was somewhat attenuated and no longer statistically significant after adjustment for IGF-I.
IGFBP-2 has been proposed as a mediator of the positive association between adiposity and risk for aggressive prostate cancer observed in several studies (41), while experimental evidence has suggested a role for IGFBP-2 in the prevention of obesity and regulation of glucose metabolism (42). It has also been suggested though that circulating IGFBP-2 might be a prostate tumour marker; several case-control studies have found circulating concentrations to be elevated in men diagnosed with prostate cancer and to increase as prostate cancer progresses (43), and IGFBP-2 can inactivate PTEN (44) and in a reciprocal manner PTEN can negatively regulate IGFBP-2 expression which thus may serve as a potential serum biomarker of PTEN status (45). While the overall positive association in the current study is consistent with this hypothesis of IGFBP2 being a tumour marker, there was no evidence that the IGFBP-2 risk association was more pronounced in men with advanced or high-grade disease or in men diagnosed soon after blood collection, and no material difference in the risk associations after adjustment for BMI. Rather, the risk association was stronger among men with early disease and varied by BMI, with an inverse association in men with a normal BMI and a positive association in men with a high BMI. However, interpretation of our results for IGFBP-2 is difficult because of the heterogeneity in findings between studies (which in part at least is likely attributable to differences in study design between the contributing studies) in that the null findings from case-control studies nested within population-based cohort studies contrast with a strong positive association from the PCPT study, a large case-control study nested in a randomised trial in which the mean BMI was relatively high (27.6 kg/m2 for controls) and the majority of cases were low-grade and localised tumours diagnosed through a routine end of study biopsy on average 7 years after blood collection.
Variation in circulating IGF concentrations between the studies contributing to this collaborative analysis may be partly due to differences in assay methodology, as well to differences in the other factors including the characteristics of the participants and the blood samples, although the majority of assays were conducted using immunoassays from one company (Diagnostic Systems Laboratories, Webster, Texas) and were enzyme-linked immunoassays (11 of 19 studies for IGF-I), conventional radioimmunoassays or immunoradiometric assays (as shown in Supplementary Table S2). However, any differences between assay methods are not expected to impact on the overall findings of these analyses because all comparisons were made within study using study-specific cut-points (46).
A potential limitation of these analyses is their reliance on a single measurement of IGF in each participant, the assumption being that the measurement of the IGF concentration in a single blood sample is a good indicator of levels of IGF in blood over the medium to long-term. Several studies with repeat samples collected up to 5 years apart have shown moderately good temporal reproducibility for IGF-I (correlations of 0.7 to 0.9) (18, 47, 48). Less is known about intra-individual variation in other IGF analytes (i.e. IGF-II, IGFBP-1, IGFBP-2 and IGFBP-3) over time but the limited published data suggest the reproducibility of these analytes may be similar to that for IGF-I; results from two studies with samples collected approximately 1 year apart reported correlations ranging from 0.6 to 0.9 (47). Intra-individual variation in IGF levels results in the observed association with prostate cancer risk being smaller than the true association. Given the intra-class correlation coefficients for IGF-I over 3 to 5 years of approximately 0.60 and an observed odds ratio of 1.29 for men in the highest compared to the lowest fifth of IGF-I, we estimate that men with the highest IGF-I levels may have an approximately 70% increase in risk for prostate cancer. With the lack of other established modifiable risk factors for prostate cancer and given the evidence that IGF-I levels are to an extent modifiable, being related for example to dietary intake of protein (49, 50), the IGF axis remains an important area for further research on prostate cancer.
In summary, the results of this collaborative pooled analysis of over ten thousand cases and thirteen thousand controls support the hypothesised role of IGF-I in the development of prostate cancer. Further data from studies of risk for aggressive prostate cancer are needed to confirm the associations of IGFs and IGFBPs with clinically relevant prostate cancer and its progression, and to help us better understand whether any of the observed associations are causal.
Supplementary Material
Acknowledgements
We thank the men who participated in the collaborating studies, the research staff, collaborating laboratories and funding agencies in each of the studies. We acknowledge the large contribution of the late Professor Dimitrios Trichopoulos to the study, including the design of the study, the acquisition and interpretation of data and the drafting of article, and his broad and insightful intellectual input to the study and the field of hormones and cancer.
Funding
Centralized pooling, checking and data analysis was supported by Cancer Research UK grants C8221/A19170 and C570/A11691. Details of funding for the original studies are in the relevant publications and in the Supplementary Methods section.
Abbreviations
- CI
confidence interval
- OR
odds ratio
Footnotes
Conflict of interest statement:
The authors disclose no potential conflicts of interest.
References
- 1.Roddam AW, Allen NE, Appleby P, Key TJ, Ferrucci L, Carter HB, et al. Insulin-like growth factors, their binding proteins, and prostate cancer risk: analysis of individual patient data from 12 prospective studies. Annals of Internal Medicine. 2008;149(7):461–471. doi: 10.7326/0003-4819-149-7-200810070-00006. W483-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowlands MA, Holly JM, Gunnell D, Donovan J, Lane JA, Hamdy F, et al. Circulating insulin-like growth factors and IGF-binding proteins in PSA-detected prostate cancer: the large case-control study ProtecT. Cancer Research. 2012;72(2):503–515. doi: 10.1158/0008-5472.CAN-11-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neuhouser ML, Platz EA, Till C, Tangen CM, Goodman PJ, Kristal A, et al. Insulin-like growth factors and insulin-like growth factor-binding proteins and prostate cancer risk: results from the prostate cancer prevention trial. Cancer Prev Res (Phila) 2013;6(2):91–99. doi: 10.1158/1940-6207.CAPR-12-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100(3):170–183. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodson K, Tangrea JA, Pollak M, Copeland TD, Taylor PR, Virtamo J, et al. Serum insulin-like growth factor I: tumor marker or etiologic factor? A prospective study of prostate cancer among Finnish men. Cancer Research. 2003;63(14):3991–3994. [PubMed] [Google Scholar]
- 6.Harman SM, Metter EJ, Blackman MR, Landis PK, Carter HB. Serum levels of insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-3, and prostate-specific antigen as predictors of clinical prostate cancer. The Journal of Clinical Endocrinology and Metabolism. 2000;85(11):4258–4265. doi: 10.1210/jcem.85.11.6990. [DOI] [PubMed] [Google Scholar]
- 7.Morris JK, George LM, Wu T, Wald NJ. Insulin-like growth factors and cancer: no role in screening. Evidence from the BUPA study and meta-analysis of prospective epidemiological studies. British Journal of Cancer. 2006;95(1):112–117. doi: 10.1038/sj.bjc.6603200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C, Lewis SK, Voigt L, Fitzpatrick A, Plymate SR, Weiss NS. Prostate carcinoma incidence in relation to prediagnostic circulating levels of insulin-like growth factor I, insulin-like growth factor binding protein 3, and insulin. Cancer. 2005;103(1):76–84. doi: 10.1002/cncr.20727. [DOI] [PubMed] [Google Scholar]
- 9.Lacey JV, Jr., Hsing AW, Fillmore CM, Hoffman S, Helzlsouer KJ, Comstock GW. Null association between insulin-like growth factors, insulin-like growth factor-binding proteins, and prostate cancer in a prospective study. Cancer Epidemiol Biomarkers Prev. 2001;10(10):1101–1102. [PubMed] [Google Scholar]
- 10.Allen NE, Key TJ, Appleby PN, Travis RC, Roddam AW, Rinaldi S, et al. Serum insulin-like growth factor (IGF)-I and IGF-binding protein-3 concentrations and prostate cancer risk: results from the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1121–1127. doi: 10.1158/1055-9965.EPI-06-1062. [DOI] [PubMed] [Google Scholar]
- 11.Janssen JA, Wildhagen MF, Ito K, Blijenberg BG, Van Schaik RH, Roobol MJ, et al. Circulating free insulin-like growth factor (IGF)-I, total IGF-I, and IGF binding protein-3 levels do not predict the future risk to develop prostate cancer: results of a case-control study involving 201 patients within a population-based screening with a 4-year interval. The Journal of Clinical Endocrinology and Metabolism. 2004;89(9):4391–4396. doi: 10.1210/jc.2004-0232. [DOI] [PubMed] [Google Scholar]
- 12.Nimptsch K, Platz EA, Pollak MN, Kenfield SA, Stampfer MJ, Willett WC, et al. Plasma insulin-like growth factor 1 is positively associated with low-grade prostate cancer in the Health Professionals Follow-up Study 1993-2004. International Journal of Cancer. 2011;128(3):660–667. doi: 10.1002/ijc.25381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pham TM, Fujino Y, Nakachi K, Suzuki K, Ito Y, Watanabe Y, et al. Relationship between serum levels of insulin-like growth factors and subsequent risk of cancer mortality: findings from a nested case-control study within the Japan Collaborative Cohort Study. Cancer Epidemiology. 2010;34(3):279–284. doi: 10.1016/j.canep.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Schaefer C, Friedman GD, Quesenberry CP, Jr., Orentreich N, Vogelman JH. IGF-I and Prostate Cancer. Science. 1998;282:199a. [Google Scholar]
- 15.Severi G, Morris HA, MacInnis RJ, English DR, Tilley WD, Hopper JL, et al. Circulating insulin-like growth factor-I and binding protein-3 and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1137–1141. doi: 10.1158/1055-9965.EPI-05-0823. [DOI] [PubMed] [Google Scholar]
- 16.Gill JK, Wilkens LR, Pollak MN, Stanczyk FZ, Kolonel LN. Androgens, growth factors, and risk of prostate cancer: the Multiethnic Cohort. The Prostate. 2010;70(8):906–915. doi: 10.1002/pros.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stattin P, Bylund A, Rinaldi S, Biessy C, Dechaud H, Stenman UH, et al. Plasma insulin-like growth factor-I, insulin-like growth factor-binding proteins, and prostate cancer risk: a prospective study. J Natl Cancer Inst. 2000;92(23):1910–1917. doi: 10.1093/jnci/92.23.1910. [DOI] [PubMed] [Google Scholar]
- 18.Chan JM, Stampfer MJ, Ma J, Gann P, Gaziano JM, Pollak M, et al. Insulin-like growth factor-I (IGF-I) and IGF binding protein-3 as predictors of advanced-stage prostate cancer. J Natl Cancer Inst. 2002;94(14):1099–1106. doi: 10.1093/jnci/94.14.1099. [DOI] [PubMed] [Google Scholar]
- 19.Weiss JM, Huang WY, Rinaldi S, Fears TR, Chatterjee N, Chia D, et al. IGF-1 and IGFBP-3: Risk of prostate cancer among men in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. International Journal of Cancer. 2007;121(10):2267–2273. doi: 10.1002/ijc.22921. [DOI] [PubMed] [Google Scholar]
- 20.Oliver SE, Gunnell D, Donovan J, Peters TJ, Persad R, Gillatt D, et al. Screen-detected prostate cancer and the insulin-like growth factor axis: results of a population-based case-control study. International Journal of Cancer. 2004;108(6):887–892. doi: 10.1002/ijc.11631. [DOI] [PubMed] [Google Scholar]
- 21.Meyer F, Galan P, Douville P, Bairati I, Kegle P, Bertrais S, et al. A prospective study of the insulin-like growth factor axis in relation with prostate cancer in the SU.VI.MAX trial. Cancer Epidemiol Biomarkers Prev. 2005;14(9):2269–2272. doi: 10.1158/1055-9965.EPI-05-0303. [DOI] [PubMed] [Google Scholar]
- 22.Finne P, Fallah M, Hakama M, Ciatto S, Hugosson J, de Koning H, et al. Lead-time in the European Randomised Study of Screening for Prostate Cancer. Eur J Cancer. 2010;46(17):3102–3108. doi: 10.1016/j.ejca.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 23.Crowe FL, Appleby PN, Travis RC, Barnett M, Brasky TM, Bueno-de-Mesquita HB, et al. Circulating fatty acids and prostate cancer risk: individual participant meta-analysis of prospective studies. J Natl Cancer Inst. 2014;106(9) doi: 10.1093/jnci/dju240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Key T, Appleby P, Barnes I, Reeves G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 25.Borugian MJ, Spinelli JJ, Sun Z, Kolonel LN, Oakley-Girvan I, Pollak MD, et al. Prostate cancer risk in relation to insulin-like growth factor (IGF)-I and IGF-binding protein-3: a prospective multiethnic study. Cancer Epidemiol Biomarkers Prev. 2008;17(1):252–254. doi: 10.1158/1055-9965.EPI-07-2694. [DOI] [PubMed] [Google Scholar]
- 26.Zu K, Martin NE, Fiorentino M, Flavin R, Lis RT, Sinnott JA, et al. Protein Expression of PTEN, Insulin-Like Growth Factor I Receptor (IGF-IR), and Lethal Prostate Cancer: A Prospective Study. Cancer Epidemiol Biomarkers Prev. 2013;22(11):1984–1993. doi: 10.1158/1055-9965.EPI-13-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grimberg A. Mechanisms by which IGF-I may promote cancer. Cancer Biology & Therapy. 2003;2(6):630–635. [PMC free article] [PubMed] [Google Scholar]
- 28.Eeles RA, Olama AA, Benlloch S, Saunders EJ, Leongamornlert DA, Tymrakiewicz M, et al. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nature Genetics. 2013;45(4):385–391. doi: 10.1038/ng.2560. 391e381-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chokkalingam AP, Gao YT, Deng J, Stanczyk FZ, Sesterhenn IA, Mostofi FK, et al. Insulin-like growth factors and risk of benign prostatic hyperplasia. The Prostate. 2002;52(2):98–105. doi: 10.1002/pros.10096. [DOI] [PubMed] [Google Scholar]
- 30.Neuhouser ML, Schenk J, Song YJ, Tangen CM, Goodman PJ, Pollak M, et al. Insulin-like growth factor-I, insulin-like growth factor binding protein-3 and risk of benign prostate hyperplasia in the prostate cancer prevention trial. The Prostate. 2008;68(13):1477–1486. doi: 10.1002/pros.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantzoros CS, Tzonou A, Signorello LB, Stampfer M, Trichopoulos D, Adami HO. Insulin-like growth factor 1 in relation to prostate cancer and benign prostatic hyperplasia. British Journal of Cancer. 1997;76(9):1115–1118. doi: 10.1038/bjc.1997.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocrine Reviews. 1995;16(1):3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 33.Guler HP, Zapf J, Schmid C, Froesch ER. Insulin-like growth factors I and II in healthy man. Estimations of half-lives and production rates. Acta Endocrinologica. 1989;121(6):753–758. doi: 10.1530/acta.0.1210753. [DOI] [PubMed] [Google Scholar]
- 34.Yamada PM, Lee KW. Perspectives in mammalian IGFBP-3 biology: local vs. systemic action. American Journal of Physiology - Cell Physiology. 2009;296(5):C954–976. doi: 10.1152/ajpcell.00598.2008. [DOI] [PubMed] [Google Scholar]
- 35.Cole SR, Platt RW, Schisterman EF, Chu H, Westreich D, Richardson D, et al. Illustrating bias due to conditioning on a collider. International Journal of Epidemiology. 2010;39(2):417–420. doi: 10.1093/ije/dyp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eeles RA, Kote-Jarai Z, Al Olama AA, Giles GG, Guy M, Severi G, et al. Identification of seven new prostate cancer susceptibility loci through a genome-wide association study. Nature Genetics. 2009;41(10):1116–1121. doi: 10.1038/ng.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribarska T, Bastian KM, Koch A, Schulz WA. Specific changes in the expression of imprinted genes in prostate cancer--implications for cancer progression and epigenetic regulation. Asian Journal of Andrology. 2012;14(3):436–450. doi: 10.1038/aja.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wheatcroft SB, Kearney MT. IGF-dependent and IGF-independent actions of IGF-binding protein-1 and -2: implications for metabolic homeostasis. Trends in Endocrinology and Metabolism. 2009;20(4):153–162. doi: 10.1016/j.tem.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Katz LE, DeLeon DD, Zhao H, Jawad AF. Free and total insulin-like growth factor (IGF)-I levels decline during fasting: relationships with insulin and IGF-binding protein-1. The Journal of Clinical Endocrinology and Metabolism. 2002;87(6):2978–2983. doi: 10.1210/jcem.87.6.8601. [DOI] [PubMed] [Google Scholar]
- 40.Ngo TH, Barnard RJ, Tymchuk CN, Cohen P, Aronson WJ. Effect of diet and exercise on serum insulin, IGF-I, and IGFBP-1 levels and growth of LNCaP cells in vitro (United States) Cancer Causes & Control. 2002;13(10):929–935. doi: 10.1023/a:1021911517010. [DOI] [PubMed] [Google Scholar]
- 41.Rowlands MA, Holly JM, Gunnell D, Gilbert R, Donovan J, Lane JA, et al. The relation between adiposity throughout the life course and variation in IGFs and IGFBPs: evidence from the ProtecT (Prostate testing for cancer and Treatment) study. Cancer Causes & Control. 2010;21(11):1829–1842. doi: 10.1007/s10552-010-9610-x. [DOI] [PubMed] [Google Scholar]
- 42.Wheatcroft SB, Kearney MT, Shah AM, Ezzat VA, Miell JR, Modo M, et al. IGF-binding protein-2 protects against the development of obesity and insulin resistance. Diabetes. 2007;56(2):285–294. doi: 10.2337/db06-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoeflich A, Reisinger R, Lahm H, Kiess W, Blum WF, Kolb HJ, et al. Insulin-like growth factor-binding protein 2 in tumorigenesis: protector or promoter? Cancer Research. 2001;61(24):8601–8610. [PubMed] [Google Scholar]
- 44.Uzoh CC, Holly JM, Biernacka KM, Persad RA, Bahl A, Gillatt D, et al. Insulin-like growth factor-binding protein-2 promotes prostate cancer cell growth via IGF-dependent or -independent mechanisms and reduces the efficacy of docetaxel. British Journal of Cancer. 2011;104(10):1587–1593. doi: 10.1038/bjc.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehrian-Shai R, Chen CD, Shi T, Horvath S, Nelson SF, Reichardt JK, et al. Insulin growth factor-binding protein 2 is a candidate biomarker for PTEN status and PI3K/Akt pathway activation in glioblastoma and prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(13):5563–5568. doi: 10.1073/pnas.0609139104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Key TJ, Appleby PN, Allen NE, Reeves GK. Pooling biomarker data from different studies of disease risk, with a focus on endogenous hormones. Cancer Epidemiol Biomarkers Prev. 2010;19(4):960–965. doi: 10.1158/1055-9965.EPI-10-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muti P, Quattrin T, Grant BJ, Krogh V, Micheli A, Schunemann HJ, et al. Fasting glucose is a risk factor for breast cancer: a prospective study. Cancer Epidemiol Biomarkers Prev. 2002;11(11):1361–1368. [PubMed] [Google Scholar]
- 48.Kaaks R, Toniolo P, Akhmedkhanov A, Lukanova A, Biessy C, Dechaud H, et al. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst. 2000;92(19):1592–1600. doi: 10.1093/jnci/92.19.1592. [DOI] [PubMed] [Google Scholar]
- 49.Crowe FL, Key TJ, Allen NE, Appleby PN, Roddam A, Overvad K, et al. The association between diet and serum concentrations of IGF-I, IGFBP-1, IGFBP-2, and IGFBP-3 in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1333–1340. doi: 10.1158/1055-9965.EPI-08-0781. [DOI] [PubMed] [Google Scholar]
- 50.Young NJ, Metcalfe C, Gunnell D, Rowlands MA, Lane JA, Gilbert R, et al. A cross-sectional analysis of the association between diet and insulin-like growth factor (IGF)-I, IGF-II, IGF-binding protein (IGFBP)-2, and IGFBP-3 in men in the United Kingdom. Cancer Causes & Control. 2012;23(6):907–917. doi: 10.1007/s10552-012-9961-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.